Abstract
(−)-Gossypol, a natural BH3-mimetic and small-molecule Bcl-2 inhibitor, shows promise in ongoing phase II clinical trials for human cancers. However, whether (−)-gossypol plays functional roles in tumor angiogenesis has not been directly elucidated yet. In this study, we demonstrated that (−)-gossypol dose-dependently inhibited the expression of vascular endothelial growth factor (VEGF), Bcl-2 and Bcl-xL in human prostate cancer cells (PC-3 and DU 145) and human primary cultured umbilical vein endothelial cells (HUVECs) in vitro. Notably, the growth of human prostate tumor PC-3 xenografts in mice was significantly suppressed by (−)-gossypol at dosage of 15 mg/kg/d. This inhibitory action of (−)-gossypol in vivo was largely dependent on suppression of angiogenesis in the solid tumors, where VEGF expression and microvessel density were remarkably decreased. Furthermore, (−)-gossypol inhibited VEGF-induced chemotactic motility and tubulogenesis in HUVECs and human microvascular endothelial cells, and suppressed microvessel sprouting from rat aortic rings ex vivo. When examined for the mechanism, we found that (−)-gossypol blocked the activation of VEGF receptor 2 kinase with the half maximal inhibitory concentration of 2.38 μmol/L in endothelial cells. Consequently, VEGF-triggered phosphorylation of key intracellular proangiogenic molecules, such as Src family kinase, focal adhesion kinase, extracellular signal-related kinase and AKT kinase, were all suppressed by the treatment. Taken together, the present study demonstrates that (−)-gossypol potently inhibits human prostate tumor growth through modulating VEGF signaling pathway, which further validates its great potential in clinical practice.
Keywords: (−)-Gossypol, tumor angiogenesis, VEGF, VEGF receptor 2 (KDR/Flk-1), Bcl-2
Introduction
Solid tumors recruit new blood vessels for their growth, maintenance, and metastasis (1, 2). Discovering drugs that suppress tumor-induced development of new blood vessels (angiogenesis) is an important strategy for cancer treatment. So far, angiogenesis inhibition has come off the bench and entered into clinical application. Many targets of endogenous angiogenesis inhibitors reflect the complexity of the process; however, current clinical therapies mainly target the vascular endothelial growth factor (VEGF) system (3). Different agents including antibodies, aptamers, peptides, and small molecules have been extensively investigated to block VEGF and its proangiogenic functions (4). The VEGF signaling events relevant to tumor angiogenesis is mainly mediated by VEGF receptor 2 (VEGFR2, KDR/Flk-1) (5, 6). Mechanistically, activating VEGFR2 at specific tyrosine sites results in the phosphorylation of various intracellular signaling molecules, such as Src family kinase (7), focal adhesion kinase (FAK) (8), phosphatidylinositol 3-kinases/AKT kinase (9, 10), extracellular signal-related kinase (ERK1/2) (11), mammalian target of rapamycin kinase (12), and signal transducer and activator of transcription (13) in endothelial cells. All of these pivotal molecules collaboratively promote proliferation, migration, invasion and differentiation to capillary-like structure of endothelial cells in the preexisting vasculature.
(−)-Gossypol, a bioactive phytochemical produced by cotton plants, has been considered as a natural BH3 mimetic (Fig. 1A). Through potent inhibition of Bcl-2/Bcl-xL/Mcl-1, gossypol potentiates apoptosis in numerous human cancer cells, including prostate (14), colon, breast, lung, pancreatic, hepatoma, and head and neck cancers (15). In addition, (−)-gossypol can radiosensitize prostate cancer in vitro and in vivo without augmenting toxicity (16). Multiple molecular investigations reveal that gossypol and its derivatives modulate TGF-beta/Akt signaling (17), activate P53 (18) and SAPK/JUK pathway (19), suppress the c-Myc signaling (20), inhibit NF-κB activity and NF-κB-mediated gene expression (21), regulate ROS-dependent mitochondria and death receptor 5 pathway (22, 23) and intracellular Ca2+ (24). Recent studies showed that (−)-gossypol and its enantiomer (AT-101) could affect proangiogenic molecules released from cancer cells at mRNA and protein levels either alone or in combination (25–27), suggesting the potential role of (−)-gossypol in antiangiogenesis. Additionally, it has been shown that Bcl-2 gene expression is significantly higher in the tumor-associated endothelial cells as compared with normal endothelial cells (28), and up-regulated Bcl-2 expression in microvascular endothelial cells was sufficient to enhance intratumoral angiogenesis and to accelerate tumor growth (29, 30). However, whether (−)-gossypol, known as a potent Bcl-2 inhibitor, can directly modulate the biological functions of endothelial cells remains obscure.
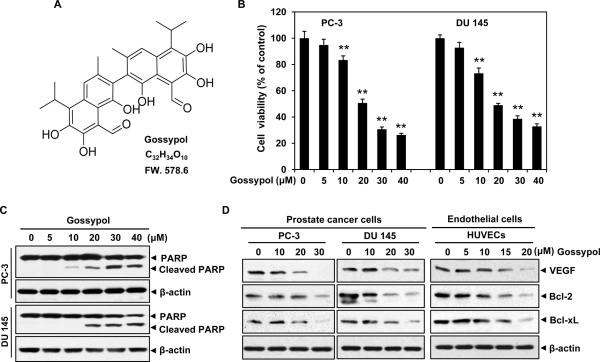
Figure 1. (−)-Gossypol decreases cell viability via apoptosis induction and inhibits Bcl-2/Bcl-xL/VEGF signaling in prostate cancer cells and endothelial cells.
A, the chemical structure of (−)-gossypol. B, (−)-gossypol inhibited prostate cancer cell viability in a dose-dependent manner. PC-3 and DU 145 cells (5~6×103 cells) were directly treated with or without various concentrations of (−)-gossypol for 48 h. Cell viability was quantified by MTS assay. Columns, mean; bars, standard error; **, P < 0.01 vs. untreated group. C, (−)-gossypol induced potent apoptosis in prostate cancer cells. PC-3 and DU 145 cells were treated with (−)-gossypol for 24 h. The whole cell protein was applied to western blotting analysis. The full length of PARP was cleaved into 89 KD form as indicated. D, (−)-gossypol suppressed the expression of VEGF, Bcl-2 and Bcl-xL in human prostate cancer cells and endothelial cells. PC-3, DU 145 and HUVECs were incubated with (−)-gossypol for 24 h. The whole cell protein was harvested and probed with specific antibodies.
Therefore, in the present study, we investigated the biological roles of (−)-gossypol in tumor angiogenesis, and our results revealed that (−)-gossypol significantly inhibited angiogenesis and the growth of prostate tumor xenografts by targeting VEGF signaling pathway.
