Abstract
The ability to make accurate judgments about the mental states of others, sometimes referred to as theory of mind (ToM), is often impaired following traumatic brain injury (TBI), and this deficit may contribute to problems with interpersonal relationships. The present study used an animated social attribution task (SAT) with functional magnetic resonance imaging (fMRI) to examine structures mediating ToM in adolescents with moderate to severe TBI. The study design also included a comparison group of matched, typically developing (TD) adolescents. The TD group exhibited activation within a number of areas that are thought to be relevant to ToM, including the medial prefrontal and anterior cingulate cortex, fusiform gyrus, and posterior temporal and parietal areas. The TBI subjects had significant activation within many of these same areas, but their activation was generally more intense and excluded the medial prefrontal cortex. Exploratory regression analyses indicated a negative relation between ToM-related activation and measures of white matter integrity derived from diffusion tensor imaging, while there was also a positive relation between activation and lesion volume. These findings are consistent with alterations in the level and pattern of brain activation that may be due to the combined influence of diffuse axonal injury and focal lesions.
Keywords: Traumatic brain injury, fMRI, Social cognition, Adolescents, Diffusion tensor imaging
Traumatic brain injury (TBI) is a common cause of disability among children and adolescents (Babikian & Asarnow, 2009) which can have long-term effects on academic achievement (Ewing-Cobbs et al., 2004), cognition (Levin et al., 1997), and quality of life (Sesma, Slomine, Ding, & McCarthy, 2008). Those with moderate to severe TBI often exhibit persistent impairments in executive functions (e.g., Levin et al., 1997), and studies have also revealed problems with social functioning (e.g., Janusz, Kirkwood, Yeates, & Taylor, 2002; Yeates et al., 2007). Adolescence, in particular, is an especially challenging period with multiple social transitions, and individuals who are injured before or during this stage may fail to develop social competence (Max et al., 2006; Turkstra, Dixon, & Baker, 2004).
Social cognition is the ability to recognize, manipulate, and respond appropriately to socially relevant information, and it includes components of perception, motivation, and emotion, as well as intentionality and perspective-taking (Adolphs, 2001). Deficits in processing intentions and emotions or in taking the perspectives of others may contribute to difficulties with social adjustment (Schmidt, Hanten, Li, Orsten, & Levin, 2010). Within the broader domain of social cognition, the ability to make accurate judgments about the mental state of people, including their intentions, desires, and beliefs, is important for predicting the behavior of others and for facilitating positive social interactions (Blakemore, 2008). These particular skills and the associated mental processes have often been referred to collectively as mentalizing or theory of mind (ToM) (Blakemore, 2008). Mentalizing encompasses the states in which an individual has a mental representation about another person's mental representation; that is, meta-representation. The vast literature on ToM addresses a variety of skills requiring meta-representation that include, but are not limited to, first- and second-order false belief (e.g., Baron-Cohen, Leslie, & Frith, 1985; Bowler, 1992; Hughes, Ensor, & Marks, 2010), comprehension of irony, sarcasm, and deception (e.g., Dennis, Purvis, Barnes, Wilkinson, & Winner, 2001), faux pas recognition (e.g., Geraci, Surian, Ferraro, & Cantagallo, 2010; Stone, Baron-Cohen, & Knight, 1998), and various forms of perspective taking (e.g., Aldrich, Tenenbaum, Brooks, Harrison, & Sines, 2011; Chevallier, Noveck, Happé, & Wilson, 2011; Stocks, Lishner, Waits, & Downum, 2011; van den Bos, van Dijk, Westenberg, Rombouts, & Crone, 2011).
Studies of adult TBI have found impairments on tests of ToM requiring the inferring of others’ thoughts or emotional states from stories, pictures, and animations (e.g., Henry, Phillips, Crawford, Ietswaart, & Summers, 2006; Muller et al., 2010), and on identification of social faux pas (Channon & Crawford, 2010; Geraci et al., 2010). Relative to healthy subjects, the findings generally suggest sparing on simple, first-order ToM tasks, but increasing impairment with the complexity of the tasks and the skills needed to complete them (for meta-analysis, see Martín-Rodríguez & León-Carrión, 2010).
Much the same story is found in children. Snodgrass and Knott (2006) found that children with moderate to severe TBI performed as well as control subjects on a simple, first-order ToM task that required the recognition of a character's false belief, but more advanced aspects of ToM were impaired. Maureen Dennis and her colleagues examined understanding of irony and deceptive praise in children with mild and severe TBI, as compared to typically developing (TD) uninjured children, and found that these groups did not differ on first-order tasks (Dennis et al., 2001). However, older children with severe TBI were significantly impaired on second-order intentionality tasks. More recently, Dennis, Agostino, Roncadin, and Levin (2009) reported finding ToM deficits in children with TBI that were related to reductions in cognitive inhibition. Thus, some of the impairments in social cognition following brain injury may be due, at least in part, to impairments in more general executive functions. It is not clear from the literature, though, to what extent problems with social cognition following TBI (e.g., impaired ToM) are domain-specific or whether they reflect more general executive deficits in cognitive control.
Functional neuroimaging research has identified brain structures that appear to be part of a network that mediates ToM in healthy individuals, including the temporoparietal junction, superior temporal sulcus, precuneus, and medial prefrontal cortex (Carrington & Bailey, 2009; Dodrell-Feder, Koster-Hale, Bedny, & Saxe, 2011). A study by Schultz et al. (2003) also identified the fusiform face area as being an additional structure that exhibits activation during mentalizing. Research has started to explore the role of these various network components, and Saxe and colleagues (e.g., Saxe & Powell, 2006) have suggested that the medial prefrontal cortex is generally involved in social cognition, rather than ToM specifically, while the superior temporal sulcus may be involved in the detection of motion cues that are useful for understanding another person's mental state (Blakemore et al., 2003; Gobbini, Koralek, Bryan, Montgomery, & Haxby, 2007). There is some evidence, however, that the temporoparietal junction is recruited for thinking about the thoughts of others (i.e., meta-representation) and that it may have a specific and central role in mentalizing (Saxe & Kanwisher, 2003; Saxe & Powell, 2006).
Little is currently known about how activation during social cognition, including ToM, changes in response to acquired neurological disorders. Most functional imaging studies utilizing nonsocial cognitive tasks have found that individuals with moderate to severe TBI have activation that is more intense and diffuse (e.g., Christodoulou et al., 2001; Hillary, 2008; Scheibel et al., 2009). These alterations in the level and pattern of brain activation during cognitive activity may reflect decreases in neural resources or neural inefficiency due to diffuse axonal injury (DAI) (e.g., Huang et al., 2009; Scheibel et al., 2009). However, research examining injury-related alterations in activation during social cognition has been limited. Newsome et al. (2010) reported that adolescents with moderate to severe TBI had posterior brain activation that was greater and more diffuse, relative to control subjects, when they evaluated trait attributions about the self from a third-person perspective. According to these previous findings, ToM-related activation following TBI might be expected to be more intense and to include brain areas that are not typically activated in uninjured individuals.
Animated geometric shapes that move in ways that suggest social interaction and personal agency have also been used to study brain activation associated with ToM (Castelli, Frith, Happé, & Frith, 2002; Heider & Simmel, 1944; Schultz et al., 2003). In normal adults, Schultz et al. (2003) found that such a procedure engaged the right and left dorsal medial prefrontal cortex, the right and left inferior frontal gyri, the orbital frontal cortex, the right temporal pole and amygdala, the right fusiform gyrus, and tissue around the right and left superior temporal sulci. Similarly, Moriguchi, Ohnishi, Mori, Matsuda, and Komaki (2007) used a ToM paradigm depicting interactions among two animated triangles and found that normally developing children and adolescents had activation bilaterally around the superior temporal sulcus, the temporal pole, the amygdala, and the medial prefrontal cortex. Their data also showed that activation shifted from the ventral to the dorsal area of the medial prefrontal cortex during late childhood and adolescence. Moriguchi et al. (2007) interpreted these age-related findings as being consistent with the maturation of prefrontal cortex and the associated development of cognitive functions.
The present study utilized an animated social attribution task (SAT), modified from the version used by Schultz et al. (2003), to examine alterations in ToM-related brain activation associated with TBI. The term ‘ToM’ is used here in the broader sense to indicate meta-representation, but not necessarily restricted to meta-representation of beliefs and knowledge. The study design included adolescents with moderate to severe TBI and a comparison group of matched, TD adolescents. We hypothesized that the SAT would activate brain structures that previous research has identified as being relevant to mentalizing (Carrington & Bailey, 2009; Moriguchi et al., 2007; Schultz et al., 2003), and that, in subjects with TBI, activation would be greater and would include structures that are not normally activated during ToM. In addition, we hypothesized that there would be significant correlations between ToM-related activation and variables reflecting TBI neuropathology, including lesion volume and measures of white matter integrity derived from the results of diffusion tensor imaging (DTI).
