Abstract
Fear of cancer recurrence (FCR) is a common and persistent concern among breast cancer survivors. Little is known about factors associated with FCR in women with ductal carcinoma in situ (DCIS) or early invasive breast cancer (EIBC). Women with first primary DCIS, or stages I–IIA breast cancer were prospectively enrolled in a quality-of-life study and completed interviews at 4–6 weeks, 6 months, and 2 years after definitive surgical treatment. In three stepwise multivariable linear regression models, including both time-independent and time-varying variables measured at each respective interview, we identified independent correlates of mean FCR scores (range 1–6) using four items from the Concern About Recurrence Scale (CARS) at 2-year follow-up. Of 506 disease-free patients at 2-year follow-up (mean [SD] age, 58 [10] years; 81% White; 34% DCIS), the average FCR score of 2.0 was low. However, 145 (29%) reported moderate-to-high levels of FCR (scores 3.0–6.0). All three models showed that younger age, stage IIA breast cancer (vs. DCIS), lower social support, and elevated anxiety were consistently associated with higher FCR at 2-year follow-up (each P < 0.05; final models R2 = 0.25–0.32). DCIS patients reported lower FCR than stage IIA patients (each P ≤ 0.01) but had similar FCR as stage I patients. Although mean FCR was low, 29% of DCIS and EIBC survivors reported moderate-to-high levels of FCR at 2-year follow-up. Management of anxiety, provision of social support, and patient education may help reduce FCR among DCIS and EIBC survivors, especially among younger survivors.
Keywords: Breast cancer, Ductal carcinoma in situ, Cancer risk perception, Fear of cancer recurrence, Anxiety, Social support
Introduction
Widespread utilization of mammography for routine breast cancer screening and increased use of adjuvant therapies have led to a growing number of breast cancer survivors during the past two decades, especially among women with ductal carcinoma in situ (DCIS) and early-invasive breast cancer (EIBC). Most women with early-stage breast cancer have a good overall health-related quality of life after completion of treatment [1, 2]. However, fear of cancer recurrence (FCR), defined as the fear or worry that cancer will come back in the same organ or spread to another part of the body [3], is a common and persistent stressor reported by breast cancer survivors [3-7]. Among cancer survivors who have completed cancer treatment, FCR is the most frequently endorsed unmet supportive care need [8].
FCR has been associated with psychological distress [3, 9, 10] and lower quality of life [4, 6]. In addition, FCR has been reported to influence patients’ decisions about surgical treatments and health behaviors. Specifically, breast cancer patients who were worried about developing recurrent disease were more likely to prefer mastectomy [11] and be hyper-vigilant about breast self-examination [12]. These findings highlight the importance of understanding factors associated with FCR in breast cancer survivors, especially in women with DCIS, who are at relatively low risk of recurrence but more likely than EIBC patients to overestimate their risk [13]. Breast cancer patients’ overestimation of their risk of recurrence was found to be associated with elevated anxiety [13, 14], and might also have an impact on adherence to treatment and to post-treatment surveillance [15].
However, little is known about factors associated with FCR in women with DCIS and EIBC. Among demographic and medical variables examined as predictors of FCR, only younger age at diagnosis was consistently associated with greater FCR [3-6, 16]. Few studies have evaluated psychosocial factors in relation to FCR among cancer survivors. Since low levels of social support have been associated with poor health outcomes in breast cancer patients [17, 18], perceived availability of social support may influence their emotional responses to breast cancer diagnosis and treatment.
Emotional distress is hypothesized to influence FCR [19], and higher levels of anxiety were found to be associated with higher perceived risk of recurrence among breast cancer survivors [14, 20-22]. In a previous analysis, we found that DCIS and EIBC patients reported similar levels of perceived risk of recurrence [14]. But DCIS patients were more likely than EIBC patients to overestimate their risk [13], although DCIS patients generally have a lower risk of recurrence after treatment. According to Protection Motivation Theory, risk perceptions may increase both the level of fear arousal and the likelihood of performing protective health behaviors [23]. However, it remains unknown whether the accuracy of perceived risk of recurrence is related to FCR among women with DCIS or EIBC and whether their FCR differs by cancer stage. Therefore, we sought to identify psychosocial, demographic, disease-related, and treatment-related correlates of FCR in a cohort of women diagnosed with DCIS and EIBC.
Methods
Patients and procedure
Data were collected as part of a longitudinal quality-of-life study in women with a pathologically confirmed first primary diagnosis of DCIS or EIBC (stages I or IIA). The sample included newly diagnosed patients between October 2003 and June 2007 at the Siteman Cancer Center at Barnes-Jewish Hospital and Washington University School of Medicine and at Saint Louis University School of Medicine, both in St. Louis, Missouri. Patients were eligible for the study if they were English-speaking, completed definitive surgical treatment, and were 40 years of age or older, as most cases of DCIS are diagnosed following routine screening mammography, and annual mammography is recommended for this age group [24]. Potential participants were excluded if they had a history of DCIS or invasive breast cancer, received neoadjuvant chemotherapy for locally advanced breast cancer, or demonstrated cognitive impairment based on weighted scores>10 on the Orientation-Memory-Concentration Test [25], which was administered to all women 65 years of age and older.
The Institutional Review Boards at Washington University and Saint Louis University Schools of Medicine approved the study. After obtaining informed consent, we abstracted patients’ breast cancer stage and treatment data from their medical records. Data for demographic and psychosocial variables of interest were collected using computer-assisted telephone interviews administered 4–6 weeks, 6 months, and 2 years following patients’ definitive surgery.
Measures
Fear of cancer recurrence was measured only at the 2-year follow-up interview using the first four items of the Concern About Recurrence Scale (CARS), which address frequency, consistency, intensity, and potential for upset caused by fear of breast cancer recurrence [3]. Items were coded on a 6-point scale, ranging from (1) “Not at all” to (6) “All the time.” A mean score measuring patients’ overall FCR (range 1–6) was computed. Higher scores indicate greater FCR (Cronbach’s alpha = 0.87), with scores rounded to 3 or 4 indicating moderate levels and scores rounded to 5 or 6 indicating high levels of FCR [3, 4].
