Abstract
Background
Since its beginnings newborn screening for cystic fibrosis (CF) using an assay for immunoreactive trypsinogen (IRT) has been plagued by a high number of false positive results, i.e., screen-positive / diagnosis negative, despite attempts to reduce them through use of altered cutoffs and 2nd tier DNA testing. IRT exists as two isoforms: IRT1 and IRT2, with IRT2 being more closely aligned with pancreatic disease, including CF. Standardization among programs using current IRT assays recognizing either one or both isoforms is a continuing problem. Here we report the development of a multiplexed assay for both forms of IRT simultaneously.
Methods
Using two different Luminex bead sets, assays for each IRT isoform were developed separately and then combined. Using the sum of IRT1 and 2 values, comparisons were made with a CF kit currently in use.
Results
In a sample set consisting of 16 cases confirmed positive for CF, a cut-off was established at >97 ng/mL total IRT. Seven of eight carriers with one CF mutation screen positive by the standard method also were screen positive by the IRT1-IRT2. Of 32 cases screen positive by the standard IRT, 11 were screen negative by the IRT1-IRT2. None of these cases had CF mutations as identified by the screening program.
Conclusions
This data indicates that the multiplex method with specificity for two isoforms of IRT has comparable performance to a standard IRT method and the advantage of improved standardization by detection of the two isoforms.
Keywords: newborn screening, cystic fibrosis, multiplex immunoassay
Introduction
Newborn screening for cystic fibrosis (CF) has evolved following the report in 1979 by Crossly et al. (1) that blood immunoreactive trypsinogen (IRT) levels are elevated in newborn infants with CF. There are several molecular forms of IRT; the two major forms secreted by exocrine cells of the pancreas are trypsinogen 1 (cationic trypsinogen, IRT1) and trypsinogen 2 (anionic trypsinogen, IRT2) (2–3). Normally the IRT1 form is present in higher levels; however, in pathological conditions such as pancreatitis the IRT2 form becomes predominant (4). Today 46 states provide NBS for CF, all using IRT for the initial screen. In New York state in 2008, the most recent year with complete data, 1585 infants were screen positive of which 53 were confirmed with CF, a ratio of 30:1 screen positive to confirmed CF. Second tier testing after an initial screen positive can use a number of different protocols (12) in an effort to minimize the number of false positive results, such as IRT positives tested again on newly collected specimens, for example, IRT test followed by DNA analysis on that same first specimen, and others.
A number of investigators have developed immunoassays to IRT, and commercial assays currently in use have employed both monoclonal and polyclonal antibodies for IRT (5–7). The heterogeneous nature of IRT and differing specificity of antibodies to the various components have raised issues with the standardization and external QC of the assay. As noted by Li et al. (8), the lack of a universally acceptable IRT standard has made the comparison of absolute IRT values among commercial immunoassays difficult. As reported by Lafont (11) trypsin exists in many forms in the serum but is not recognized equally among immunoassays, thus contributing to the discordant results when comparing one assay with another.
In the present study we report development of a suspension array multiplexed immunoassay for the two specific isoforms of trypsinogen IRT1 and IRT2. The specificity of the assay for the two isoforms allows development of external QC for the heterogeneous forms of IRT and allows for analysis of the IRT1 to IRT 2 ratio as a potential added parameter before referral for mutation analysis.
Materials and Methods
Antibody Reagents
Anti-trypsin isoform specific monoclonal antibodies were coupled to Luminex xMAP microspheres according to the instructions provided by Luminex (http://Luminexcorp.custhelp.com) with 100 µg of IRT1 capture monoclonal antibody (HYB 021-08-02, Affinity Bioreagents) of Luminex carboxy microspheres, region 177 (L-100-C177-04). Similarly, 100 µg of IRT2 capture monoclonal (8607, Medix Biochemica), was coupled to 5×106 beads of the Luminex carboxy microspheres, region 183 (L-100-C183-04).
