Abstract
Epidemiological studies have associated traffic-related airborne pollution with adverse cardiovascular outcomes. Nitric oxide (NO) is a common component of fresh diesel and gasoline engine emissions that rapidly transforms both in the atmosphere and once inhaled. Because of this rapid transformation, limited information is available in terms of potential human exposures and adverse health effects. Young rats were exposed to whole diesel emissions (DE) adjusted to 300 µg/m3 of particulate matter (containing 3.5 ppm NO) or 0, 3, or 10 ppm NO as a positive control. Animals were also pre-injected (i.p.) with either saline or n-acetyl-cysteine (NAC), a precursor of glutathione. Predictably, pure NO exposures led to a concentration-dependent increase in plasma nitrates compared to controls, which lasted for roughly 4 hr post-exposure. Whole DE exposure for 1 hr also led to a doubling of plasma NOx. NAC injection increased the levels of plasma nitrates and nitrites (NOx) in the DE exposure group. Inhibition of NOS by L-NNA did not block the rise in plasma NOx, demonstrating that the increase was entirely due to exogenous sources. Both DE and pure NO exposures paradoxically led to elevated eNOS expression in aortic tissue. Furthermore, coronary arterioles from NO-exposed animals exhibited greater constriction to endothelin-1 compared to controls, consistent with a derangement of the NOS system. Thus, NO may be an important contributor to traffic-related cardiovascular morbidity although further research is necessary for proper hazard identification.
Keywords: Nitric Oxide, air pollution, cardiovascular, diesel exhaust, eNOS, n-acetyl-cysteine
INTRODUCTION
Air pollution has been associated with increased risk of myocardial infarction and ischemic stroke (Cheng et al. 2009; Chiu and Yang 2009) and recent studies indicate a role for disruption of vascular homeostasis as a potential mechanism (Briet et al. 2007; Shah et al. 2008). Several studies found that nitric oxide (NO) and the enzyme that creates endogenous NO, nitric oxide synthase (NOS), may be vulnerable components of vascular function (Knuckles et al. 2008; Nurkiewicz et al. 2009). Among the more compelling studies, Mills et al (2005) found that diesel emissions exerted a potent effect in driving vascular effects in healthy human subjects (and electrocardiographic markers of risk in coronary arterial disease subjects), but this effect was not recapitulated in subsequent studies of concentrated ambient particulate or NO2 (Langrish et al. 2010; Mills et al. 2008).
Lund et al. (2009) recently described that diesel exposures in humans rapidly increased plasma nitrates/nitrites that diminished within 24 hr following exposure. The dynamics suggest that the plasma levels may reflect uptake of exogenous NO, rather than formation of endogenous NO. In those exposures, NO concentrations were roughly 4 ppm (McDonald et al. 2004) in the diesel exhaust system used in the various studies (Cherng et al. 2009; Knuckles et al. 2008; Lund et al. 2009). Such levels are extraordinarily high compared to measured ambient levels, which are infrequently above 50 ppb (Brunekreef et al. 2009). However, NO data from municipal or federally-controlled ambient monitoring are likely inadequate for assessing near-roadway conditions as such monitors are intentionally located at a distance from traffic to minimize vehicular influence on readings. NO transforms so rapidly in the atmosphere that large discrepancies in hourly averages have been reported between proximal monitors and municipal monitors (Restrepo et al. 2004). Indeed, some near-roadway monitoring studies documented extremes in the hourly concentrations of NO approaching 1 ppm (De et al. 1994; Fujita et al. 2003; Restrepo et al. 2004).
Given the difficulties in monitoring this gas that transforms so rapidly, NO is not readily incorporated into epidemiological studies of traffic-related adverse health effects. The present study, therefore, is an initial toxicologic investigation into the basic systemic disposition of inhaled NO, whether as a sole component of ambient air or within the whole diesel emissions mixture. The relative levels of nitrates/nitrites (NOx) in plasma and vascular tissue were examined, as well as the influence on concentrations of S-nitrosothiols (SNO), which are postulated to be a benevolent form of transformed NO. Added to this are permutations testing 1) the impact of N-acetylcysteine (NAC), as a precursor to glutathione (GSH) designed to sequester NO, and 2) the use of a nitric oxide synthase (NOS) inhibitor, NG-nitro-L-arginine (LNNA), to confirm that changes in plasma NOx are related to exogenous sources, rather than increased endogenous NOS activity. Subsequently, the impact of NO was examined on coronary vascular function, as a comparison with previous studies of diesel emissions (Cherng et al., 2009).
METHODS
Animals
Rats (male, Sprague-Dawley, 10–14 week, were obtained from a commercial vendor (Charles River) weighing approximately 300 g. Animals were housed in an Association for Assessment and Accreditation of Laboratory Animal Care International-approved rodent housing facility that was maintained at constant temperature (20–24°C) and humidity (30–60% relative humidity) conditions. All procedures were approved by the Lovelace Respiratory Research Institute’s Animal Care and Use Committee.