METHODS
Subjects
Nine adolescents with chronic moderate to severe TBI, as defined by a post-resuscitation score of 3–12 on the Glasgow Coma Scale (GCS) (Teasdale & Jennett, 1974), were selected from a larger cohort of TBI patients followed in Houston and Dallas (see Table 1). Their mean GCS score was 5.56 (SD = 3.13), and the mean interval between time of injury and the fMRI study was 3.65 years (SD = 0.56). Selection criteria included availability for participation, age, severity of injury, and the ability to follow the task instructions and restrain movement during scanning. Nine TD adolescents served as comparison subjects, and they were individually matched to the TBI patients on gender (5 boys) and age. The Wilcoxon two-sample test with normal approximation indicated no significant between-group differences for age at the time of assessment (p < .86, TBI mean = 16.32 years, SD = 2.50, range = 12.38–19.70; TD mean = 16.84, SD = 2.24, range = 13.19–19.94 years) or the mother's level of education (p < .62, TBI mean = 13.11 years, SD = 3.52; TD mean = 14.22 years, SD = 2.05). Fisher's exact test indicated no significant between-group differences for ethnicity (p < .47, TBI = 5 Caucasian, 1 Caucasian-Asian, 3 Hispanic; TD = 1 African-American, 3 Caucasian, 5 Hispanic). All subjects were right-handed (Oldfield, 1971), none were taking psychoactive medications at the time of assessment, and none had a history of previous neurologic or psychiatric disorder. Eight TBI patients had focal frontal lobe lesions, and six had temporal lesions on structural MRI (see Table 1). The lesions were measured by the neuroradiologist on coronal T2-weighted fluid attenuated inversion recovery (FLAIR) images at the time the anatomical scans were reviewed, and these often consisted of relatively small hemosiderin deposits or areas of gliosis. Total lesion volume within brain areas thought to be especially relevant to social cognition was calculated for each subject, and the mean was 7.7 cc (SD = 12.0) (see Table 1). Child assent and parental consent were obtained, and the study was approved by the institutional review boards at Baylor College of Medicine, the University of Texas Southwestern Medical School at Dallas, and the University of Texas at Dallas.
TABLE 1.
Demographic and injury characteristics of nine adolescents with traumatic brain injury
| Age at testing | Age at injury | Post-injury interval(years) | Mother's education | Gender | Mechanism of injury | GCS score | Total lesion volume* (cc) | Lesion sites |
|---|---|---|---|---|---|---|---|---|
| 19.7 | 16.7 | 3.0 | 16 | M | MVA | 10 | 1.7 | R MFG, L MedFG, B SFG, B MTG, B Temp pole, L operculum |
| 15.4 | 11.3 | 4.2 | GED | M | Bicycle accident | 3 | 20.2 | B GyrRect, B OrbG, B MFG, B SFG, B CC (body), L MTG, L ITG, L Temp pole |
| 18.0 | 14.7 | 3.3 | 14 | F | Fall | 9 | 0 | L MTG |
| 17.0 | 12.9 | 4.2 | 16 | M | Fall | 3 | 1.2 | B SFG, L IPC, L hippocampus |
| 19.7 | 16.0 | 3.8 | 14 | M | MVA | 3 | 0.6 | L IFG, B MFG, R SFG, B SPC, L putamen, R Thal |
| 13.8 | 9.4 | 4.4 | 6 | F | Fall | 9 | 0.6 | B OrbG, R IFG, R MFG, L Cblm |
| 12.4 | 9.2 | 3.2 | 15 | F | MVA | 3 | 6.2 | B IFG, R MFG, R SFG, R STG, R MTG, R ITG, R Temp stem, R IPC, L caudate, L CC (body), L Cblm |
| 15.6 | 12.7 | 2.9 | 16 | F | MVA | 7 | 34.7 | B OrbG, L MFG, B SFG, L STG, R MTG, R ITG, R Temp Pole, B Ant Temp Pole, midline Ant CC |
| 15.4 | 11.5 | 3.9 | 14 | M | MVA | 3 | 4.2 | L IFG, B MFG, R SFG, B SPC, R Thal, R putamen |
Notes: Ant = anterior, B = both sides, L = left, Mid = middle, R = right; Cblm = cerebellum, CC = corpus callosum, GyrRect = gyrus rectus, IFG = inferior frontal gyrus, IPC = inferior parietal cortex, ITG = inferior temporal gyrus, MedFG = medial frontal gyrus, MFG = middle frontal gyrus, MTG = medial temporal gyrus, OccLobe = occipital lobe, OrbG = orbital gyrus, SFG = superior frontal gyrus, SPC = superior parietal cortex, Temp = temporal, Thal = thalamus, GED = graduate equivalency degree, MVA = motor vehicle accident.
Total lesion volume within brain areas thought to be especially important for social cognition, including the IFG, MFG, SFG, Temp Pole, MedFG, GyrRect, OrbG, and SPC. Note that one subject had a single lesion in the MTG, but this was not included in the lesion volume variable because it was within a portion of the temporal lobe that is not thought to be especially relevant to social cognition.
Behavioral measures
All of the subjects were assessed by the Wechsler Abbreviated Scale of Intelligence (WASI) (Wechsler, 1999) to provide an intelligence quotient (IQ) and to assess verbal knowledge and spatial processing. The Gray Oral Reading Test (GORT) (Weiderholt & Bryant, 2001) was also administered.
fMRI task
The SAT was similar to the fMRI paradigm used by Schultz et al. (2003), with films illustrating interactions among geometric shapes programmed in E-Prime (www.pstnet.com/eprime) and presented by an MRA fMRI stimulus delivery system (www.mra1.com), but each film had a duration of 17 s instead of 15.1 s. There were eight films for each of two conditions, and each film contained the same three white geometric figures (i.e., triangle, circle, diamond) that moved against a black background. During the social condition, there was a box in the center of this background, with one side that opened as if it were a door, and the shapes moved as if they were able to open or shut the door, enter the box, and chase or drag other shapes inside. Subjects viewed the film and then pressed a button with their right index finger if they judged that the shapes were all friends and pressed with their left finger if the shapes were not friends. In this case, the implied question for the condition of interest (social condition) is, “Do you think the figures are friends?” To answer this question, the subject must engage in meta-representational thought, although not necessarily restricted to knowledge or belief. That is, the subject creates a representation of the figures’ representation of each other. However, because the design does not allow for examining the exact process the subject uses to determine whether or not the figures are friends, we cannot know the nature or complexity of the meta-representation. Therefore, in reference to this study and this task, we use the term “ToM” to mean, broadly, engaging in meta-representation. There was also a “bumper car” control condition in which the same shapes moved around the same box in the center of the background, as if they were bumper cars. After viewing the film, the subjects pressed the button on the right if they thought the objects represented in the film were all the same weight. They pressed the left button if they thought the weights differed. Prescan training, including an explanation of the instructions and a single practice run, was performed outside the scanner environment to familiarize subjects with the task.
Block-design fMRI was performed in the scanner, using the SAT with four runs that each consisted of two films from each condition. Order of presentation of the conditions (social versus “bumper car”) was counter-balanced across the four runs and all started with a 3-s screen that identified the condition for the film that was to follow (“PEOPLE, ALL FRIENDS?” or “BUMPER CARS, SAME WEIGHT?”), and then there was a17-s film, and this was followed by a 13-s fixation cross. Subjects had been instructed during prescan training to withhold their response until the cross appeared. The amount of time to present one combination of the instructions, film, and fixation cross was 33 s, and the total duration of each run was 132 s. Both accuracy and reaction time (RT) were recorded.
Scanning protocol
Whole-brain imaging data were acquired with a multichannel SENSE headcoil on a 3.0 T Philips Achieva scanner. Blood-oxygen-level-dependent (BOLD) T2* weighted, single-shot, gradient-echo echoplanar images (EPI) were acquired in 32 axial slices of 3.75 mm thickness with a 1.0-mm gap, using a 240 mm × 240 mm field of view (FOV), 64 × 64 matrix, a repetition time (TR) of 1500 ms, echo time (TE) of 25 ms, 60° flip angle, and a SENSE factor of 2.0. After the functional scans, a set of high-resolution, T1-weighted, 3D-Turbo Field Echo (TFE) anatomical images was acquired in 132 axial slices of 1.0-mm thickness (no gap) with 240 mm × 240 mm FOV, 256 × 256 matrix, TR of 9.9 ms, TE of 4.6 ms, 8.0° flip angle, and a SENSE factor of 1.5. These parameters produced 1-mm isotropic voxels for the anatomical data. Weisskoff stability measurements (Weisskoff, 1996) (minimum 1/signal to noise ratio index, peak-to-peak and root mean square stability) taken on the day of each scan indicated stability of the scanner over time. The DTI data were acquired with transverse, multislice spin echo, single-shot, EPI sequences (TR = 6161 ms; TE = 51; 2.0-mm slices; no gap). A 224-mm field of view (FOV) (rectangular field of view, RFOV = 100%) was used with a measured voxel size of 1.75 × 1.75 × 2.00 mm and a reconstructed voxel size of 2.00 × 2.00 × 2.00 mm. Diffusion was measured along 32 directions (number of b-value = 2, low b-value = 0, and high b-value = 1000 s/mm2). To improve the signal to noise ratio, high-b images were acquired twice and were averaged. Each DTI acquisition took approximately 5 min, and 70 slices were obtained. For lesion analysis, a coronal T2-weighted FLAIR sequence was used (1100-ms TR, 140-ms TE, 5.0-mm slices). This sequence had a 220-mm FOV with a reconstructed voxel size of 0.86 × 0.86 × 5.0 mm.