Polyclonal detector antibody (K50900R, Biodesign International, http://meridianlifescience.com) was biotinylated using the Fluoreporter biotin-XX labeling kit (Invitrogen, http://www.invitrogen.com/site/us/en/home.html) according to manufacturer’s instructions. A sheep polyclonal anti-trypsin with the biotin label from the manufacturer (BAF3586, R&D Systems) was used. The two antibodies were combined to make the detector mix, with K50900 at a concentration of 5.0 µ/mL and BAF3586 at 1.25 µg/mL.
The performance of the antibodies was determined by titer to evaluate affinity and sensitivity, and by cross-reaction tests to evaluate their specificity. The concentration of each antibody was titrated to achieve optimal performance.
Assay Calibrators
IRT1 calibrators from the standard method kit (MP Biomedical) were used. These calibrators were prepared with IRT1 only (personal communication with MPB). IRT2 calibrators were prepared from recombinant IRT2 (R&D Systems). Serum was treated with activated charcoal according to the method of Li et al. (8) and combined with washed red blood cells and the hematocrit was adjusted to 55%. Aprotinin (Sigma Chemical) a trypsin protease inhibitor was added at a concentration of 1 mg/L. The reconstituted whole blood was enriched with the recombinant IRT2 and dispensed to make the dried blood spot calibrators. The spots were air dried overnight, packed in plastic bags with desiccant and stored frozen at −20°C.
Assay Procedure
Assay buffer (pH 7.4) was prepared containing phosphate buffered saline, 0.055% Tween 20, 0.05% sodium azide, 0.2% gelatin. Aprotinin, 1mg/L, (Sigma Chemical Co.) was added to the assay buffer to prepare the spot elution buffer. The dried blood spots (a single 3 mm punch per well) were eluted overnight at room temperature in 100 µl of elution buffer with gentle shaking. For the assay, 75 µl of the sample eluate was combined with 25 µl of the trypsin 1 and trypsin 2 bead mix in order to obtain 2000 microspheres per well for each of the analytes. The capture incubation was for 3 h at 37°C with gentle shaking. Microspheres were washed three times in 100 µl assay buffer, then 100 µl of the anti trypsin detector antibody mix was added to each well. The detector antibodies were incubated for 1 h at 37°C with gentle shaking, and the microspheres were again washed three times with 100 µl assay buffer. For detection, 100 µl of streptavidin phycoerithrin (Invitrogen, S-866) was added at 4µg/mL, and incubated for 30 min at 37°C. The assay plate was aspirated and the microspheres re-suspended in 100 µl of Luminex sheath fluid for analysis.
Analysis and data collection were performed in multiplex acquisition mode on the Luminex 100 instrument. Luminex software LX100 IS 2.3 calculated the results expressed in median fluorescence intensity (MFI) of 100 microspheres of each set. The software LiquiChip Analyser 1.0 (Qiagen) was used to analyze the data.
Samples
All newborn specimens assayed were provided by the New York State Department of Health Newborn Screening Laboratory (NBS) under Institutional Review Board (IRB) Protocol number 07-016. In compliance with the IRB number identifying information was transferred with the specimens, only the CF screening results (IRT and DNA status) were maintained. The IRT screening results were determined with the Blood Spot Trypsin MW ELISA (cat #07596307, MP Biomedicals, Inc., www.mpbio.com). In the NBS IRT assay, and in our assay, all samples were measured in singlicate.
Results
We prepared calibrators with and without aprotinin and determined that calibrators without aprotinin had less than 60% recovery compared with the calibrators with aprotinin. Also, it was noted that the calibrators and buffer of the standard method (MP Biologicals kit) contain aprotinin.