Exposures
Rats were allowed to acclimatize to whole body DE exposure chambers at least 3 days prior to exposure. Following acclimatization, animals were exposed to either filtered air (FA) or whole DE for 1 or 2 hr at an approximate concentration of 300 µg PM/m3; the inhalation system was previously characterized and described (McDonald et al. 2004). In those characterizations under full operating load, the NOx levels are roughly 3.6 ppm, with 90% of that level attributable to NO (3.24 ppm) and approximately 10% attributable to NO2/3 (0.36 ppm). Real-time measurements of total NOx allowed us to titrate concentrations to match these previous characterizations.
NO was supplied by a commercial vendor (Matheson Trigas, Albuquerque, NM) and diluted with filtered air to desired concentrations (3 or 10 ppm). Immediately prior to exposure, some animals were pre-injected with NAC (200 µM/kg, i.p.), which enhances tissue and plasma levels of GSH. In a separate study, rats were pretreated with the eNOS inhibitor L-NNA (5 mg/kg, ip) then exposed to 0 or 3 ppm NO for 1 hr (N=4 per group). For vascular constriction experiments, rats were exposed in whole body exposure chambers for 5 hr at 10 ppm NO. Animals were immediately sacrificed following exposure and a 15 min off-gassing period.
Biochemical Assays
Whole blood was taken following exposures to either NO or DE via cardiac puncture. The whole blood was centrifuged at 4000 g for 10 min and the resulting plasma was filtered to remove protein > 10 kD, aliquoted and stored at −80°C. Plasma NOx levels were measured using a Nitrate/Nitrite colorimetric kit (#9001, Cayman Chemical), per manufacturer instructions. Nitrosothiols were measured by a commercial collaborator (Dr. Anna Leone; Oxonon, San Francisco, CA) using standardized HPLC methods with electrochemical detection. Glutathione-SNO was assessed as a specific indicator to better characterize the responses observed.
Western Blot
Aortic cytosolic protein was isolated from frozen tissues. Aorta segments were homogenized in cold Tris·HCl homogenization buffer containing a complete protease inhibitor cocktail (Pierce) using a motorized homogenizer (Fisher Scientific). Homogenates were sonicated then spun at 4,000 g for 4 min at 4°C. The supernatant was analyzed for protein concentration using the Bradford method (Pierce). Samples were heat denatured in 4x Lamella buffer (Bio-Rad) and then separated in a 4–20% Tris·HCl polyacrylamide gradient gel (Bio-Rad) containing samples (50 µg protein/lane) and molecular weight markers with each lane of the gel representing one animal. After transfer to nitrocellulose membrane (GE Healthcare), blots were blocked overnight in Blotto (Tris Buffered Saline [TBS], with 0.5% Tween-20, and 10% milk) and then washed with TBS and incubated with monoclonal antibodies specific for eNOS (1:3,000, Transduction Labs) in Blotto with 5% bovine serum albumin. After washing with TBS, blots were incubated with anti-IgG secondary antibody conjugated to HRP in TBS with 0.5% Tween-20. Blots were rinsed with TBS and developed with enhanced chemiluminescence reagents (GE Healthcare). Blots were exposed to X-OMAT Blue film (Kodak) and analyzed using Image J software (NIH).
Isolated Coronary Arteries
Coronary artery function was assessed as previously described (Cherng et al. 2009). Briefly, following exposures, rats (N=6/gp) were euthanized with sodium pentobarbital (200 mg/kg, i.p.) and hearts immediately removed to isolate intraseptal coronary arteries. Both ends of the arteries were cannulated onto glass micropipettes in a tissue chamber (Living Systems, CH-1) and secured with silk sutures within 30 min of isolation from the heart. Arteries were stretched to approximate in situ length and pressurized to 60 mmHg with PSS in the lumen absent of flow and superfused at a rate of 5 ml/min with 37°C oxygenated PSS. Endothelin-1 (ET-1; Sigma-Aldrich) was then administered in increasing concentration to induce constriction, measured by contrast imaging.
Statistical Analyses
Data expressed as mean +/−SEM. One-way analysis of variance (ANOVA) with Tukey’s post-hoc test was used to compare groups for most assays. A two-way ANOVA was used for vascular constriction data, comparing dose of ET-1 and exposure group. Data were tested for normality and this assumption was valid in all cases. A p<0.05 was considered statistically significant.