Materials and Methods
Reagents
(−)-Gossypol was supplied by Tocris Bioscience (St. Louis, MO). A 100-mmol/L stock solution was prepared in dimethyl sulfoxide (DMSO) and then stored at −20°C as small aliquots until needed. Bacteria-derived recombinant human VEGF (rhVEGF) was a gift from National Institutes of Health (NIH; Bethesda, MD). Growth factor–reduced Matrigel was purchased from BD Biosciences (San Jose, CA). Antibodies against ERK1, Bcl-2, VEGF and β-actin were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Poly (ADP-ribose) polymerase (PARP), Bcl-xL, VEGFR2, AKT, Src, FAK and phospho-specific anti-VEGFR2 (Tyr1175) and anti-VEGFR2 (Tyr996), anti-Src (Tyr416), anti-FAK (Tyr397), anti-AKT (Ser473), anti-pERK1/2 (Thr202/Tyr204) were purchased from Cell Signaling Technology (Danvers, MA). Antibody against CD31was bought from Epitomics (Burlingame, CA).
Cell lines and cell culture
Primary human umbilical vascular endothelial cells (HUVECs) kindly gifted from Dr. Xinli Wang (Cardiothoracic Surgery Division of Michael E. DeBakey Department of Surgery at Baylor College of Medicine in Houston) in 2008 were cultured in endothelial cell culture medium (ECM) as described previously (31). Human microvascular endothelial cells (HMEC-1), human prostate cancer PC-3 cells and human prostate cancer DU 145 cells were got from American Type Culture Collection (ATCC; Manassas, VA) in 2009. HMEC-1 was cultured with MCDB 131 medium (Sigma; St Louis, MO) supplemented with 10% fetal bovine serum (FBS; Hyclone, Logan, UT), 2 mmol/L L-glutamine, 100 IU/mL of penicillin, 100 mg/mL of streptomycin, 10 ng/mL of epidermal growth factor and 1 mg/ml of hydrocortisone (Sigma). HUVECs and HMEC-1 were confirmed by their typical microscopic morphology: homogeneous, large, polygonal and cobblestone-like. PC-3 cells were cultured in RPMI 1640 medium (Hyclone) supplemented with 10% FBS, and DU 145 cells were cultured in Dulbecco's Modified Eagle Medium (Hyclone) supplemented with 10% FBS. Western blotting using epithelial markers authenticated that they were of epithelial origin before experiments. All these cells were tested for mycoplasma-free by PCR methods before use and maintained at 37°C under a humidified 95%:5% (v/v) mixture of air and CO2.
Animal studies
Animals used in the present study were maintained according to the NIH standards established in the Guidelines for the Care and Use of Experimental Animals. All of the experimental protocols were approved by the Animal Investigation Committee of East China Normal University.
Xenograft human prostate tumor mouse model
Xenograft mouse model was conducted as previously described (31). Five- to 6-week-old male BALB/cA nude mice (National Rodent Laboratory Animal Resources, Shanghai, China) were randomly divided into each group of 6~7 mice. PC-3 cells were grown to 80–90% confluence, harvested, prepared at 5×106 cells/100 μL cell suspensions, and inoculated on the flank region of nude mice. After tumors grew to about 50 mm3, mice were treated with or without (−)-gossypol (15 mg/kg) by daily intralesional injections for consecutive 50 days. (−)-Gossypol (dissolved in DMSO) was delivered through one or two injection sites around the tumors, depending on tumor size at the time of injection. The control mouse group was administrated with the control solution containing the same amount of DMSO without the drug. The body weight of each mouse was recorded every 5 days. The volume of solid tumors were determined using Vernier caliper measurement and calculated according to the formula of A×B2×0.52, where A is the longest diameter of the tumor and B is the shortest. After 50 d, mice were sacrificed.
Histology and immunohistochemistry
Solid tumors were fixed with 10% formaldehyde and embedded in paraffin. Antibodies against CD31, VEGFR2 and VEGF were applied to indicate infiltrating blood vessel and detect VEGF expression on 5-μm tumor sections. Images were taken using a Leica DM 4000B photo microscope (Solms, Germany; magnification, 400×). The microvessel density was calculated statistically by using Image-Pro Plus 6.0 software (Media Cybernetics, Bethesda, MD) according to CD31 immunohistochemistry (n=5).
Cell viability assay
PC-3 and DU 145 (5~6×103 cells/well) cells were directly incubated with indicated concentrations of (−)-gossypol for 48 h. HUVECs (6~7×103 cells/well) were treated with or without VEGF (50 ng/mL) and various concentrations of (−)-gossypol for 48 h. To determine cell viability, we used a CellTiter 96 AQueous One Solution Cell Proliferation kit (Promega; Madison, WI) and a VERSAmax microplate reader (Molecular Devices; Sunnyvale, CA).
Endothelial cell migration assay
Transwell migration assay was performed as described previously (32). Briefly, HUVECs (2×104 cells/well) or HMEC-1 (2×104 cells/well) along with the indicated concentrations of (−)-gossypol were seeded into the upper chambers. The bottom chambers were filled with 500 μL basal endothelial cell culture medium supplemented with 0.5% FBS and 30 ng/mL VEGF. After 6–8 h incubation, migrated cells were fixed with 4% paraformaldehyde and stained with 1% crystal violet. Images were taken using an OLYMPUS inverted microscope (Olympus; magnification, 160×). Three independent experiments were performed.
Endothelial cell capillary-like tube formation assay
Tube formation was assessed as described previously (32). Briefly, HUVECs or HMEC-1 were pretreated with various dilutions of (−)-gossypol for 2 h and then seeded onto the Matrigel layer in 24-well plates at a density of 4~7×104 cells. ECM (0.5% FBS) with or without 30 ng/mL of VEGF was added into wells. After 4~6 h, tubulogenesis was fixed and photographed using an inverted microscope (Olympus; original magnification, 100×). Three independent experiments were performed.
Rat aortic ring assay
In brief, 48-well plates were coated with 120 μL of Matrigel per well and polymerized in an incubator. Aorta isolated from 5-week-old male Sprague-Dawley rats was cut into rings of 1~1.5 mm in circumference, randomized into wells and sealed with a 100 μL–overlay of Matrigel. VEGF in 500 μL ECM (0.5% FBS) with or without (−)-gossypol was added into the wells. Fresh medium was replaced every 2 d. After a week, microvessel sprouting was fixed and photographed using an inverted microscope (Olympus, Center Valley, PA; magnification 100×). The assay was scored from 0 (least positive) to 5 (most positive) in a double-blind manner.