Perceived availability of social support was measured using the Medical Outcomes Study Social Support Survey (Cronbach’s alpha = 0.97) [26]. Severity of symptoms of anxiety was measured with the validated Beck Anxiety Inventory® (BAI®; Cronbach’s alpha = 0.89) [27]. Elevated anxiety was defined by a total score ≥10 on the BAI®, a level indicative of a possible anxiety disorder [27]. The severity of depressive symptoms “during the past week” was measured using the validated Center for Epidemiologic Studies-Depression Scale (CES-D; Cronbach’s alpha = 0.76) [28]. Elevated depressive symptoms were defined as total scores on the CES-D of 16 or more, a level indicative of clinical depression [28]. Based on the literature [29] and surgeons’ anecdotal reports of patients’ complaints after surgery, we developed an eight-item measure of the severity of surgical side effects with higher scores (range 1–5) indicating more severe side effects related to breast surgery (Cronbach’s alpha = 0.84) [30]. Katz’s validated adaptation of the Charlson Comorbidity Index was used to measure the presence/history of several comorbid conditions [31, 32]. We also asked patients whether or not they had a family history of breast cancer in their first-degree relatives.
Patients’ perceived risk of breast cancer recurrence was measured using the question, “What do you think the chances are that you will have this disease again some-day?” [33]. Responses ranged from 0%, meaning they definitely will not develop recurrent disease, to 100%, meaning they definitely will develop recurrent disease. We defined “recurrence” broadly as a recurrence in the same breast or in other organs or a metachronous contralateral breast cancer. We categorized their responses into one of six groups: 0%, 1–9%, 10–24%, 25–49%, ≥50%, and uncertain.
To evaluate the accuracy of patients’ perceived risk of recurrence, a patient’s ‘actual’ risk of recurrence was calculated as described previously [13]. Briefly, for DCIS patients, the 10-year risk of recurrence, including local and distant recurrence and contralateral breast cancer, was estimated by type of surgical treatment based on results of randomized trials [34-38]. For EIBC patients, the 10-year estimated risk of local recurrence varied with type of surgical treatment, types of adjuvant therapy, and nodal status [39]. The 10-year risk of distant recurrence among EIBC patients was estimated using Adjuvant! Online, a web-based program that predicts 10-year risks of mortality and recurrence for EIBC patients [40]. The risks of contralateral breast cancer for EIBC patients were estimated based on the literature for patients with an intact contralateral breast [41] and patients who underwent bilateral mastectomy [42]. The estimated 10-year risk of recurrence was the sum of estimates of local recurrence, distant recurrence, and contralateral breast cancer. The Adjuvant!-derived proportional risk reductions were used to estimate the efficacy of adjuvant systemic therapy on all types of recurrence [40]. In a 20-year follow-up of EIBC patients, 89% of first recurrences had occurred within 10 years of diagnosis [43]; therefore our assumption that the estimated 10-year cumulative risk of recurrence in early-stage breast cancer approximates the estimated lifetime risk is likely valid.
Since receipt of adjuvant therapies was included in the calculation of a patient’s actual risk of recurrence and since 37–44% of patients who were supposed to receive chemotherapy and/or radiation therapy had not completed their therapy until 6 months after surgery, the accuracy of each patient’s perceived risk of recurrence was assessed at the 6-month and 2-year interviews. We contrasted patients’ perceived risk-of-recurrence categories with their respective calculated risk categories at the 6-month and 2-year follow-ups, creating four categories of accuracy of perceived risk: underestimated (perceived < calculated risk), accurate (patient’s perceived risk fell in the same category as her calculated risk), overestimated (perceived >calculated risk), or uncertain (patients reported not knowing their risk) [13].
Statistical analysis
The potential correlates of FCR that we evaluated in the study included age at diagnosis (continuous), race (white vs. non-white), education (high school or less vs. more than high school), marital status (married or partnered vs. non-married or non-partnered), family history of breast cancer among first-degree relatives, cancer stage (IIA, I vs. DCIS), type of breast surgery (BCS vs. mastectomy), chemotherapy, radiation therapy, adjuvant hormone therapy, accuracy of patients’ perceived risk of recurrence (underestimated, overestimated, uncertain vs. accurate), surgical side effects (continuous), comorbidity (score >0 vs. score = 0), perceived availability of social support (continuous), elevated anxiety (BAI® ≥ 10 vs. BAI® < 10), and elevated depressive symptoms (CES-D ≥ 16 vs. CES-D < 16).
Student’s t test was used to test for differences in FCR scores according to demographic, medical, and psychosocial characteristics. For each potential correlate, Cohen’s d effect size was computed for the standardized mean difference in FCR. Analysis of differences between multiple groups was performed using one-way analysis of variance with Dunnett’s post hoc test. Pearson correlation coefficients were used to measure the correlations between FCR and each of age at diagnosis, surgical side effects, and perceived availability of social support. Multivariable stepwise linear regression analyses were performed to identify the significant correlates of FCR, with an entry criterion of 0.20 and a stay criterion of 0.05. We developed three multivariable linear regression models, each including time-independent variables (e.g., age at diagnosis, race, education, cancer stage), time-varying variables (including surgical side effects, comorbidity, social support, elevated anxiety, and elevated depressive symptoms) measured at 4–6 weeks (Model 1), 6 months (Model 2), and 2 years (Model 3) after surgery, and accuracy of perceived risk of recurrence measured 6 months (Models 1 and 2) and 2 years (Model 3) after surgery. As cancer stage and patients’ accuracy of perceived risk of recurrence were the two predictor variables of primary interest, these two explanatory variables were forced into the regression models. The presence of multicollinearity in the regression analysis was indicated by a variance inflation factor >4 [44]. Analyses were performed using SAS version 9.12 (SAS Institute, Cary, NC). A two-tailed P value <0.05 was considered significant.
Results
In all, 772 patients meeting the inclusion criteria were identified prospectively using the medical record and surgical pathology reports. With permission from each patient’s treating physician, study recruitment letters and consent forms were mailed 2–3 weeks following a patient’s definitive surgical treatment; 587 patients consented to participate, but 38 were subsequently determined not to be eligible based on exclusion criteria. Thus, 549 (71%) eligible patients completed the first telephone interview a mean 6 weeks following definitive surgery. Participants and non-participants did not differ significantly by marital status (P = 0.07), pathological stage (P = 0.84), or type of surgery (P = 0.10). Compared with non-participants, participants were younger (58 years vs. 61 years, P = 0.01) and were more likely to be white (79% vs. 64%, P < 0.001). Of the 549 participants, 514 (94%) completed the 2-year follow-up interview at which time patients’ FCR was measured. Eight patients who experienced recurrent or contralateral breast cancer at a follow-up were excluded from the analysis.