RESULTS
Diesel and Nitric Oxide Inhalation Increase Plasma NOx
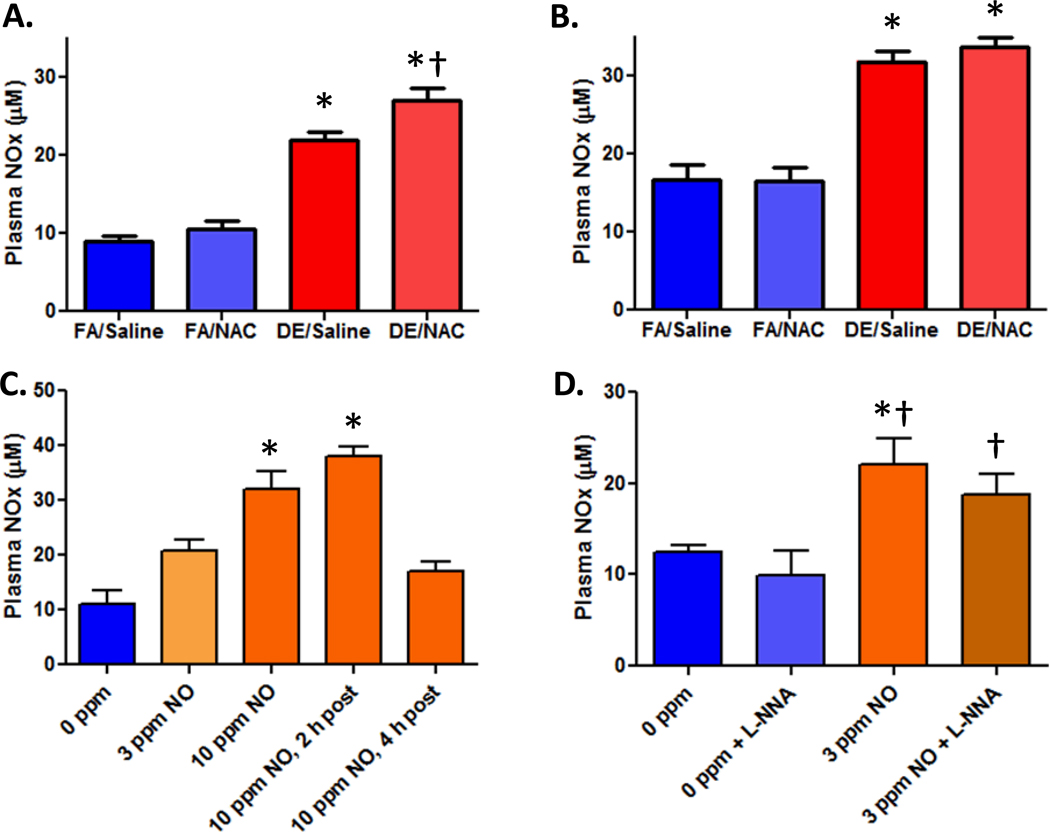
Whole DE exposure for 1 or 2 hr increased total plasma NOx levels approximately 2-fold (Figure 1A, 1B). Supplementation of the animals with NAC immediately prior to whole diesel exposures increased NOx concentrations in the plasma following 1 hr of exposure, but not after 2 hr (Figures 1A, 1B). The loss of this presumed GSH sequestration effect at 2 hr is likely due to the half-life (T1/2) of NAC being approximately 45 min in the body (McLellan et al. 1995); thus, the increased thiol sink for NO would have already returned to baseline by 2 hr post-injection. The concentration of injected NAC, 200 µmol/kg is more than sufficient, stoichiometrically, to account for the elevated plasma NOx levels in the DE+NAC group compared to DE alone (approximately 5 µM).
Figure 1. NAC enhances plasma NOx concentrations following DE exposures.
A) Animals exposed to whole DE (approximately 3.2 ppm NO) with or without NAC for 1 hr (N=6/gp). B) Plasma NOx levels from animals exposed to whole DE with or without NAC for 2 hr (N=6/gp). C) Plasma NOx concentrations in rat plasma following 1 hr exposure to a pure NO (0, 3 and 10 ppm) atmosphere (N=6–10/gp). In addition, in a smaller cohort of rats, NOx levels were determined at 2 hr post exposure to 10 ppm NO (N=2) and 4 hr post exposure to 10 ppm NO (N=4). D) Pretreatment with NOS inhibitor L-NNA in filtered air or 10 ppm NO-exposed rats. Panels A and B, * p<0.05 vs FA/Saline and FA/NAC control,
† p<0.05 vs. DE/Saline; Panel C,
* p<0.05 vs Filtered Air and 4 hr post-exposure; Panel D,
* p<0.05 vs 0 ppm, † p<0.05 vs. 0 ppm + L-NNA.
In order to determine if the rise in plasma NOx might result from the NO component of the whole DE, rats were exposed to NO at 0, 3, or 10 ppm for 1 hr. Immediately following exposure to inhaled NO, a similar rise in plasma NOx was noted (Figure 1C). The 3 ppm NO exposure led to a 70% increase in plasma NOx and the 10 ppm level produced a 190% elevation in plasma NOx. Data also suggest that a linear dose response between NO concentration and plasma NOx levels exists since the approximate increases in fold plasma NOx is equivalent to the fold elevation in ppm of NO.
