Abstract
The actin cytoskeleton plays a significant role in changes of cell shape and motility, and interactions between the actin filaments and the cell membrane are crucial for a variety of cellular processes. Several adaptor proteins, including talin, maintain the cytoskeleton-membrane linkage by binding to integral membrane proteins and to the cytoskeleton. Layilin, a recently characterized transmembrane protein with homology to C-type lectins, is a membrane-binding site for talin in peripheral ruffles of spreading cells. To facilitate studies of layilin's function, we have generated a layilin-Fc fusion protein comprising the extracellular part of layilin joined to human immunoglobulin G heavy chain and used this chimera to identify layilin ligands. Here, we demonstrate that layilin-Fc fusion protein binds to hyaluronan immobilized to Sepharose. Microtiter plate-binding assays, coprecipitation experiments, and staining of sections predigested with different glycosaminoglycan-degrading enzymes and cell adhesion assays all revealed that layilin binds specifically to hyaluronan but not to other tested glycosaminoglycans. Layilin's ability to bind hyaluronan, a ubiquitous extracellular matrix component, reveals an interesting parallel between layilin and CD44, because both can bind to cytoskeleton-membrane linker proteins through their cytoplasmic domains and to hyaluronan through their extracellular domains. This parallelism suggests a role for layilin in cell adhesion and motility.
INTRODUCTION
Cell shape and movement form the basis for several biological phenomena such as morphogenesis, invasion, and metastasis. The actin cytoskeleton is a major determinant of changes in cell shape, and interactions between actin filaments and the cell membrane are essential for cell adhesion, spreading, and migration, as well as for signal transduction (Hall, 1998; Schoenwaelder and Burridge, 1999). Several molecules have been recognized as linkers between the actin cytoskeleton and the cell membrane. For example, members of the band 4.1/ERM superfamily (band 4.1, talin, ezrin, radixin, moesin, and merlin, the product of the neurofibromatosis type 2 tumor suppressor gene) are characterized by the presence of a conserved N-terminal membrane-binding domain, which is able to bind to the cytoplasmic region of several transmembrane receptors, including CD44, CD43, ICAM-2, and ICAM-3 (Sainio et al., 1997; Heiska et al., 1998; Yonemura et al., 1998). The C-terminal regions of ERM proteins are thought to bind to actin filaments, thereby linking these transmembrane receptors to the cytoskeleton (reviewed by Mangeat et al., 1999).
Layilin is a recently cloned ∼55-kDa membrane-binding partner for talin (Borowsky and Hynes, 1998) that is widely expressed in different cell types and tissue extracts. It is found in peripheral ruffles of spreading cells and is recruited to membrane ruffles in cells induced to migrate in in vitro wounding experiments. Layilin colocalizes with talin in ruffles and binds to talin's ∼50-kDa head domain (amino acids 280–435). Other parts of talin (the ∼220-kDa tail fragment) can bind β-integrin cytoplasmic tails, vinculin, and F-actin and hence form an integrin-cytoskeleton linkage at sites of cell-substratum contact (reviewed by Critchley, 2000). Talin's head domain has been proposed to have a role in cell motility and morphology (Hemmings et al., 1996; Bolton et al., 1997) and an actin-binding site in the head domain may provide a direct link between layilin and F-actin. Layilin therefore represents a membrane-binding site for talin in ruffles, whereas integrins anchor talin in focal contacts where no layilin expression can be found. Although layilin's exact role in ruffles is currently unclear, one tempting model is that layilin recruits talin to ruffles but is displaced by integrins as more stable adhesions are established. This idea is supported by the finding of Calderwood et al. (1999) that β1-cytoplasmic domains can bind both to the head and rod domain of talin and that the β1-binding site overlaps with the layilin-binding site in talin's head domain.
Layilin's extracellular domain is homologous with the carbohydrate-recognition domains (CRD) of C-type lectins. Because layilin is a type I transmembrane protein, it has the potential to mediate signals from extracellular matrix (ECM) to the cell cytoskeleton, and, based on layilin's homology to E-selectin's ligand-binding region (Graves et al., 1994; Borowsky and Hynes, 1998), one could speculate that layilin may act in a manner analogous to selectins in mediating transient interactions with the ECM. However, because no binding partners for layilin's extracellular part have been described, we were interested to identify layilin ligands. For this purpose, we have developed a chimeric protein containing the layilin extracellular region and the hinge and constant regions of human immunoglobulin (Ig)G1. In this paper, we report that this soluble form of layilin binds to hyaluronan (HA), an abundant component of the ECM, and that the binding is specific for this glycosaminoglycan (GAG). Because there is no sequence homology with any previously known HA receptors, including CD44, RHAMM (receptor for HA-mediated motility), or LYVE-1 (lymphatic vessel endothelial HA receptor-1), layilin represents a novel member of the HA-binding protein family (Stamenkovic et al., 1989; Hardwick et al., 1992; Banerji et al., 1999). Thus, by binding to HA, layilin may facilitate cell migration by mediating early interactions between spreading cells and the ECM.
MATERIALS AND METHODS
Chemicals, Cells, and Antibodies
DNA-manipulating enzymes were from New England Biolabs (Beverly, MA). Oligonucleotides and sequencing service were purchased from Massachusetts Institute of Technology Cancer Center Biopolymer Facility (Cambridge, MA). Chemicals were purchased from Sigma Chemical (St. Louis, MO) unless otherwise indicated. Biotinylated HA (bHA)-binding protein was from Seikagaku (Tokyo, Japan). HA dodecasaccharides were a gift from Pipetten Biotech (Uppsala, Sweden) and were >98% homogenous with regard to size as judged from mass spectrometry and contained no contaminating protein or nucleic acid.
Chinese hamster ovary (CHO) and NIH3T3 cells were grown as described previously (Bono et al., 1998; Bloom et al., 1999). MCF-7 breast carcinoma cells were grown in DMEM supplemented with 10% fetal bovine serum. The preparation and purification of polyclonal antisera against layilin have been described in detail (Borowsky and Hynes, 1998). Briefly, a synthetic peptide from the 20 carboxy-terminal amino acids was used to immunize rabbits, and the resulting antisera were affinity purified with the peptide covalently coupled to thiopropyl-Sepharose 6B. Peroxidase-conjugated F(ab′)2 fragment of rabbit anti-human IgG was purchased from DAKO (Glostrup, Denmark). Biotin-conjugated goat anti-human IgG (Fc) was from Rockland (Gilbertsville, PA), and peroxidase-conjugated goat anti-human IgG (Fc-specific) was from Sigma.
Fc Fusion Plasmid Constructions
The extracellular region of layilin was amplified from layilin cDNA by polymerase chain reaction using oligonucleotides IgGF1 (5′-TCC CGA ATT CTC TGC CTT AGT CCC G-3′) and IgGR1 (5′-TCC GCT CGA GGC TTT CTT TGA ATG TTT C-3′) as primers. Reaction conditions for the amplification were 94°C for 1 min, 58°C for 1 min, and 72°C for 1 min for 30 cycles. The amplified fragment was digested with EcoRI and XhoI and introduced in-frame, upstream of the hinge and Fc region of human IgG1 in a derivative of pCDM8 (pCDM8/Fc; Chen and Nelson, 1996) also cleaved with EcoRI and XhoI. The integrity of the construct was determined by sequencing. Thereafter, the layilin-Fc cDNA was excised from pCDM8 using NotI and HindIII and ligated into expression vector pCEP4 (Invitrogen, Carlsbad, CA) cleaved with NotI and HindIII. Finally, the layilin-Fc construct in the expression vector was sequenced before making a large-scale preparation of DNA for transfections.