DTI procedures
The Philips diffusion affine registration tool was used to remove shear and eddy current distortion and head motion prior to calculating fractional anisotropy (FA) maps with Philips fiber-tracking 4.1V3 Beta 4 software (Netsch & van Musiwinkel, 2004). A quantitative DTI tractography approach was used where mean FA of the fiber system was used as the measure for DTI variables. The algorithm for fiber tracking is based upon the fiber assignment by the continuous tracking (FACT) method (Mori, Crain, Chacko, & van Zijl, 1999). For each region of interest (ROI), standard parameters were used where tracking terminated if the FA in the voxels decreased below 0.2 or if the angle between adjacent voxels along the tract was greater than 6.75°. Tracts or ROIs were selected by their reproducibility, using the specified protocols, coverage of major white matter regions of the brain, and/or their association with cortical regions implicated in social cognition. Tracts or ROIs included: (1) ventromedial and dorsolateral frontal regions (containing white matter underlying the medial prefrontal areas and inferior frontal gyrus); (2) temporal lobe regions, including the arcuate fasiculus, inferior fronto-occipital longitudinal fasciculus (connecting the ventromedial areas to the occipital regions via the temporal stem), inferior longitudinal fasiculus (connecting the temporal pole to the parietal and occipital areas, and underlying the superior temporal sulcus), and uncinate fasciculus (connecting the frontal and temporal poles); (3) corpus callosum (connecting the hemispheres); (4) cingulum bundle (which underlies the cingulate cortex and contains projections to several areas including the frontal, temporal, and parietal regions; and (5) anterior (connecting the thalamus to the frontal areas) and posterior (connecting the parietal areas to the brain stem) limbs of the internal capsule (Levin et al., 2008; Oni et al., 2010; Wakana et al., 2007; Wilde et al., 2006, 2009, 2010). Estimates of intraoperator and interoperator reproducibility were obtained for each of these ROIs, using intraclass correlation coefficients. All intraclass correlations exceeded .97.
We elected to investigate the impact of overall white matter integrity by creating a composite of all DTI tracts or regions of interest (whole-brain FA composite): This was created by averaging FA z-scores from each of the ROIs. A second composite score, the social brain FA composite, was created from ROIs that were thought to be especially relevant for social functions (i.e., genu, bilateral uncinate, and inferior longitudinal fasciculi), because these connect cortical areas that have been implicated in social cognition (Blakemore, 2008), including ToM (Carrington & Bailey, 2009).
Functional image processing and analysis
The fMRI data were subjected to voxel by voxel analyses using Statistical Parametric Mapping (SPM) 5 software (Wellcome Department of Cognitive Neurology, University College, London, UK) implemented in Matlab (Mathworks, Inc., Sherborn, MA, USA). Anatomical and EPI functional image data were first imported into SPM5. Slice timing correction was applied, and the EPI data sets were then realigned and checked for excessive head movement. There were no runs with head motion greater than 2 mm translation or 2° rotation. Each subject's own high-resolution, anatomical T1-weighted scan was then coregistered (mutual information coregistration) to their EPI images, and then the anatomical and EPI images were spatially normalized to MNI space, using the unified segmentation approach within SPM5. This method implicitly models lesions as part of the segmentation and normalization process, and it provides superior normalization results when compared to explicit lesion-masking procedures, such as cost function masking (Crinion et al., 2007). Spatial smoothing was performed by convolving the EPI data with a 6-mm, full-width at half-maximum (FWHM) Gaussian filter.
The first six images in each fMRI time series were eliminated to allow equilibrium in magnetization to occur. The BOLD hemodynamic response to each behavioral condition was then modeled in a boxcar design convolved with the SPM5 canonical hemodynamic response function. The SPM5 autocorrelation correction of the time series was conducted and a 128-s, high-pass, temporal filter was used to reduce low-frequency noise. After specifying the appropriate design matrix, the effects were estimated for each individual subject according to the general linear model (GLM) at each voxel. The contrast of interest for this first-level (fixed effects) analysis was the SAT social block minus the “bumper car” block. Thus, for these and all subsequent analyses, brain activation was defined as greater differential activation during the social interaction condition, relative to the “bumper car” control condition. Then SPM5 second-level random effects procedures utilized the contrast images from the individual subjects to perform within- and between-group analyses. These second-level analyses consisted of within-group t-tests for the TD and TBI groups, a between-group t-test, and separate SPM5 simple regression analyses relating SAT brain activation to total lesion volume within areas relevant to social cognition (see Table 1) and to DTI measures reflecting white matter integrity within the whole brain (i.e., whole-brain FA composite score), within white matter tracts that may be especially relevant to ToM (i.e., social brain FA composite score), and within the genu of the corpus callosum.
In SPM image analysis, a cluster is a spatially contiguous group of voxels that all exceed a statistical probability threshold (Friston et al., 1995). The cluster-defining (height) threshold for all analyses was initially set at voxel-level t = 1.79. All reported clusters were statistically significant (corrected p < .05) at the cluster level of inference, using the random field theory family-wise error (FWE) correction for multiple comparisons over the whole-brain volume. When the cluster size exceeded 2000 voxels, a more stringent cluster-level (height) threshold was used to reduce cluster sizes to 2000 voxels or less. All coordinates from statistically significant clusters were extracted and transformed to Talairach space (Talairach & Tournoux, 1988), using a Matlab script. The Talairach Daemon (http://ric.uthscsa.edu/projects/talairachdaemon.html) with the single-coordinate query option was then used to provide an anatomical label for each of the transformed coordinates.
RESULTS
Behavioral findings
The WASI IQ score and oral reading performance did not differ between the groups (see Table 2). There were also no significant between-group differences for accuracy or RT for either the social interaction or “bumper car” conditions of the SAT. However, both groups had mean accuracy scores that were under 60% for the “bumper car” portion of the fMRI task (TD mean = 58.33, TBI mean = 44.44).
TABLE 2.
Behavioral data for the traumatic brain injury and typically developing groups
| TD group |
TBI group |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| n | Mean | SD | Median | n | Mean | SD | Median | Probability* | |
| WASI IQ | 9 | 111.56 | 13.06 | 109.00 | 9 | 105.11 | 11.39 | 108.00 | .307 |
| GORT | 6 | 24.00 | 4.38 | 23.50 | 6 | 16.33 | 6.19 | 16.00 | .052 |
| SAT bumper condition | |||||||||
| Accuracy | 9 | 58.33 | 20.73 | 62.50 | 9 | 44.44 | 18.86 | 37.50 | .161 |
| Reaction Time | 9 | 1326.66 | 277.92 | 1292.00 | 9 | 1198.77 | 644.73 | 1050.67 | .331 |
| SAT social interaction condition | |||||||||
| Accuracy | 9 | 74.60 | 6.30 | 71.43 | 9 | 74.60 | 15.61 | 71.43 | .635 |
| Reaction time | 9 | 1571.65 | 579.79 | 1561.86 | 9 | 1115.71 | 305.94 | 1178.00 | .077 |
Notes: GORT = Gray Oral Reading Test, SAT = Social Attribution Task, TBI = traumatic brain injury, TD = typically developing, WASI = Wechsler Abbreviated Scale of Intelligence.
Wilcoxon two-sample test (two-sided probability, normal approximation).
DTI and lesion volume findings
Between-group comparisons were performed for each of the DTI ROIs while correcting for age, but due to the relatively small sample size, these results were not corrected for multiple comparisons. Least squares means (corrected for age), standard errors, F statistics, p values, and effect sizes (i.e., Cohen's f ) are reported in Table 3. Briefly, significant group differences were found in the FA of several ROIs, including the genu of the corpus callosum, the total corpus callosum, the left ventromedial frontal, and the right and left dorsolateral frontal areas. Of these regions, the magnitude of the between-group difference was greatest for the genu of the corpus callosum, and the relation between this measure and the fMRI data was examined further in an SPM regression analysis (see ‘Brain activation and white matter integrity’).
TABLE 3.
Between-group DTI results for individual regions of interest
| Region | TBI FA LSM (SE) | TD FA LSM (SE) | F-statistic | p value | Effect size (f) |
|---|---|---|---|---|---|
| Right ventromedial frontal | 0.365 (0.007) | 0.383 (0.007) | 3.34 | .088 | 0.47 |
| Left ventromedial frontal | 0.370 (0.006) | 0.398 (0.006) | 12.91 | .003* | 0.93 |
| Right dorsolateral frontal | 0.359 (0.006) | 0.383 (0.006) | 7.58 | .015* | 0.71 |
| Left dorsolateral frontal | 0.370 (0.006) | 0.394 (0.006) | 6.79 | .020* | 0.67 |
| Right arcuate fasciculus | 0.383 (0.013) | 0.389 (0.013) | 0.09 | .773 | 0.08 |
| Left arcuate fasciculus | 0.435 (0.007) | 0.455 (0.007) | 4.34 | .055 | 0.54 |
| Right inferior fronto-occipital longitudinal fasciculus | 0.419 (0.010) | 0.444 (0.010) | 2.33 | .148 | 0.39 |
| Left inferior fronto-occipital longitudinal fasciculus | 0.438 (0.008) | 0.460 (0.008) | 3.44 | .083 | 0.48 |
| Right inferior longitudinal fasciculus | 0.413 (0.010) | 0.400 (0.010) | 0.72 | .410 | 0.22 |
| Left inferior longitudinal fasciculus | 0.431 (0.009) | 0.442 (0.009) | 0.61 | .445 | 0.20 |
| Right uncinate fasciculus | 0.355 (0.007) | 0.361 (0.007) | 0.35 | .561 | 0.15 |
| Left uncinate fasciculus | 0.367 (0.009) | 0.381 (0.009) | 1.30 | .274 | 0.30 |
| Right cingulum bundle | 0.403 (0.007) | 0.419 (0.007) | 2.76 | .117 | 0.43 |
| Left cingulum bundle | 0.427 (0.011) | 0.447 (0.011) | 1.76 | .204 | 0.34 |
| Right anterior limb of the internal capsule | 0.363 (0.006) | 0.380 (0.006) | 4.01 | .064 | 0.52 |
| Left anterior limb of the internal capsule | 0.375 (0.006) | 0.386 (0.006) | 1.60 | .226 | 0.33 |
| Right posterior limb of the internal capsule | 0.443 (0.006) | 0.452 (0.006) | 1.20 | .290 | 0.28 |
| Left posterior limb of the internal capsule | 0.467 (0.007) | 0.476 (0.007) | 0.71 | .414 | 0.22 |
| Genu corpus callosum | 0.428 (0.006) | 0.459 (0.006) | 14.44 | .002* | 0.98 |
| Body corpus callosum | 0.444 (0.010) | 0.472 (0.010) | 4.34 | .055 | 0.54 |
| Splenium corpus callosum | 0.501 (0.007) | 0.518 (0.007) | 3.04 | .102 | 0.45 |
| Total corpus callosum | 0.453 (0.006) | 0.483 (0.006) | 13.36 | .002* | 0.94 |
Notes: For Cohen's f, 0.1 = small, 0.25 = moderate and 0.40 = large effect size.
