Abstract
Nonsteroidal anti-inflammatory drugs such as sulindac have shown promising antineoplastic activity, although toxicity from cyclooxygenase (COX) inhibition and the suppression of prostaglandin synthesis limits their use for chemoprevention. Previous studies have concluded that the mechanism responsible for their antineoplastic activity may be COX independent. To selectively design out the COX inhibitory activity of sulindac sulfide (SS), in silico modeling studies were done that revealed the crucial role of the carboxylate moiety for COX-1 and COX-2 binding. These studies prompted the synthesis of a series of SS derivatives with carboxylate modifications that were screened for tumor cell growth and COX inhibitory activity. A SS amide (SSA) with a N,N-dimethylethyl amine substitution was found to lack COX-1 and COX-2 inhibitory activity, yet potently inhibit the growth of human colon tumor cell lines, HT-29, SW480, and HCT116 with IC50 values of 2 to 5 µmol/L compared with 73 to 85 µmol/L for SS. The mechanism of growth inhibition involved the suppression of DNA synthesis and apoptosis induction. Oral administration of SSA was well-tolerated in mice and generated plasma levels that exceeded its in vitro IC50 for tumor growth inhibition. In the human HT-29 colon tumor xenograft mouse model, SSA significantly inhibited tumor growth at a dosage of 250 mg/kg. Combined treatment of SSA with the chemotherapeutic drug, Camptosar, caused a more sustained suppression of tumor growth compared with Camptosar treatment alone. These results indicate that SSA has potential safety and efficacy advantages for colon cancer chemoprevention as well as utility for treating malignant disease if combined with chemotherapy.
Epidemiologic, clinical, and experimental studies have shown that certain nonsteroidal anti-inflammatory drugs (NSAID) and cyclooxygenase (COX)-2 selective inhibitors, display convincing evidence of chemopreventive efficacy. Population-based studies, for example, have shown that aspirin and to a greater extent, nonaspirin NSAIDs can significantly reduce the incidence of colorectal cancer (1, 2). Additionally, clinical studies have reported that certain NSAIDs such as sulindac can cause the regression of adenomas in patients with either familial or sporadic adenomatous polyposis (3–6). Unfortunately, the depletion of physiologically important prostaglandins resulting from COX-1 or COX-2 inhibition is associated with gastrointestinal, renal, or cardiovascular toxicities (7–9), which limits the use of NSAIDs or COX-2 selective inhibitors for cancer chemoprevention.
The mechanism responsible for the antineoplastic activity of NSAIDs is poorly understood, although COX-2 inhibition and the suppression of prostaglandin biosynthesis are generally believed to account for their chemopreventive efficacy. However, some investigators have concluded that a COX-independent mechanism may be involved and suggest that an off-target effect may either contribute to or be fully responsible for their antineoplastic activity (10–16). A number of COX-independent targets have been implicated including 15-lipoxygenase (17), Ras (18), PPAR (19), nuclear factor-κB (20), PDK-1/Akt (21), phosphodiesterase (22), as well as others (23, 24). The pharmacologic effects of NSAIDs are undoubtedly complex and may involve both COX-dependent and COX-independent effects that lead to direct effects on tumor cells to suppress proliferation or induce apoptosis, as well as indirect effects such as inhibition of angiogenesis.
Sulindac metabolites have provided useful drug probes to determine if COX inhibition is required for the chemopreventive properties of NSAIDs. As a sulfoxide prodrug, sulindac requires metabolism to a sulfide that inhibits COX-1 and COX-2, which is responsible for its anti-inflammatory activity (25, 26). A sulfone metabolite is also generated by oxidation of the sulfoxide, which does not inhibit COX (27) or contribute to the anti-inflammatory activity of sulindac (28). As evidence for a COX-independent mechanism, sulindac sulfone (exisulind) was reported to inhibit colon tumor cell growth and induce apoptosis in vitro in a manner similar to sulindac sulfide (SS), albeit with less potency (11, 14). Sulindac sulfone inhibited tumorigenesis in the azoxymethane-induced rat colon model without suppressing prostaglandin levels (29), and was also effective in chemically induced models of mammary (30, 31), lung (32), and bladder (33) carcinogenesis. In clinical trials, sulindac sulfone caused regression of adenomas in patients with either familial (34) or sporadic polyposis (35) with modest efficacy but did not receive Food and Drug Administration approval due, in part, to hepatotoxicity. Together, these studies suggest that COX inhibition is not necessary for the antineoplastic activity of sulindac, although the molecular target(s) remain to be determined.
As a strategy to develop safer derivatives of sulindac for cancer chemoprevention, we conducted computational studies to determine specific structural properties of SS that are required for COX-1 and COX-2 binding. These studies revealed that the carboxylic acid moiety is crucial for binding COX-1 and COX-2, which prompted the synthesis of a series of derivatives with chemical modifications to the carboxylic acid moiety. Certain analogs bearing a basic amino group that will protonate at physiological pH were found to have reduced COX-1 and COX-2 inhibitory activity but unexpectedly were found to display appreciably higher potency to inhibit colon tumor cell growth and induce apoptosis compared with SS. Here, we describe a prototypic amide derivative of SS that is orally bioavailable and strongly suppresses tumor growth in the HT-29 colon tumor xenograft mouse model alone and in combination with the chemotherapeutic drug, Camptosar.