Western blotting analysis
To examine the apoptotic effects of (−)-gossypol on prostate cancer cells, PC-3 and DU 145 were directly treated with various concentrations of (−)-gossypol for 24 h. To detect the expression of VEGF, Bcl-2 and Bcl-xL in treated cancer cells and endothelial cells, PC-3, DU 145 and HUVECs were incubated with (−)-gossypol for 24 h. To determine the molecular basis of (−)-gossypol in angiogenesis signaling, HUVECs were first starved in serum-free ECM for 4–6 h and then pretreated with or without various concentrations of (−)-gossypol for 30 min, followed by stimulation with 50 ng/mL of VEGF for 2~20 min. The whole-cell extracts were prepared in RIPA buffer (20 mmol/L Tris, 2.5 mmol/L EDTA, 1% Triton X-100, 1% deoxycholate, 0.1% SDS, 40 mmol/L NaF, 10 mmol/L Na4P2O7, and 1 mmol/L PMSF) supplemented with proteinase inhibitor cocktail (Calbiochem, San Diego, CA). About 40~50 μg of cellular protein from each sample was applied to 6%–12% SDS-polyacrylamide gels and probed with specific antibodies, followed by exposure to a horseradish peroxidase-conjugated goat anti-mouse or goat anti-rabbit antibody (Cell Signaling Technology). Protein concentration was determined using bicinchoninic acid assay and equalized before loading. Relative optical density of blotting bands was qualified by Image J software (NIH; Bethesda, MD).
In vitro VEGFR2 kinase Inhibition assay
VEGFR2 kinase assay was performed using an HTScan VEGFR2 kinase kit from Cell Signaling Technology (Danvers, MA) combined with colorimetric ELISA detection as described previously (33). The final reaction system contained 60 mmol/L HEPES (pH 7.5), 5 mmol/L MgCl2, 5 mmol/L MnCl2, 3 μmol/L Na3VO4, 1.25 mmol/L DTT, 20 μmol/L ATP, 1.5 μmol/L substrate peptide, 100 ng of VEGF receptor kinase and different concentrations of (−)-gossypol.
Statistical analysis
Statistical comparisons between groups were performed using one-way analysis of variance (ANOVA) followed by Student's t-test. Data were presented as means ± standard error. P values ≤ 0.05 were considered statistically significant.
Results
(−)-Gossypol decreases cell viability and induces apoptosis in human prostate cancer cells
Prostate cancer continues to represent a burgeoning medical problem in the United States. In our study, the cytotoxic effects of (−)-gossypol were first examined on PC-3 and DU 145 cancer cells. The MTS results showed that (−)-gossypol inhibited cell viability in a dose-dependent manner, with the half maximal inhibitory concentrations of ~20 μmol/L (Fig.1B). Western blotting analysis further revealed that (−)-gossypol induced potent apoptosis in PC-3 and DU 145 cells, where the full length of nuclear poly (ADP-ribose) polymerase (PARP) were cleaved from the intact form (116 KD) into cleaved from (89 KD) (Fig.1C). These results were consistent with previous finding that (−)-gossypol suppressed the proliferation of prostate cancer cells in vitro (34).
(−)-Gossypol suppresses the expression of VEGF, Bcl-2 and Bcl-xL in human prostate cancer cells and endothelial cells
VEGF is a major tumor-associated growth factor that potently stimulates endothelial cell proliferation, chemotaxis, angiogenesis and vascular permeability. Bcl-2 has been shown to active nuclear factor-κB (NF-κB) in cancer cells, which regulates expression of chemokines and proangiogenic factors involved in inflammation and angiogenesis (35, 36). Thus, we examined whether (−)-gossypol could downregulate the expression of VEGF while blocking Bcl-2. As shown in Fig. 1D, treatment with (−)-gossypol resulted in a dose-dependent inhibition of VEGF and Bcl-2/Bcl-xL in both cancer cells and endothelial cells, indicating its great function in tumor angiogenesis.
(−)-Gossypol suppresses tumor growth and angiogenesis in a human prostate tumor xenograft mouse model
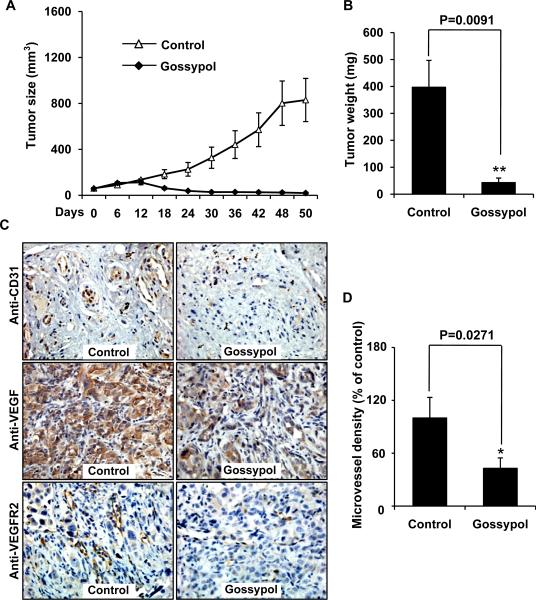
To investigate the effect of (−)-gossypol on tumor growth and tumor angiogenesis in vivo, we conducted a prostate tumor (PC-3) xenograft mouse model. We found that administration of (−)-gossypol (15 mg/kg/d) for 50 d substantially suppressed tumor volume (Fig. 2A) and reduced tumor weight (Fig. 2B) compared to the control groups injected with the same solution without the drug. The average tumor volume of the control group increased from 61.19 ± 6.96 mm3 to 829.83 ±187.91 mm3 at the end of the experiments, whereas that in the (−)-gossypol-treated group decreased from 58.46 ± 3.25 mm3 to 19.74 ± 7.71 mm3. Additionally, the average tumor weight of the control group was 397.12 ± 99.69 mg, whereas that in the (−)-gossypol-treated group was only 43.10 ± 16.83 mg (Fig. 1B), suggesting a significant inhibition of tumor growth by (−)-gossypol. In our experimental system, low dosage of (−)-gossypol at 5 mg/kg/d was also tested; however, little effect was observed in mice. During treatment, the (−)-gossypol-treated mice appeared healthy and (−)-gossypol had little effect on the body weight of mice (data not shown). In addition, pathologic analysis at autopsy revealed no (−)-gossypol-induced tissue changes in the organs, suggesting that (−)-gossypol had little toxicity at the tested dosage.
Figure 2. (−)-Gossypol suppresses tumor growth and angiogenesis of human prostate tumor xenografts.
PC-3 cells were injected into 5- to 6-week-old BALB/cA nude mice (5×106 cells per mouse). After solid tumors established, the mice were subcutaneously treated with or without (−)-gossypol at dosage of 15 mg/kg daily. A, (−)-gossypol inhibited tumor growth as measured by tumor volume. B, the weight of solid tumors in the (−)-gossypol-treated mice was significantly lower than that of the control group. C, anti-CD 31, anti-VEGF and anti-VEGFR2 immunohistochemistry revealed that (−)-gossypol inhibited neovascularization and VEGF expression in solid tumors. D, microvessel density was analyzed by Image-Pro Plus 6.0 software. Columns, mean; bars, standard error; *, P < 0.05 vs. the control group.