The cloning and detailed structures of CD44-Fc and E-cadherin-Fc fusion plasmids were described earlier (Aruffo et al., 1990; Higgins et al., 1998). CD44-Fc fusion protein (the extracellular region of CD44 fused to the hinge and Fc region of human IgG1) was a generous gift of Dr. Ivan Stamenkovic (Massachusetts General Hospital, Charlestown, MA).
Production of Fc Fusion Proteins
CHO cells (5 × 106/transfection) were stably transfected with 20 μg of plasmid DNA using electroporation (0.3 kV, 960 μF, 0.4-cm cuvette in RPMI plus 1 mM sodium pyruvate, 2 mM l-glutamine without serum; Gene Pulser Apparatus, Bio-Rad, Hercules, CA). After transfections, the cells were selected in the presence of 400 μg/ml hygromycin. After ∼20 d, supernatants from wells containing resistant colonies were assayed for fusion proteins by Western blot analysis.
To produce layilin-Fc fusion proteins on a large scale, stably transfected CHO cells were grown in 175-cm2 flasks in 10% Ultralow IgG fetal bovine serum (Life Technologies, Paisley, UK) and 400 μg/ml hygromycin. After the cells reached confluency, the medium was harvested and purified with protein A-Sepharose CL-4B beads (Amersham Pharmacia Biotech, Uppsala, Sweden). After the sample was washed, the fusion proteins were eluted with 100 mM glycine, pH 3.0, and neutralized with one-tenth volume of 1 M Tris-HCl, pH 8.0. The purified fusion proteins were dialyzed into phosphate-buffered saline (PBS) and stored at −20°C. Finally, the concentrations of the purified proteins were determined with Micro-BSA (bovine serum albumin) protein assay kit (Pierce, Rockford, IL) using BSA as a standard, and the purity of the fusion proteins was analyzed by SDS-PAGE and Coomassie staining.
Fc Fusion Protein Binding to HA Immobilized to Sepharose
HA-EDA-Sepharose 4B (McCourt and Gustafson, 1997) was kindly provided by Dr. Paraskevi Heldin (Uppsala University, Sweden). Unsubstituted Sepharose 4B was used as a control in the binding reactions. For the binding experiments, 0.5 μg of purified layilin-Fc or E-cadherin-Fc was incubated in the presence of 5 mM Ca2+ for 2 h at 4°C. After the sample was centrifuged, the beads were washed three times with PBS/Ca2+ and then eluted with 2 mg/ml HA-specific dodecasaccharides or with 170 μg/ml N-acetyl-d-glucosamine (2-acetamido-2-deoxy-d-glucose; Sigma). The elutions were boiled in SDS-PAGE sample-loading buffer and loaded onto SDS-PAGE gels. The eluted material was detected with horseradish peroxidase (HRP)-conjugated goat anti-human IgG (Fc-specific) with an enhanced chemiluminescence system.
Cetylpyridinium Chloride (CPC) Precipitations
Confluent plates of NIH 3T3 cells (∼4 × 106 cells) were washed three times with PBS and lysed in 1 ml of PBS containing 0.5% Nonidet P-40. After addition of protease inhibitors, lysates were incubated on ice for 15 min and subsequently centrifuged for 10 min to remove insoluble material. Aliquots of the lysate (50 μl) were used to perform CPC precipitations essentially as described by Lee et al. (1992), using 50 μl of 1 mg/ml aqueous solutions of different GAGs (HA [Sigma H5388], heparin [heparan sulfate sodium salt from porcine intestinal mucosa; Sigma H9902], or chondroitin sulfate sodium salt [Seikagaku] or N-acetyl glucosamine or H2O as negative controls). After the sample was incubated at room temperature for 1 h, CPC was added to the GAG/lysate mixture to a final concentration of 1% (vol/vol) and incubated for 1 h. Precipitates containing GAGs and bound proteins were pelleted by centrifugation, washed three times with 1 ml of 1% CPC, 30 mM NaCl, and finally dissolved in 50 μl of sample-loading buffer and analyzed on a 10% SDS-PAGE polyacrylamide gel under reducing conditions (100 mM dithiothreitol).
SDS-PAGE and Western Blot Analysis
After proteins were electrophoretically resolved by size, they were electrically transferred to nitrocellulose membranes, which were stained using a Western blot chemiluminescence reagent from NEN Life Science Products (Boston, MA) according to the manufacturer's recommendations. The detected signals were quantitated using AlphaImager 1220 documentation and analysis system (Alpha Innotech, San Leandro, CA).
Binding of Layilin-Fc to Immobilized HA
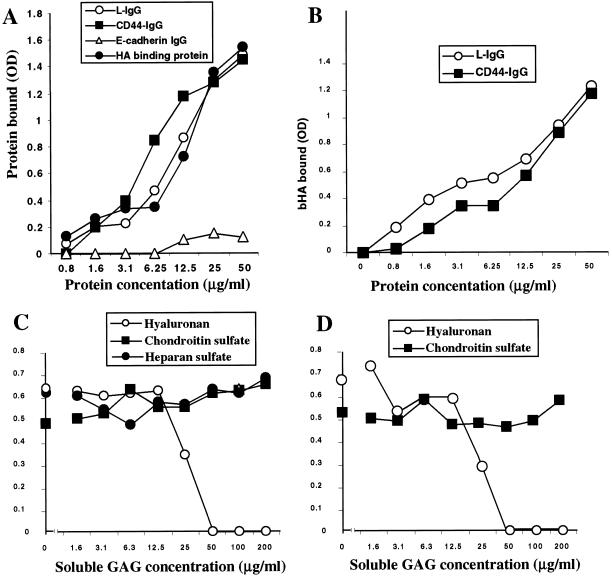
Binding of layilin-Fc fusion protein to immobilized HA (from rooster comb; Sigma H5388) was measured in 96-well microtiter plates (Maxisorp plate, Nalge Nunc International, Rochester, NY). The wells were coated overnight at 4°C with 2 mg/ml HA in coating buffer (15 mM sodium carbonate and 34 mM sodium bicarbonate, pH 9.3) and blocked for 2 h in 0.25% BSA and 0.05% Tween-20 in PBS. After the plates were washed three times with PBS, they were incubated with purified fusion proteins for 2 h at room temperature. After the unbound proteins were washed from the plates, the plates were incubated with HRP-conjugated anti-human IgG antibody (or streptavidin-HRP for bHA-binding protein control reactions), the reactions were developed using tetramethylbenzidine substrate (DAKO TMB One-Step Substrate system), and the absorbances were measured at 650 nm in a standard microplate reader. The values expressed in Figure 5 represent averages of duplicate determinations in at least three experiments with an SD ≤ 10%.
Figure 5.