Development and optimization of assay
Each immunoassay was developed separately and optimized for affinity, sensitivity and specificity. The assays were then combined into a multiplex format. The specificity was tested for IRT1 and IRT2; as capture antibodies we used anti-trypsin isoform specific monoclonal antibodies; in the multiplex assay, we detected no cross-reactivity between the antibodies to IRT1 and IRT2, as determined by calibration curves. Although the lower limit of quantification is not important in these assays (the program screens for greatly elevated IRT), antibody concentrations were optimized to obtain sensitivity at 13ng/ml for IRT1 and at 8 ng/ml for IRT2. Using the mean of eight independent values for each concentration of calibrators, we examined the assays’ precision profiles; the CVs were approximately 7% for the lower concentrations of IRT2 and 12% for the lower concentrations of IRT1. Standard curves were linear up to the highest standard concentration used in the IRTS assay (250 ng/ml). Intra- and inter-assay variations were calculated from controls with three levels of IRT concentrations (75, 195, 284 ng/ml for IRT1; 31, 62, 125 ng/ml for IRT2); intra-assay evaluations were performed over a three week period using 14 plates, with the inter-assay variation calculated from each plate. At all concentrations, the inter-assay CV were from 6% to 14% for IRT1 and from 9% to 10% for IRT2. The intra-assay CV for both ITR1 and IRT2 were from 0% to 18% (data not shown).
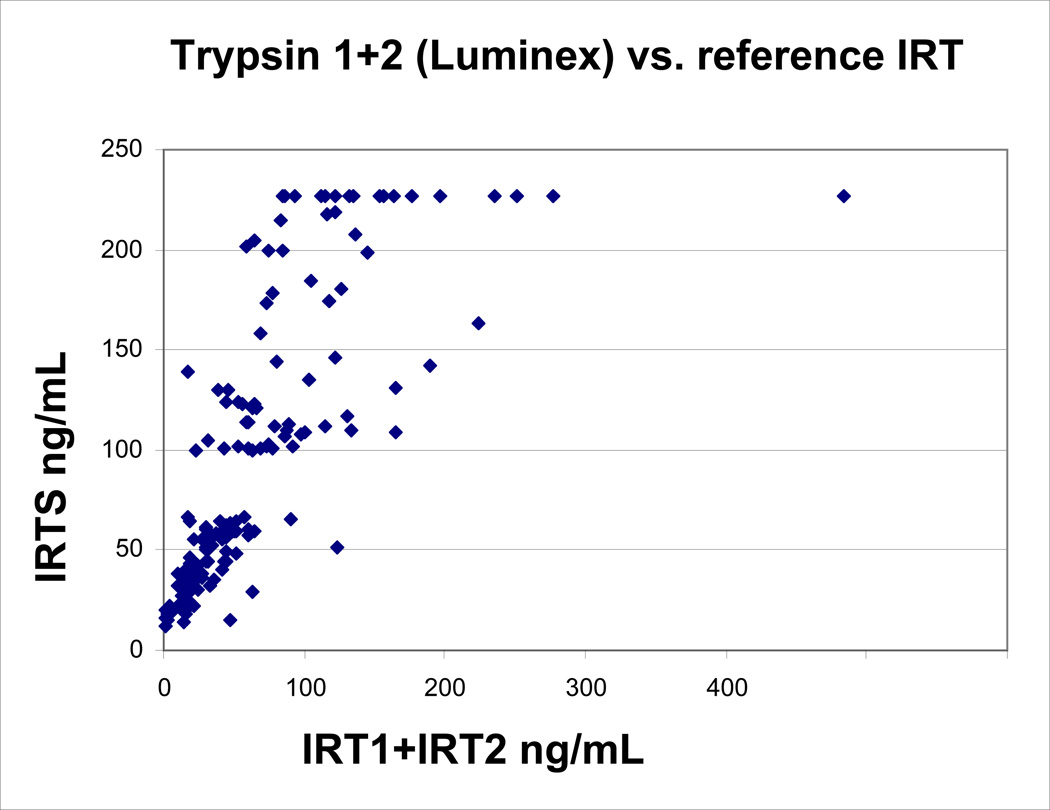
Correlation study
We compared samples analyzed in the IRT1-IRT2 assay with the values obtained from the standard method. The selection criteria for the NBS samples analyzed in the correlation study were specified for low to high IRT values: 3 mm punches from 168 blood spots with values determined by the NBS laboratory were divided into 5 groups as shown in TABLE 1. IRT+IRT2 assay values were compared with the standard method (IRTS) and had a correlation coefficient of 0.75 (FIGURE 1), with a mean IRT+IRT2 value lower than the IRTS, IRT1+IRT2 (X=63.8+/−SD 62.6), and IRTS (X=92.9+/−SD 69.0). This is not surprising due to the different formats of the assays and different antibodies (11). Of the 133 samples that were screen negative by the IRTS assay, 11 were screen positive using the IRT1+IRT2 assay. Having no link to the original specimen, it was impossible to retest and verify these findings. Of the 35 cases screen positive by the IRTS, 11 cases were screen negative by the IRT1+IRT2 method. Each of these screen negative cases had been confirmed to have no CF mutations by the screening program in its second tier mutation analysis and was reported as negative for CF.