Table 1 summarizes the characteristics of 506 women with DCIS or EIBC included in this study. Univariate analysis showed that patients who were diagnosed with stage IIA breast cancer, had completed chemotherapy, or had elevated anxiety or elevated depressive symptoms at baseline reported greater FCR 2 years after definitive surgery (each P < 0.01). Higher FCR scores were moderately correlated with younger age, more severe surgical side effects, and lower social support reported at baseline (Table 2).
Table 1.
Characteristics of 506 women with early-stage breast cancer and their univariate associations with fear of cancer recurrence measured 2 years after definitive breast surgery
| N (%) | Fear of recurrence |
|||
|---|---|---|---|---|
| Mean score (SD) |
P value | Effect size |
||
| Race | ||||
| White | 408 (80.6) | 2.05 (0.99) | ||
| Non-white | 98 (19.4) | 2.00 (1.35) | 0.71 | −0.04 |
| Marital status | ||||
| Married/partnered | 313 (61.9) | 2.09 (1.01) | ||
| Non-married/non-partnered | 193 (38.1) | 1.98 (1.15) | 0.26 | -0.10 |
| Education | ||||
| High school or less | 153 (30.2) | 2.15 (1.21) | ||
| More than high school | 353 (69.8) | 2.00 (1.00) | 0.18 | -0.14 |
| First-degree relative history of breast cancera | ||||
| No | 373 (75.8) | 2.07 (1.11) | ||
| Yes | 119 (24.2) | 1.95 (0.89) | 0.22 | −0.12 |
| Missing | 14 | |||
| Pathological stage of breast cancer | <0.001 | |||
| DCIS | 173 (34.2) | 1.95 (0.99) | ||
| Stage I | 258 (51.0) | 1.99 (1.03) | 0.90d | 0.04 |
| Stage IIA | 75 (14.8) | 2.47 (1.26) | <0.001d | 0.46 |
| Surgical treatment | ||||
| Mastectomy | 176 (34.8) | 2.12 (1.18) | ||
| Breast conserving surgery | 330 (65.2) | 2.01 (1.00) | 0.31 | −0.10 |
| Chemotherapyb | ||||
| No | 380 (75.1) | 1.95 (1.01) | ||
| Yes | 126 (24.9) | 2.33 (1.17) | <0.01 | 0.35 |
| Radiation therapyb | ||||
| No | 178 (35.2) | 2.04 (1.14) | ||
| Yes | 328 (64.8) | 2.05 (1.03) | 0.87 | 0.01 |
| Hormonal therapyb | ||||
| No | 233 (48.7) | 2.13 (1.13) | ||
| Yes | 245 (51.3) | 1.95 (0.96) | 0.06 | −0.17 |
| Missing | 28 | |||
| Comorbidity score | ||||
| 0 | 340 (67.2) | 2.06 (1.07) | ||
| >0 | 166 (32.8) | 2.02 (1.06) | 0.67 | −0.04 |
| Elevated anxiety (BAI® ≥ 10 in the past week)c | ||||
| No | 394 (77.9) | 1.85 (0.90) | ||
| Yes | 112 (22.1) | 2.72 (1.31) | <0.0001 | 0.77 |
| Elevated depressive symptoms (CES-D ≥ 16 in the past week)c | ||||
| No | 426 (84.2) | 1.88 (0.90) | ||
| Yes | 80 (15.8) | 2.90 (1.43) | <0.0001 | 0.85 |
| Accuracy of perceived risk of recurrenceb | <0.0001 | |||
| Accurate | 81 (16.5) | 2.06 (1.04) | ||
| Underestimated | 220 (44.9) | 1.86 (0.92) | 0.26e | −0.20 |
| Overestimated | 109 (22.2) | 2.37 (1.15) | 0.11e | 0.28 |
| Uncertain | 80 (16.3) | 1.92 (1.06) | 0.66e | −0.13 |
| Undetermined | 16 | |||
The variable was measured 1 year after definitive breast surgery
The data were collected 6 months after definitive breast surgery
The variable was measured at baseline (4–6 weeks after definitive breast surgery)
The P value of the post hoc test for the comparison with DCIS
The P value of the post hoc test for the comparison with ‘accurate’
Table 2.
Pearson correlations of fear of cancer recurrence measured 2 years after definitive breast surgery with age at diagnosis, surgical side effects, and perceived availability of social support, which were measured 4–6 weeks after definitive breast surgery, in 506 women with early-stage breast cancer
| Mean (range) | 1 | 2 | 3 | 4 | |
|---|---|---|---|---|---|
| 1. Fear of cancer recurrence | 2.0 (1.0–6.0) | 1.00 | −0.31* | −0.21* | 0.23* |
| 2. Age at diagnosis | 58.3 (40.0–89.0) | 1.00 | 0.03 | −0.33* | |
| 3. Perceived availability of social support | 4.5 (1.4–5.0) | 1.00 | −0.08 | ||
| 4. Surgical side effects | 1.69 (1.0–4.6) | 1.00 |
P < 0.0001
The average FCR score was low in our sample with a mean of 2.0 on a 1–6 scale (Table 2). However, 24.8% (123/506) of patients reported moderate levels, and 4% (22/506) reported high levels of FCR. Figure 1 shows the prevalence of moderate and high levels of FCR 2 years after surgery by cancer stage. Moderate-to-high FCR (range 3.0–6.0) was reported by 29.0% of DCIS patients, which was not significantly different from FCR reported by stage IIA patients (38.7%, P = 0.13) or stage I patients (26.0%, P = 0.50).
Fig. 1.