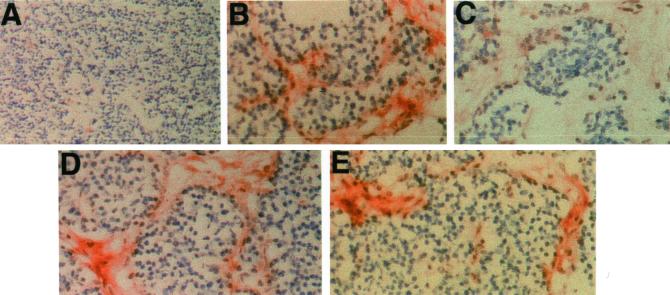
Histochemical staining of tumors derived from pancreata of RIP-Tag2 mice. Cryostat sections of mouse pancreas tumors were reacted with chimeras (0.5 μg/ml) and processed for histochemistry as described in MATERIALS AND METHODS. Original magnification 50× in A and 400× in B–E. (A) Control fusion protein (E-cadherin IgG) staining of tumor section. The ECM lacks staining. (B) Layilin-IgG stains positively the ECM, and the staining is sensitive to hyaluronidase treatment before incubation with layilin-IgG (C). Similar pretreatment of sections with chondroitinase (D) or heparitinase (E) did not abolish layilin-Fc reactivity.
To determine possible layilin-Fc binding to other GAGs, layilin-Fc (10 μg/ml, a concentration from the linear part of the binding curve, see Figure 5A) was preincubated with free GAGs (1.5–200 μg/ml) for 30 min on ice before incubation with HA immobilized to the wells as described above (Figure 5, C and D).
Binding of Soluble HA to Immobilized Layilin
Purified HA was biotinylated according to the method of Yu and Toole (1995). Briefly, purified HA (40–60 kDa, from Seikagaku) was dissolved in PBS at 5 mg/ml and dialyzed against 0.1 M 2-(N-morpholino)ethanesulfonic acid, pH 5.5 (Sigma). Biotin-LC-hydrazide (Pierce) in dimethyl sulfoxide was added to give a final concentration of 1 mM in the presence of 10 mM 1,ethyl-3-[3-dimethylaminopropyl]carbodiimide buffer (Pierce), dissolved in 2-(N-morpholino)ethanesulfonic acid buffer, and stirred overnight at room temperature, followed by dialysis against PBS and a 15-h centrifugation at 4°C to remove any precipitates. The biotinylation reaction leads to a maximum of 1 in 10–20 carboxyl groups in HA becoming labeled, which has been reported to be sufficient for sensitive detection and to preserve full binding activity of the labeled HA.
Ninety-six–well polystyrene microtiter plates (Costar, Cambridge, MA) were coated with Fc fusion proteins (1–50 μg/ml) overnight at 4°C, washed three times to remove unbound material, blocked, and incubated with bHA (10 μg/ml) for a further 2 h. Bound bHA was detected by incubation with HRP-conjugated streptavidin (Amersham Pharmacia Biotech) and tetramethylbenzidine as a substrate. Absorbance was measured at 650 nm as described above.
Layilin-Fc Staining of Tissue Sections
Tissue samples were freshly prepared from pancreata of RIP1-Tag2 transgenic mice that spontaneously develop solid tumors at the age of 14 wk (Hanahan, 1985; Folkman et al., 1989). Sections were cut from liquid nitrogen-frozen tissue samples and stored at −20°C. The sections were air dried, fixed with acetone, and blocked for 30 min with 4% goat serum in PBS/0.05% Tween-20. After a brief PBS/Tween-20 wash, sections were incubated at room temperature for 45 min with 0.5 μg/ml purified layilin or E-cadherin-Fc fusion proteins diluted in 4% goat serum including 5 mM Ca2+. After the sections were stained with the primary reagent, they were washed and incubated for 45 min with biotinylated goat anti-human Fc antibody diluted 1:200 in 2% mouse serum. The staining was visualized by the avidin-biotin complex technique using Vectastain ABC kit (Vector Laboratories, Burlingame, CA), and the sections were finally slightly counterstained with hematoxylin.
For GAG digestions the sections were incubated for 45 min at 37°C with 100 μg/ml hyaluronidase (from bovine testes, type I; Sigma) or heparitinase (gift from A. Lander; University of California, Irvine, CA) or with 0.5 U/ml chondroitinase (chondroitinase ABC lyase from Proteus vulgaris; Sigma), followed by washes and blocking steps as described above.
Adhesion Assays
Adhesion assays were conducted essentially as described previously (Li et al., 1999). The wells of Linbro 96-well microtiter plates (ICN-Flow Laboratories) were coated with 100 μl/well of 0.5 mg/ml BSA or 1 mg/ml human umbilical cord HA and blocked overnight with 1% (wt/vol) BSA in Tris-buffered saline. The wells were subsequently washed two times with 20 mM HEPES, pH 7.4, 137 mM NaCl, and 3 mM KCl (HBS) before assays. Expression construct consisting of layilin cDNA in pLEN/NEO vector (Borowsky and Hynes, 1998) was used to generate stably transfected MCF-7 cells after selection for 4 wk in the presence of 0.5 mg/ml Geneticin (Life Technologies). Layilin transfectants or mock controls were released from culture dishes using 0.02% (wt/vol) trypsin, 2 mM CaCl2, and HBS to minimize proteolysis. Cells were labeled with 5 μg/μl calcein AM (Molecular Probes, Eugene, OR) by adding the fresh label to the trypsin medium. After 2 volumes of 0.04% (wt/vol) soy bean trypsin inhibitor in HBS were added to the sample and the sample was washed twice with HBS, the cells were resuspended in HBS containing 0.1% (wt/vol) BSA and 1 mM CaCl2, added to coated microtiter plate wells, and incubated for 90 min at 37°C. After one to six washings with HBS and 1 mM CaCl2 the fluorescence was measured with a 96-well plate reader. In each assay, the percentage of adherent cells was determined by comparison with wells containing cells treated identically but not subjected to the washings.
RESULTS
Preparation of Soluble Layilin-Fc Fusion Protein
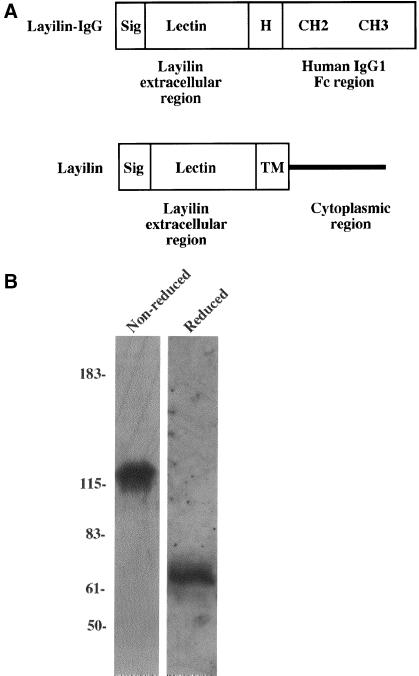
The soluble layilin-globulin was generated by in-frame fusion of the cDNA segment encoding the extracellular domain of layilin with a construct encoding the Fc region of human IgG1 (including the hinge, CH2, and CH3 domains; Figure 1A). Stably transfected cells expressing soluble layilin-Fc protein were generated by selection of the transfectants with hygromycin, and the secreted layilin-Fc chimeras were purified with protein A-Sepharose. Western blot analysis of SDS-PAGE gels revealed the presence of protein of the expected size (∼64 kDa in reduced conditions and ∼128 kDa in nonreduced conditions; Figure 1B). The purified layilin fusion protein migrated as a single band when subjected to SDS-PAGE and Coomassie blue staining (Bono, Rubin, Higgins, and Hynes, unpublished results), which further confirmed the identity and purity of the recombinant protein. The purified and Coomassie-stained CD44-Fc and E-cadherin-Fc migrated as described earlier (Bono, Rubin, Higgins, and Hynes, unpublished results; Aruffo et al., 1990; Higgins et al., 1998).