To ascertain whether increased levels of plasma NOx were due to exogenous uptake or activation of endogenous NO synthesis, rats were pretreated with L-NNA to inhibit NOS then exposed to 3 ppm NO for 1 hr. The L-NNA treatment led to a quantitative reduction in mean plasma NOx levels at baseline. However, L-NNA did not prevent the rise in plasma NOx due to exposure to inhaled NO, suggesting that the increased plasma NOx is entirely exogenous in origin. Notably, baseline levels of plasma NOx vary somewhat, possibly due to differences in the standard curves generated or chow and water supply. However, the relative impact of the exposures is consistent.
Formation of Nitrosothiols Following Exposure to DE and NO
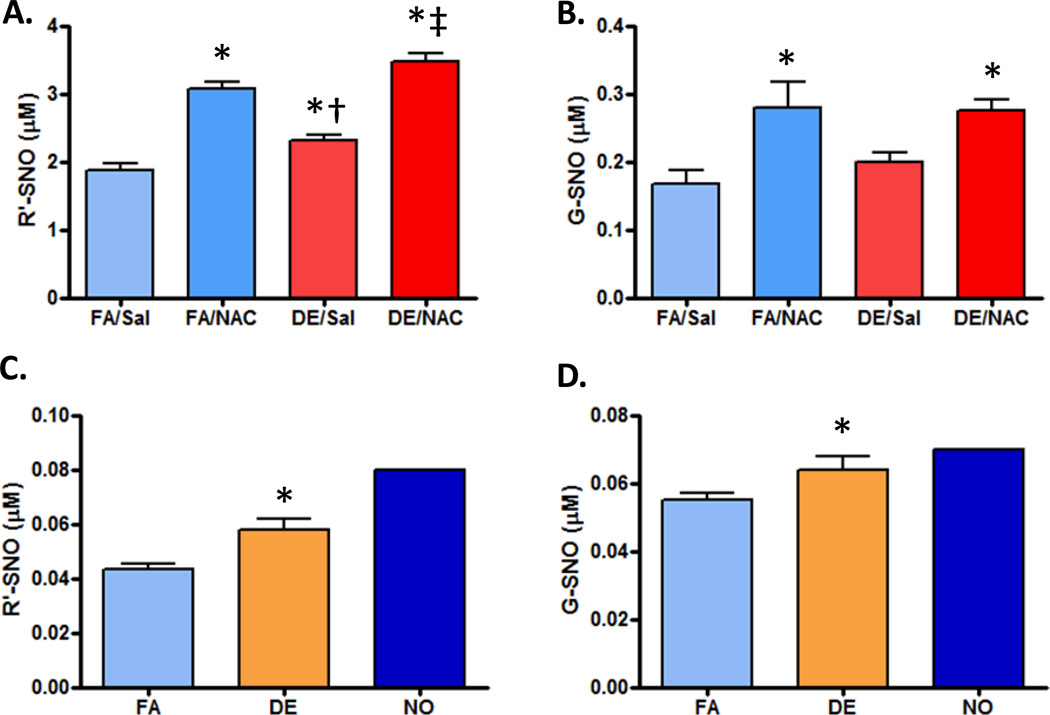
NO interacts and transforms rapidly in a biological system, being converted into a stable compound or interacting with various moieties on existing molecules. One such moiety is the sulfhydryl, or thiol group, common to many small molecules and proteins and a ready acceptor for NO, which leads to the formation of an SNO group. Analysis for SNO containing compounds was performed on the 1 hr whole DE exposure plasma samples in saline and NAC pretreated animals. All plasma SNO containing compounds (R'-SNO) and specifically glutathione-SNO (GSNO) were increased with NAC treatment regardless of filtered air (FA) or DE exposure (Figure 2A, 2B). R'-SNO content in the plasma was quantitatively elevated following DE exposure in both saline and NAC pretreatment (Figure 2A). Notably, all exposure groups in Figure 2A are statistically distinct, indicating that DE exposure affected total R-SNO levels in both vehicle- and NAC-treated rats. However, exposure to DE failed to increase plasma levels of G-SNO compounds (Figure 2B).
Figure 2. S-nitrosothiols (SNO) levels in the plasma and aortas from exposed animals.
A) Plasma levels for all SNO groups (R’-SNO) in control and exposed animals with or without NAC (N=6/gp). B) Plasma concentrations of glutathione-s-nitrosothiols (G-SNO) in the normal and exposed animals (N=6/gp). C) Aortic levels of all SNO moieties (R’-SNO, from cellular components <10 kD; N=6/gp, except NO, where N=1) D) Aortic G-SNO levels following FA, DE or NO exposure (N=6/gp, except NO, where N=1;
- p<0.05 vs FA/Saline and FA/NAC control,
- † p<0.01 vs. DE/NAC and FA/NAC;
- ‡ p<0.01 vs. DE/Sal and FA/NAC. Note: too few samples in NO-exposed group for inclusion in test).