TABLE 1.
Correlation Study Sample Selection Criteria
| IRTS value range ng/mL | Sample N |
|---|---|
| IRT < 35 | 32 |
| IRT 35–55 | 32 |
| IRT 55–100 | 32 |
| IRT 100–170 | 40 |
| IRT ≥ 170 * | 32 |
IRT ≥170 ng/ml indicate screen positives by standard method
FIGURE 1.
Correlation of IRT1+IRT2 concentrations with IRTS concentrations
Also, we tested the IRT2 spiked DBS calibrators in the standard method, none of the calibrators (ranging from 0 to 1000 ng/ml IRT2) had IRT2 measurement above background. Therefore, we conclude that this ELISA kit does not detect IRT2. This is of some concern, given that IRT2 has been reported to be increased in CF patients (1, 4).
Screen Positive Sample Evaluation
The samples screened positive by IRT/DNA analysis in NY NBS laboratory consisted of a total of 19 confirmed positive cases with two CF mutations, eight confirmed positive cases with one CF mutation, and 137 cases confirmed as non-cystic fibrosis with no CF mutations detected. The screen positive cut-off established by the NY NBS laboratory for the standard method is a value ≥ 170ng/mL. The screen positive sample evaluation shown in TABLE 2 indicated that a total trypsin (sum of IRT1 and IRT2) cut-off of >97ng/mL would be necessary to achieve 100% sensitivity for the confirmed disease population.
TABLE 2.
Screen Positive Sample Evaluation
| Two Mutations | IRT1 ng/mL |
IRT2 ng/mL |
ITR1+IRT2* ng/mL |
IRT1 to IRT2 Ratio |
IRTS ng/mL |
|---|---|---|---|---|---|
| 1. Confirmed Disease | |||||
| F508del/3121+G>A | 121 | 129 | 250 | 0.938 | 248 |
| F508del/F508del | 63.6 | 93.2 | 156.8 | 0.682 | 183.5 |
| F508del/F508del | 117 | 184 | 301 | 0.636 | 248 |
| F508del/R553X | 104 | 269 | 373 | 0.387 | 248 |
| F508del/F508del | 96.6 | 103 | 199.6 | 0.938 | 248 |
| F508del/W1282X | 265 | 326 | 591 | 0.813 | 248 |
| F508del/F508del | 131 | 129 | 260 | 1.016 | 248 |
| F508del/N1303K | 111 | 130 | 241 | 0.854 | 248 |
| F508del/Undetected | 154 | 354 | 508 | 0.435 | 248 |
| F508del/R117H, 7T, 9T, var | 67.6 | 33.6 | 101.2 | 2.012 | 194.3 |
| F508del/F508del | 95.4 | 119 | 214.4 | 0.802 | 248 |
| E60delx/F508del | 40.1 | 57.1 | 97.2 | 0.702 | 248 |
| S549/c387delA | 76.8 | 107 | 183.8 | 0.718 | 248 |
| R117H/D1152H, 7T,7T | 107 | 364 | 471 | 0.294 | 226.5 |
| F508del/F508del | 78.4 | 62.4 | 140.8 | 1.256 | 226.5 |
| G85E/F508del | 252 | 884 | 1136 | 0.285 | 226.5 |
cut-off >97 ng/ml
TABLE 3 shows the analysis of eight single mutation CF carriers that were screen positive as established by the ≥170ng/ml cut-off for the IRTS assay. Seven of these carriers would also screen positive by use of the IRT1+IRT2 with cut-off of >97ng/mL.