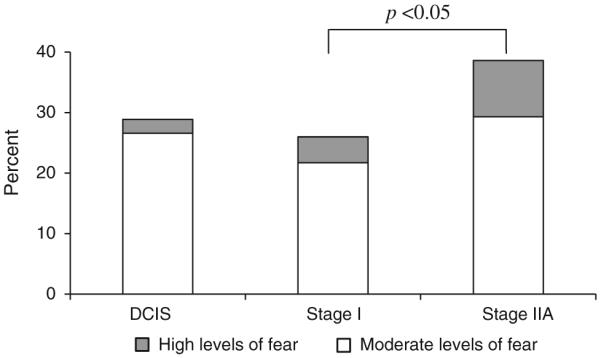
Prevalence of moderate and high levels of fear of cancer recurrence by cancer stage in women with ductal carcinoma in situ (DCIS) and early invasive breast cancer (stages I and IIA). Moderate levels of fear were defined as scores rounded to 3 or 4 and high levels were defined as scores rounded to 5 or 6 on the CARS [4, 5]. Overall chi-square P = 0.06
Results from the regression models are presented in Table 3. All three models showed that younger age, stage IIA breast cancer (vs. DCIS), lower social support, and elevated anxiety were consistently associated with higher FCR 2 years after diagnosis. When time-varying variables that were measured at baseline were included in the analysis, we found that more highly educated patients reported less FCR and patients who had elevated depressive symptoms at baseline reported higher FCR (Model 1). The effects of education and elevated depressive symptoms on FCR were not observed at the 6-month and 2-year follow-ups. BCS and more severe surgical side effects at 6-month and 2-year follow-ups were significantly correlated with higher FCR (Models 2 and 3), but were not correlated with FCR at baseline. Compared with patients who accurately perceived their risk of recurrence, patients who overestimated their risk of recurrence at the 2-year follow-up reported greater FCR, and patients who underestimated their risk of recurrence at the 2-year follow-up reported less FCR. Accuracy of perceived risk of recurrence was not a significant predictor of FCR in either Model 1 or Model 2. Variables retained in the three final models accounted for 25–32% of the variance of FCR.
Table 3.
Multivariable stepwise linear regression analyses of factors associated with fear of cancer recurrence in 506 women with early-stage breast cancer
| Model 1a |
Model 2b |
Model 3c |
||||
|---|---|---|---|---|---|---|
| Standardized β | P | Standardized β | P | Standardized β | P | |
| Age at diagnosis | −0.25 | <0.0001 −0.26 | <0.0001 −0.21 | <0.0001 | ||
| Education | – | – | ||||
| High school or less | Reference | |||||
| More than high school | −0.09 | 0.02 | ||||
| Cancer stage | ||||||
| DCIS | Reference | Reference | Reference | |||
| I | 0.04 | 0.44 | 0.05 | 0.29 | 0.04 | 0.33 |
| IIA | 0.14 | <0.01 | 0.14 | <0.01 | 0.11 | 0.01 |
| Type of surgery | – | |||||
| Mastectomy | Reference | Reference | ||||
| Breast-conserving surgery | 0.09 | 0.04 | 0.11 | <0.01 | ||
| Side effects of breast surgery | – | 0.22 | <0.0001 0.24 | <0.01 | ||
| Social support | −0.11 | <0.01 | −0.13 | <0.01 | −0.12 | <0.01 |
| Elevated anxiety (BAI® ≥ 10 in the past week) | 0.16 | <0.001 | 0.15 | <0.01 | 0.15 | <0.01 |
| Elevated depressive symptoms (CES-D ≥ 16 in the past week) 0.14 | <0.01 | – | – | |||
| Accuracy of perceived risk of recurrence | ||||||
| Accurate | Reference | Reference | Reference | |||
| Underestimated | −0.10 | 0.08 | −0.11 | 0.06 | −0.13 | 0.01 |
| Overestimated | 0.08 | 0.13 | 0.06 | 0.31 | 0.14 | <0.01 |
| Uncertain | −0.01 | 0.81 | 0.00 | 0.93 | 0.02 | 0.59 |
| Adjusted R2 | 0.25 | 0.27 | 0.32 | |||
The stepwise linear regression analysis included time-independent variables (age at diagnosis, race, education, marital status, family history of breast cancer among first-degree relatives, cancer stage, and type of breast surgery), time-varying variables (including surgical side effects, comorbidity, chemotherapy, radiation therapy, and adjuvant hormonal therapy, social support, elevated anxiety, and elevated depressive symptoms) that were measured at the baseline (4–6 weeks after definitive surgery) interview, and accuracy of perceived risk of recurrence evaluated at the 6-month follow-up interview
The analysis was similar as Model 1 except time-varying variables were measured at the 6-month follow-up interview
The analysis was similar as Model 1 except time-varying variables were measured at the 2-year follow-up interview
The variable was not retained in the final model
FCR was not associated with race, marital status, family history of breast cancer, comorbidity, or adjuvant therapy.
Discussion
Although mean FCR at 2-year follow-up was low in our sample of DCIS and EIBC survivors, 29% of participants expressed moderate-to-high levels of FCR. We observed a lower prevalence of moderate-to-high FCR among our participants than the prevalence of 46–85% reported previously in studies that included patients with more advanced disease an average 2–3 years after diagnosis [3, 4, 7, 45], but we consider a prevalence of 29% to be high given the large proportion women in our sample with DCIS and stage I disease. DCIS patients reported similar levels of FCR as stage I patients, consistent with their comparable perceived risk of recurrence reported previously [14, 22, 46]. These similar findings for DCIS and EIBC patients’ FCR might be explained by a lack of knowledge about DCIS patients’ better prognosis generally and/or the similarity in treatment options for DCIS and most stage I patients.
We identified other prognostic factors, such as age and surgical treatment, associated with FCR. Older age at diagnosis predicted less FCR at 2-year follow-up, which is consistent with other studies [3-6, 16]. The literature is inconsistent regarding the impact of type of surgery on FCR. FCR did not differ significantly by type of surgery in some studies [3, 5, 7], but other studies reported that patients with mastectomy were more confident that their cancer had been cured and less concerned about recurrence than patients who received BCS [47, 48], which supports our finding that patients with BCS reported higher FCR than patients with mastectomy.