Figure 1.
Structure and purification of recombinant layilin-Fc fusion protein. (A) The protein domains of the layilin-Fc and wild-type layilin are shown. Layilin's extracellular part was cloned immediately N-terminal to the hinge domain (H) of the human IgG1 so that the chimera contains two cysteine residues (not shown) within the hinge domain responsible for Ig dimerization. Sig, NH2-terminal signal sequence, Lectin, layilin's extracellular part, which is homologous with C-type lectins; TM, transmembrane domain; CH2 and CH3, constant regions of the human IgG. (B) Purified layilin-Fc fusion protein was analyzed on an SDS-PAGE gel under both reduced and nonreduced conditions and detected by immunoblotting with the anti-human IgG (Fc-specific) antibody. Molecular masses (in kDa) are shown at the left.
HA Is a Ligand for Layilin
We chose to test layilin binding to HA for several reasons. First, layilin is widely expressed on different cell types and tissues (Borowsky and Hynes, 1998); therefore, it seemed plausible that a ligand for layilin might be a ubiquitous component of ECM. Second, HA can promote cell adhesion and motility (Knudson and Knudson, 1993; Entwistle et al., 1996), which are processes in which layilin is likely to play a role. Third, layilin's extracellular domain is a C-type lectin, and the three-dimensional structure of link domain (the HA-binding domain found in many HA-binding proteins; Neame and Barry, 1993; Kohda et al., 1996) has revealed an interesting structural homology between C-type lectins and the link module. The structure of the link domain showed that the predicted HA-binding site in the link domain is at an analogous position to the carbohydrate-binding pocket in the CRD of E-selectin, and layilin's CRD includes an identical insertion in the CRD loop as does E-selectin. Therefore, we decided to test the binding of layilin-IgG to HA immobilized on Sepharose beads.
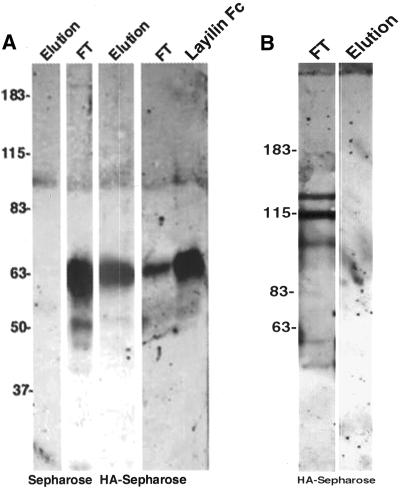
HA is a linear polysaccharide, which can be immobilized, for example, to EDA-Sepharose (McCourt and Gustafson, 1997). Because HA-binding proteins can be purified by affinity chromatography on immobilized HA (Tengblad, 1979), we incubated layilin-IgG as well as control IgG with HA-Sepharose beads. The bound material was eluted with HA dodecasaccharides, separated on SDS-PAGE gels, and detected with anti-human IgG (Fc specific) antibody. As shown in Figure 2A, layilin-IgG binds to HA-Sepharose but not to control Sepharose beads. The bound layilin-IgG could be released from the HA-Sepharose beads with HA dodecasaccharides (Figure 2A) and also with glycine or EDTA (Bono, Rubin, Higgins, and Hynes, unpublished results). HA octasaccharides were less effective in eluting layilin-IgG (Bono, Rubin, Higgins, and Hynes, unpublished results). The E-cadherin control fusion protein did not show any binding to HA-Sepharose; all the E-cadherin IgG was found in the flow-through fraction (Figure 2B), confirming that the layilin part of the IgG fusion protein (and not the Fc region) is binding specifically to HA.
Figure 2.
Purified layilin-Fc fusion protein binds to immobilized HA. (A) Layilin-Fc fusion protein was incubated with HA-Sepharose or with Sepharose control beads, and HA-specific dodecasaccharides were used to elute the bound material from the beads. The flow-through fractions (FT) and elutions were subjected to SDS-PAGE and immunoblotting with anti-human IgG antibody. Note that in Sepharose control lanes all the signal is found in the FT fraction. A small aliquot of the purified (unbound) layilin Fc was run on a parallel lane to show the location of the fusion protein in reduced conditions. (B) In a similar binding experiment with the E-cadherin Fc fusion protein, elution with HA oligosaccharides does not result in any detectable signal, and all the fusion protein is found in the FT lane. Molecular masses (in kDa) are shown at the left.
Layilin Binds to HA but Not to Other GAGs
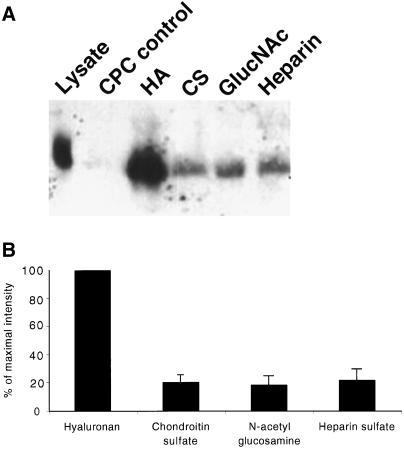
To address further the specific interaction between layilin and HA, we performed coprecipitation experiments with HA, heparin, chondroitin sulfate or N-acetyl glucosamine. In these experiments, NIH 3T3 cells were lysed in Nonidet P-40 buffer before addition of GAGs, bound proteins were precipitated by addition of 1% CPC, and the precipitates were resolved on SDS-PAGE gels and probed using an anti-layilin antibody. As shown in Figure 3A, layilin was coprecipitated only with HA. Other GAGs tested did not significantly precipitate layilin, as indicated by much weaker signals in the precipitation reactions. The results from two separate experiments were quantitated to compare the binding of layilin to different GAGs, and the quantitation clearly shows that layilin binding is specific for HA (Figure 3B).
Figure 3.
Layilin can be precipitated only in the presence of HA but not other GAGs. (A) Total cell lysates of 3T3 cells were incubated with different GAGs (50 μg each) and subjected to CPC precipitation. Precipitates and total cell lysate were resolved on an SDS-PAGE gel and stained with anti-layilin antibody after blotting onto membrane. Control reactions were precipitated with CPC without addition of any GAG or after addition of N-acetyl glucosamine. The intensities of the precipitated layilin bands in two similar experiments were quantitated, and the result is shown in B.
Layilin Binds Both Immobilized and Soluble HA
To study the interaction between layilin and HA, a binding assay was developed in which soluble fusion proteins were incubated with HA immobilized on 96-well plates. Bound fusion proteins were detected by adding HRP-conjugated anti-human IgG antibodies, which recognized the human IgG portion of the fusion protein. Layilin-IgG bound to HA in a concentration-dependent manner similar to the binding of CD44-IgG or HA-binding protein (Figure 4A). The control E-cadherin-IgG fusion protein did not show any significant binding at similar concentrations. For all three HA-binding proteins, the binding curves were biphasic, indicating complex binding kinetics. Nevertheless, because the binding curves showed similar patterns, it is possible to conclude that the respective affinities are similar, differing not more than two- to threefold. Estimates of Kd from the linear parts of the binding curves gave values on the order of 10−7 M.
Figure 4.