To further examine whether (−)-gossypol inhibited angiogenesis (new blood vessel formation), we carried out immunohistochemistry with anti-CD31, anti-VEGFR2 and anti-VEGF antibodies on tumor sections with or without the treatment of (−)-gossypol. The results showed that VEGF expression was remarkably inhibited by (−)-gossypol. The microvessel density in (−)-gossypol-treated group was 42.84% of the control group (Fig. 2C), indicating that addition of (−)-gossypol significantly inhibited neovascularization besides its direct cytotoxic effect on prostate tumor cells.
(−)-Gossypol inhibits VEGF-induced endothelial cell migration and differentiation to capillary-like structure in vitro
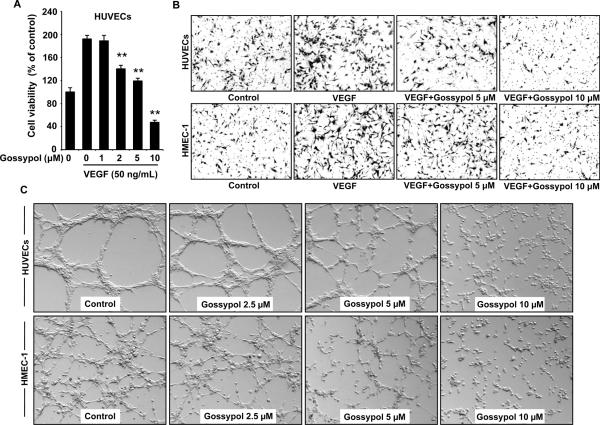
To assess the detailed activities of (−)-gossypol on angiogenesis in vitro, we examined whether (−)-gossypol modulated the VEGF-induced proliferation of endothelial cells by MTS assay. As shown in figure 3A, about 2 μmol/L of (−)-gossypol significantly decreased VEGF-induced cell viability in HUVECs after 48 h incubation. We further examined its inhibitory function on the chemotactic motility by the Boyden chamber assay in two kinds of endothelial cells, HUVECs and HMEC-1. Our results showed that invasive endothelial cells in the (−)-gossypol-treated group were dramatically less than that of the VEGF alone group, suggesting a potent inhibitory effect of (−)-gossypol on VEGF-induced endothelial cell motility (Fig. 3B).
Figure 3. (−)-Gossypol inhibits VEGF-induced viability, chemotactic motility and capillary-like structure formation of endothelial cells.
A, (−)-gossypol dose-dependently inhibited VEGF-induced cell viability. HUVECs (6~7×103 cells/well) were starved with serum-free medium and then treated with or without VEGF (50 ng/mL) and various concentrations of (−)-gossypol for 48 h. Cell viability was quantified by MTS assay. Columns, mean; bars, standard error; **, P < 0.01 vs. VEGF alone group. B, (−)-gossypol inhibited cell migration in HUVECs and HMEC-1. Endothelial cells were seeded in the upper chamber of Transwells and treated with different concentrations of (−)-gossypol. The bottom chamber was filled with medium supplemented with 30 ng/mL VEGF. Cells that migrated through the membrane were photographed (magnification, 160×). C, (−)-gossypol inhibited the capillary-like structure formation in endothelial cells. Pretreated HUVECs and HMEC-1 were placed in 24-well plates coated with Matrigel. After 4~6 h, cells were fixed, and tubular structures were photographed (original magnification, 100×).
Tubulogenesis is the event mimicking one of the last steps of angiogenesis. Therefore, we examined whether (−)-gossypol regulated capillary tube formation in HUVECs and HMEC-1. As shown in figure 3C, when endothelial cells were seeded on two-dimensional Matrigel, robust tubular-like structures were formed. However, treatment with (−)-gossypol (5~10 μmol/L) could abolish this process to a great extent. The number, length and area of capillary-like structures were significantly decreased. Together, these results indicated that (−)-gossypol could block angiogenesis in vitro by inhibiting VEGF-induced cell proliferation, chemotaxis, and capillary-like structure formation of endothelial cells.
(−)-Gossypol inhibits VEGF-induced microvessel sprouting ex vivo
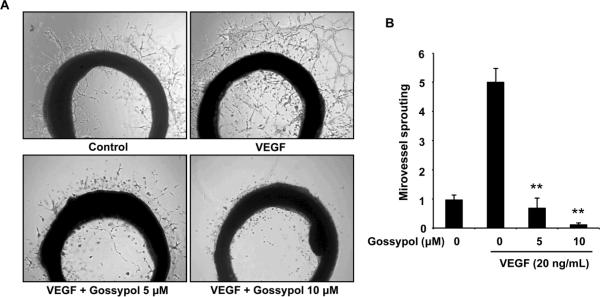
To study whether (−)-gossypol could affect angiogenesis ex vivo, we examined the sprouting of microvessels from aortic rings in the presence or absence of (−)-gossypol. As shown in figure 4A, the presence of VEGF significantly triggered the microvessel sprouting around the aortic rings. Addition of different concentrations of (−)-gossypol antagonized the VEGF-induced sprouting in a dose-dependent manner, and 10 μmol/L of (−)-gossypol completely abolished those microvessel sprouts (Fig. 4B).
Figure 4. (−)-Gossypol inhibits VEGF-induced microvessel sprouting ex vivo.
Aortic segments isolated from Sprague-Dawley rats were placed in Matrigel-covered wells and treated with VEGF in the presence or absence of (−)-gossypol for 6 d. A, representative photographs of endothelial cell sprouts from aortic rings. B, sprouts were scored from 0 (least positive) to 5 (most positive) in a double-blinded manner. Columns, mean; bars, standard error; **, P < 0.01 vs. VEGF alone group.
(−)-Gossypol blocks VEGFR2 kinase in vascular endothelial cells
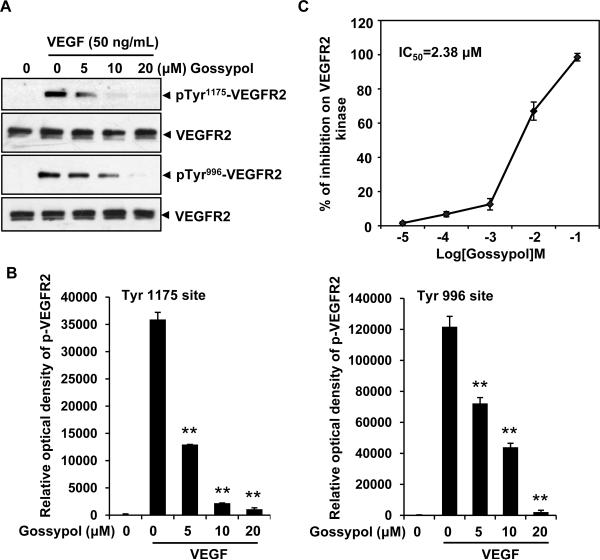
To understand the molecular basis of (−)-gossypol-mediated antiangiogenesis, we examined whether (−)-gossypol could inhibit the activation of VEGFR2, a critical receptor tyrosine kinase on the cell surface of endothelial cells. As shown in figure 5A and 5B, (−)-gossypol (5 μmol/L) strongly inhibited VEGF-activated VEGFR2 phosphorylation at both Tyr 1175 site and Tyr 996 site. To confirm this result, we performed in vitro kinase assay using a kinase kit. Our data demonstrated that (−)-gossypol inhibited VEGFR2 kinase activity in a dose-dependent manner with the half maximal inhibitory concentration of 2.38 μmol/L (Fig. 5C).