Materials and Methods
Molecular modeling
Modeling studies were done using the Schrödinger Suite 2007 Induced Fit Docking Protocol (Glide version 4.5; Schrödinger, LLC). COX-1 and COX-2 crystal structures (PDB entries 2OYE and 6COX, respectively) were preprocessed using the Protein Preparation Wizard. SS was docked into the COX-1 and COX-2 structures using Induced-Fit. Because Trp387 altered docking poses, this side chain was removed in the initial stages of docking and later reinserted. All other parameters were set at default (refinement of residues within 5 Å from ligand, Van der Waals scaling 0.70, ligand scaling 0.50).
Drugs and reagents
SS amide (SSA) was synthesized from sulindac by reaction with N, N-dimethylaminoethylamine using N,N'-dicyclohexylcarbodiimide as the coupling agent followed by reduction of the intermediate sulfoxide using triphenylphosphine/iodine as the oxygen acceptor. The product was then characterized by proton magnetic resonance spectroscopy, fast atom bombardment mass spectrometry, and elemental analysis before biological testing. All other drugs and reagents were purchased from Sigma-Aldrich unless otherwise stated.
COX assays
COX-1 and COX-2 activities were measured using purified ovine COX-1 and COX-2 with colorimetric assay kits obtained from Cayman Chemical Co. The activities of COX-1 and COX-2 were measured after the addition of arachidonic acid and incubation at 25°C for 5 min by absorbance at 590 nm as specified by the manufacturer.
Cell culture
Human HT-29, SW480, and HCT116 colon tumor cell lines were obtained from the American Type Culture Collection and grown under standard cell culture conditions in RPMI 1640 containing 5% fetal bovine serum at 37°C in a humidified atmosphere with 5% CO2. Cell counts were determined with a Coulter Model Z1 cell counter, and viability was measured by propidium iodide staining followed by analysis on a Beckman Coulter EPICS XL flow cytometer. Only cultures displaying >95% viability were used for experiments.
Growth assay
Growth inhibitory activity was determined by a reduction of viable cell number as measured by ATP levels using the CellTiterGlo assay (Promega) according to manufacturer's specifications. In brief, cells were seeded in tissue culture microtiter 96-well plates at a density of 5,000 cells per well and incubated 16 h before dosing. Cells were treated for a total of 72 h while incubating at 37°C.
Proliferation assay
The antiproliferative activity of SSA and sulindac metabolites was determined by a reduction of DNA synthesis, which was measured by a radioactive thymidine incorporation assay. Cells were seeded and dosed as described above for the growth assay. Once dosing was complete, cells were incubated at 37°C for ~56 h at which time a 1 µCi aliquot of 3H-thymidine (Amersham Biosciences) was added to each well. Cells were incubated another 16 h to allow sufficient time for thymidine incorporation into DNA for a total of 72 h of treatment. Plates were harvested with a semiautomatic cell harvester onto filters that bind the DNA. Samples were counted in an automated scintillation counter to determine the amount of 3H-thymidine uptake.
Apoptosis assays
Apoptosis was measured by caspase activation and DNA strand breaks, which are early- and late-stage biochemical markers of apoptosis, respectively. For measuring caspase activation, cells were seeded in tissue culture microtiter 96-well plates at a density of 10,000 cells per well. Cells were incubated for 18 to 24 h before treatment and an additional 2 to 8 h after treatment. Activity of caspases 3 and 7 was measured by the Caspase-Glo 3/7 Assay (Promega), which is a luminescent assay that measures substrate cleavage by caspases 3 and 7. The assay was done according to manufacturer's specifications. Apoptosis was also measured by terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL). Cells were seeded in 10 cm tissue culture dishes at a density of 2 × 106 cells per dish. After incubating for 48 h, the cell media was replaced with media containing the specified compound or vehicle control. After 24 h of incubation with SSA or SS, cells were collected and fixed with 4% formalin on ice for 15 min. Samples were stained for DNA strand breaks using the APO-BrdUrd TUNEL Assay kit (Invitrogen) in which strand breaks are labeled with BrdUrd and detected with anti–BrdUrd-Alex-aFluor 488 and nuclei with propidium iodide. The assay was done according to the manufacturer's specifications. The percentage of TUNEL-positive cells was quantified using a Guava EasyCyte Plus flow cytometer. A minimum of 10,000 events were collected for each treatment group with use of minimal electronic compensation. Data were analyzed using CytoSoft 5.0 software (Guava Technologies).