p < .05. LSM = least squares mean (corrected for age); SE = standard error.
Total lesion volume was not significantly correlated with the whole-brain FA composite score (rho = –.10, p < .80), the social brain FA composite score (rho = .02, p < .97), or genu FA (rho = –.13, p < .73).
fMRI results
Tables 4 and 5 present a summary of the fMRI findings in relation to major anatomical structures, while the text includes selected Brodmann areas (BA). Anatomical information presented within the following text is based upon detailed output representing all voxels within each significant cluster, but to conserve space a general description is provided for each cluster's location.
TABLE 4.
Summary of fMRI findings for within- and between-group comparisons organized according to major anatomical regiona
| Within-group t-tests |
Between-group t-test |
||
|---|---|---|---|
| TD group | TBI group | TBI group > TD group | |
| Cluster-defining thresholdb | 3.66 | 3.92 | 1.93 |
| Number of clusters | 11 | 7 | 3 |
| Anatomical region | |||
| Frontal lobe | |||
| Inferior frontal gyrus | R | B | R |
| Medial frontal gyrus | B | ||
| Middle frontal gyrus | R | B | R |
| Superior frontal gyrus | B | R | |
| Precentral gyrus | R | R | |
| Paracentral lobule | |||
| Temporal lobe | |||
| Inferior temporal gyrus | B | R | R |
| Middle temporal gyrus | B | B | B |
| Superior temporal gyrus | B | B | R |
| Transverse temporal gyrus | R | ||
| Fusiform gyrus | B | L | B |
| Parietal lobe | |||
| Postcentral gyrus | R | ||
| Paracentral lobule | |||
| Angular gyrus | B | ||
| Supramarginal gyrus | R | R | |
| Inferior parietal lobule | R | R | R |
| Superior parietal lobule | |||
| Precuneus | B | B | B |
| Occipital lobe | |||
| Cuneus | B | ||
| Precuneus | B | B | |
| Fusiform gyrus | L | L | L |
| Inferior occipital gyrus | B | B | |
| Middle occipital gyrus | L | B | B |
| Superior occipital gyrus | L | ||
| Lingual gyrus | L | B | |
| Cingulate gyrus | |||
| Anterior cingulate gyrus | R | ||
| Posterior cingulate gyrus | B | ||
| Other | B | L | |
| Parahippocampal gyrus | B | ||
| Insula | R | R | R |
| Thalamus | |||
| Claustrum | R | ||
| Basal ganglia | |||
| Caudate (head) | B | ||
| Caudate (tail) | R | ||
| Cerebellum | B | L | B |
| Brainstem | |||
Notes: B = both sides, L = left side, R = right side, TBI = traumatic brain injury, TD = typically developing.
Results reported in this table reflect the location of voxels within significant clusters, as labeled by the Talairach Daemon, but with a focus on major anatomical areas to reduce length. For gyral locations, only those coordinates that are within gray matter are reported here (i.e., subgyral while matter coordinates were excluded from this table).
T value threshold that was used to define the cluster. This T value was conservatively increased to reduce the size of all significant clusters to 2000 voxels or less.
TABLE 5.
Summary of fMRI findings for the regression of SAT activation with DTI measures and lesion volume within the TBI group organized according to major anatomical regiona
| Whole-brain FA Negative regression | Social brain FA Negative regression | Genu FA Negative regression | Lesion volumeb Positive regression | |
|---|---|---|---|---|
| Cluster-defining thresholdc | 3.07 | 2.93 | 3.00 | 2.29 |
| Number of clusters | 12 | 14 | 11 | 4 |
| Anatomical region | ||||
| Frontal lobe | ||||
| Inferior frontal gyrus | R | R | ||
| Medial frontal gyrus | B | B | R | |
| Middle frontal gyrus | B | B | ||
| Superior frontal gyrus | L | B | R | |
| Precentral gyrus | B | B | B | B |
| Paracentral lobule | B | B | B | |
| Temporal lobe | ||||
| Inferior temporal gyrus | L | L | R | L |
| Middle temporal gyrus | R | B | R | B |
| Superior temporal gyrus | R | B | ||
| Transverse temporal gyrus | R | R | ||
| Hippocampus | L | L | L | |
| Fusiform gyrus | L | B | B | L |
| Parietal lobe | ||||
| Postcentral gyrus | B | B | B | B |
| Paracentral lobule | B | R | ||
| Angular gyrus | L | |||
| Supramarginal gyrus | ||||
| Inferior parietal lobule | L | B | B | |
| Superior parietal lobule | L | B | ||
| Precuneus | B | B | B | R |
| Occipital lobe | ||||
| Cuneus | B | L | B | B |
| Precuneus | B | B | B | B |
| Fusiform gyrus | L | B | B | |
| Inferior occipital gyrus | B | R | ||
| Middle occipital gyrus | B | L | R | B |
| Superior occipital gyrus | R | L | ||
| Lingual gyrus | B | B | R | B |
| Cingulate gyrus | ||||
| Anterior cingulate gyrus | R | B | ||
| Posterior cingulate gyrus | B | B | B | B |
| Other | B | B | ||
| Parahippocampal gyrus | B | B | B | B |
| Insula | B | |||
| Thalamus | B | B | ||
| Basal ganglia | ||||
| Caudate (head) | L | |||
| Caudate (body) | L | |||
| Caudate (tail) | L | |||
| Putamen | R | R | ||
| Globus pallidus | R | R | ||
| Cerebellum | B | B | B | B |
| Brainstem | B | B | B |
Notes: B = both sides, L = left side, R = right side, FA = fractional anisotropy.
Results reported in this table reflect the location of voxels within significant clusters, as labeled by the Talairach Daemon, but with a focus on major anatomical areas to reduce length. For gyral locations, only those coordinates that are within gray matter are reported here (i.e., subgyral while matter coordinates were excluded from this table).
Total lesion volume within brain areas thought to be especially important for social cognition (see Table 1).
T value threshold that was used to define the cluster. This T value was conservatively increased to reduce the size of all significant clusters to 2000 voxels or less.
Within-group analysis for control subjects
The TD control subjects had extensive activation during the SAT (social interaction minus “bumper car” contrast) with 11 clusters that included a number of brain areas that have been implicated in ToM (see Table 4 and Figure 1, panel A). There was a bilateral cluster within medial prefrontal areas that included the medial (right BA 10, bilateral BA 11) and superior frontal (right BA 10, left BA 11) gyri. A second medial frontal cluster was more superior in location and also included the medial and superior frontal gyri. Both medial prefrontal clusters were centered further anterior than the dorsal medial prefrontal ROIs of Schultz et al. (2003), but this activation appeared to overlap some with the location of a medial prefrontal cluster (–4, 60, 32) that was reported by Castelli, Happé, Frith, and Frith (2000) for a similar animated ToM task. Two other clusters were located within a more lateral area of the right frontal lobe, the first of which included part of the inferior (BA 11, BA 47) and middle frontal (BA 11) gyri, and the second was located within the middle frontal (BA 8) and superior frontal gyri. There were also seven other clusters that, in combination, included the right anterior cingulate gyrus (BA 25, BA 32) and bilateral parahippocampal and fusiform gyri (e.g., BA 20, BA 36, BA 37), the right temporal pole, and an area around the superior temporal sulcus (e.g., bilateral superior temporal gyri) and temporoparietal junction of both hemispheres. The posterior regions included several areas that Schultz et al. (2003) had also identified as exhibiting activation, such as the right fusiform gyrus and the right and left superior temporal gyri. Both studies also found activation within the right temporal pole, but Schultz et al. (2003) did not report a full set of coordinates for their results, and it is difficult to determine whether our activation includes precisely the same portions of that structure. The current study found no activation within the amygdala.