More severe surgical side effects reported 6 months after surgery predicted higher FCR in our sample, although breast surgery-related symptoms reported 4–6 weeks after surgery had no predictive effect on subsequent FCR. Lingering somatic symptoms might be viewed by patients as a constant reminder of their breast cancer, or be misinterpreted as an indicator of breast cancer recurrence. Breast cancer patients reportedly have limited knowledge about treatment side effects and often are confused about a lingering physical symptom (e.g., pain, fever, lymphedema, fatigue) [49, 50]. These results point to the need for patient education about common treatment side effects, both before and after surgical treatment, to reduce FCR. This type of education typically includes information regarding the illness or symptoms, symptom management, and/or discussion of treatment options. But the effects of such patient education on FCR remain unknown.
The psychosocial factors that were consistently associated with greater FCR included lower social support and elevated anxiety symptoms. Evidence suggests that social support helps breast cancer survivors cope with uncertainty [51] and moderates the impact of breast cancer-related intrusive thoughts on quality of life [52]. Consistent with the literature that social support works as a buffer against poorer psychological adjustment to stressful events [53, 54], we found that greater perceived social support predicted both lower perceived risk of recurrence [14] and less FCR. Disclosure of one’s thoughts and feelings to a supportive social network could improve emotional well-being after a traumatic or distressing event [55]. Patients with more social support may be more likely to know (or to have been made aware of) long-term disease-free survival among early-stage breast cancer survivors, and therefore base their own perceived risk of recurrence on the experiences of other survivors or on what their friends and family say to survivors to allay their FCR.
We found that elevated anxiety at baseline and 6-month follow-up predicted greater FCR at 2-year follow-up in women with DCIS and EIBC. Elevated depressive symptoms at baseline also predicted greater FCR, although this association was not observed at 6-month follow-up. Thus, identification of patients with elevated anxiety and depressive symptoms as soon as possible after their surgery, and timely management of their symptoms may help prevent greater long-term FCR among patients with DCIS or EIBC. In addition, our findings suggest that elevated anxiety has a greater impact on FCR than depressed mood after patients complete their adjuvant therapies. A recent longitudinal study examined bi-directional relations between total mood disturbance and fear of the future among women with locoregional or metastatic breast cancer [9]. In that study, reductions in mood disturbance (including anxiety and depressed mood among the six mood factors assessed) over 15 months after diagnosis led to decreases in fear of the future; however, the reverse was not true—a reduction in fear of the future did not lead to a reduction in mood disturbance. The measures for anxiety and depressed mood were not reported separately in that study.
While accuracy of patients’ perceived risk of recurrence at baseline and 6-month follow-up was not significantly predictive of higher FCR, we found that accuracy of recurrence-risk perceptions was correlated with concurrently measured FCR. Compared with patients who accurately perceived their risk of recurrence, patients who overestimated their recurrence risk 2 years after surgery reported greater FCR at that time and those who underestimated their recurrence risk reported less FCR. Higher recurrence-risk perception has been associated with greater cancer-specific worries in women with invasive breast cancer [56]. Our study extends the literature by including patients with DCIS, a premalignant breast lesion and by evaluating the relationship between accuracy of recurrence-risk perceptions and FCR.
In our study, education levels were inversely associated with FCR. More highly educated women with breast cancer have been found to have greater knowledge of the disease and treatment prior to undergoing definitive surgery than less-educated patients [57]. Therefore, breast cancer patients with low educational attainment might especially benefit from patient education designed to reduce FCR.
There were several limitations in this study. First, we included only women aged 40 and older with DCIS or EIBC, and our results may not be generalizable to younger women or to women with more advanced breast cancer. Second, because we asked about patients’ FCR only once, we were unable to assess changes in FCR over time. Breast cancer patients’ worry about their future health was reported to decrease over the first 3 months after surgical treatment, and stabilize afterward [16]. However, most prior studies did not find a change in FCR over time, even 5 years or more after diagnosis of invasive breast cancer [3-6, 45, 47, 49]. Other unmeasured variables, such as known BRCA 1 and 2 mutations, also might be associated with FCR and require further study.
In conclusion, although mean FCR was low among DCIS and EIBC survivors, a substantial proportion of these survivors reported moderate-to-high levels of FCR and overestimated their risk of recurrence 2 years following definitive surgical treatment. These findings underline the need for specific psychosocial interventions addressing FCR for this subset of patients with DCIS and EIBC. Management of anxiety and depression, providing social support, and educating patients about how breast cancer stage and various treatments affect risk of recurrence and treatment side effects may improve recurrence-risk perceptions and help manage FCR in women with DCIS and EIBC, especially in younger patients.
Acknowledgments
This study was supported by grants from the National Cancer Institute and Breast Cancer Stamp Fund (#R01CA102777), and the National Cancer Institute Cancer Center Support Grant (#P30 CA91842) to the Alvin J. Siteman Cancer Center. We thank our patient participants, the interviewers, and the Siteman Cancer Center’s Health Behavior, Communication and Outreach Core and Biostatistics Core (Dr. Yan Yan) for data management and statistical services, and Pam Hunborg, RN, BSN, CCRC, at Saint Louis University School of Medicine. We also greatly appreciate the many physicians who helped us recruit their patients for this study, including Drs. Barbara Monsees, Jill Dietz, Julie Margenthaler, Virginia Herrmann, Timothy Eberlein, Matthew Ellis, Imran Zoberi, Marie Taylor, Michael Naughton, Antonella Rastelli, Donald Lombardi, Cynthia Ma, Loren Michel, and Rama Suresh at Washington University School of Medicine and Dr. Eddie Hsueh at Saint Louis University School of Medicine. The Beck Anxiety Inventory® and BAI® (copyright 1990, 1993 by Aaron T. Beck), are trademarks of The Psychological Corporation, a Harcourt Assessment Company. The BAI® was adapted and used by permission of the publisher, The Psychological Corporation. All rights reserved.
Contributor Information
Ying Liu, Division of Public Health Sciences, Department of Surgery, Washington University School of Medicine, 660 South Euclid Avenue, Campus Box 8100, St. Louis, MO 63110, USA.
Maria Pérez, Division of Health Behavior Research, Department of Internal Medicine, Washington University School of Medicine, St. Louis, MO, USA.