Binding of layilin-IgG (L-IgG) to immobilized and soluble HA. (A) Binding of varying amounts of purified chimeras to immobilized HA in the absence of competing GAGs. The bound layilin-IgG, CD44-IgG (positive control), and E-cadherin-IgG (negative control) fusion proteins were detected with an anti-Fc antibody in conjunction with HRP and the bound bHA-binding protein was detected with HRP-conjugated streptavidin. (B) Binding of soluble bHA to layilin-IgG (L-IgG) or CD44-IgG immobilized in microtiter wells. Varying amounts of fusion proteins were used to precoat the wells, which were then incubated with 10 μg/ml bHA. (C) Binding of 10 μg/ml layilin-Fc in the presence of free HA, chondroitin sulfate, or heparin. (D) Similar competition as in C but with CD44-IgG binding instead of layilin-IgG. Results in A–D each represent an average of duplicate determinations from the same experiment. Data presented are representative of three individual binding experiments.
The specificity of the layilin-IgG binding was examined by competition experiments in which free GAGs were incubated with layilin-IgG before the mixture was added to the wells coated with HA (Figure 4C). The results showed that neither heparin nor chondroitin sulfate could block the binding at the tested concentrations (1.6–200 μg/ml). In contrast, preincubation of layilin-IgG with soluble HA efficiently competed with the binding of layilin-IgG to immobilized HA in a manner almost identical to its competition of CD44-IgG binding to HA (Figure 4D). Thus, the affinity of layilin for HA is of similar range as the affinity of CD44 for HA.
Reciprocally, layilin's ability to bind soluble HA was tested with soluble bHA. Ninety-six–well plates were coated with layilin-IgG, blocked, and incubated with bHA, and the bound bHA was detected with streptavidin-HRP secondary reagent (Figure 4B). The results show that layilin-Fc immobilized to polystyrene wells bound to bHA in a concentration-dependent manner, and the binding pattern resembled closely the binding of bHA to CD44-Fc.
Layilin-Fc Binding to Tissue Sections Is Abolished by Hyaluronidase
To corroborate the results of the HA-Sepharose and microtiter binding experiments, we stained frozen tissue sections using layilin-Fc fusion protein as a histochemical reagent in conjunction with biotinylated anti-human IgG and the HRP-ABC reagent. Because tumors are frequently characterized by local accumulation of HA in vivo (Toole et al., 1979; Knudson et al., 1984), we stained sections from pancreata of RIP1-Tag2 transgenic mice, which spontaneously develop pancreatic tumors.
Incubation of frozen sections from the pancreata of RIP-Tag2 mice with layilin-Fc showed positive staining of the ECM of the tumors (Figure 5B), whereas no staining was observed with the E-cadherin-Fc control (Figure 5A). Positive staining with E-cadherin-Fc was observed in the epithelial cells of the surrounding mucosal tissues (Bono, Rubin, Higgins, and Hynes, unpublished results), as expected, because E-cadherin is abundantly expressed on epithelial cells (Nose and Takeichi, 1986).
To examine the specificity of the layilin-Fc staining, sections were pretreated with different GAG-degrading enzymes before incubation with the fusion protein. Pretreatment of the sections with hyaluronidase significantly reduced the layilin-Fc reactivity (Figure 5C), confirming that the staining of the ECM in Figure 5B is due to layilin-Fc binding to HA present in the matrix. Treatment of sections with chondroitinase or heparitinase (Figure 5, D and E, respectively) did not significantly affect the layilin-Fc binding, indicating that HA is the predominant layilin ligand on these tumor sections.
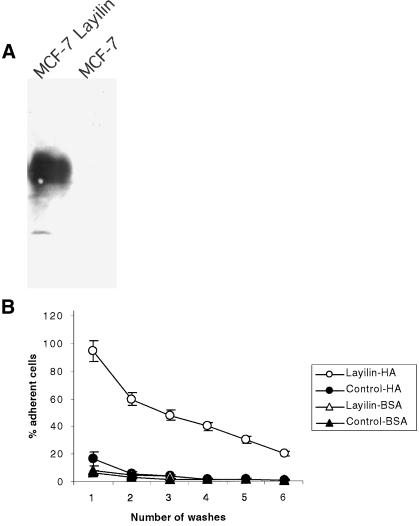
Layilin-transfected Cells Adhere to HA Substrates
Human MCF-7 breast cancer cells bind poorly to HA-coated wells, and Western blotting with anti-layilin antibody (Figure 6A) revealed that these cells do not express endogenous layilin. MCF-7 cells stably transfected with layilin cDNA were compared with mock-transfected controls in cell-substratum adhesion assays. Figure 6B shows that layilin-expressing cells adhered to HA-coated wells much better than did control cells, and the enhanced binding of layilin transfectants could be detected even after repeated washes of cells. Thus, layilin is a functional HA receptor on cells and can mediate cell adhesion.
Figure 6.
Layilin-transfected MCF-7 cells adhere to HA. (A) Expression of layilin on MCF-7 cells stably transfected with layilin cDNA or mock control. After selection, total cell lysates were subjected to SDS-PAGE and detection with anti-layilin antibody. (B) Layilin-expressing MCF-7 cells bind to HA. Microtiter plates were coated with HA or BSA, and the adhesion of layilin-MCF-7 or mock transfectants was determined after 90 min of adhesion using a fluorogenic assay. Data are presented as percentages of adherent cells. Means ± SD are shown. Data are representative of at least three separate experiments. Note that, even after repeated washes, considerably more layilin-MCF-7 cells than mock control cells bind to HA.
DISCUSSION
HA is a negatively charged high molecular mass polysaccharide composed of repeated disaccharides of d-glucuronic acid and N-acetyl glucosamine (Goa and Benfield, 1994). It is ubiquitously expressed in the extracellular space, represents a major constituent of the ECM, and has a central role in stability of ECM (Knudson and Knudson, 1993). Numerous cellular processes including cell migration (Chen et al., 1989), adhesion (Klein et al., 1996), and proliferation (Mast et al., 1993; Wiig et al., 1996) are influenced by HA. Thus, HA has an important role in such processes as morphogenesis (Toole, 1997), wound healing (Nishida et al., 1991), inflammation (Weigel et al., 1988), and immune cell trafficking (Mohamadzadeh et al., 1998), as well as in many aspects of tumor biology (Delpech et al., 1997).
Many of the effects of HA are mediated via its interaction with HA-binding proteins and receptors. The majority of the HA-binding proteins contain a common protein module termed link module, which is a structural domain of ∼100 amino acids in length (Neame and Barry, 1993; Kohda et al., 1996). Link modules have been described in several ECM molecules (link protein, aggrecan, versican, neurocan, and brevican) as well as on cell surface receptors. However, not all HA-binding proteins contain a link domain, namely, RHAMM, Cdc37, inter-α-trypsin inhibitor, plasma HA-binding protein, fibroblast HA-binding protein (reviewed by Day, 1999), and intracellular hyaluronate-binding protein (Hofmann et al., 1998). The best characterized HA cell surface receptors are CD44, RHAMM, ICAM-1, and LYVE-1 (Entwistle et al., 1996; Banerji et al., 1999), although there is some controversy as to whether ICAM-1 is a genuine HA receptor (McCourt and Gustafson, 1997).