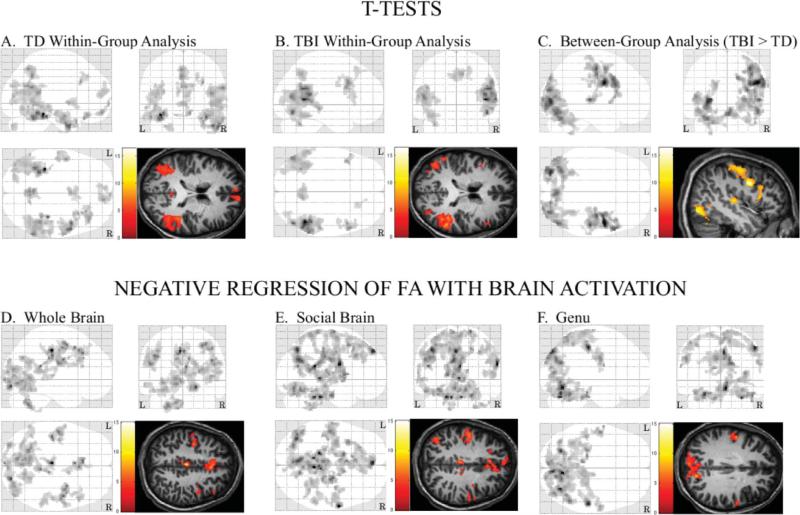
Figure 1.
Three-plane maximum intensity projections and image overlays for t-test contrasts and simple regression analyses. (A) Activation (social interaction minus “bumper car” contrast) for the TD adolescents. (B) Activation for adolescents with TBI. (C) Areas where SAT-related activation was greater within adolescents with TBI, relative to the TD comparison group. (D) Areas where there was a significant negative relation between activation during the SAT and the whole brain FA composite score. (E) Areas where there was a significant negative relation between SAT-related activation and the social brain FA composite score. (F) Areas where there was a significant negative relation between whole brain activation and FA within the genu of the corpus callosum. L = left side, R = right side.
Within-group analysis for TBI subjects
The TBI subjects had seven significant clusters with SAT activation (see Table 4 and Figure 1, panel B). A right-sided cluster included part of the middle and superior frontal gyri and was centered more laterally than the right medial prefrontal cluster reported by Schultz et al. (2003). There were also two lateral frontal clusters and one of these included the right precentral gyrus and the inferior (BA 6, BA 9, BA 44, BA 45, BA 46) and middle frontal gyri (BA 6, BA 8, BA 9, BA 46). The other lateral frontal cluster was located within the left inferior (BA 9) and middle frontal gyri (BA 9, BA 46). There was a large left-sided, posterior cluster that included the cerebellum and a portion of the occipital (e.g., inferior and middle occipital gyri) and temporal lobes (e.g., fusiform gyrus and the middle and superior temporal gyri). A right-sided cluster included the insula and parts of the occipital (e.g., inferior and middle occipital gyri), temporal (inferior, middle, and superior temporal gyri), and parietal lobes (e.g., inferior parietal lobule and supramarginal gyri). The other posterior clusters were smaller in size and were located primarily within the right occipital lobe (e.g., middle occipital gyrus) and bilaterally within the precuneus (BA 7).
Between-group comparison
There were no areas where the TD subjects had greater activation than the TBI subjects, but there were three clusters where the TBI group had greater activation than the TD group (see Table 4 and Figure 1, panel C). This included a right-sided cluster located within a posterior and lateral portion of the frontal lobe (i.e., precentral, inferior frontal, and middle frontal gyri), the postcentral gyrus and inferior parietal lobule (BA 40), the insula, the claustrum, and the transverse temporal and superior temporal gyri (BA 13, BA 22, BA 41). Another cluster included the cerebellum and portions of the right occipital (e.g., cuneus, lingual gyrus, and the inferior and middle occipital gyri), parietal (e.g., precuneus), and temporal lobes (e.g., fusiform gyrus and the inferior and middle temporal gyri). There was also a bilateral cluster that was primarily located within the left occipital lobe (e.g., cuneus, lingual gyrus, and the inferior and middle occipital gyri) and that extended into the left temporal lobe (e.g., fusiform and middle temporal gyri), the lingual gyrus of the right occipital lobe, and the cerebellum.
Brain activation and white matter integrity
Separate exploratory SPM5 simple regression analyses were conducted to relate activation during the SAT to (1) the whole-brain FA composite score, which was considered an index for overall white matter integrity; (2) the social brain FA composite score, which was included to contain a limited set of specific tracts considered to be most important for ToM; and (3) the genu of the corpus callosum FA. The genu was selected because this region exhibited the greatest between-group difference in FA, and also because of its potential role in connection of medial prefrontal regions important in social cognition.
There was no significant positive correlation between SAT activation and the whole-brain FA composite score in TBI subjects. However, there were 12 clusters where there was a significant negative relation, including a bilateral medial frontal cluster that included the cingulate (right BA 24, bilateral BA 32), medial (e.g., BA 6, BA 8, right BA32), and superior frontal gyri (left BA 8) (see Table 5 and Figure 1, panel D). Three other clusters were located primarily within more lateral areas of the right frontal lobe and, together, these included the precentral gyrus and the inferior (BA 9, BA 44) and middle frontal gyri (BA 6, BA 8, BA 9, BA 46). There was a large bilateral cluster within both occipital lobes that extended into the cerebellum and part of the left temporal lobe (e.g., fusiform and inferior temporal gyri), the posterior cingulate gyrus (e.g., BA 30), and the parahippocampal gyrus. Together, the other seven clusters were scattered throughout posterior brain areas and included structures within the cerebellum, the right temporal lobe, and both occipital and parietal lobes.
In the TBI subjects, there were 14 significant clusters where there was a negative relation between SAT activation and the social brain FA composite score. In general, these clusters included many of the same structures that were identified in the regression analysis for the whole-brain FA composite score (see Table 5 and Figure 1, panel E). However, a significant negative association between activation and genu FA had a distribution that was more restricted and excluded a number of anterior brain regions identified in the other analyses (see Figure 1, panel F).
Brain activation and lesions
An SPM5 simple regression analysis indicated no significant negative relation between SAT activation and the lesion volume measure, but there were four clusters where there was a significant positive correlation, and one of these was located primarily within the right parietal lobe. A second cluster included parts of the cerebellum, brainstem, diencephalon, and structures within the inferior portion of the left cerebrum (e.g., parahippocampal and fusiform gyri), while another was located primarily within the left parietal lobe (e.g., postcentral gyrus, inferior parietal lobule). There was also a large bilateral cluster that included deep brain structures, such as the midbrain, and that extended into the posterior cingulate gyrus, both occipital lobes (e.g., cuneus, lingual gyrus), and the right middle temporal gyrus (see Table 5).
DISCUSSION
During the SAT, the TD adolescents exhibited an activation pattern that was generally similar to that reported by previous investigations of ToM (Carrington & Bailey, 2009), including structures such as the medial prefrontal cortex, cingulate cortex, and posterior temporal and parietal areas. The TBI subjects had significant activation within many of these same areas, but their activation was generally more intense and excluded medial prefrontal areas that were activated in previous studies of ToM with healthy subjects (e.g., Castelli et al., 2000; Schultz et al., 2003). Brain activation during the SAT was also related to variables reflecting TBI neuropathology, including lesion volume and DTI measures of white matter integrity. As expected, greater neuropathology (i.e., greater lesion volume, decreased white matter integrity as indicated by lower FA) was associated with higher activation levels, and this is generally consistent with previous reports of a relationship between greater TBI severity and increased activation during cognitive tasks (e.g., Scheibel et al., 2009). Overall, these findings provide initial information about differences in activation during ToM in adolescents who have sustained TBI, relative to matched TD adolescents, as well as some preliminary results indicating that both DAI and focal lesions contribute to the activation changes.
Typically developing adolescents had SAT-related activation in many of the same brain areas that activated in normal adults studied by Schultz et al. (2003), but examination of our cluster coordinates in relation to that study's ROIs indicated only partial convergence. For example, our TD adolescents had medial prefrontal clusters that were further anterior than the activation reported by Schultz et al. (2003), but these appear to overlap more with the location of a medial prefrontal cluster from another ToM study (Castelli et al., 2000). The location and role of some components of the ToM network may vary among individuals, perhaps reflecting differences in experience or development (Bird, Castelli, Malik, Frith, & Husain, 2004; Moriguichi et al., 2007), and these factors may account for some minor differences in the precise location of brain activation within various studies. In general, however, the pattern observed in the current study is consistent with prior reports of ToM-related brain activation, and replicated findings of medial prefrontal and superior temporal activation (Carrington & Bailey, 2009; Castelli et al., 2000; Schultz et al., 2003).
Carrington and Bailey (2009) reviewed the findings from functional neuroimaging research and concluded that the medial prefrontal area is the region that is most consistently activated in studies using ToM tasks. However, there is some evidence that the temporoparietal junction has a more specific and central role in ToM (Saxe & Kanwisher, 2003; Saxe & Powell, 2006). Many studies have also found task-related activation in structures around the superior temporal sulcus and, in combination with medial prefrontal cortex, these structures may function as part of a neural network that mediates ToM reasoning (Carrington & Bailey, 2009). Other brain areas (e.g., insula) may also activate during various types of ToM tasks, but activation in these areas has been observed with less consistency and their recruitment may reflect ancillary aspects of the particular task (Carrington & Bailey, 2009). In the within-group analysis, our TBI subjects had significant activation within a number of the same areas that activated in our TD adolescents, such as the posterior portion of the superior temporal gyrus, but their prefrontal activation had a more lateral distribution.