Tolerance studies
The maximum tolerated dose (MTD) of sulindac and SSA was determined using 5 to 6-wk-old male NCr-nu/nu mice. Sulindac or SSA were administered once daily by gastric gavage with increasing dosages for up to 20 or 17 d, respectively. Both compounds were formulated as a suspension in 0.05% carboxymethylcellulose, 0.25% Tween 80 in water. Mice were observed daily and body weights measured twice weekly.
Pharmacokinetic studies
Sulindac (50 mg/kg) and SSA (200 mg/kg) were administered as a single treatment by gastric gavage to 5 to 6-wk-old male NCr-nu/nu athymic mice. Blood was collected after the indicated time after treatment. Plasma was processed by protein precipitation followed by solvent evaporation. Solvents were reconstituted with 50% methanol in 5 mmol/L ammonium acetate. The samples were analyzed using reverse phase chromatography (Perkin-Elmer Series 200 autosampler and micropumps) with MS/MS (Perkin-Elmer Sciex API 3000) detection operating in the positive ion mode using multiple reaction monitoring.
Antitumor efficacy studies
The antitumor efficacy of SSA and Camptosar (irinotecan HCl) was evaluated in a mouse model using human HT-29 colon tumor fragments that were implanted s.c. in 5 to 6-wk-old male NCr-nu/nu athymic mice as previously described (36). SSA at a dosage of 250 mg/kg was administered by gastric gavage using a twice daily schedule in which injections were spaced 8 h apart. Camptosar was administered i.v. once every 4 d for 3 injections at a dosage of 40 mg/kg. Mice were observed daily for mortality. Tumor dimensions and body weights were measured twice weekly starting on the first day of treatment. Tumor volume was determined by caliper measurements (mm) and using the formula for an ellipsoid sphere: L × W2/2 = mm3, where L and W refer to the larger and smaller perpendicular measurements, respectively. This formula was also used to calculate tumor weight, assuming unit density (1 mm3 = 1 mg). Toxicity was assessed based on observed deaths and mean body weight loss compared with mean body weight on the first day of the treatment. For all animal studies described above, mice were housed in microisolator cages, provided filtered tap water and sterile pelleted diet ad libitum. Mice reaching a maximum tumor weight of 4,000 mg were euthanized as per Institutional Animal Care and Use Committee guidelines. All animal protocols were reviewed by the Southern Research Institutional Animal Care and Use Committee before experimentation. For graphical representation of the results, the mean or median tumor weights for the group included the tumor weights from mice that reached maximum tumor size, which were euthanized before the completion of the study. Tumor growth inhibition was compared between groups by the t test or Mann-Whitney Rank Sum Test of tumor weights from the number of mice that survived the entire duration of the study.
Results
Lack of COX binding and inhibitory activity of SSA
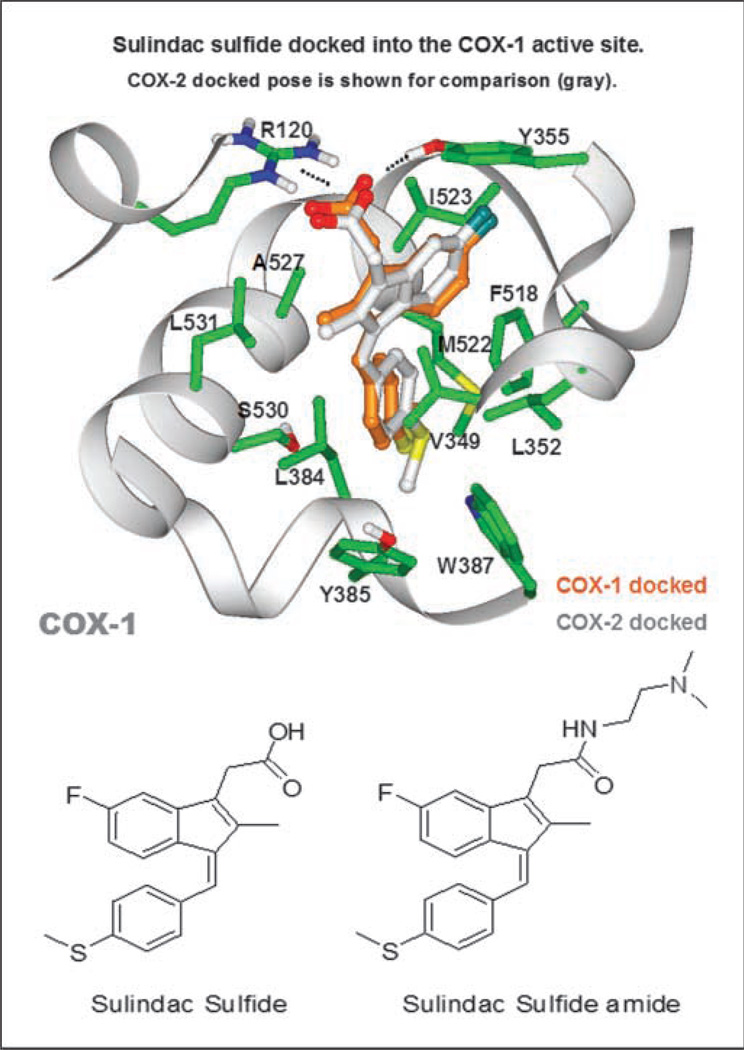
Molecular modeling studies were done to identify specific structural features of SS that are important for binding to COX-1 and COX-2. Using InducedFit modeling software to dock SS into the binding pockets within COX-1 and COX-2, complex binding structures were generated in silico using published structures of COX-1 and COX-2 obtained from X-ray diffraction studies. The orientation of SS bound to COX-1 and COX-2 was found to be very similar. Superimposing COX-1 and COX-2, the root mean square deviation between docked SS poses is 0.86 between heavy atoms, which represents a single favored ligand pose. The binding mode of SS to COX-1 and COX-2 as shown in Fig. 1 (top) reveals that the carboxylic acid moiety forms a salt bridge with the positively charged guanidinium moiety of arginine 120 (R120), which constrains the position of the rest of the molecule in a region that is rich in nonpolar amino acids. Based on this model, we hypothesized that modifications which substitute a positive charge for a negatively charged carboxylate of SS would effectively reduce or eliminate COX-1 and COX-2 binding.