Mario Schootman, Division of Health Behavior Research, Department of Internal Medicine, Washington University School of Medicine, St. Louis, MO, USA; Alvin J. Siteman Cancer Center at Barnes-Jewish Hospital and Washington University School of Medicine, St. Louis, MO, USA.
Rebecca L. Aft, Alvin J. Siteman Cancer Center at Barnes-Jewish Hospital and Washington University School of Medicine, St. Louis, MO, USA; Department of Surgery, Washington University School of Medicine, St. Louis, MO, USA; John Cochran Veterans Administration Hospital, St. Louis, MO, USA
William E. Gillanders, Alvin J. Siteman Cancer Center at Barnes-Jewish Hospital and Washington University School of Medicine, St. Louis, MO, USA; Department of Surgery, Washington University School of Medicine, St. Louis, MO, USA
Donna B. Jeffe, Division of Health Behavior Research, Department of Internal Medicine, Washington University School of Medicine, St. Louis, MO, USA; Alvin J. Siteman Cancer Center at Barnes-Jewish Hospital and Washington University School of Medicine, St. Louis, MO, USA
References
- 1.Mols F, Vingerhoets AJ, Coebergh JW, van de Poll-Franse LV. Quality of life among long-term breast cancer survivors: a systematic review. Eur J Cancer. 2005;41:2613–2619. doi: 10.1016/j.ejca.2005.05.017. [DOI] [PubMed] [Google Scholar]
- 2.Ganz PA, Coscarelli A, Fred C, Kahn B, Polinsky ML, Petersen L. Breast cancer survivors: psychosocial concerns and quality of life. Breast Cancer Res Treat. 1996;38:183–199. doi: 10.1007/BF01806673. [DOI] [PubMed] [Google Scholar]
- 3.Vickberg SM. The Concerns About Recurrence Scale (CARS): a systematic measure of women’s fears about the possibility of breast cancer recurrence. Ann Behav Med. 2003;25:16–24. doi: 10.1207/S15324796ABM2501_03. [DOI] [PubMed] [Google Scholar]
- 4.van den Beuken-van Everdingen MH, Peters ML, de Rijke JM, Schouten HC, van Kleef M, Patijn J. Concerns of former breast cancer patients about disease recurrence: a validation and prevalence study. Psychooncology. 2008;17:1137–1145. doi: 10.1002/pon.1340. [DOI] [PubMed] [Google Scholar]
- 5.Mehnert A, Berg P, Henrich G, Herschbach P. Fear of cancer progression and cancer-related intrusive cognitions in breast cancer survivors. Psychooncology. 2009;18:1273–1280. doi: 10.1002/pon.1481. [DOI] [PubMed] [Google Scholar]
- 6.Simard S, Savard J, Ivers H. Fear of cancer recurrence: specific profiles and nature of intrusive thoughts. J Cancer Surviv. 2010;4:361–371. doi: 10.1007/s11764-010-0136-8. [DOI] [PubMed] [Google Scholar]
- 7.Curran D, van Dongen JP, Aaronson NK, Kiebert G, Fentiman IS, Mignolet F, Bartelink H. Quality of life of early-stage breast cancer patients treated with radical mastectomy or breast-conserving procedures: results of EORTC Trial 10801. The European Organization for Research and Treatment of Cancer (EORTC), Breast Cancer Co-operative Group (BCCG) Eur J Cancer. 1998;34:307–314. doi: 10.1016/s0959-8049(97)00312-2. [DOI] [PubMed] [Google Scholar]
- 8.Armes J, Crowe M, Colbourne L, Morgan H, Murrells T, Oakley C, Palmer N, Ream E, Young A, Richardson A. Patients’ supportive care needs beyond the end of cancer treatment: a prospective. J Clin Oncol. 2009;27:6172–6179. doi: 10.1200/JCO.2009.22.5151. [DOI] [PubMed] [Google Scholar]
- 9.Lebel S, Rosberger Z, Edgar L, Devins GM. Emotional distress impacts fear of the future among breast cancer survivors not the reverse. J Cancer Surviv. 2009;3:117–127. doi: 10.1007/s11764-009-0082-5. [DOI] [PubMed] [Google Scholar]
- 10.Deimling GT, Bowman KF, Sterns S, Wagner LJ, Kahana B. Cancer-related health worries and psychological distress among older adult, long-term cancer survivors. Psychooncology. 2006;15:306–320. doi: 10.1002/pon.955. [DOI] [PubMed] [Google Scholar]
- 11.Molenaar S, Oort F, Sprangers M, Rutgers E, Luiten E, Mulder J, de Haes H. Predictors of patients’ choices for breast-conserving therapy or mastectomy: a prospective study. Br J Cancer. 2004;90:2123–2130. doi: 10.1038/sj.bjc.6601835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brain K, Norman P, Gray J, Mansel R. Anxiety and adherence to breast self-examination in women with a family history of breast cancer. Psychosom Med. 1999;61:181–187. doi: 10.1097/00006842-199903000-00010. [DOI] [PubMed] [Google Scholar]
- 13.Liu Y, Perez M, Aft RL, Massman K, Robinson E, Myles S, Schootman M, Gillanders WE, Jeffe DB. Accuracy of perceived risk of recurrence among patients with early-stage breast cancer. Cancer Epidemiol Biomarkers Prev. 2010;19:675–680. doi: 10.1158/1055-9965.EPI-09-1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu Y, Perez M, Schootman M, Aft RL, Gillanders WE, Ellis MJ, Jeffe DB. A longitudinal study of factors associated with perceived risk of recurrence in women with ductal carcinoma in situ and early-stage invasive breast cancer. Breast Cancer Res Treat. 2010;124:835–844. doi: 10.1007/s10549-010-0912-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosenstock IM, Irwin M. The Health Belief Model: explaining health behavior through expectancies. In: Glanz K, Lewis FM, Rimer BK, editors. Health behavior and health education: theory, research, and practice. Jossey-Bass; San Francisco: 1990. pp. 39–62. [Google Scholar]
- 16.Wade TD, Nehmy T, Koczwara B. Predicting worries about health after breast cancer surgery. Psychooncology. 2005;14:503–509. doi: 10.1002/pon.866. [DOI] [PubMed] [Google Scholar]
- 17.Courtens AM, Stevens FC, Crebolder HF, Philipsen H. Longitudinal study on quality of life and social support in cancer patients. Cancer Nurs. 1996;19:162–169. doi: 10.1097/00002820-199606000-00002. [DOI] [PubMed] [Google Scholar]