In this work we searched for a ligand for layilin, a novel talin-binding protein localized in membrane ruffles and showed that layilin is a novel HA-binding cell surface receptor based on the following criteria: 1) layilin-Fc fusion protein binds to HA immobilized on Sepharose, and the bound material can be eluted with highly purified HA dodecasaccharides; 2) HA, but not other tested GAGs, precipitates layilin in the presence of 1% CPC; 3) layilin binds to HA immobilized in microtiter wells, and soluble HA binds to immobilized layilin; 4) layilin-IgG stains HA on frozen tissue sections in a hyaluronidase-sensitive manner, whereas chondroitinase or heparitinase treatment of the sections did not affect the staining intensity; 5) layilin-negative cells that do not bind to HA in adhesion assays become adherent after transfection with layilin. Thus, layilin is a member of the family of HA-binding proteins and can serve as an HA-binding cell adhesion receptor.
Layilin does not have obvious sequence identity with previously cloned HA receptors and does not contain a link module or a Bx7B motif (an α-helical sequence with clusters of basic amino acids), which is the HA-binding sequence in RHAMM (Yang et al., 1994), another HA receptor without a link domain. However, the extracellular domain of layilin is homologous with C-type lectins. The structural homology between the link domain and C-type lectins probably accounts for layilin's ability to bind HA (Brissett and Perkins, 1996; Kohda et al., 1996).
Although layilin and CD44, a known HA receptor, do not share any sequence homology, there is an interesting parallel between layilin and CD44. They both bind to HA via their extracellular part (link domain in CD44, lectin in layilin), they both can bind to molecules of the ERM superfamily with their intracellular parts because CD44 has been reported to bind ezrin and merlin (Sainio et al., 1997; Heiska et al., 1998; Yonemura et al., 1998), and layilin can bind talin and radixin (Borowsky and Hynes, 1998; Cordero, unpublished data). For CD44, the ERM-binding site has been mapped to a positively charged 19-amino acid cluster in the juxtamembrane region of the cytoplasmic domain (Yonemura et al., 1998). However, layilin does not have an obvious positively charged amino acid cluster next to the membrane-spanning region, and we have previously reported that the shortest talin-binding motif is in the C terminus of layilin's cytoplasmic domain (Borowsky and Hynes, 1998). However, similar extracellular and intracellular binding partners suggest possible shared functions between these molecules, and therefore, it will be of interest to investigate whether layilin also has a role in the processes of leukocyte migration to inflamed sites, cell adhesion and migration, and tumor metastasis (Gunthert et al., 1991; Stamenkovic et al., 1991; DeGrendele et al., 1996, 1997), all processes in which CD44 is known to play a role.
CD44 is known to have several isoforms due to alternative splicing (Haynes et al., 1991), although not all CD44 variants can bind HA and/or mediate lymphocyte homing (Berg et al., 1989; Stamenkovic et al., 1991). Although layilin genomic clones have not yet been analyzed, it will be of interest to study whether there also exist different layilin isoforms. Based on a Northern blot analysis of CHO cell RNA (Borowsky and Hynes, 1998), there is so far no evidence for multiple layilin forms, although it should be remembered that there may be species-specific differences.
Layilin's binding to HA is not remarkably strong; the affinity of layilin for HA is on the order of 10−7 M. Based on the similarities in the binding curves (Figure 4) layilin's affinity for HA appears similar to that of CD44. In comparison with other cell adhesion mechanisms such as those involving cadherins or integrins, the detected layilin affinity for HA is fairly weak. However, there are situations in which such weak binding may be an advantage. For example, in transient interactions, strong binding can be a disadvantage, and it is tempting to speculate that layilin's function is to mediate early cell-matrix interactions followed by more stable binding mediated, for example, by integrins.
Layilin's binding to HA may be affected by several factors including layilin's interactions with the cytoskeleton. The cytoskeleton may indirectly control layilin's binding to HA, for example by controlling the distribution and clustering of layilin on the cell surface. This could lead to possible multiple and higher affinity interactions between layilin and HA, which may increase the binding avidity over that of a monovalent interaction. Such multivalent interactions, in which several molecules of CD44 bind to the same HA molecule, have been reported (Underhill and Toole, 1980; Underhill, 1992), and the importance of proper cytoskeletal connection for CD44 binding to HA has also been suggested by experiments in which cells transfected with CD44 with a truncated cytoplasmic domain bind soluble HA less well than do cells transfected with intact CD44 (Lesley et al., 1992). Hence, it will be of interest to study whether cytoskeleton can regulate layilin's distribution on the cell surface and in this way regulate layilin's binding to HA.
In conclusion, we have identified layilin as a novel receptor for HA. Layilin binds specifically to HA but not to heparin or chondroitin sulfate under the binding conditions used in this study. Layilin's binding to this ECM component suggests a role for layilin in processes in which HA is known to be involved, including cell adhesion and motility. The identification of HA as a ligand for layilin defines this talin-binding partner as a novel member of the diverse family of HA-binding proteins and should facilitate attempts to find the true biological role for layilin.
ACKNOWLEDGMENTS
We thank Dr. Evi Heldin for providing the HA-EDA-Sepharose, Dr. Anders Uhlin for HA oligosaccharides, and Dr. Ivan Stamenkovic for the CD44-Fc construct. We are grateful to Denise Crowley for histology and Daniela Taverna for comments on the manuscript. This work has been supported by a grant from National Cancer Institute (RO1 CA17007). K. Rubin was on sabbatical leave from Uppsala University and supported by Swedish Cancer Foundation. P. Bono is an associate and R.O. Hynes is an investigator of the Howard Hughes Medical Institute.