Fig. 1.
Molecular modeling of SS binding at the active site pocket of COX-1 or COX-2. Top, SS docked into the crystal structures of COX-1 and COX-2. Amino acids that participate in binding of SS to both COX-1 and COX-2 are illustrated through side chain heavy atoms and α carbons. Bottom, the two-dimensional chemical structure of SS and SSA.
A series of SS derivatives were therefore synthesized with various modifications to the carboxylic acid moiety and screened for COX and tumor cell growth inhibitory activity. A subgroup of analogs bearing a basic amino group that will protonate at physiological pH was identified, which displayed higher potency to inhibit HT-29 colon tumor cell growth compared with SS but lacked COX-1 and COX-2 inhibitory activity. A SS derivative that contained a N,N-dimethylethyl amine group was selected for further study that is shown in Fig. 1 (bottom) along side SS and is referred to here as SSA for simplicity. From the binding model, we predicted that the two-carbon spacer in SSA would position the positively charged N,N-dimethylethyl amine group ~4 Å or less away from the center of mass of the positively charged R120 guanidinium into a highly unfavorable environment to greatly reduce COX-1 and COX-2 binding affinity.
To confirm reduced COX inhibitory activity of SSA, COX-1 and COX-2 inhibitory activity was measured using an enzymatic assay with purified isozymes. As summarized in Table 1, SS nonselectively inhibited COX-1 and COX-2 with IC50 values of 1.8 and 6.3 µmol/L, respectively, which is comparable with IC50 values reported previously by other investigators (26). SSA did not inhibit COX-1 at concentrations as high as 300 µmol/L and only weakly inhibited COX-2 with an IC50 value of 164.5 µmol/L, which is unlikely to be pharmacologically relevant. As controls, indomethacin was confirmed to potently inhibit COX-1 and COX-2 with IC50 values of 0.02 and 1 µmol/L, respectively, whereas rofecoxib selectively inhibited COX-2 with an IC50 value of 2.7 µmol/L but did not inhibit COX-1. Sulindac sulfoxide and sulfone were also confirmed to lack both COX-1 and COX-2 inhibitory activity as previously reported (31). Consistent with these observations, SSA was appreciably less effective than SS to reduce prostaglandin synthesis in COX-2–overexpressing cells and did not affect the expression of COX-2 in SW480 cells (data not shown).
Table 1.
COX inhibitory activity of NSAIDs and derivatives
| Compound | COX-1 IC50 (µmol/L) | COX-2 IC50 (µmol/L) |
|---|---|---|
| Rofecoxib | >300 | 2.7 |
| Indomethacin | 0.02 | 1.0 |
| SS | 1.8 | 6.3 |
| Sulindac sulfoxide | >300 | >300 |
| Sulindac sulfone | >300 | >300 |
| SSA | >300 | 164.5 |
NOTE: COX activity was measured using an enzyme assay with purified COX-1 and COX-2 isozymes as described under “Materials and Methods.”