Following inhalation exposure to whole DE for 1 hr, homogenized aortas were evaluated for SNO moieties. DE increased all SNO-containing compounds and specifically elevated GSNO levels in aortas of exposed animals (Figure 2C,D). With a single sample, results demonstrated that inhaled NO (10 ppm) exposure increased SNO levels in aortas greater than inhalation of whole DE, which is likely a dosing effect. Because of the limited sample size, this was not statistically comparable.
Vascular Function Effects of Inhaled NO and DE
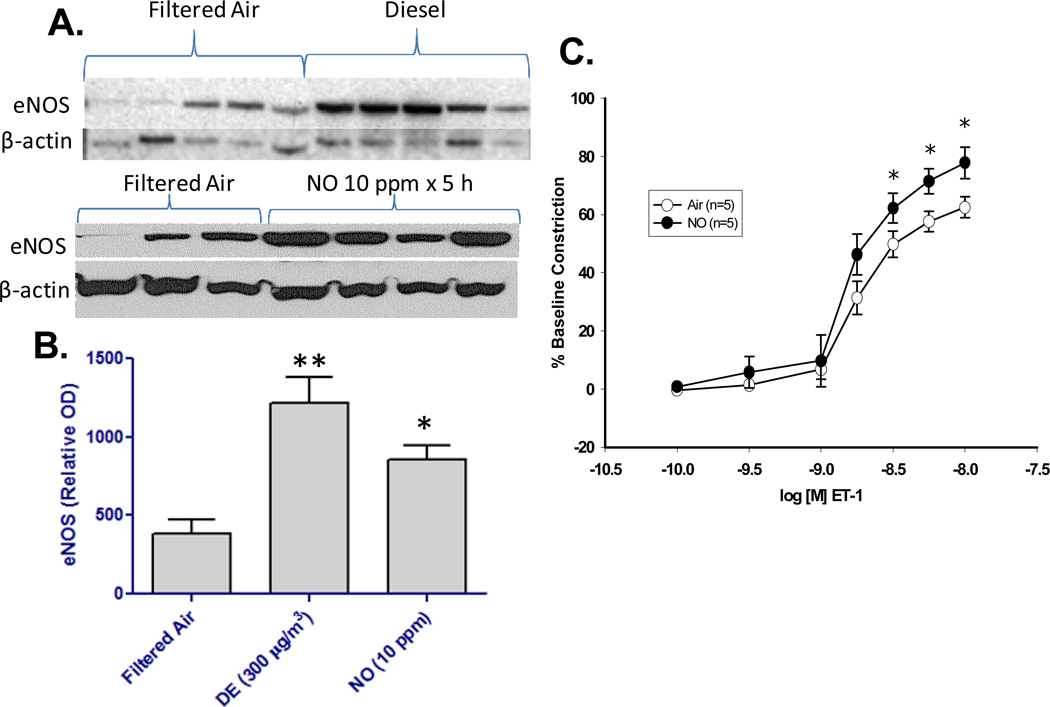
Lastly, the impact of DE and NO alone was examined on aortic eNOS protein levels and coronary vascular constriction to ET-1. Interestingly, inhalation of either DE or NO alone increased eNOS protein levels in harvested aortas following a 5 hr exposure (Figure 3A,B). Densitometry measures of the western blots determined that pure NO exposure induced an approximate 3-fold rise in eNOS protein expression following exposure (Figure 3B). As previously reported for DE inhalation (Cherng et al. 2009), NO inhalation led to a significant enhancement of ET-1-induced constriction (Figure 3C). Because recently reported effects of DE appear to involve the uncoupling of eNOS (Cherng et al., 2010), it is likely that the increased protein production is in response to the incapacitation of this enzyme system.
Figure 3. Inhalation of NO increases eNOS protein and enhances constriction to ET-1.
A. Western blots for eNOS are shown for aortic samples obtained from rats exposed to FA, DE, or NO (each band represents one animal). B. Quantitative densitometry for the eNOS immunoblot results are shown for FA (N=8), DE (N=5), and NO (N=4), (*p<0.05). C. Coronary arteriolar constriction to endothelin-1 (ET-1) following exposure to NO at 10 ppm (N=6/gp). Asterisks (*) indicate difference from FA controls (p<0.05).
DISCUSSION
The present study characterized the systemic disposition of NO-related species from single exposures to diesel emissions and NO alone, along with the resultant vascular effects in healthy rodents. Of interest, it was found that both DE and pure NO inhalation produced elevations in several classes of nitrosative chemical species in the plasma and aorta, including NOx and SNO. This is the first report, to our knowledge, that inhalation of diesel emissions may lead to elevated systemic nitrosothiol levels, which complements our recent findings of elevated plasma NOx in DE-exposed humans (Lund et al. 2009). It was notable that inhaled NO and DE led to relatively minimal increases in SNO compared to the impact on NOx. NAC pretreatment elevated the levels of plasma NOx and SNO resulting from DE exposure, potentially due to an enhanced gradient for NO uptake related to the pool of free thiols. Combined with the NO-induced alterations in eNOS and the enhanced response to ET-1, data provide evidence that NO, as an exogenous entity, may play a role in air pollution-related systemic vascular dysregulation.