Figure 5. (−)-Gossypol is a VEGFR2 kinase inhibitor.
A, (−)-gossypol suppressed the activation of VEGFR2 triggered by VEGF in endothelial cell. HUVECs were starved in serum-free medium for 4~6 h, pretreated with (−)-gossypol for 30 min, and then stimulated with 50 ng/mL VEGF for 2 min. The activation of VEGFR2 from different treatments was analyzed by western blotting and probed with anti-phospho-VEGFR2 antibody at Tyr 1175 and Tyr 996 sites. B, the relative optical density was qualified by Image J software. C, (−)-gossypol inhibited VEGFR2 kinase activity in vitro. Dots, mean; Bars, standard error.
(−)-Gossypol inhibits the activation of key intracellular proangiogenic kinases
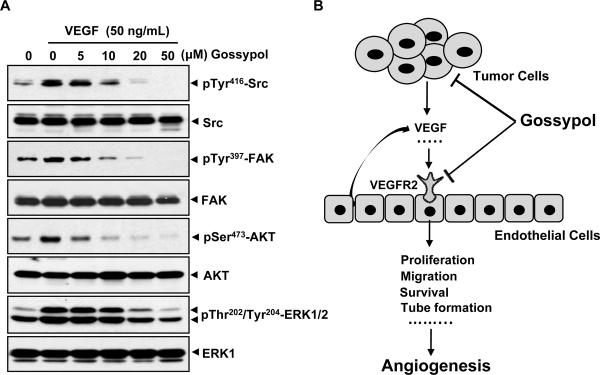
VEGFR2 activation induced by VEGF leads to the phosphorylation of various downstream signaling molecules that are responsible for endothelial cell migration, proliferation and survival. To determine whether (−)-gossypol inhibited the intracellular angiogenic signaling, we examined several key kinases involved in the process of VEGFR2-mediated angiogenesis. We found that 5~10 μmol/L of (−)-gossypol significantly suppressed the phosphorylation of Src, FAK, AKT, and ERK induced by VEGF (50 ng/mL) in HUVECs (Fig. 6A), suggesting that (−)-gossypol exerted its antiangiogenic function through blockade of VEGF/VEGFR2 signaling cascade in endothelial cells.
Figure 6. (−)-Gossypol inhibits activation of key proangiogenic molecules involved in VEGF signaling.
A, (−)-gossypol inhibited VEGF-induced activation of Src, FAK, AKT and ERK kinase in endothelial cells. Several key signaling molecules that mediate angiogenesis were analyzed by western blotting assay. HUVECs were starved in serum-free medium for 4~6 h, pretreated with (−)-gossypol for 30 min, and then stimulated with 50 ng/mL VEGF for 5~20 min. Protein was harvested for the analysis. B, schematic model depicted the (−)-gossypol-mediated antiangiogenic signaling pathway. (−)-Gossypol potentially affected the production of VEGF released from tumor cells and endothelial cells by inhibition of Bcl-2 family protein, and further it blocked the activation of VEGFR2 and intracellular kinases in vascular endothelial cells.
Discussion
Prostate cancer continues to represent a burgeoning medical problem in males. Recent studies show that (−)-gossypol treatment induces DNA damage in metastatic (37), hormone-resistant, drug-resistant and castrate-resistant prostate cancer cells (38, 39) and prostate tumor-initiating cells (18). Notably, there are a number of clinical trials that (−)-gossypol and its derivatives show promising efficacy against some refractory human cancers (38). And recently, (−)-gossypol has also been selected as an adjuvant agent for human prostate cancer (14). In the present study, we show for the first time that the suppression of prostate tumor in vivo medicated by (−)-gossypol is partially dependent on angiogenesis inhibition, and our results further reveal that (−)-gossypol modulates multiple steps of VEGF signaling-mediated angiogenesis.
It was shown that different hormone- and drug-resistant prostate cancers constitutively express some important angiogenic cytokines, which are known to regulate tumorigenicity and angiogenesis. Previous studies on (−)-gossypol had shown that there were 1.6- and 1.8-fold decreases in VEGF and interleukin-8 levels after treatment with 10 μmol/L of (−)-gossypol in human prostate or ovarian cancer cells (26, 27), indicating (−)-gossypol could affect the profile of proangiogenic factors released from tumors. This information provide us significant clue to study the direct antiangiogenic role of (−)-gossypol in vitro and in vivo. In the present study, we found that (−)-gossypol functioned as a potent angiogenesis inhibitor. It not only inhibited VEGF expression of prostate cancer cells and endothelial cells in vitro (Fig. 1C) and in vivo (Fig. 2C), but blocked multiple steps in VEGF-activated biological events of endothelial cells, including endothelial cell proliferation, migration and differentiation (Fig. 3). As evidenced by the human prostate tumor xenograft mouse model, tumor growth was significantly inhibited when (−)-gossypol antagonized angiogenesis (Fig.2).