In vitro tumor cell growth inhibitory activity of SSA
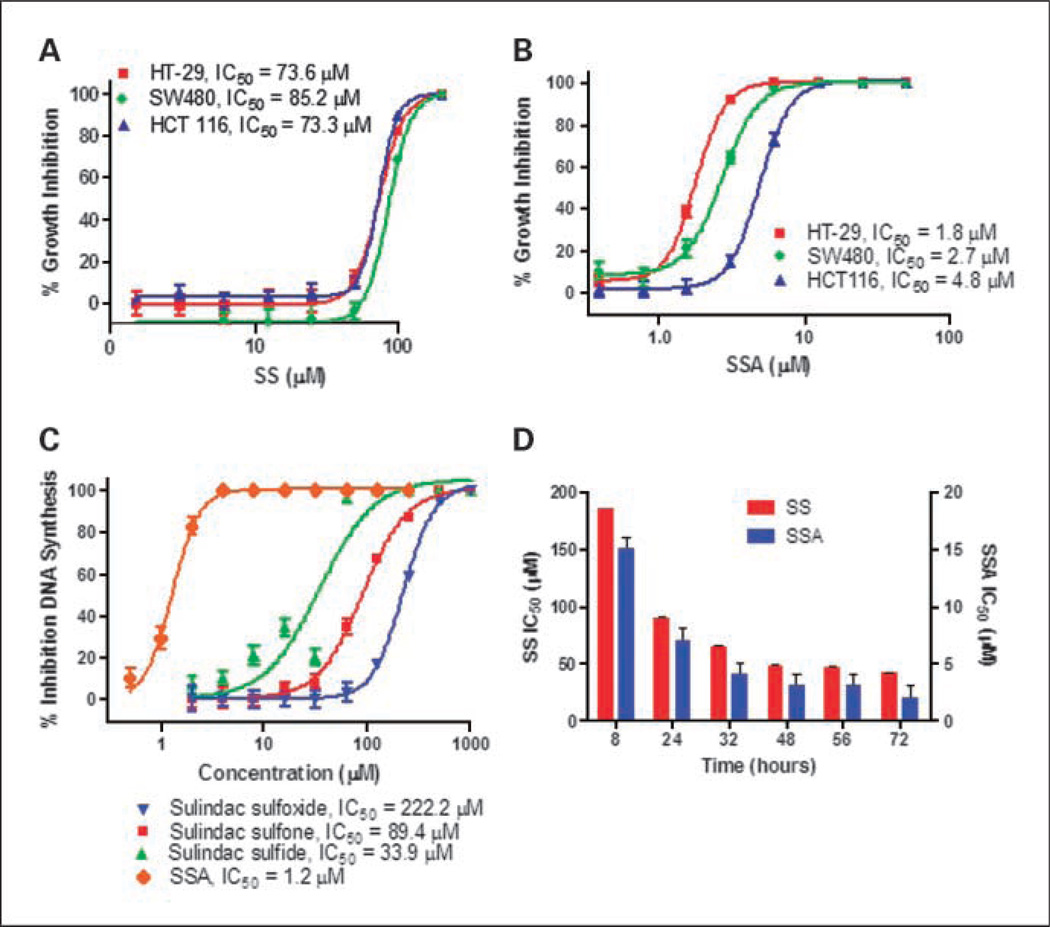
The tumor cell growth inhibitory activity of SS and SSA was measured with an ATP sensitive luminescence assay using the human colon tumor cells lines, HT-29, SW480, and HCT116, after 72 hours of continuous treatment. As shown in Fig. 2A, SS inhibited colon tumor cell growth with IC50 values of 73.3 to 85.2 µmol/L, which is consistent with potency values reported by other investigators (37). Despite lacking COX-1 and COX-2 inhibitory activities, SSA displayed appreciably more potent colon tumor cell growth inhibitory activity with IC50 values of 1.8 to 4.8 µmol/L (Fig. 2B). The HT-29 colon tumor cell line was highly sensitive to SSA, showing an approximate 40-fold increase in potency compared with SS. As shown in Fig. 2C, the tumor cell growth inhibitory activity of SSA was associated with suppression of DNA synthesis as measured by 3H-thymidine incorporation using HT-29 colon tumor cells. The IC50 value of SSA to inhibit DNA synthesis was determined to be 1.2 µmol/L and appreciably more potent that SS, sulindac sulfone, or sulindac sulfoxide, which resulted in IC50 values of 33.9, 89.4, and 222.2 µmol/L, respectively. Time course studies using HT-29 colon tumor cells as shown in Fig. 2D demonstrated that SS and SSA displayed similar kinetics of growth inhibition in which both compounds required ~2 days of continuous treatment to reach their maximum potency.
Fig. 2.
Inhibition of colon tumor cell growth and DNA synthesis by SS and SSA. A and B, dose-dependent growth inhibitory activity of SS (A) and SSA (B) in human HT-29, HCT116, and SW480 colon tumor cells as measured by the CellTiterGlo assay described under “Materials and Methods.” C, the potency of SSA and SS, sulfone and sulfoxide to inhibit DNA synthesis in human HT-29 colon tumor cells. D, a time course experiment of SSA and SS to inhibit HT-29 tumor cell growth in which cell viability was measured using the CellTiterGlo assay. Columns, mean; bars, SE.