Abbreviations used:
- bHA
biotinylated hyaluronan
- BSA
bovine serum albumin
- CHO
Chinese hamster ovary
- CPC
cetylpyridinium chloride
- CRD
carbohydrate recognition domain
- ERM
ezrin, radixin, moesin
- GAG
glycosaminoglycan
- HA
hyaluronan
- HRP
horseradish peroxidase
- Ig
immunoglobulin
- LYVE-1
lymphatic vessel endothelial hyaluronan receptor-1
- PBS
phosphate-buffered saline
- RHAMM
receptor for HA-mediated motility
REFERENCES
- Aruffo A, Stamenkovic I, Melnick M, Underhill CB, Seed B. CD44 is the principal cell surface receptor for hyaluronate. Cell. 1990;61:1303–1313. doi: 10.1016/0092-8674(90)90694-a. [DOI] [PubMed] [Google Scholar]
- Banerji S, Ni J, Wang SX, Clasper S, Su J, Tammi R, Jones M, Jackson DG. LYVE-1, a new homologue of the CD44 glycoprotein, is a lymph-specific receptor for hyaluronan. J Cell Biol. 1999;144:789–801. doi: 10.1083/jcb.144.4.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg EL, Goldstein LA, Jutila MA, Nakache M, Picker LJ, Streeter PR, Wu NW, Zhou D, Butcher EC. Homing receptors and vascular addressins: cell adhesion molecules that direct lymphocyte traffic. Immunol Rev. 1989;108:5–18. doi: 10.1111/j.1600-065x.1989.tb00010.x. [DOI] [PubMed] [Google Scholar]
- Bloom L, Ingham KC, Hynes RO. Fibronectin regulates assembly of actin filaments and focal contacts in cultured cells via the heparin-binding site in repeat III13. Mol Biol Cell. 1999;10:1521–1536. doi: 10.1091/mbc.10.5.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton SJ, Barry ST, Mosley H, Patel B, Jockusch BM, Wilkinson JM, Critchley DR. Monoclonal antibodies recognizing the N- and C-terminal regions of talin disrupt actin stress fibers when microinjected into human fibroblasts. Cell Motil Cytoskeleton. 1997;36:363–376. doi: 10.1002/(SICI)1097-0169(1997)36:4<363::AID-CM6>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Bono P, Salmi M, Smith DJ, Jalkanen S. Cloning and characterization of mouse vascular adhesion protein-1 reveals a novel molecule with enzymatic activity. J Immunol. 1998;160:5563–5571. [PubMed] [Google Scholar]
- Borowsky ML, Hynes RO. Layilin, a novel talin-binding transmembrane protein homologous with C- type lectins, is localized in membrane ruffles. J Cell Biol. 1998;143:429–442. doi: 10.1083/jcb.143.2.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brissett NC, Perkins SJ. The protein fold of the hyaluronate-binding proteoglycan tandem repeat domain of link protein, aggrecan and CD44 is similar to that of the C-type lectin superfamily. FEBS Lett. 1996;388:211–216. doi: 10.1016/0014-5793(96)00576-5. [DOI] [PubMed] [Google Scholar]
- Calderwood DA, Zent R, Grant R, Rees DJ, Hynes RO, Ginsberg MH. The talin head domain binds to integrin beta subunit cytoplasmic tails and regulates integrin activation. J Biol Chem. 1999;274:28071–28074. doi: 10.1074/jbc.274.40.28071. [DOI] [PubMed] [Google Scholar]
- Chen WY, Grant ME, Schor AM, Schor SL. Differences between adult and fetal fibroblasts in the regulation of hyaluronate synthesis: correlation with migratory activity. J Cell Sci. 1989;94:577–584. doi: 10.1242/jcs.94.3.577. [DOI] [PubMed] [Google Scholar]
- Chen YT, Nelson WJ. Continuous production of soluble extracellular domain of a type-I transmembrane protein in mammalian cells using an Epstein-Barr virus Ori-P-based expression vector. Anal Biochem. 1996;242:276–278. doi: 10.1006/abio.1996.0466. [DOI] [PubMed] [Google Scholar]
- Critchley DR. Focal adhesions: the cytoskeletal connection. Curr Opin Cell Biol. 2000;12:133–139. doi: 10.1016/s0955-0674(99)00067-8. [DOI] [PubMed] [Google Scholar]
- Day AJ. The structure and regulation of hyaluronan-binding proteins. Biochem Soc Trans. 1999;27:115–121. doi: 10.1042/bst0270115. [DOI] [PubMed] [Google Scholar]
- DeGrendele HC, Estess P, Picker LJ, Siegelman MH. CD44 and its ligand hyaluronate mediate rolling under physiologic flow: a novel lymphocyte-endothelial cell primary adhesion pathway. J Exp Med. 1996;183:1119–1130. doi: 10.1084/jem.183.3.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeGrendele HC, Estess P, Siegelman MH. Requirement for CD44 in activated T cell extravasation into an inflammatory site. Science. 1997;278:672–675. doi: 10.1126/science.278.5338.672. [DOI] [PubMed] [Google Scholar]
- Delpech B, Girard N, Bertrand P, Courel MN, Chauzy C, Delpech A. Hyaluronan: fundamental principles and applications in cancer. J Intern Med. 1997;242:41–48. doi: 10.1046/j.1365-2796.1997.00172.x. [DOI] [PubMed] [Google Scholar]
- Entwistle J, Hall CL, Turley EA. HA receptors: regulators of signaling to the cytoskeleton. J Cell Biochem. 1996;61:569–577. doi: 10.1002/(sici)1097-4644(19960616)61:4<569::aid-jcb10>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Folkman J, Watson K, Ingber D, Hanahan D. Induction of angiogenesis during the transition from hyperplasia to neoplasia. Nature. 1989;339:58–61. doi: 10.1038/339058a0. [DOI] [PubMed] [Google Scholar]
- Goa KL, Benfield P. Hyaluronic acid: a review of its pharmacology and use as a surgical aid in ophthalmology, and its therapeutic potential in joint disease and wound healing. Drugs. 1994;47:536–566. doi: 10.2165/00003495-199447030-00009. [DOI] [PubMed] [Google Scholar]
- Graves BJ, Crowther RL, Chandran C, Rumberger JM, Li S, Huang KS, Presky DH, Familletti PC, Wolitzky BA, Burns DK. Insight into E-selectin/ligand interaction from the crystal structure and mutagenesis of the lec/EGF domains. Nature. 1994;367:532–538. doi: 10.1038/367532a0. [DOI] [PubMed] [Google Scholar]
- Gunthert U, Hofmann M, Rudy W, Reber S, Zoller M, Haussmann I, Matzku S, Wenzel A, Ponta H, Herrlich P. A new variant of glycoprotein CD44 confers metastatic potential to rat carcinoma cells. Cell. 1991;65:13–24. doi: 10.1016/0092-8674(91)90403-l. [DOI] [PubMed] [Google Scholar]
- Hall A. Rho GTPases. and the actin cytoskeleton. Science. 1998;279:509–514. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- Hanahan D. Heritable formation of pancreatic beta-cell tumors in transgenic mice expressing recombinant insulin/simian virus 40 oncogenes. Nature. 1985;315:115–122. doi: 10.1038/315115a0. [DOI] [PubMed] [Google Scholar]
- Hardwick C, Hoare K, Owens R, Hohn HP, Hook M, Moore D, Cripps V, Austen L, Nance DM, Turley EA. Molecular cloning of a novel hyaluronan receptor that mediates tumor cell motility [published erratum appears in J. Cell Biol. 1992; 18, 753] J Cell Biol. 1992;117:1343–1350. doi: 10.1083/jcb.117.6.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes BF, Liao HX, Patton KL. The transmembrane hyaluronate receptor (CD44): multiple functions, multiple forms. Cancer Cells. 1991;3:347–350. [PubMed] [Google Scholar]
- Heiska L, Alfthan K, Gronholm M, Vilja P, Vaheri A, Carpen O. Association of ezrin with intercellular adhesion molecule-1 and -2 (ICAM-1 and ICAM-2): regulation by phosphatidylinositol 4,5-bisphosphate. J Biol Chem. 1998;273:21893–21900. doi: 10.1074/jbc.273.34.21893. [DOI] [PubMed] [Google Scholar]