TABLE 3.
Carriers (1 CF mutation) Screen Positive by IRTS
| One Mutation | IRT1 | IRT2 | IRT1+IRT2* | IRT1 to IRT2 | standard IRT |
|---|---|---|---|---|---|
| ng/mL | ng/mL | ng/mL | ratio | ||
| R553X | 45.4 | 70.7 | 116.1 | 0.642 | 197.9 |
| F508del | 159 | 289 | 448 | 0.550 | 226.5 |
| D1152H | 223 | 187 | 410 | 1.193 | 226.5 |
| 3120+1G>A | 254 | 790 | 1044 | 0.322 | 226.5 |
| A455E | 59 | 130 | 189 | 0.454 | 186.3 |
| F508del | 87.4 | 111 | 198.4 | 0.787 | 213.7 |
| 711+1G>T | 44.3 | 51 | 95.3 | 0.869 | 174.7 |
| F508del | 57.5 | 70.1 | 127.6 | 0.820 | 226.5 |
cut-off >97 ng/ml
In the correlation study plus the population study 137 cases that were screen positive by the IRTS assay with no CF mutations detected, 26 would be screen negative using the IRT1+IRT2 cut-off of >97ng/mL, a reduction of 19% in the false positive rate in this selected study population.
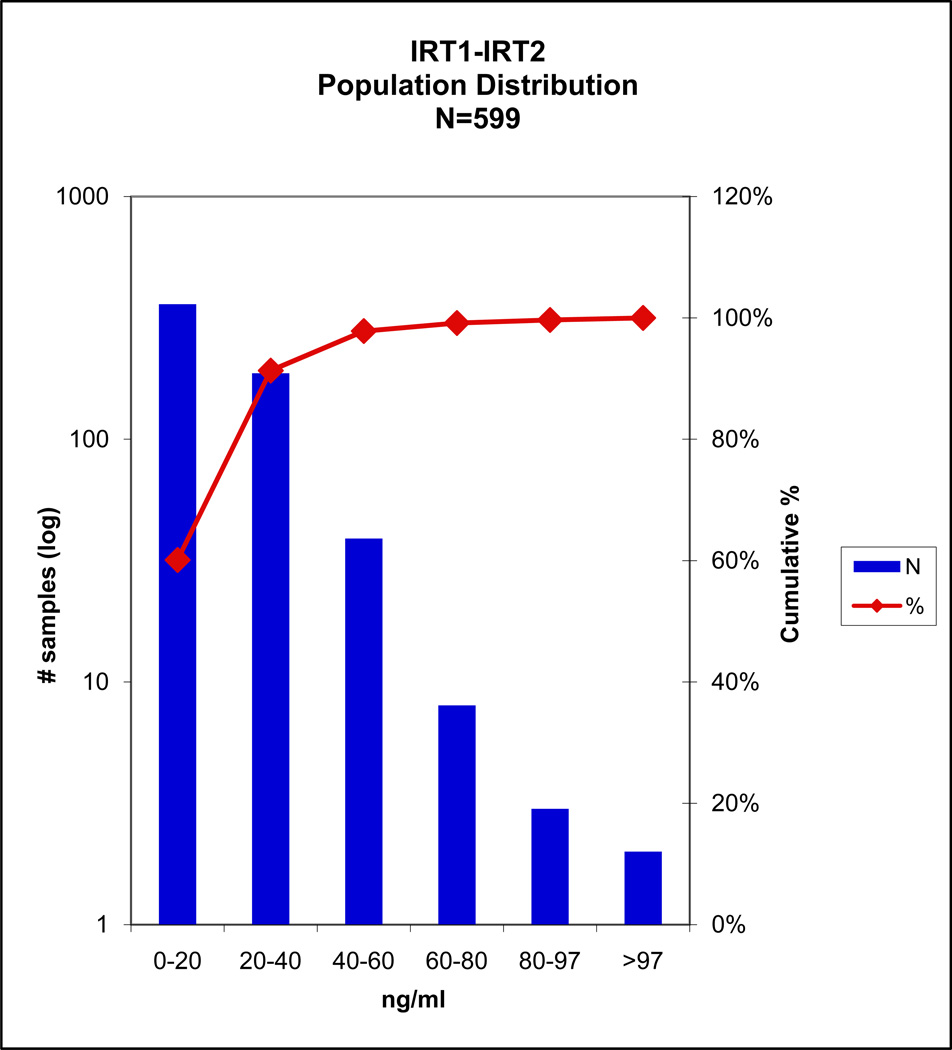
Population study
A total of 597 population study samples were analyzed, the distribution is shown in FIGURE 2. Two cases in this population were screen positive by the IRT1+IRT2 criteria; of these, one case fell within the top 5% of the IRTS method and had one CF mutation detected, the second case was screen negative by the IRTS.