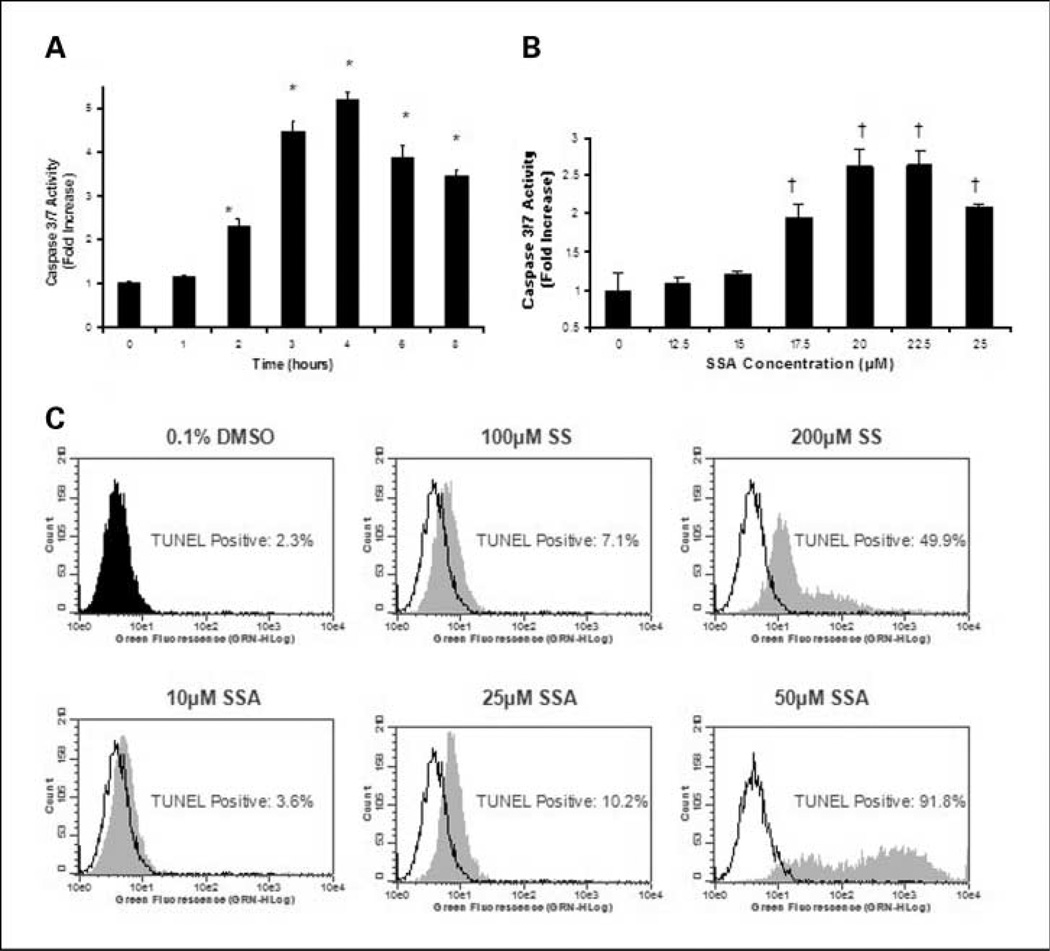
The ability of SSA to induce apoptosis was determined by measuring caspase activation and DNA strand breaks, which represent early and late stage biochemical markers of apoptosis, respectively. As shown in Fig. 3A, SSA at a concentration of 25 µmol/L caused a time-dependent increase in caspase 3 and 7 activities in human HT-29 colon tumor cells after 2 to 8 hours of treatment, reaching levels 2- to 5-fold above caspase activity levels in vehicle-treated cells. The optimum treatment time for caspase induction was ~4 hours. SSA also caused a dose-dependent increase in caspases 3 and 7 activities with significant changes apparent at concentrations of 17.5 to 25 µmol/L after 5 hours of treatment as shown in Fig. 3B. Apoptosis induction by SSA was confirmed and compared with SS by measuring DNA strand breaks using TUNEL after 24 hours of treatment. Compared with vehicle-treated cultures in which 2.3% of the cells were apoptotic, SSA increased the percentage of apoptotic cells to 3.6, 10.2, and 91.8% at concentrations of 10, 25, and 50 µmol/L, respectively (Fig. 3C). By comparison, SS increased the percentage of apoptotic cells to 7.1% and 49.9% at concentrations of 100 and 200 µmol/L, respectively.
Fig. 3.
Apoptosis induction by SSA and SS in human HT-29 colon tumor cells. A and B, time- (A) and dose-dependent (B) induction of caspases 3 and 7 activities upon treatment with SSA measured by the Caspase-Glo 3/7 assay described under “Materials and Methods.” For time course studies, SSA was tested at a concentration of 25 µmol/L, whereas dose-response studies involved treatment for 5 h. C, a dose-dependent increase in DNA strand breaks by SSA and SS as measured by the TUNEL assay as described under “Materials and Methods.” Representative histograms (C) depict a dose-dependent increase in green fluorescence in each treatment group (gray) when compared with the vehicle control (black outline). Treatment values are significantly different than those for vehicle control with P value of ≤0.001 (†) or P value of ≤0.005 (*). Data represent mean ± SE.