It has already been validated that racemic (−)-gossypol and its enantiomer (AT-101) are natural BH3 mimetics that bind to the BH3 binding pocket of Bcl-2 and Bcl-xL to inhibit antiapoptotic functions (40–42) or induce autophagic cell death in apoptosis-resistant cancer cells (43). In agreement, we also found that treatment with (−)-gossypol led to inhibition of cell viability (Fig. 1B) and induction of apoptosis in different kinds of prostate cancer cells (Fig. 1C). However, recent work identifies a new function for Bcl-2 in cancer biology that is beyond its classic role in cell survival by its close relationship with VEGF (28, 44). VEGF from paracrine/autocrine of tumor cells and endothelial cells induces expression of Bcl-2 in tumor-associated microvascular endothelial cells (45). Up-regulated Bcl-2 expression in microvascular endothelial cells is sufficient to enhance intratumoral angiogenesis and to accelerate tumor growth (29). Interestingly, Bcl-2 in turn functions as a proangiogenic molecule through its ability to activate the NF-κB signaling pathway and to induce expression of the proangiogenic CXCL8 and CXCL1 chemokines from endothelial cells to affect nearby tumor cells (30). Therefore, the VEGF–Bcl-2–CXCL8 pathway suggests new targets for the development of anti-angiogenic strategies. And nowadays, short interfering RNA and small molecule inhibitors of Bcl-2 are being developed to inhibit solid tumors (46–48). In our present investigation, we demonstrated that treatment of (−)-gossypol led to obvious downregulation of VEGF in both cancer cells and endothelial cells (Fig. 1C), which help to significantly decrease VEGF concentration in tumor microenvironment in vivo. As shown in Fig. 1D, the suppression on Bcl-2/Bcl-xL by (−)-gossypol paralleled with its inhibition on VEGF, which partially suggested that the Bcl-2/VEGF signaling pathway could be blocked by (−)-gossypol. Consequently, the biological dysfunctions of activated endothelial cells with higher Bcl-2 expression can be rectified by (−)-gossypol's treatment (Fig. 3). Previous study revealed (−)-gossypol inhibited NF-κB activity and NF-κB-mediated gene expression (21). Although we did not examine the CXC chemokine production in the treated cells, combination of these observations confirmed the antiangiogenic effect of (−)-gossypol in cancer treatment.
Further, we investigated the molecular events associated with the antiangiogenic activity of (−)-gossypol in endothelial cells. It is shown that Bcl-2 gene expression is significantly higher in the tumor–associated endothelial cells as compared with normal endothelial cells (28, 45). Tumor cell–derived or endothelial cell–derived VEGF signals to modulate endothelial cell proliferation, migration and differentiation in a pathway that requires its binding to VEGFR2 and activation of downstream signaling (9, 45). In the present study, we found that (−)-gossypol dose-dependently inhibited VEGFR2 kinase activity, with the half maximal inhibitory concentration of 2.38 μmol/L (Fig. 5C). Although there are three tyrosine receptor kinases, VEGFR-1, -2, and -3, expressed in endothelial cells, the VEGF signaling events relevant to tumor angiogenesis are mainly mediated by VEGFR2 (49). Conversely, VEGFR1 (Flt-1) is a dual regulator of angiogenesis with very low activity in endothelial cells, even in VEGFR1-overexpressing primary endothelial cells in culture, and VEGFR3 is the critical modulator of lymphangiogenesis. With a specific pattern, VEGFR2 activation results in activation of diverse intracellular substrates in endothelial cells. Our data revealed that the phosphorylation of Src, FAK, AKT, and ERK kinases induced by VEGF were all suppressed by (−)-gossypol (Fig.6A). Src kinase has been reported to participate in tumor angiogenesis via regulating gene expression of proangiogenic growth factors and cytokines, especially VEGF and interleukin 8 (50). As evidenced by previously report that (−)-gossypol had ability to decrease VEGF and IL-8 expression in cancer cells (27), we reason that this effect is partially due to the inhibition of (−)-gossypol on activation of these intracellular kinases.
When compared with the inhibitory effect of (−)-gossypol in endothelial cells and prostate cancer cells, we found that the effective concentration in activated endothelial cells was much lower than that in cancer cells, suggesting that biological alterations of endothelial cells (angiogenesis) might be primary target of (−)-gossypol in tumor inhibition in vivo at relative low dosage. It is noteworthy that 5 μmol/L of (−)-gossypol is sufficient to inhibit VEGF-induced angiogenic responses in vitro (Fig. 3, 5 and 6) and ex vivo angiogenesis assays (Fig. 4) while 10 μmol/L of (−)-gossypol completely blocks microvessel sprouting (Fig. 4). However, higher concentrations of gossypol are required to inhibit cancer cell viability and to induce cancer cell apoptosis (Fig. 1B and 1C). These data suggest that (−)-gossypol's antiangiogenic activity in vivo is probably much earlier than its toxic effects on tumor cells.
In conclusion, we found that (−)-gossypol potently inhibited angiogenesis-mediated tumor growth by modulating VEGF signaling (Fig. 6B), which suggested the therapeutic potential in the treatment of human cancers in clinical settings.
Acknowledgments
We thank the Biological Resources Branch, National Cancer Institute, NIH, for the rhVEGF.
Financial support: This work is supported by the Innovation Program of East China Normal University (78210021 to X. Pang), the Chenguang Program of Shanghai Municipal Education Commission (10CG25 to X. Pang), the Research Platform for Cell Signaling Networks (06DZ22923 to M. Liu), the Pujiang Program of the Science and Technology Commission of Shanghai Municipality (09PJ1403900 to M. Liu), and the NIH grants (1R01CA106479 and 1R01CA134731 to M. Liu).
Abbreviations
- VEGF
vascular endothelial growth factor
- VEGFR2
vascular endothelial growth factor receptor 2
- HUVECs
human umbilical vein endothelial cells
- HMEC-1
human microvascular endothelial cells
- ERK
extracellular signal-regulated protein kinase
- FAK
focal adhesion kinase
- NF-κB
nuclear factor-kappa B
- PARP
Poly (ADP-ribose) polymerase
- ECM
endothelial cell culture medium
- DMSO
dimethyl sulfoxide
Footnotes
Disclosure Statement: No potential conflicts of interest were disclosed.