- 18.Reynolds P, Boyd PT, Blacklow RS, Jackson JS, Greenberg RS, Austin DF, Chen VW, Edwards BK, National Cancer Institute Black/White Cancer Survival Study Group The relationship between social ties and survival among black and white breast cancer patients. Cancer Epidemiol Biomarkers Prev. 1994;3:253–259. [PubMed] [Google Scholar]
- 19.Lee-Jones C, Humphris G, Dixon R, Hatcher MB. Fear of cancer recurrence—a literature review and proposed cognitive formulation to explain exacerbation of recurrence fears. Psychooncology. 1997;6:95–105. doi: 10.1002/(SICI)1099-1611(199706)6:2<95::AID-PON250>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 20.Cameron LD, Booth RJ, Schlatter M, Ziginskas D, Harman JE. Changes in emotion regulation and psychological adjustment following use of a group psychosocial support program for women recently diagnosed with breast cancer. Psychooncology. 2007;16:171–180. doi: 10.1002/pon.1050. [DOI] [PubMed] [Google Scholar]
- 21.Partridge A, Adloff K, Blood E, Dees EC, Kaelin C, Golshan M, Ligibel J, de Moor JS, Weeks J, Emmons K, Winer E. Risk perceptions and psychosocial outcomes of women with ductal carcinoma in situ: longitudinal results from a cohort study. J Natl Cancer Inst. 2008;100:243–251. doi: 10.1093/jnci/djn010. [DOI] [PubMed] [Google Scholar]
- 22.Rakovitch E, Franssen E, Kim J, Ackerman I, Pignol JP, Paszat L, Pritchard KI, Ho C, Redelmeier DA. A comparison of risk perception and psychological morbidity in women with ductal carcinoma in situ and early invasive breast cancer. Breast Cancer Res Treat. 2003;77:285–293. doi: 10.1023/a:1021853302033. [DOI] [PubMed] [Google Scholar]
- 23.Ripptoe PA, Rogers RW. Effects of components of protection-motivation theory on adaptive and maladaptive coping with a health threat. J Pers Soc Psychol. 1987;52:596–604. doi: 10.1037//0022-3514.52.3.596. [DOI] [PubMed] [Google Scholar]
- 24.Leitch AM, Dodd GD, Costanza M, Linver M, Pressman P, McGinnis L, Smith RA. American Cancer Societyguidelines for the early detection of breast cancer: update 1997. CA Cancer J Clin. 1997;47:150–153. doi: 10.3322/canjclin.47.3.150. [DOI] [PubMed] [Google Scholar]
- 25.Katzman R, Brown T, Fuld P, Peck A, Schechter R, Schimmel H. Validation of a short Orientation-Memory-Concentration Test of cognitive impairment. Am J Psychiatry. 1983;140:734–739. doi: 10.1176/ajp.140.6.734. [DOI] [PubMed] [Google Scholar]
- 26.Sherbourne CD, Stewart AL. The MOS social support survey. Soc Sci Med. 1991;32:705–714. doi: 10.1016/0277-9536(91)90150-b. [DOI] [PubMed] [Google Scholar]
- 27.Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: psychometric properties. J Consult Clin Psychol. 1988;56:893–897. doi: 10.1037//0022-006x.56.6.893. [DOI] [PubMed] [Google Scholar]
- 28.Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Measur. 1977;1:385–401. [Google Scholar]
- 29.Schag CA, Ganz PA, Polinsky ML, Fred C, Hirji K, Petersen L. Characteristics of women at risk for psychosocial distress in the year after breast cancer. J Clin Oncol. 1993;11:783–793. doi: 10.1200/JCO.1993.11.4.783. [DOI] [PubMed] [Google Scholar]
- 30.Perez M, Liu Y, Schootman M, Aft RL, Schechtman KB, Gillanders WE, Jeffe DB. Changes in sexual problems over time in women with and without early-stage breast cancer. Menopause. 2010;17:924–937. doi: 10.1097/gme.0b013e3181d5dd26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 32.Katz JN, Chang LC, Sangha O, Fossel AH, Bates DW. Can comorbidity be measured by questionnaire rather than medical record review? Med Care. 1996;34:73–84. doi: 10.1097/00005650-199601000-00006. [DOI] [PubMed] [Google Scholar]
- 33.Andersen MR, Urban N. Involvement in decision-making and breast cancer survivor quality of life. Ann Behav Med. 1999;21:201–209. doi: 10.1007/BF02884834. [DOI] [PubMed] [Google Scholar]
- 34.Fisher B, Dignam J, Wolmark N, Wickerham DL, Fisher ER, Mamounas E, Smith R, Begovic M, Dimitrov NV, Margolese RG, Kardinal CG, Kavanah MT, Fehrenbacher L, Oishi RH. Tamoxifen in treatment of intraductal breast cancer: National Surgical Adjuvant Breast and Bowel Project B-24 randomised controlled trial. Lancet. 1999;353:1993–2000. doi: 10.1016/S0140-6736(99)05036-9. [DOI] [PubMed] [Google Scholar]
- 35.Goodwin A, Parker S, Ghersi D, Wilcken N. Post-operative radiotherapy for ductal carcinoma in situ of the breast. Cochrane Database Syst Rev. 2009:CD000563. doi: 10.1002/14651858.CD000563.pub5. [DOI] [PubMed] [Google Scholar]
- 36.Houghton J, George WD, Cuzick J, Duggan C, Fentiman IS, Spittle M. Radiotherapy and tamoxifen in women with completely excised ductal carcinoma in situ of the breast in the UK, Australia, and New Zealand: randomised controlled trial. Lancet. 2003;362:95–102. doi: 10.1016/s0140-6736(03)13859-7. [DOI] [PubMed] [Google Scholar]
- 37.Lee LA, Silverstein MJ, Chung CT, Macdonald H, Sanghavi P, Epstein M, Holmes DR, Silberman H, Ye W, Lagios MD. Breast cancer-specific mortality after invasive local recurrence in patients with ductal carcinoma-in situ of the breast. Am J Surg. 2006;192:416–419. doi: 10.1016/j.amjsurg.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 38.Viani GA, Stefano EJ, Afonso SL, De Fendi LI, Soares FV, Leon PG, Guimaraes FS. Breast-conserving surgery with or without radiotherapy in women with ductal carcinoma in situ: a meta-analysis of randomized trials. Radiat Oncol. 2007;2:28. doi: 10.1186/1748-717X-2-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Clarke M, Collins R, Darby S, Davies C, Elphinstone P, Evans E, Godwin J, Gray R, Hicks C, James S, MacKinnon E, McGale P, McHugh T, Peto R, Taylor C, Wang Y. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;366:2087–2106. doi: 10.1016/S0140-6736(05)67887-7. [DOI] [PubMed] [Google Scholar]
- 40. [Accessed July 3, 2010]; Adjuvant! Online. Available from https://www.adjuvantonline.com/online.jsp.