Tolerance and pharmacokinetic studies
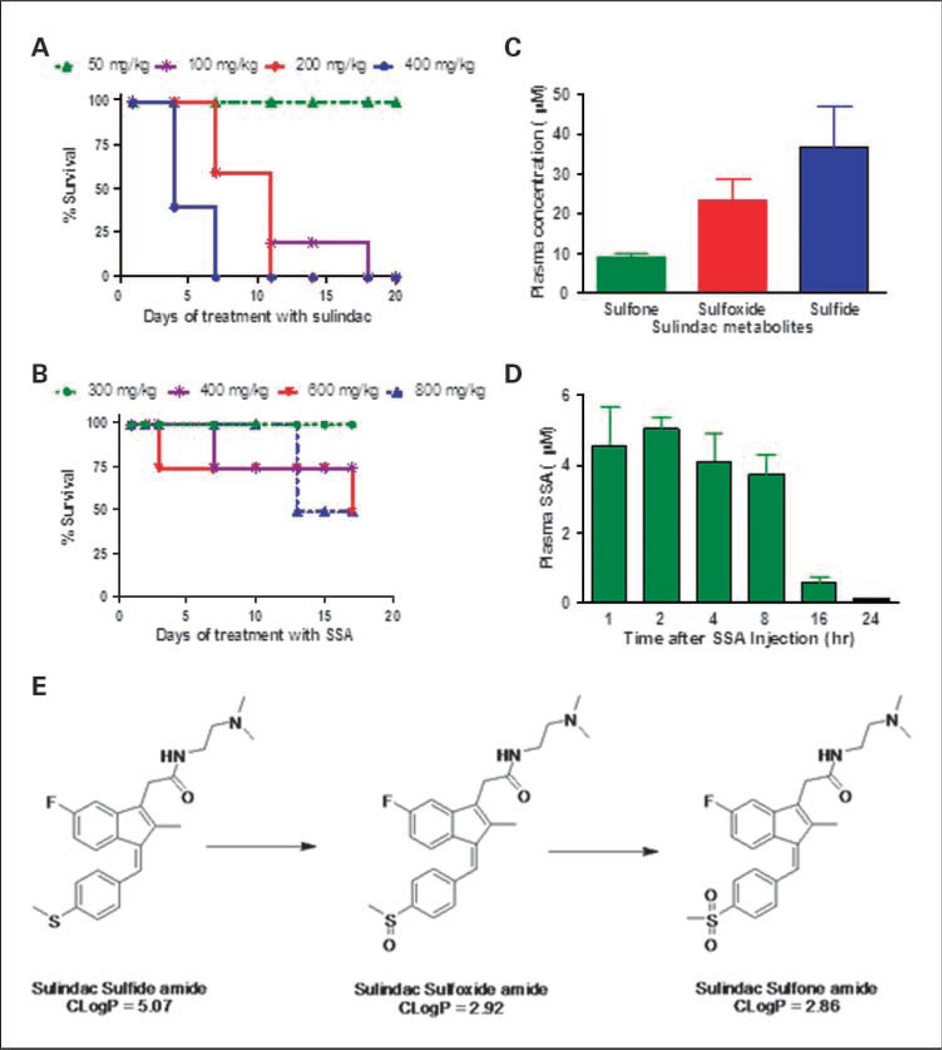
Studies in mice were conducted to determine the MTD of SSA and sulindac after once daily administration by gastric gavage. As shown in Fig. 4A, sulindac was tolerated up to a dosage of 50 mg/kg for 20 days of treatment. Consistent with its reduced COX inhibitory activity, appreciably higher dosages of SSA up to 300 mg/kg were tolerated for 17 days of treatment (Fig. 4B). Given that differences in tolerance could be attributed to differences in bioavailability, pharmacokinetic studies were done to measure plasma levels of SSA and SS after a single dose administration of SSA or sulindac by gastric gavage. As shown in Fig. 4C, the sulfide was the major metabolite detected in plasma 6 hours after administering 50 mg/kg sulindac, which generated concentrations of 36.7 µmol/L that were approximately half of its IC50 value to inhibit colon tumor cell growth in vitro. Appreciably lower levels of the sulfoxide and sulfone were measured within this time period, which did not approach levels required to affect tumor cell growth in vitro. By comparison, a 200 mg/kg dose of SSA generated plasma levels that were greater than or comparable with its IC50 value for inhibiting colon tumor cell growth in vitro (Fig. 4D). The calculated plasma half-life of SSA was 9.9 hours and similar to the half-life of sulindac as previously reported (25). The major metabolite of SSA was a sulfoxide amide, which was absorbed and cleared within the same time period as the sulfide amide (Table 2). Lesser amounts of a sulfone amide were also detected, which suggest a similar mode of oxidative metabolism of SSA as sulindac as illustrated in Fig. 4E. To confirm that SSA is not converted to sulindac, plasma levels of sulindac sulfoxide were measured in plasma of mice treated with SSA but were found to be very low to undetectable.
Fig. 4.