References
- 1.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 2.Ferrara N, Kerbel RS. Angiogenesis as a therapeutic target. Nature. 2005;438:967–74. doi: 10.1038/nature04483. [DOI] [PubMed] [Google Scholar]
- 3.Albini A, Indraccolo S, Noonan DM, Pfeffer U. Functional genomics of endothelial cells treated with anti-angiogenic or angiopreventive drugs. Clin Exp Metastasis. 2010;27:419–39. doi: 10.1007/s10585-010-9312-5. [DOI] [PubMed] [Google Scholar]
- 4.Niu G, Chen X. Vascular endothelial growth factor as an anti-angiogenic target for cancer therapy. Curr Drug Targets. 2010;11:1000–17. doi: 10.2174/138945010791591395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–76. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 6.Olsson AK, Dimberg A, Kreuger J, Claesson-Welsh L. VEGF receptor signalling - in control of vascular function. Nat Rev Mol Cell Biol. 2006;7:359–71. doi: 10.1038/nrm1911. [DOI] [PubMed] [Google Scholar]
- 7.Schlessinger J. New roles for Src kinases in control of cell survival and angiogenesis. Cell. 2000;100:293–6. doi: 10.1016/s0092-8674(00)80664-9. [DOI] [PubMed] [Google Scholar]
- 8.Le Boeuf F, Houle F, Huot J. Regulation of vascular endothelial growth factor receptor 2-mediated phosphorylation of focal adhesion kinase by heat shock protein 90 and Src kinase activities. J Biol Chem. 2004;279:39175–85. doi: 10.1074/jbc.M405493200. [DOI] [PubMed] [Google Scholar]
- 9.Gerber HP, McMurtrey A, Kowalski J, Yan M, Keyt BA, Dixit V, et al. Vascular endothelial growth factor regulates endothelial cell survival through the phosphatidylinositol 3'-kinase/Akt signal transduction pathway. Requirement for Flk-1/KDR activation. J Biol Chem. 1998;273:30336–43. doi: 10.1074/jbc.273.46.30336. [DOI] [PubMed] [Google Scholar]
- 10.Dayanir V, Meyer RD, Lashkari K, Rahimi N. Identification of tyrosine residues in vascular endothelial growth factor receptor-2/FLK-1 involved in activation of phosphatidylinositol 3-kinase and cell proliferation. J Biol Chem. 2001;276:17686–92. doi: 10.1074/jbc.M009128200. [DOI] [PubMed] [Google Scholar]
- 11.Takahashi T, Yamaguchi S, Chida K, Shibuya M. A single autophosphorylation site on KDR/Flk-1 is essential for VEGF-A-dependent activation of PLC-gamma and DNA synthesis in vascular endothelial cells. EMBO J. 2001;20:2768–78. doi: 10.1093/emboj/20.11.2768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu LZ, Zheng JZ, Wang XR, Jiang BH. Endothelial p70 S6 kinase 1 in regulating tumor angiogenesis. Cancer Res. 2008;68:8183–8. doi: 10.1158/0008-5472.CAN-08-0819. [DOI] [PubMed] [Google Scholar]
- 13.Yahata Y, Shirakata Y, Tokumaru S, Yamasaki K, Sayama K, Hanakawa Y, et al. Nuclear translocation of phosphorylated STAT3 is essential for vascular endothelial growth factor-induced human dermal microvascular endothelial cell migration and tube formation. J Biol Chem. 2003;278:40026–31. doi: 10.1074/jbc.M301866200. [DOI] [PubMed] [Google Scholar]
- 14.Meng Y, Tang W, Dai Y, Wu X, Liu M, Ji Q, et al. Natural BH3 mimetic (−)-gossypol chemosensitizes human prostate cancer via Bcl-xL inhibition accompanied by increase of Puma and Noxa. Mol Cancer Ther. 2008;7:2192–202. doi: 10.1158/1535-7163.MCT-08-0333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wolter KG, Wang SJ, Henson BS, Wang S, Griffith KA, Kumar B, et al. (−)-gossypol inhibits growth and promotes apoptosis of human head and neck squamous cell carcinoma in vivo. Neoplasia. 2006;8:163–72. doi: 10.1593/neo.05691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu L, Yang D, Wang S, Tang W, Liu M, Davis M, et al. (−)-Gossypol enhances response to radiation therapy and results in tumor regression of human prostate cancer. Mol Cancer Ther. 2005;4:197–205. [PubMed] [Google Scholar]
- 17.Jiang J, Ye W, Lin YC. Gossypol inhibits the growth of MAT-LyLu prostate cancer cells by modulation of TGFbeta/Akt signaling. Int J Mol Med. 2009;24:69–75. [PubMed] [Google Scholar]
- 18.Volate SR, Kawasaki BT, Hurt EM, Milner JA, Kim YS, White J, et al. Gossypol induces apoptosis by activating p53 in prostate cancer cells and prostate tumor-initiating cells. Mol Cancer Ther. 2010;9:461–70. doi: 10.1158/1535-7163.MCT-09-0507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zerp SF, Stoter R, Kuipers G, Yang D, Lippman ME, van Blitterswijk WJ, et al. AT-101, a small molecule inhibitor of anti-apoptotic Bcl-2 family members, activates the SAPK/JNK pathway and enhances radiation-induced apoptosis. Radiat Oncol. 2009;4:47. doi: 10.1186/1748-717X-4-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hu ZY, Sun J, Zhu XF, Yang D, Zeng YX. ApoG2 induces cell cycle arrest of nasopharyngeal carcinoma cells by suppressing the c-Myc signaling pathway. J Transl Med. 2009;7:74. doi: 10.1186/1479-5876-7-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moon DO, Kim MO, Lee JD, Kim GY. Gossypol suppresses NF-kappaB activity and NF-kappaB-related gene expression in human leukemia U937 cells. Cancer Lett. 2008;264:192–200. doi: 10.1016/j.canlet.2008.01.030. [DOI] [PubMed] [Google Scholar]
- 22.Ko CH, Shen SC, Yang LY, Lin CW, Chen YC. Gossypol reduction of tumor growth through ROS-dependent mitochondria pathway in human colorectal carcinoma cells. Int J Cancer. 2007;121:1670–9. doi: 10.1002/ijc.22910. [DOI] [PubMed] [Google Scholar]
- 23.Sung B, Ravindran J, Prasad S, Pandey MK, Aggarwal BB. Gossypol induces death receptor-5 through activation of ROS-ERK-chop pathway and sensitizes colon cancer cells to trail. J Biol Chem. 2010;285:35418–27. doi: 10.1074/jbc.M110.172767. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 24.Cheng JS, Lo YK, Yeh JH, Cheng HH, Liu CP, Chen WC, et al. Effect of gossypol on intracellular Ca2+ regulation in human hepatoma cells. Chin J Physiol. 2003;46:117–22. [PubMed] [Google Scholar]
- 25.Atmaca H, Gorumlu G, Karaca B, Degirmenci M, Tunali D, Cirak Y, et al. Combined gossypol and zoledronic acid treatment results in synergistic induction of cell death and regulates angiogenic molecules in ovarian cancer cells. Eur Cytokine Netw. 2009;20:121–30. doi: 10.1684/ecn.2009.0159. [DOI] [PubMed] [Google Scholar]
- 26.Varol U, Karaca B, Tunali D, Degirmenci M, Cirak Y, Purcu DU, et al. The effect of racemic gossypol and at-101 on angiogenic profile of ovcar-3 cells: a preliminary molecular framework for gossypol enantiomers. Exp Oncol. 2009;31:220–5. [PubMed] [Google Scholar]