Fig 2.
Two cases screen positive by IRT1+IRT2. One fell within the top 5% by the MPB standard method and had one mutation. The second case was screen negative.
Analysis of three cases of confirmed disease with an IRTS value below the 170 ng/mL cut-off is shown in TABLE 4. Two of the three cases would be screen positive using the IRT1+IRT2 assay criteria, with values greater than 97ng/ml.
TABLE 4.
Confirmed disease with IRTS < 170 ng/mL
| Confirmed Disease |
IRT1 | IRT2 | IRT1+IRT2* | IRT1 to IRT2 | IRTS |
|---|---|---|---|---|---|
| 2 mutations | ng/ml | ng/ml | ng/ml | ratio | ng/ml |
| W128X/N130K | 55.2 | 49.8 | 105 | 1.108 | 147.6 |
| F508DelL/F508Del | 28.1 | 48.7 | 76.8 | 0.577 | 67.9 |
| F508DelL/F508Del | 38.5 | 73.8 | 112.3 | 0.522 | 111.8 |
cut-off >97 ng/ml
Discussion
The false positive rate in newborn screening for CF has remained persistently high, despite numerous attempts to lower it (12). To reduce this, one unexplored approach, use of the two isoforms of trypsin, was examined in these studies. The goal in this study was the development of a multiplexed assay for CF using the two major trypsinogen isoforms that would meet screening standards for clinical accuracy when compared with current commercial NBS IRT assays.
The correlation study showed substantially equivalent performance of the assays in segregation of a screen positive population. Importantly for the 11 discrepant cases that were screen positive in the IRTS but reported as screen negative since no mutations had been detected in them by the screening program, all were screen negative in the IRT1+IRT2 assay, suggesting a greater specificity for the multiplex assay. [The current protocol of New York state NBS program considers samples with IRT values ≥170 ng/ml as screen positive regardless of mutation analysis results.] Using the IRT1+IRT2 cut-off of >97 ng/ml we achieved a reduction of 19% in the false positive rate in this selected study population.
Analysis of a screen positive population with confirmed disease indicated that a cut-off of ≥97 ng/mL in the IRT1+IRT2 assay would be needed to achieve 100% sensitivity for these samples. Although this cut-off is substantially lower than the one developed for the IRTS method of 170 ng/mL, it is nearer to one of 112 ng/mL cut-off reported for a monoclonal antibody based method for total IRT (7). It is likely that more specific immunoreactivity is observed when measuring the two isoforms as reported here.
Cystic fibrosis carriers have been shown to have higher IRT values than the normal population (9–10). In a screening program in which the goal is detect disease and not carrier status, discrimination of disease states from carried states could be of great help. In these studies use of the sum of IRT1+ IRT2 was unable to discriminate the carrier population, with seven of eight carriers screen positive by the IRTS assay also screen positive by the IRT1-IRT2 criteria.