When directly compared to TD adolescents, the TBI subjects had greater activation within right lateral frontal and parietal areas, and bilateral increases within posterior brain regions. Some of these posterior areas have been implicated in social reasoning, such as the fusiform and superior temporal gyri (Blakemore, 2008; Schultz et al., 2003), while others are not typically considered to be involved in social cognition. A similar finding was reported by Newsome et al. (2010), who found increased posterior activation when children with TBI were asked to think about their own traits from another person's perspective. Based upon the current findings and those of Newsome et al. (2010), it appears that the neural resources utilized for social cognition may be greater following brain injury and that these alterations are not restricted to areas that typically mediate such functions in uninjured individuals. Activation increases during cognitive fMRI paradigms are frequently observed in association with neuropathology, and proposed interpretations of this finding have included the disinhibition of duplicate neural systems, learning-related neuroplasticity, and cognitive reorganization (Price & Friston, 2002). When task performance is equated in comparisons with a control group, the overactivation may reflect a higher level of effort, perhaps as a consequence of inefficient processing or as a form of compensation involving the allocation of additional cognitive and neural resources (Price & Friston, 2002; Ricker, Hillary, & DeLuca, 2001). There has also been some speculation that such alterations in the level and pattern of brain activation following TBI may reflect decreases in neural resources or neural inefficiency due to DAI (e.g., Huang et al., 2009; Scheibel et al., 2009).
A large number of studies have documented changes in white matter following TBI, including postmortem findings (e.g., Adams, Mitchell, Graham, & Doyle, 1977) and the in vivo examination of white matter by imaging techniques such as DTI (e.g.,Wilde et al., 2006). The present study also used DTI and found evidence for reduced white matter integrity in the current sample of adolescents with moderate to severe TBI, including reduced FA within the genu of the corpus callosum and the total corpus callosum, and left ventromedial and left and right dorsolateral frontal areas. White matter traversing the genu of the corpus callosum connects the right and left medial prefrontal areas, regions previously reported to be important in social cognition (Blakemore, 2008; Carrington & Bailey, 2009). Additionally, the dorsolateral frontal area, which includes the inferior frontal gyrus, has also been implicated in social cognition. These regions are also known to be vulnerable to TBI-related injury (Povlishock & Katz, 2005). Because FA was examined within a large number of regions, further analysis of these findings was conducted using only the single region where the changes were greatest (i.e., genu) and two composite scores, one of which reflected damage to white matter within the entire brain and another that was specific for regions that are related to ToM.
Regression analyses using the FA composite scores indicated a relation between diffuse decreases in white matter integrity and activation increases within a large posterior portion of the brain, as well as the anterior cingulate cortex and medial prefrontal areas. In contrast, reductions in genu FA were related to increased activation within posterior areas, and there was no significant correlation between genu FA and ToM-related activation within many of the more anterior brain structures. This latter finding was unexpected since, anatomically, the medial prefrontal cortex is in relatively close proximity to the genu and because interhemispheric prefrontal connections pass through the genu and anterior body of the corpus callosum (Zarei et al., 2006). Possibly, the same traumatic forces that injured the genu white matter also produced local injury to the prefrontal cortex, thus forcing greater reliance upon posterior brain areas to perform the SAT. However, another possibility is that damage to white matter in and around the genu may have caused some disconnection among components of the social brain network that are relevant to ToM, including tracts between the medial prefrontal cortex and posterior areas such as the superior temporal gyrus. Disrupted functional connectivity has been proposed as a mechanism for decreased ToM performance in individuals with autism or Asperger's syndrome (Castelli et al., 2002), including the loss of top-down modulation by more anterior brain areas upon the extrastriate cortex. Similarly, decreased top-down modulation may also contribute to the activation increases noted among posterior areas following TBI, and, based upon the current DTI findings, physical injury to the white matter appears to contribute to the changes in brain activation that were observed during the SAT.
The neuropathology associated with TBI is heterogeneous and, in addition to DAI, focal lesions in gray and white matter are also common in individuals with moderate to severe TBI (Povlishock & Katz, 2005). Many lesions in the current TBI sample were relatively small, however, and most subjects had multiple areas of focal pathology, and all but one had at least one frontal lobe lesion. These lesion characteristics and the small sample size made the analysis of lesion location in relation to brain activation or SAT performance variables impractical, but total lesion volume within task-relevant brain areas was used as a variable reflecting the overall severity of focal injury. The regression analysis with lesion volume indicated increased activation, possibly reflecting increased recruitment, of nonfrontal brain structures in association with greater focal neuropathology. However, lesion volume was not correlated with the FA measures, and, since greater lesion volume and reduced white matter integrity were both associated with activation increases, it appears that these different types of neuropathology make separate contributions to the activation changes that occur following TBI.
Although the use of DTI measures and lesion volume to examine the relationship between the severity of TBI neuropathology and brain activation is relatively new, previous studies have reported that activation during cognitive fMRI tasks is associated with other severity measures in individuals with neurological disorders. For example, Mainero et al. (2004) reported that patients with multiple sclerosis had increased activation on attention and memory tasks that was positively correlated with lesion load. Similarly, Scheibel et al. (2009) found that the level of activation during a visual stimulus-response compatibility task was greater when adults had more severe TBI, as indicated by lower initial scores on the Glasgow Coma Scale. In that study the activation pattern changed with increasing severity so that additional brain areas, such as the left lateral frontal cortex, exhibited significant increases only when the TBI was more severe. The current results are generally consistent with those previous findings, since changes in brain activation were noted in association with the degree of injury, but in the present investigation the design focused on ToM. Alterations in the level and pattern of activation that were observed in association with neuropathology may reflect higher resource utilization to perform the ToM task, perhaps as a form of compensation for reduced neural efficiency (Scheibel et al., 2009). However, it is also possible that some activation increases and diffusivity associated with TBI reflect pathological processes, such as the loss of functional connectivity and decreased modulatory control (Castelli et al., 2002).
Performance on ToM tasks is related to deficits in social behavior following TBI (Hynes, Stone, & Kelso, 2011 this issue). However, in the present study, the prescan training allowed both groups to complete the social interaction condition of the SAT with \hbox{accuracy} that exceeded 70%. Accuracy during the “bumper car” control condition was under 60% for both groups despite the fact that the overall level of intellectual functioning, as reflected by the WASI IQ, was within the average to high-average range and did not differ between the groups. This accuracy level for the control condition is lower than that found by Schultz et al. (2003), who reported 74% correct for the “bumper car” condition with a nearly identical task. The sample used by Schultz et al. (2003) consisted entirely of normal adults, however, and the bumper car condition can be relatively challenging. It is possible that adolescents in the current study had not yet acquired the skills required to accurately comprehend the relationships among velocity, direction, and mass that are needed to perform well during this control condition (Jacobs, Michaels, & Runeson, 2000).
The current study's design has several limitations and produced some unexpected findings. The first of these, as noted above, was lower than expected accuracy during the control (i.e., “bumper car”) condition of the fMRI task. However, the activation pattern within the group of TD adolescents was generally consistent with the results from previous studies of ToM function (Carrington & Bailey, 2009), including research using similar animated stimuli (e.g., Castelli et al., 2000). Amygdala activation was observed in some previous studies (e.g., Schultz et al., 2003), and this was not found in the current investigation, but a limitation of BOLD imaging methods is the weak signal within the amygdala (LaBar, Gitelman, Mesulam, & Parrish, 2001), and activation during ToM has not been reported consistently within this brain area (Carrington & Bailey, 2009). In addition, one study examining ToM in normal individuals found greater amygdala activation in adults than in a group of children (Kobayashi, Glover, & Temple, 2007), suggesting that lack of amygdala activation within the current sample may reflect maturational differences.
Another limitation of the current study is that the sample size did not allow for corrections for multiple comparisons, and the design was not optimized for exploring relationships among brain activation, neuropathology variables (e.g., lesion location), SAT performance, and additional measures of cognitive function and social competence. Thus, it is not clear from the present results how focal injury to different brain structures relates to the SAT or to what degree the fMRI findings reflect alterations in executive functions and other general cognitive skills, as opposed to abilities more specially associated with ToM. A larger study is currently underway to explore these issues further.
Acknowledgments
This research was supported by National Institutes of Health grant NS021889. We thank the adolescents and their families for their participation, Xiaoqi Li for statistical assistance, and Dr Sandra Chapman for advice regarding the design of this study. Recruitment and imaging of the subjects were facilitated by the General Clinical Research Center at Texas Children's Hospital and Ben Taub General Hospital in Houston, as well as the Children's Medical Center and Our Children's House at the Baylor Medical Center in Dallas and the Advanced Imaging Center at the University of Texas Southwestern Medical Center. The South Central Mental Illness Research, Education, and Clinical Center (MIRECC) and the Michael E. DeBakey Veteran's Affairs Medical Center provided access to laboratory facilities used for the analysis of the image data.
Footnotes
Publisher's Disclaimer: Full terms and conditions of use: http://www.tandfonline.com/page/terms-and-conditions This article may be used for research, teaching, and private study purposes. Any substantial or systematic reproduction, redistribution, reselling, loan, sub-licensing, systematic supply, or distribution in any form to anyone is expressly forbidden. The publisher does not give any warranty express or implied or make any representation that the contents will be complete or accurate or up to date. The accuracy of any instructions, formulae, and drug doses should be independently verified with primary sources. The publisher shall not be liable for any loss, actions, claims, proceedings, demand, or costs or damages whatsoever or howsoever caused arising directly or indirectly in connection with or arising out of the use of this material.