Because of the complex nature of combustion-source mixtures, the specific driver(s) of adverse cardiovascular health effects remain clouded. A recent head-to-head study of vascular effects from various combustion atmospheres and individual components thereof demonstrated some intriguing responses to inhaled NO (Campen et al. 2010). These responses included induction of aortic ET-1 and MMP-9 mRNA, as well as enhanced vascular gelatinase activity. NO inhalation recapitulated some (but not all) of the responses induced by gasoline engine emissions, but stood in contrast to effects of NO2 inhalation, which were negligible. The United States Environmental Protection Agency set a standard for a summative exposure to NOx species, but the contribution of NO toxicity in setting this regulation is negligible compared to the established and well-characterized impact of NO2 exposure. The current findings suggest that further inquiry into the biological effects and public health impact of NO is warranted.
Dosing with NAC before exposures led to significant changes in the observed tissue uptake for NO species (Figure 1A). However, this effect was lost for the 2 hr exposure. This dynamic is temporally congruent with the kinetics of NAC-to-glutathione conversion following a single intraperitoneal injection which peaks at about 45 min and has a fairly rapid decay thereafter (McLellan et al. 1995). Three potential mechanisms may explain the phenomenon of the NAC-nduced rise in systemic NOx and SNO species following 1 hr of DE. First, it is conceivable that the additional cysteine residue acted as a sink, thereby increasing the thermodynamic gradient for NO uptake. Second, NAC may stimulate endogenous production/release of NOx, a mechanism that is enhanced in the presence of DE components. Third, several lines of evidence suggest that nitrosothiol species enhance respiratory drive in the brainstem (Gozal et al. 1997; Lipton et al. 2001). While this is somewhat more speculative, there have been, to our knowledge, no reports of the impact of NAC on respiratory physiology and, as ventilatory parameters were not measured in the present study; thus, one can not rule out such an effect.
The role of nitrosothiols as signaling molecules has been of recent interest in respiratory and cardiovascular physiology. Free thiol groups are likely vital cytoprotective and homeostatic moieties as they scavenge NO and, in a controlled manner, shuttle the molecule to more benign pathways (Gaston et al. 2006). This may explain in part the protection afforded by highly functional glutathione synthesis or transport proteins and explain why certain polymorphisms lead to added risk. In the present study, NAC injection markedly increased plasma SNO, especially compared to the effect of DE alone (Figure 2A,2B). Palmer et al (2007) noted that NAC may sequester NO away from hemoglobin under certain physiological conditions. As the large proteins were removed from our samples prior to HPLC analysis, it is not known if SNO were produced and released, or simply transferred from a pool bound to large proteins to the small molecule NAC. Future studies are needed to resolve the origin of the small molecular weight SNO. Similarly, DE may have liberated such groups from large proteins, although with the increased level of free NOx post-exposure, it is more likely that the exposure-related SNO are exogenously-derived.
Increased protein expression of eNOS in aortic tissue after NO or DE exposure was associated with elevated plasma NOx/SNO and tissue SNO levels. Based on our study with LNNA, evidence indicates that the elevated plasma NOx are not a result of increased eNOS levels, as the NO inhalation still led to a significant elevation in plasma NOx when NOS was inhibited. Previous studies reported that DE inhalation leads to endothelial dysfunction in a variety of models (Cherng et al. 2009; 2010; Knuckles et al. 2008) and, this phenomenon was also reported in humans (Knuckles et al. 2008; Mills et al. 2005). In the present study, inhalation of NO was sufficient to recapitulate the previously reported enhancement of ET-1-induced coronary vasoconstriction, mediated by endothelial ETB receptors, produced by DE inhalation (Cherng et al. 2009). This paradoxical increase in eNOS expression following NO and DE inhalation suggests a compensatory response by endothelial cells to upregulate the dysfunctional dilatory pathways.
These studies support our finding that NO alone is sufficient to alter vasoconstriction in the absence of other DE related compounds (Figure 4C). Knuckles et al. (2008) previously noted that the enhanced vasoconstriction induced by DE exposure was mediated by eNOS uncoupling, which appears to also be the case in this study since a rise in eNOS led to an enhanced constriction. Previous studies utilizing eNOS overexpressing transgenic mice showed a lack of vasodilation with increased eNOS protein levels through uncoupling (Adlam et al. 2007). In a similar manner, a mouse model of constitutively active eNOS, produced by a caveolin knockout, leads to significant impairments in vascular function and induces pulmonary hypertension (Zhao et al. 2009). Thus, it is possible that the increased eNOS protein either reflects dysfunction of that pathway or is itself contributing to vascular impairments.