Moreover, the ratio of IRT1 to IRT2 was unable to distinguish carriers from unaffected, as proposed by Itkonen et al. (4).
In three cases identified with confirmed disease that had an IRTS below the cut-off [per NBS protocol], two were screen positive by our assay criteria (TABLE 3). More studies are needed to determine whether these results indicate that the IRT1+IRT2 assay has greater sensitivity.
This study demonstrates that the IRT1+IRT2 multiplexed assay for CF has substantial equivalence in detecting screen positive specimens compared with the standard IRT method. Thus, this multiplexed assay for the two IRT isoforms has equal sensitivity in detecting CF and could offer improved specificity over the standard single-analyte methods used in newborn screening. The specificity of the antibodies for the two isoforms might also provide advantages in the standardization and in the preparation of external QC materials (9).
Perhaps most importantly, the multiplex format allows additional biomarkers e.g., pancreatitis associated protein (13), to be added in the future to improve the specificity of this assay. Building on our previous work with multiplex assays (14–16), the assay described here brings us one step closer to even more comprehensive multiplex assays for newborn screening; the combination of this CF assay with immunoassays for congenital hypothyroidism, and congenital adrenal hyperplasia into a single assay for the three current immunoassays used by NBS, thereby saving time in the screening laboratory, specimen usage, and perhaps at a lower cost.
Acknowledgments
This work was supported by NIH Contract ADB-NO1-DK-6-3430 (HHSN267200603430), Novel Technologies in Newborn Screening and Luminex, Corp.
We acknowledge the expertise and skills provided by Martin Sorette in these studies. We also thank the NYS Newborn Screening Program and Dr. Michele Caggana for making residual specimens available. Use of trade names is purely for discussion purposes, and does not constitute any statement about the performance of those materials.
These studies were performed under NYS DOH Institutional Review Board number: 00-402 "Evaluation of Multiplexed Newborn Screening with Luminex Technology”.
Abbreviations
- NBS
Newborn screening
- CF
Cystic fibrosis
- IRT
Immunoreactive trypsin
- IRTS
Standard IRT assay used by the NY NBS program
- IRT1
Cationic IRT
- IRT2
Anionic IRT
- IRT1+IRT2
our IRT assay
Footnotes
This is an un-copyedited authored manuscript copyrighted by The American Association for Clinical Chemistry (AACC). This may not be duplicated or reproduced, other than for personal use or within the rule of 'Fair Use of Copyrighted Materials' (section 107, Title 17, U.S. Code) without permission of the copyright owner, AACC. The AACC disclaims any responsibility or liability for errors or omissions in this version of the manuscript or in any version derived from it by the National Institutes of Health or other parties. The final publisher-authenticated version of the article will be made available at http://www.clinchem.org 12 months after its publication in Clinical Chemistry.
References
- 1.Crossley JR, Elliott RB, Smith PA. Dried blood spot screening for cystic fibrosis in the newborn. Lancet. 1979;1:742–744. doi: 10.1016/s0140-6736(79)90825-0. [DOI] [PubMed] [Google Scholar]
- 2.Guy O, Lombardo D, Bartelt DC, Amic J, Figarella C. Two human trypsinogens: purification, molecular properties and N-Terminal sequences. Biochemistry. 1978;17(9):1669–1675. doi: 10.1021/bi00602a014. [DOI] [PubMed] [Google Scholar]