REFERENCES
- Adams H, Mitchell DE, Graham DI, Doyle D. Diffuse brain damage of the immediate impact type. Its relationship to ‘primary brain-stem damage’ in head injury. Brain. 1977;100(3):489–502. doi: 10.1093/brain/100.3.489. [DOI] [PubMed] [Google Scholar]
- Adolphs R. The neurobiology of social cognition. Current Opinion in Neurobiology. 2001;11:231–239. doi: 10.1016/s0959-4388(00)00202-6. [DOI] [PubMed] [Google Scholar]
- Aldrich NJ, Tenenbaum HR, Brooks PJ, Harrison KS, Sines J. Perspective taking in children's narratives about jealousy. British Journal of Developmental Psychology. 2011;29(1):86–109. doi: 10.1348/026151010X533238. [DOI] [PubMed] [Google Scholar]
- Babikian T, Asarnow R. Neurocognitive outcomes and recovery after pediatric TBI: Meta-analytic review of the literature. Neuropsychology. 2009;23(3):283–296. doi: 10.1037/a0015268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron-Cohen S, Leslie AM, Frith U. Does the autistic child have a “theory of mind”? Cognition. 1985;21(1):37–46. doi: 10.1016/0010-0277(85)90022-8. [DOI] [PubMed] [Google Scholar]
- Bird CM, Castelli F, Malik O, Frith U, Husain M. The impact of extensive medial frontal lobe damage on “theory of mind” and cognition. Brain. 2004;127(4):914–928. doi: 10.1093/brain/awh108. [DOI] [PubMed] [Google Scholar]
- Blakemore SJ. The social brain in adolescence. Nature Reviews Neuroscience. 2008;9(4):267–277. doi: 10.1038/nrn2353. [DOI] [PubMed] [Google Scholar]
- Blakemore SJ, Boyer P, Pachot-Clouard M, Meltzoff A, Segebarth C, Decety J. The detection of contingency and animacy from simple animations in the human brain. Cerebral Cortex. 2003;13:837–844. doi: 10.1093/cercor/13.8.837. [DOI] [PubMed] [Google Scholar]
- Bowler DM. “Theory of mind” in Asperger's syndrome. Journal of Child Psychology and Psychiatry. 1992;33(5):877–893. doi: 10.1111/j.1469-7610.1992.tb01962.x. [DOI] [PubMed] [Google Scholar]
- Carrington SJ, Bailey AJ. Are there theory of mind regions in the brain? A review of the neuroimaging literature. Human Brain Mapping. 2009;30(8):2313–2335. doi: 10.1002/hbm.20671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castelli F, Frith C, Happé F, Frith U. Autism, Asperger syndrome and brain mechanisms for the attribution of mental states to animated shapes. Brain. 2002;125(8):1839–1849. doi: 10.1093/brain/awf189. [DOI] [PubMed] [Google Scholar]
- Castelli F, Happé F, Frith U, Frith C. Movement and minds: A functional imaging study of perception and interpretation of complex intentional movement patterns. NeuroImage. 2000;12(3):314–325. doi: 10.1006/nimg.2000.0612. [DOI] [PubMed] [Google Scholar]
- Channon S, Crawford S. Mentalising and social problem-solving after brain injury. Neuropsychological Rehabilitation. 2010;20(5):739–759. doi: 10.1080/09602011003794583. [DOI] [PubMed] [Google Scholar]
- Chevallier C, Noveck I, Happé F, Wilson D. What's in a voice? Prosody as a test case for the theory of mind account of autism. Neuropsychologia. 2011;49(3):507–517. doi: 10.1016/j.neuropsychologia.2010.11.042. [DOI] [PubMed] [Google Scholar]
- Christodoulou C, DeLuca J, Ricker JH, Madigan NK, Bly BM, Lange G, et al. Functional magnetic resonance imaging of working memory impairment after traumatic brain injury. Journal of Neurology, Neurosurgery, and Psychiatry. 2001;71(2):161–168. doi: 10.1136/jnnp.71.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crinion J, Ashburner J, Leff A, Brett M, Price C, Friston K. Spatial normalization of lesioned brains: Performance evaluation and impact on fMRI analyses. NeuroImage. 2007;37:866–875. doi: 10.1016/j.neuroimage.2007.04.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis M, Agostino A, Roncadin C, Levin H. Theory of mind depends on domain-general executive functions of working memory and cognitive inhibition in children with traumatic brain injury. Journal of Clinical and Experimental Neuropsychology. 2009;31(7):835–847. doi: 10.1080/13803390802572419. [DOI] [PubMed] [Google Scholar]
- Dennis M, Purvis K, Barnes MA, Wilkinson M, Winner E. Understanding of literal truth, ironic criticism, and deceptive praise following childhood head injury. Brain and Language. 2001;78(1):1–16. doi: 10.1006/brln.2000.2431. [DOI] [PubMed] [Google Scholar]
- Dodrell-Feder D, Koster-Hale J, Bedny M, Saxe R. fMRI item analysis in a theory of mind task. NeuroImage. 2011;55:705–712. doi: 10.1016/j.neuroimage.2010.12.040. [DOI] [PubMed] [Google Scholar]
- Ewing-Cobbs L, Barnes M, Fletcher JM, Levin HS, Swank PR, Song J. Modeling of longitudinal academic achievement scores after pediatric traumatic brain injury. Developmental Neuropsychology. 2004;25(1–2):107–133. doi: 10.1080/87565641.2004.9651924. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes A, Worsley KJ, Poline JP, Frith CD, Frackowiak RSJ. Statistical parametric maps in functional imaging: A general linear approach. Human Brain Mapping. 1995;2:189–210. [Google Scholar]
- Geraci A, Surian L, Ferraro M, Cantagallo A. Theory of mind in patients with ventromedial or dorso-lateral prefrontal lesions following traumatic brain injury. Brain Injury. 2010;24(7–8):978–987. doi: 10.3109/02699052.2010.487477. [DOI] [PubMed] [Google Scholar]
- Gobbini MI, Koralek AC, Bryan RE, Montgomery KJ, Haxby JV. Two takes on the social brain: A comparison of theory of mind tasks. Journal of Cognitive Neuroscience. 2007;19:1803–1814. doi: 10.1162/jocn.2007.19.11.1803. [DOI] [PubMed] [Google Scholar]
- Heider F, Simmel M. An experimental study of apparent behavior. American Journal of Psychology. 1944;57(2):243–259. [Google Scholar]
- Henry JD, Phillips LH, Crawford JR, Ietswaart M, Summers F. Theory of mind following traumatic brain injury: The role of emotion recognition and executive dysfunction. Neuropsychologia. 2006;44(10):1623–1628. doi: 10.1016/j.neuropsychologia.2006.03.020. [DOI] [PubMed] [Google Scholar]
- Hillary FG. Neuroimaging of working memory dysfunction and the dilemma with brain reorganization hypotheses. Journal of the International Neuropsychological Society. 2008;14(4):526–534. doi: 10.1017/S1355617708080788. [DOI] [PubMed] [Google Scholar]
- Huang MX, Theilmann RJ, Robb A, Angeles A, Nichols S, Drake A, et al. Integrated imaging approach with MEG and DTI to detect mild traumatic brain injury in military and civilian patients. Journal of Neurotrauma. 2009;26(8):1213–1226. doi: 10.1089/neu.2008.0672. [DOI] [PubMed] [Google Scholar]
- Hughes C, Ensor R, Marks A. Individual differences in false belief understanding are stable from 3 to 6 years of age and predict children's mental state talk with school friends. Journal of Experimental Child Psychology. 2010;108(1):96–112. doi: 10.1016/j.jecp.2010.07.012. [DOI] [PubMed] [Google Scholar]
- Hynes CA, Stone VE, Kelso LA. Social and emotional competence in traumatic brain injury: New and established assessment tools. Social Neuroscience. 2011;XX:XXX–XXX. doi: 10.1080/17470919.2011.584447. [DOI] [PubMed] [Google Scholar]
- Jacobs DM, Michaels CF, Runeson S. Learning to perceive the relative mass of colliding balls: The effects of ratio scaling and feedback. Perception and Psychophysics. 2000;62(7):1332–1340. doi: 10.3758/bf03212135. [DOI] [PubMed] [Google Scholar]
- Janusz JA, Kirkwood MW, Yeates KO, Taylor HG. Social problem-solving skills in children with traumatic brain injury: Long-term outcomes and prediction of social competence. Child Neuropsychology. 2002;8(3):179–194. doi: 10.1076/chin.8.3.179.13499. [DOI] [PubMed] [Google Scholar]
- Kobayashi C, Glover GH, Temple E. Children's and adults’ neural bases of verbal and non-verbal ‘theory of mind’. Neuropsychologia. 2007;45(7):1522–1532. doi: 10.1016/j.neuropsychologia.2006.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaBar KS, Gitelman DR, Mesulam MM, Parrish TB. Impact of signal-to-noise on functional MRI of the human amygdala. Neuroreport. 2001;12(16):3461–3464. doi: 10.1097/00001756-200111160-00017. [DOI] [PubMed] [Google Scholar]
- Levin HS, Song J, Scheibel RS, Fletcher JM, Harward H, Lilly M, et al. Concept formation and problem-solving following closed head injury in children. Journal of the International Neuropsychological Society. 1997;3(6):598–607. [PubMed] [Google Scholar]