Currently, limited evidence exists linking ambient NO exposure to morbidity/mortality, since NO is generally thought of as safe at levels encountered in the environment. However, Filleul et al (2005) showed that ambient levels of NO are associated with increased risk of non-accidental death, and more recent studies from our lab in animal models of cardiovascular disease linked exposure to NO to expression of remodeling markers of atherosclerosis and gelatinase activity (Campen et al. 2010). Combined with the present study, there is justification to consider both vascular toxicity of traffic-related NO and also to more thoroughly assess roadway patterns of NO pollution. While inhaled NO may be safely administered in select clinical populations to achieve specific therapeutic outcomes, extension of such assertions of safety to public health remains unwarranted without further study.
Acknowledgements
This project was supported by grants from the EPA (RD831860; NLK) and NIH (ES014639; MJC).
REFERENCES
- Adlam D, Bendall JK, De Bono JP, Alp NJ, Khoo J, Nicoli T, Yokoyama M, Kawashima S, Channon KM. Relationships between nitric oxide-mediated endothelial function, eNOS coupling and blood pressure revealed by eNOS-GTP cyclohydrolase 1 double transgenic mice. Exp. Physiol. 2007;92:119–126. doi: 10.1113/expphysiol.2006.035113. [DOI] [PubMed] [Google Scholar]
- Briet M, Collin C, Laurent S, Tan A, Azizi M, Agharazii M, Jeunemaitre X, Henc-Gelas F, Boutouyrie P. Endothelial function and chronic exposure to air pollution in normal male subjects. Hypertension. 2007;50:970–976. doi: 10.1161/HYPERTENSIONAHA.107.095844. [DOI] [PubMed] [Google Scholar]
- Brunekreef B, Beelen R, Hoek G, Schouten L, Bausch-Goldbohm S, Fischer P, Armstrong B, Hughes E, Jerrett M, van den BP. Effects of long-term exposure to traffic-related air pollution on respiratory and cardiovascular mortality in the Netherlands: the NLCS-AIR study. Res. Rep. Health Eff. Inst. 2009;139:5–71. [PubMed] [Google Scholar]
- Campen MJ, Lund AK, Doyle-Eisele ML, McDonald JD, Knuckles TL, Rohr AC, Knipping EM, Mauderly JL. A comparison of vascular effects from complex and individual air pollutants indicates a role for monoxide gases and volatile hydrocarbons. Environ. Health Persp. 2010;118:921–927. doi: 10.1289/ehp.0901207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng MF, Tsai SS, Yang CY. Air pollution and hospital admissions for myocardial infarction in a tropical city: Kaohsiung, Taiwan. J. Toxicol. Environ. Health A. 2009;72:1135–1140. doi: 10.1080/15287390903091756. [DOI] [PubMed] [Google Scholar]
- Cherng TW, Campen MJ, Knuckles TL, Gonzalez BL, Kanagy NL. Impairment of coronary endothelial cell ET(B) receptor function after short-term inhalation exposure to whole diesel emissions. Am. J. Physiol Regul. Integr. Comp Physiol. 2009;297:R640–R647. doi: 10.1152/ajpregu.90899.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherng TW, Jackson-Weaver O, Paffett M, walker B, Campen MJ, Kanagy NL. Mechanisms of diesel-induced endothelial NOS dysfunction in coronary arterioles. Environ. Health Persp. 2010 doi: 10.1289/ehp.1002286. [Epub Sept. 22, 2010] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu HF, Yang CY. Air pollution and emergency room visits for arrhythmias: are there potentially sensitive groups? J. Toxicol. Environ. Health A. 2009;72:817–823. doi: 10.1080/15287390902800405. [DOI] [PubMed] [Google Scholar]
- De FR, Bruynseraede P, Kretzschmar JG. Air pollution measurements in traffic tunnels. Environ. Health Persp. 1994;102 Suppl 4:31–37. doi: 10.1289/ehp.102-1566941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filleul L, Rondeau V, Vandentorren S, Le MN, Cantagrel A, nnesi-Maesano I, Charpin D, Declercq C, Neukirch F, Paris C, Vervloet D, Brochard P, Tessier JF, Kauffmann F, Baldi I. Twenty five year mortality and air pollution: results from the French PAARC survey. Occup. Environ. Med. 2005;62:453–460. doi: 10.1136/oem.2004.014746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita EM, Campbell DE, Zielinska B, Sagebiel JC, Bowen JL, Goliff WS, Stockwell WR, Lawson DR. Diurnal and weekday variations in the source contributions of ozone precursors in California's South Coast Air Basin. J. Air Waste Manage. Assoc. 2003;53:844–863. doi: 10.1080/10473289.2003.10466226. [DOI] [PubMed] [Google Scholar]