- 3.Kimland M, Russick C, Marks WH, Borgstrom A. Immunoreactive anionic and cationic trypsin in human serum. Clin Chim Acta. 1989;184:31–46. doi: 10.1016/0009-8981(89)90254-4. [DOI] [PubMed] [Google Scholar]
- 4.Itkonen O, Koivunen E, Hurme M, Alfthan H, Schroder T, Stenman U-H. Time resolved immunofluorometric assays for trypsinogen 1 and 2 in serum reveal preferential elevation of trypsinogen-2 in pancreatitis. J Lab Clin Med. 1990;115:712–718. [PubMed] [Google Scholar]
- 5.Deam SM, Ryley HC. Enzyme imunoassay of immunoreactive trypsin in serum and blood spots. Wein Klin Wochenschr. 1988;100:55–57. [PubMed] [Google Scholar]
- 6.Cabrini G, Pederzini F, Perobelli L, Mastella G. An evaluation of an enzyme linked immunoassay method for immunoreactive trypsinogen in dried blood spots. Clin Biochem. 1990;23:213–219. doi: 10.1016/0009-9120(90)90614-z. [DOI] [PubMed] [Google Scholar]
- 7.Ball CL, Montgomery MD, Bridge PJ, Lyon ME. Evaluation of the Quantase™ neonatal immunoractive trypsinogen (IRT) screening assay for cystic fibrosis. Clin Chem Lab Med. 2005;43(5):570–572. doi: 10.1515/CCLM.2005.099. [DOI] [PubMed] [Google Scholar]
- 8.Li L, Zhou Y, Bell CJ, Earley MC, Hannon WH, Mei JV. Development and characterization of dried blood spot materials for the measurement of immunoreactive trypsinogen. J Med Screening. 2006;13:79–84. doi: 10.1258/096914106777589623. [DOI] [PubMed] [Google Scholar]
- 9.Casellani C, Picci L, Scarpa M, Deshecci MC, Zanolla L, Assael BM, et al. Cystic fibrosis carriers have higher neonatal trypsinogen values than non carriers. Am J Med Genet A. 2005;135(2):142–144. doi: 10.1002/ajmg.a.30470. [DOI] [PubMed] [Google Scholar]
- 10.Lecoq I, Brouard J, Laroche D, Ferec C, Travert J. Blood immunoreactive trypsinogen concentrations are genetically determined in healthy and cystic fibrosis newborns. Acta Paediatr. 1999;88:338–341. doi: 10.1080/08035259950170141. [DOI] [PubMed] [Google Scholar]
- 11.Lafont P, Guy-Crotte G, Paulin C, Galvain D, Mertani S, Figarella C, et al. A specific immunoradiometric assay of cationic trypsin(ogen) that does not recognize trypsin-alpha-1-proteinase inhibitor complex. Clin Chim Acta. 1995;235:197–206. doi: 10.1016/0009-8981(95)06030-x. [DOI] [PubMed] [Google Scholar]
- 12.Wilcken B. Newborn screening for cystic fibrosis: Techniques and strategies. J Inherit Metab Dis. 2007;30:537–543. doi: 10.1007/s10545-007-0584-0. [DOI] [PubMed] [Google Scholar]
- 13.Sarles J, Berthe’Z`Ne P, Le Louarn C, Somma C, Perini J-M, Catheline M, Mirallie´ M, et al. Combing immunoreative trypsinogen and pancreatitis-associated protein assays: A method of newborn screening for cystic fibrosis that avoids DNA analysis. J Pediatr. 2005;147:302–305. doi: 10.1016/j.jpeds.2005.05.017. [DOI] [PubMed] [Google Scholar]
- 14.Bellisario R, Colinas R, Pass KA. Simultaneous measurement of antibodies to three HIV-1 antigens in newborn dried blood spot specimens using a multiplexed microsphere-based immunoassay. Early Hum Dev. 2001;64:21–25. doi: 10.1016/s0378-3782(01)00167-0. [DOI] [PubMed] [Google Scholar]
- 15.Bellisario R, Colinas RJ, Pass KA. Simultaneous measurement of thyroxine (T4) and thyrotropin (TSH) from newborn dried blood spot specimens using a multiplexed fluorescent immunoassay. Clin Chem. 2000;46:1422–1424. [PubMed] [Google Scholar]
- 16.Colinas RJ, Bellisario R, Pass KA. Multiplexed genotyping of beta-globin variants from polymerase chain reaction-amplified newborn bloodspot DNA by hybridization with allele-specific oligodeoxynucleotides coupled to an array of fluorescent microspheres. Clin Chem. 2000;46(7):1–3. [PubMed] [Google Scholar]