- Hemmings L, Rees DJ, Ohanian V, Bolton SJ, Gilmore AP, Patel B, Priddle H, Trevithick JE, Hynes RO, Critchley DR. Talin contains three actin-binding sites each of which is adjacent to a vinculin-binding site. J Cell Sci. 1996;109:2715–2726. doi: 10.1242/jcs.109.11.2715. [DOI] [PubMed] [Google Scholar]
- Higgins JM, Mandlebrot DA, Shaw SK, Russell GJ, Murphy EA, Chen YT, Nelson WJ, Parker CM, Brenner MB. Direct and regulated interaction of integrin alphaEbeta7 with E-cadherin. J Cell Biol. 1998;140:197–210. doi: 10.1083/jcb.140.1.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann M, Fieber C, Assmann V, Gottlicher M, Sleeman J, Plug R, Howells N, von Stein O, Ponta H, Herrlich P. Identification of IHABP, a 95 kDa intracellular hyaluronate binding protein. J Cell Sci. 1998;111:1673–1684. doi: 10.1242/jcs.111.12.1673. [DOI] [PubMed] [Google Scholar]
- Klein ES, Asculai SS, Ben-Ari GY. Effects of hyaluronic acid on fibroblast behavior in peritoneal injury. J Surg Res. 1996;61:473–476. doi: 10.1006/jsre.1996.0149. [DOI] [PubMed] [Google Scholar]
- Knudson CB, Knudson W. Hyaluronan-binding proteins in development, tissue homeostasis, and disease. FASEB J. 1993;7:1233–1241. [PubMed] [Google Scholar]
- Knudson W, Biswas C, Toole BP. Stimulation of glycosaminoglycan production in murine tumors. J Cell Biochem. 1984;25:183–196. doi: 10.1002/jcb.240250402. [DOI] [PubMed] [Google Scholar]
- Kohda D, Morton CJ, Parkar AA, Hatanaka H, Inagaki FM, Campbell ID, Day AJ. Solution structure of the link module: a hyaluronan-binding domain involved in extracellular matrix stability and cell migration. Cell. 1996;86:767–775. doi: 10.1016/s0092-8674(00)80151-8. [DOI] [PubMed] [Google Scholar]
- Lee TH, Wisniewski HG, Vilcek J. A novel secretory tumor necrosis factor-inducible protein (TSG-6) is a member of the family of hyaluronate binding proteins, closely related to the adhesion receptor CD44. J Cell Biol. 1992;116:545–557. doi: 10.1083/jcb.116.2.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesley J, He Q, Miyake K, Hamann A, Hyman R, Kincade PW. Requirements for hyaluronic acid binding by CD44: a role for the cytoplasmic domain and activation by antibody. J Exp Med. 1992;175:257–266. doi: 10.1084/jem.175.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Kim SH, Higgins JMG, Brenner M, Sacks DB. IQGAP and calmodulin modulate E-cadherin function. J Biol Chem. 1999;274:37885–37892. doi: 10.1074/jbc.274.53.37885. [DOI] [PubMed] [Google Scholar]
- Mangeat P, Roy C, Martin M. ERM proteins in cell adhesion and membrane dynamics. Trends Cell Biol. 1999;9:187–192. doi: 10.1016/s0962-8924(99)01544-5. [DOI] [PubMed] [Google Scholar]
- Mast BA, Diegelmann RF, Krummel TM, Cohen IK. Hyaluronic acid modulates proliferation, collagen and protein synthesis of cultured fetal fibroblasts. Matrix. 1993;13:441–446. doi: 10.1016/s0934-8832(11)80110-1. [DOI] [PubMed] [Google Scholar]
- McCourt PA, Gustafson S. On the adsorption of hyaluronan and ICAM-1 to modified hydrophobic resins. Int J Biochem Cell Biol. 1997;29:1179–1189. doi: 10.1016/s1357-2725(97)00058-7. [DOI] [PubMed] [Google Scholar]
- Mohamadzadeh M, DeGrendele H, Arizpe H, Estess P, Siegelman M. Proinflammatory stimuli regulate endothelial hyaluronan expression and CD44/HA-dependent primary adhesion. J Clin Invest. 1998;101:97–108. doi: 10.1172/JCI1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neame PJ, Barry FP. The link proteins. Experientia. 1993;49:393–402. doi: 10.1007/BF01923584. [DOI] [PubMed] [Google Scholar]
- Nishida T, Nakamura M, Mishima H, Otori T. Hyaluronan stimulates corneal epithelial migration. Exp Eye Res. 1991;53:753–758. doi: 10.1016/0014-4835(91)90110-z. [DOI] [PubMed] [Google Scholar]
- Nose A, Takeichi M. A novel cadherin cell adhesion molecule: its expression patterns associated with implantation and organogenesis of mouse embryos. J Cell Biol. 1986;103:2649–2658. doi: 10.1083/jcb.103.6.2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sainio M, Zhao F, Heiska L, Turunen O, den Bakker M, Zwarthoff E, Lutchman M, Rouleau GA, Jaaskelainen J, Vaheri A, Carpen O. Neurofibromatosis 2 tumor suppressor protein colocalizes with ezrin and CD44 and associates with actin-containing cytoskeleton. J Cell Sci. 1997;110:2249–2260. doi: 10.1242/jcs.110.18.2249. [DOI] [PubMed] [Google Scholar]
- Schoenwaelder SM, Burridge K. Bidirectional signaling between the cytoskeleton and integrins. Curr Opin Cell Biol. 1999;11:274–286. doi: 10.1016/s0955-0674(99)80037-4. [DOI] [PubMed] [Google Scholar]
- Stamenkovic I, Amiot M, Pesando JM, Seed B. A lymphocyte molecule implicated in lymph node homing is a member of the cartilage link protein family. Cell. 1989;56:1057–1062. doi: 10.1016/0092-8674(89)90638-7. [DOI] [PubMed] [Google Scholar]
- Stamenkovic I, Aruffo A, Amiot M, Seed B. The hematopoietic and epithelial forms of CD44 are distinct polypeptides with different adhesion potentials for hyaluronate-bearing cells. EMBO J. 1991;10:343–348. doi: 10.1002/j.1460-2075.1991.tb07955.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tengblad A. Affinity chromatography on immobilized hyaluronate and its application to the isolation of hyaluronate binding properties from cartilage. Biochim Biophys Acta. 1979;578:281–289. doi: 10.1016/0005-2795(79)90158-2. [DOI] [PubMed] [Google Scholar]
- Toole BP. Hyaluronan in morphogenesis. J Intern Med. 1997;242:35–40. doi: 10.1046/j.1365-2796.1997.00171.x. [DOI] [PubMed] [Google Scholar]
- Toole BP, Biswas C, Gross J. Hyaluronate and invasiveness of the rabbit V2 carcinoma. Proc Natl Acad Sci USA. 1979;76:6299–6303. doi: 10.1073/pnas.76.12.6299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Underhill C. CD44: the hyaluronan receptor. J Cell Sci. 1992;103:293–298. doi: 10.1242/jcs.103.2.293. [DOI] [PubMed] [Google Scholar]
- Underhill CB, Toole BP. Physical characteristics of hyaluronate binding to the surface of simian virus 40-transformed 3T3 cells. J Biol Chem. 1980;255:4544–4549. [PubMed] [Google Scholar]
- Weigel PH, Frost SJ, McGary CT, LeBoeuf RD. The role of hyaluronic acid in inflammation and wound healing. Int J Tissue React. 1988;10:355–365. [PubMed] [Google Scholar]
- Wiig M, Abrahamsson SO, Lundborg G. Effects of hyaluronan on cell proliferation and collagen synthesis: a study of rabbit flexor tendons in vitro. J Hand Surg Am. 1996;21:599–604. doi: 10.1016/S0363-5023(96)80010-4. [DOI] [PubMed] [Google Scholar]
- Yang B, Yang BL, Savani RC, Turley EA. Identification of a common hyaluronan binding motif in the hyaluronan binding proteins RHAMM, CD44 and link protein. EMBO J. 1994;13:286–296. doi: 10.1002/j.1460-2075.1994.tb06261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonemura S, Hirao M, Doi Y, Takahashi N, Kondo T, Tsukita S. Ezrin/radixin/moesin (ERM). proteins bind to a positively charged amino acid cluster in the juxta-membrane cytoplasmic domain of CD44, CD43, and ICAM-2. J Cell Biol. 1998;140:885–895. doi: 10.1083/jcb.140.4.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Q, Toole BP. Biotinylated hyaluronan as a probe for detection of binding proteins in cells and tissues. Biotechniques. 1995;19:122–124. , 126–129. [PubMed] [Google Scholar]