- Levin HS, Wilde EA, Chu Z, Yallampalli R, Hanten GR, Li X, et al. Diffusion tensor imaging in relation to cognitive and functional outcome of traumatic brain injury in children. Journal of Head Trauma Rehabilitation. 2008;23(4):197–208. doi: 10.1097/01.HTR.0000327252.54128.7c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mainero C, Caramia F, Pozzilli C, Pisani A, Pestalozza I, Borriello G, et al. fMRI evidence of brain reorganization during attention and memory tasks in multiple sclerosis. NeuroImage. 2004;21(3):858–867. doi: 10.1016/j.neuroimage.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Martín-Rodríguez JF, León-Carrión J. Theory of mind deficits in patients with acquired brain injury: A quantitative review. Neuropsychologia. 2010;48(5):1181–1191. doi: 10.1016/j.neuropsychologia.2010.02.009. [DOI] [PubMed] [Google Scholar]
- Max JE, Levin HS, Schachar RJ, Landis J, Saunders AE, Ewing-Cobbs L, et al. Predictors of personality change due to traumatic brain injury in children and adolescents six to twenty-four months after injury. Journal of Neuropsychiatry and Clinical Neurosciences. 2006;18(1):21–32. doi: 10.1176/jnp.18.1.21. [DOI] [PubMed] [Google Scholar]
- Mori S, Crain BJ, Chacko VP, van Zijl PC. Three-dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Annals of Neurology. 1999;45(2):265–269. doi: 10.1002/1531-8249(199902)45:2<265::aid-ana21>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Moriguchi Y, Ohnishi T, Mori T, Matsuda H, Komaki G. Changes of brain activity in the neural substrates for theory of mind during childhood and adolescence. Psychiatry and Clinical Neurosciences. 2007;61(4):355–363. doi: 10.1111/j.1440-1819.2007.01687.x. [DOI] [PubMed] [Google Scholar]
- Muller F, Simion A, Reviriego E, Galera C, Mazaux JM, Barat M, et al. Exploring theory of mind after severe traumatic brain injury. Cortex. 2010;46(9):1088–1099. doi: 10.1016/j.cortex.2009.08.014. [DOI] [PubMed] [Google Scholar]
- Netsch T, van Muiswinkel A. Quantitative evaluation of image-based distortion correction in diffusion tensor imaging. IEEE Transactions on Medical Imaging. 2004;23(7):789–798. doi: 10.1109/TMI.2004.827479. [DOI] [PubMed] [Google Scholar]
- Newsome MR, Scheibel RS, Hanten G, Chu Z, Steinberg JL, Hunter JV, et al. Brain activation while thinking about the self from another person's perspective after traumatic brain injury. Neuropsychology. 2010;24(2):139–147. doi: 10.1037/a0017432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia. 1971;9(1):97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Oni MB, Wilde EA, Bigler ED, McCauley SR, Wu TC, Yallampalli R, et al. Diffusion tensor imaging analysis of frontal lobes in pediatric traumatic brain injury. Journal of Child Neurology. 2010;25(8):976–984. doi: 10.1177/0883073809356034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Povlishock JT, Katz DI. Update of neuropathology and neurological recovery after traumatic brain injury. Journal of Head Trauma Rehabilitation. 2005;20:76–94. doi: 10.1097/00001199-200501000-00008. [DOI] [PubMed] [Google Scholar]
- Price CJ, Friston KJ. Functional imaging studies of neuropsychological patients: Applications and limitations. Neurocase. 2002;8:345–354. doi: 10.1076/neur.8.4.345.16186. [DOI] [PubMed] [Google Scholar]
- Ricker JH, Hillary FG, DeLuca J. Functionally activated brain imaging (O-15 PET and fMRI) in the study of learning and memory after traumatic brain injury. Journal of Head Trauma Rehabilitation. 2001;16:191–205. doi: 10.1097/00001199-200104000-00007. [DOI] [PubMed] [Google Scholar]
- Saxe R, Kanwisher N. People thinking about thinking people. The role of the temporo-parietal junction in “theory of mind.”. NeuroImage. 2003;19:1835–1842. doi: 10.1016/s1053-8119(03)00230-1. [DOI] [PubMed] [Google Scholar]
- Saxe R, Powell LJ. It's the thought that counts: Specific brain regions for one component of theory of mind. Psychological Science. 2006;17:692–699. doi: 10.1111/j.1467-9280.2006.01768.x. [DOI] [PubMed] [Google Scholar]
- Scheibel RS, Newsome MR, Troyanskaya M, Steinberg JL, Goldstein FC, Mao H, et al. Effects of severity of traumatic brain injury and brain reserve on cognitive-control related brain activation. Journal of Neurotrauma. 2009;26(9):1447–1461. doi: 10.1089/neu.2008.0736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt AT, Hanten GR, Li X, Orsten KD, Levin HS. Emotion recognition following pediatric traumatic brain injury: Longitudinal analysis of emotional prosody and facial expression. Neuropsychologia. 2010;48:2869–2877. doi: 10.1016/j.neuropsychologia.2010.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz RT, Grelotti DJ, Klin A, Kleinman J, Van der Gaag C, Marois R, et al. The role of the fusiform face area in social cognition: Implications for the pathobiology of autism. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 2003;358(1430):415–427. doi: 10.1098/rstb.2002.1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sesma HW, Slomine BS, Ding R, McCarthy ML. Executive functioning in the first year after pediatric traumatic brain injury. Pediatrics. 2008;121(6):e1686–e1695. doi: 10.1542/peds.2007-2461. [DOI] [PubMed] [Google Scholar]
- Snodgrass C, Knott F. Theory of mind in children with traumatic brain injury. Brain Injury. 2006;20(8):825–833. doi: 10.1080/02699050600832585. [DOI] [PubMed] [Google Scholar]
- Stocks EL, Lishner DA, Waits BL, Downum EM. I'm embarrassed for you: The effect of valuing and perspective taking on empathic embarrassment and empathic concern. Journal of Applied Social Psychology. 2011;41(1):1–26. [Google Scholar]
- Stone VE, Baron-Cohen S, Knight RT. Frontal lobe contributions to theory of mind. Journal of Cognitive Neuroscience. 1998;10(5):640–656. doi: 10.1162/089892998562942. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereo-taxic atlas of the human brain. Thieme Medical Publishers; New York, NY: 1988. [Google Scholar]
- Teasdale G, Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet. 1974;2(7872):81–84. doi: 10.1016/s0140-6736(74)91639-0. [DOI] [PubMed] [Google Scholar]
- Turkstra LS, Dixon TM, Baker KK. Theory of mind and social beliefs in adolescents with traumatic brain injury. NeuroRehabilitation. 2004;19(3):245–256. [PubMed] [Google Scholar]
- van den Bos W, van Dijk E, Westenberg M, Rombouts SA, Crone EA. Changing brains, changing perspectives: The neurocognitive development of reciprocity. Psychological Science. 2011;22(1):60–70. doi: 10.1177/0956797610391102. [DOI] [PubMed] [Google Scholar]
- Wakana S, Caprihan A, Panzenboeck MM, Fallon JH, Perry M, Gollub RL, et al. Reproducibility of quantitative tractography methods applied to cerebral white matter. NeuroImage. 2007;36(3):630–644. doi: 10.1016/j.neuroimage.2007.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence. Psychological Corporation; San Antonio, TX: 1999. [Google Scholar]
- Weiderholt JL, Bryant BR. Gray Oral Reading Test. 4th ed. ProEd; Austin, TX: 2001. [Google Scholar]
- Weisskoff RM. Simple measurement of scanner stability for functional NMR imaging of activation in the brain. Magnetic Resonance Medicine. 1996;36(4):643–645. doi: 10.1002/mrm.1910360422. [DOI] [PubMed] [Google Scholar]
- Wilde EA, Chu Z, Bigler ED, Hunter JV, Fearing MA, Hanten G, et al. Diffusion tensor imaging in the corpus callosum in children after moderate to severe traumatic brain injury. Journal of Neurotrauma. 2006;23(10):1412–1426. doi: 10.1089/neu.2006.23.1412. [DOI] [PubMed] [Google Scholar]
- Wilde EA, McCauley SR, Chu Z, Hunter JV, Bigler ED, Yallampalli R, et al. Diffusion tensor imaging of hemispheric asymmetries in the developing brain. Journal of Clinical and Experimental Neuropsychology. 2009;31(2):205–218. doi: 10.1080/13803390802098118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilde EA, Ramos MA, Yallampalli R, Bigler ED, McCauley SR, Chu Z, et al. Diffusion tensor imaging of the cingulum bundle in children after traumatic brain injury. Developmental Neuropsychology. 2010;35(3):333–351. doi: 10.1080/87565641003696940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeates KO, Bigler ED, Dennis M, Gerhardt CA, Rubin KH, Stancin T, et al. Social outcomes in childhood brain disorder: A heuristic integration of social neuroscience and developmental psychology. Psychological Bulletin. 2007;133(3):535–556. doi: 10.1037/0033-2909.133.3.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarei M, Johansen-Berg H, Smith S, Ciccarelli O, Thompson AJ, Mathews PM. Functional anatomy of interhemispheric cortical connections in the human brain. Journal of Anatomy. 2006;209(3):311–320. doi: 10.1111/j.1469-7580.2006.00615.x. [DOI] [PMC free article] [PubMed] [Google Scholar]