- 27.Karaca B, Kucukzeybek Y, Gorumlu G, Erten C, Gul MK, Cengiz E, et al. Profiling of angiogenic cytokines produced by hormone- and drug-refractory prostate cancer cell lines, PC-3 and DU-145 before and after treatment with gossypol. Eur Cytokine Netw. 2008;19:176–84. doi: 10.1684/ecn.2008.0139. [DOI] [PubMed] [Google Scholar]
- 28.Kaneko T, Zhang Z, Mantellini MG, Karl E, Zeitlin B, Verhaegen M, et al. Bcl-2 orchestrates a cross-talk between endothelial and tumor cells that promotes tumor growth. Cancer Res. 2007;67:9685–93. doi: 10.1158/0008-5472.CAN-07-1497. [DOI] [PubMed] [Google Scholar]
- 29.Nor JE, Christensen J, Liu J, Peters M, Mooney DJ, Strieter RM, et al. Up-Regulation of Bcl-2 in microvascular endothelial cells enhances intratumoral angiogenesis and accelerates tumor growth. Cancer Res. 2001;61:2183–8. [PubMed] [Google Scholar]
- 30.Karl E, Warner K, Zeitlin B, Kaneko T, Wurtzel L, Jin T, et al. Bcl-2 acts in a proangiogenic signaling pathway through nuclear factor-kappaB and CXC chemokines. Cancer Res. 2005;65:5063–9. doi: 10.1158/0008-5472.CAN-05-0140. [DOI] [PubMed] [Google Scholar]
- 31.Pang X, Yi T, Yi Z, Cho SG, Qu W, Pinkaew D, et al. Morelloflavone, a biflavonoid, inhibits tumor angiogenesis by targeting rho GTPases and extracellular signal-regulated kinase signaling pathways. Cancer Res. 2009;69:518–25. doi: 10.1158/0008-5472.CAN-08-2531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pang X, Yi Z, Zhang X, Sung B, Qu W, Lian X, et al. Acetyl-11-keto-beta-boswellic acid inhibits prostate tumor growth by suppressing vascular endothelial growth factor receptor 2-mediated angiogenesis. Cancer Res. 2009;69:5893–900. doi: 10.1158/0008-5472.CAN-09-0755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yi T, Yi Z, Cho SG, Luo J, Pandey MK, Aggarwal BB, et al. Gambogic acid inhibits angiogenesis and prostate tumor growth by suppressing vascular endothelial growth factor receptor 2 signaling. Cancer Res. 2008;68:1843–50. doi: 10.1158/0008-5472.CAN-07-5944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang XQ, Huang XF, Mu SJ, An QX, Xia AJ, Chen R, et al. Inhibition of proliferation of prostate cancer cell line, PC-3, in vitro and in vivo using (−)-gossypol. Asian J Androl. 2010;12:390–9. doi: 10.1038/aja.2009.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Regula KM, Ens K, Kirshenbaum LA. IKK beta is required for Bcl-2-mediated NF-kappa B activation in ventricular myocytes. J Biol Chem. 2002;277:38676–82. doi: 10.1074/jbc.M206175200. [DOI] [PubMed] [Google Scholar]
- 36.Ricca A, Biroccio A, Del Bufalo D, Mackay AR, Santoni A, Cippitelli M. bcl-2 over-expression enhances NF-kappaB activity and induces mmp-9 transcription in human MCF7(ADR) breast-cancer cells. Int J Cancer. 2000;86:188–96. doi: 10.1002/(sici)1097-0215(20000415)86:2<188::aid-ijc7>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 37.Huang YW, Wang LS, Dowd MK, Wan PJ, Lin YC. (−)-Gossypol reduces invasiveness in metastatic prostate cancer cells. Anticancer Res. 2009;29:2179–88. [PubMed] [Google Scholar]
- 38.Liu G, Kelly WK, Wilding G, Leopold L, Brill K, Somer B. An open-label, multicenter, phase I/II study of single-agent AT-101 in men with castrate-resistant prostate cancer. Clin Cancer Res. 2009;15:3172–6. doi: 10.1158/1078-0432.CCR-08-2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sanli UA, Gorumlu G, Erten C, Gul MK, Cengiz E, Kucukzeybek Y, et al. Targeting apoptosis in the hormone- and drug-resistant prostate cancer cell line, DU-145, by gossypol/zoledronic acid combination. Cell Biol Int. 2009;33:1165–72. doi: 10.1016/j.cellbi.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 40.Kang MH, Reynolds CP. Bcl-2 inhibitors: targeting mitochondrial apoptotic pathways in cancer therapy. Clin Cancer Res. 2009;15:1126–32. doi: 10.1158/1078-0432.CCR-08-0144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Balakrishnan K, Wierda WG, Keating MJ, Gandhi V. Gossypol, a BH3 mimetic, induces apoptosis in chronic lymphocytic leukemia cells. Blood. 2008;112:1971–80. doi: 10.1182/blood-2007-12-126946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Balakrishnan K, Burger JA, Wierda WG, Gandhi V. AT-101 induces apoptosis in CLL B cells and overcomes stromal cell-mediated Mcl-1 induction and drug resistance. Blood. 2009;113:149–53. doi: 10.1182/blood-2008-02-138560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lian J, Wu X, He F, Karnak D, Tang W, Meng Y, et al. A natural BH3 mimetic induces autophagy in apoptosis-resistant prostate cancer via modulating Bcl-2-Beclin1 interaction at endoplasmic reticulum. Cell Death Differ. 2010 doi: 10.1038/cdd.2010.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zeitlin BD, Nor JE. Small-Molecule Inhibitors Reveal a New Function for Bcl-2 as a Proangiogenic Signaling Molecule. Curr Top Microbiol Immunol. 2010 doi: 10.1007/82_2010_109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nor JE, Christensen J, Mooney DJ, Polverini PJ. Vascular endothelial growth factor (VEGF)-mediated angiogenesis is associated with enhanced endothelial cell survival and induction of Bcl-2 expression. Am J Pathol. 1999;154:375–84. doi: 10.1016/S0002-9440(10)65284-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zeitlin BD, Spalding AC, Campos MS, Ashimori N, Dong Z, Wang S, et al. Metronomic small molecule inhibitor of Bcl-2 (TW-37) is antiangiogenic and potentiates the antitumor effect of ionizing radiation. Int J Radiat Oncol Biol Phys. 2010;78:879–87. doi: 10.1016/j.ijrobp.2010.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jain HV, Nor JE, Jackson TL. Modeling the VEGF-Bcl-2-CXCL8 pathway in intratumoral agiogenesis. Bull Math Biol. 2008;70:89–117. doi: 10.1007/s11538-007-9242-9. [DOI] [PubMed] [Google Scholar]
- 48.Wang L, Sloper DT, Addo SN, Tian D, Slaton JW, Xing C. WL-276, an antagonist against Bcl-2 proteins, overcomes drug resistance and suppresses prostate tumor growth. Cancer Res. 2008;68:4377–83. doi: 10.1158/0008-5472.CAN-07-6590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kowanetz M, Ferrara N. Vascular endothelial growth factor signaling pathways: therapeutic perspective. Clin Cancer Res. 2006;12:5018–22. doi: 10.1158/1078-0432.CCR-06-1520. [DOI] [PubMed] [Google Scholar]
- 50.Kim LC, Song L, Haura EB. Src kinases as therapeutic targets for cancer. Nat Rev Clin Oncol. 2009;6:587–95. doi: 10.1038/nrclinonc.2009.129. [DOI] [PubMed] [Google Scholar]