- 41.Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365:1687–1717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 42.Herrinton LJ, Barlow WE, Yu O, Geiger AM, Elmore JG, Barton MB, Harris EL, Rolnick S, Pardee R, Husson G, Macedo A, Fletcher SW. Efficacy of prophylactic mastectomy in women with unilateral breast cancer: a cancer research network project. J Clin Oncol. 2005;23:4275–4286. doi: 10.1200/JCO.2005.10.080. [DOI] [PubMed] [Google Scholar]
- 43.Fisher B, Anderson S, Bryant J, Margolese RG, Deutsch M, Fisher ER, Jeong JH, Wolmark N. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. 2002;347:1233–1241. doi: 10.1056/NEJMoa022152. [DOI] [PubMed] [Google Scholar]
- 44.Van Steen K, Curran D, Kramer J, Molenberghs G, Van Vreckem A, Bottomley A, Sylvester R. Multicollinearity in prognostic factor analyses using the EORTC QLQ-C30: identification and impact on model selection. Stat Med. 2002;21:3865–3884. doi: 10.1002/sim.1358. [DOI] [PubMed] [Google Scholar]
- 45.Bluman LG, Borstelmann NA, Rimer BK, Iglehart JD, Winer EP. Knowledge, satisfaction, and perceived cancer risk among women diagnosed with ductal carcinoma in situ. J Womens Health Gend Based Med. 2001;10:589–598. doi: 10.1089/15246090152543175. [DOI] [PubMed] [Google Scholar]
- 46.van Gestel YR, Voogd AC, Vingerhoets AJ, Mols F, Nieuwenhuijzen GA, van Driel OJ, van Berlo CL, van de Poll-Franse LV. A comparison of quality of life, disease impact and risk perception in women with invasive breast cancer and ductal carcinoma in situ. Eur J Cancer. 2006;43:549–556. doi: 10.1016/j.ejca.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 47.Hartl K, Janni W, Kastner R, Sommer H, Strobl B, Rack B, Stauber M. Impact of medical and demographic factors on long-term quality of life and body image of breast cancer patients. Ann Oncol. 2003;14:1064–1071. doi: 10.1093/annonc/mdg289. [DOI] [PubMed] [Google Scholar]
- 48.Hall A, Fallowfield L. Psychological outcome of treatment for early breast cancer: a review. Stress Med. 1989;5:167–175. [Google Scholar]
- 49.Gil KM, Mishel M, Belyea M, Germino B, Germino LS, Porter L, LaNey IC, Stewart J. Triggers of uncertainty about recurrence and long-term treatment side effects in older African American and Caucasian breast cancer survivors. Oncol Nurs Forum. 2004;31:633–639. doi: 10.1188/04.onf.633-639. [DOI] [PubMed] [Google Scholar]
- 50.Gray RE, Fitch M, Greenberg M, Hampson A, Doherty M, Labrecque M. The information needs of well, longer-term survivors of breast cancer. Patient Educ Couns. 1998;33:245–255. doi: 10.1016/s0738-3991(98)00024-x. [DOI] [PubMed] [Google Scholar]
- 51.Sammarco A. Quality of life among older survivors of breast cancer. Cancer Nurs. 2003;26:431–438. doi: 10.1097/00002820-200312000-00002. [DOI] [PubMed] [Google Scholar]
- 52.Lewis JA, Manne SL, DuHamel KN, Vickburg SM, Bovbjerg DH, Currie V, Winkel G, Redd WH. Social support, intrusive thoughts, and quality of life in breast cancer survivors. J Behav Med. 2001;24:231–245. doi: 10.1023/a:1010714722844. [DOI] [PubMed] [Google Scholar]
- 53.Kornblith AB, Herndon JE, II, Zuckerman E, Viscoli CM, Horwitz RI, Cooper MR, Harris L, Tkaczuk KH, Perry MC, Budman D, Norton LC, Holland J. Social support as a buffer to the psychological impact of stressful life events in women with breast cancer. Cancer. 2001;91:443–454. doi: 10.1002/1097-0142(20010115)91:2<443::aid-cncr1020>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 54.Ringdal GI, Ringdal K, Jordhoy MS, Kaasa S. Does social support from family and friends work as a buffer against reactions to stressful life events such as terminal cancer? Palliat Support Care. 2007;5:61–69. doi: 10.1017/s1478951507070083. [DOI] [PubMed] [Google Scholar]
- 55.Lepore SJ, Silver RC, Wortman CB, Wayment HA. Social constraints, intrusive thoughts, and depressive symptoms among bereaved mothers. J Pers Soc Psychol. 1996;70:271–282. doi: 10.1037//0022-3514.70.2.271. [DOI] [PubMed] [Google Scholar]
- 56.Rothrock NE, Matthews AK, Sellergren SA, Fleming G, List M. State anxiety and cancer-specific anxiety in survivors of breast cancer. J Psychosoc Oncol. 2004;22:93–109. [Google Scholar]
- 57.Fagerlin A, Lakhani I, Lantz PM, Janz NK, Morrow M, Schwartz K, Deapen D, Salem B, Liu L, Katz SJ. An informed decision? Breast cancer patients and their knowledge about treatment. Patient Educ Couns. 2006;64:303–312. doi: 10.1016/j.pec.2006.03.010. [DOI] [PubMed] [Google Scholar]