- Gaston B, Singel D, Doctor A, Stamler JS. S-nitrosothiol signaling in respiratory biology. Am. J. Respir. Crit Care Med. 2006;173:1186–1193. doi: 10.1164/rccm.200510-1584PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gozal D, Gozal E, Torres JE, Gozal YM, Nuckton TJ, Hornby PJ. Nitric oxide modulates ventilatory responses to hypoxia in the developing rat. Am. J. Respir. Crit Care Med. 1997;155:1755–1762. doi: 10.1164/ajrccm.155.5.9154888. [DOI] [PubMed] [Google Scholar]
- Knuckles TL, Lund AK, Lucas SN, Campen MJ. Diesel exhaust exposure enhances venoconstriction via uncoupling of eNOS. Toxicol. Appl. Pharmacol. 2008;230:346–351. doi: 10.1016/j.taap.2008.03.010. [DOI] [PubMed] [Google Scholar]
- Langrish JP, Lundback M, Barath S, Soderberg S, Mills NL, Newby DE, Sandstrom T, Blomberg A. Exposure to nitrogen dioxide is not associated with vascular dysfunction in man. Inhal. Toxicol. 2010;22:192–198. doi: 10.3109/08958370903144105. [DOI] [PubMed] [Google Scholar]
- Lipton AJ, Johnson MA, Macdonald T, Lieberman MW, Gozal D, Gaston B. S-nitrosothiols signal the ventilatory response to hypoxia. Nature. 2001;413:171–174. doi: 10.1038/35093117. [DOI] [PubMed] [Google Scholar]
- Lund AK, Lucero J, Lucas S, Madden MC, McDonald JD, Seagrave JC, Knuckles TL, Campen MJ. Vehicular emissions induce vascular MMP-9 expression and activity associated with endothelin-1-mediated pathways. Arterioscler. Thromb. Vasc. Biol. 2009;29:511–517. doi: 10.1161/ATVBAHA.108.176107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald JD, Barr EB, White RK. Design, characterization, and evaluation of a small-scale diesel exhaust exposure system. Aerosol Sci. Technol. 2004;38:62–78. [Google Scholar]
- McLellan LI, Lewis AD, Hall DJ, Ansell JD, Wolf CR. Uptake and distribution of N-acetylcysteine in mice: tissue-specific effects on glutathione concentrations. Carcinogenesis. 1995;16:2099–2106. doi: 10.1093/carcin/16.9.2099. [DOI] [PubMed] [Google Scholar]
- Mills NL, Robinson SD, Fokkens PH, Leseman DL, Miller MR, Anderson D, Freney EJ, Heal MR, Donovan RJ, Blomberg A, Sandstrom T, MacNee W, Boon NA, Donaldson K, Newby DE, Cassee FR. Exposure to concentrated ambient particles does not affect vascular function in patients with coronary heart disease. Environ. Health Persp. 2008;116:709–715. doi: 10.1289/ehp.11016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills NL, Tornqvist H, Robinson SD, Gonzalez M, Darnley K, MacNee W, Boon NA, Donaldson K, Blomberg A, Sandstrom T, Newby DE. Diesel exhaust inhalation causes vascular dysfunction and impaired endogenous fibrinolysis. Circulation. 2005;112:3930–3936. doi: 10.1161/CIRCULATIONAHA.105.588962. [DOI] [PubMed] [Google Scholar]
- Nurkiewicz TR, Porter DW, Hubbs AF, Stone S, Chen BT, Frazer DG, Boegehold MA, Castranova V. Pulmonary nanoparticle exposure disrupts systemic microvascular nitric oxide signaling. Toxicol. Sci. 2009;110:191–203. doi: 10.1093/toxsci/kfp051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer LA, Doctor A, Chhabra P, Sheram ML, Laubach VE, Karlinsey MZ, Forbes MS, Macdonald T, Gaston B. S-Nitrosothiols signal hypoxia-mimetic vascular pathology. J. Clin. Invest. 2007;117:2592–2601. doi: 10.1172/JCI29444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Restrepo C, Zimmeran R, Thurston G, Clemente J, Gorczynski J, Zhong M, Blaustein M, Chen LC. A comparison of ground-level air quality data with New York State Department of Environmental Conservation monitoring stations data in South Bronx, New York. Atmos Eviron. 2004;38:5295–5304. [Google Scholar]
- Shah AP, Pietropaoli AP, Frasier LM, Speers DM, Chalupa DC, Delehanty JM, Huang LS, Utell MJ, Frampton MW. Effect of inhaled carbon ultrafine particles on reactive hyperemia in healthy human subjects. Environ. Health Persp. 2008;116:375–380. doi: 10.1289/ehp.10323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao YY, Zhao YD, Mirza MK, Huang JH, Potula HH, Vogel SM, Brovkovych V, Yuan JX, Wharton J, Malik AB. Persistent eNOS activation secondary to caveolin-1 deficiency induces pulmonary hypertension in mice and humans through PKG nitration. J. Clin. Invest. 2009;119:2009–2018. doi: 10.1172/JCI33338. [DOI] [PMC free article] [PubMed] [Google Scholar]