Tolerance and pharmacokinetics of SSA and sulindac in mice. A and B, the survival of mice treated with sulindac or SSA by gastric gavage, respectively. Mice were treated once daily with sulindac for 20 d and once daily with SSA for 17 d. C, plasma levels of SS, sulfone, and sulfoxide from mice 6 h after gastric gavage of sulindac at a dosage of 50 mg/kg. D, plasma levels of SSA from mice after various times after gastric gavage of SSA at a dosage of 200 mg/kg. Plasma levels of sulindac metabolites and SSA were measured as described under “Materials and Methods.” Columns, mean; bars, SE. The proposed metabolic pathway of SSA is shown in E.
Table 2.
Metabolites of SSA in plasma after oral administration of SSA
| Analyte | Hours post gastric gavage administration of SSA (200 mg/kg) | |||||
|---|---|---|---|---|---|---|
| 1 | 2 | 4 | 8 | 16 | 24 | |
| Sulfide amide | 4.55 ± 1.91 | 5.02 ± 0.55 | 4.08 ± 1.39 | 3.70 ± 0.99 | 0.56 ± 0.29 | 0.09 ± 0.05 |
| Sulfone amide | 0.19 ± 0.04 | 0.27 ± 0.03 | 0.47 ± 0.04 | 0.56 ± 0.03 | 0.04 ± 0.02 | 0.01 ± 0.004 |
| Sulfoxide amide | 3.33 ± 0.8 | 3.18 ± 0.36 | 2.81 ± 0.53 | 2.96 ± 1.19 | 0.24 ± 0.09 | 0.05 ± 0.02 |
| Sulindac | 0.05 ± 0.04 | 0.06 ± 0.01 | 0.05 ± 0.01 | 0.05 ± 0.014 | BLD | BLD |
NOTE: Levels in µmol/L units are shown ± SD.
Abbreviation: BLD, below detection limit.
In vivo antitumor activity
SSA was evaluated for in vivo antitumor efficacy in a mouse xenograft model using s.c. implanted HT-29 colon tumor fragments. Mice were treated with SSA at a dosage of 250 mg/kg administered twice daily by gastric gavage. Treatment was initiated on the day of tumor implantation and continued for 55 days. As shown in Fig. 5A, SSA was tolerated for the duration of treatment. The mean body weight loss in the treatment group did not exceed 6% on any given day and averaged 1.1% over the duration of treatment. Additionally, the final body weight of the treatment group was comparable with the control group. As shown in Fig. 5B, the mean tumor weight in treated mice was reduced by ~60% or greater compared with the control group beginning on approximately day 27 and persisted until the study was terminated. For example, on day 36, the mean tumor weight of the control group was 1,151 and 448 mg in the treatment group (P = <0.001, Mann-Whitney Rank Sum Test). On day 55, the mean weight of the control group was 2,534 and 1,141 mg in the treatment group (P = <0.05, t test), which shows a sustained reduction of tumor growth as a result of treatment with SSA.
Fig. 5.
In vivo antitumor efficacy of SSA and Camptosar alone and in combination as determined using the human HT-29 colon tumor xenograft mouse model. A, body weights of mice treated with vehicle or SSA administered twice daily by gastric gavage (day 1–55) at a dosage of 250 mg/kg. B, tumor growth of mice treated with vehicle or SSA at a dosage and schedule as described in A. C, tumor growth of mice treated with vehicle, SSA twice daily by gastric gavage (day 14–70) at a dosage of 250 mg/kg, Camptosar (day 14, 18, and 22) at a dosage of 40 mg/kg, i.v., or SSA and Camptosar (same dosages as single agent treatments). Treatment was initiated on the day of tumor implantation (day 0) for the study shown in A and B. For the study shown in C, treatment was initiated once the tumors reached a median size of 200 mg (day 14). Treatment values are significantly different than those for vehicle control with P value of ≤0.001 (**) or P ≤ 0.05 (*). Points, mean (A and B), or median (C) values; bars, SE.
To determine potential therapeutic benefits of SSA, treatment was initiated after tumors were established. As shown in Fig. 5C, SSA did not affect tumor growth rate if treatment was delayed until after the tumors were established. For comparison, the topoisomerase inhibitor and chemotherapeutic drug, Camptosar, was also evaluated under identical conditions at a dosage of 40 mg/kg administered i.v. for 3 injections once every 4 d. Despite testing at a dosage that was close to its MTD, Camptosar was also only marginally effective in this model in which tumor growth resumed a rate comparable with the control group soon after treatment was discontinued. However, combined treatment of SSA with Camptosar resulted in a more sustained suppression of tumor growth. By day 70, there was no significant difference bet-ween the vehicle, SSA, or Camptosar treatment groups, while the SSA plus Camptosar treatment group was 46% less than the control group (P = <0.05, t test). Calculation of the median number of days to reach a tumor weight of 2,000 mg for the control, SSA, Camptosar, and SSA plus Camptosar groups were 42.8, 40.8, 48.2, and 58.6 days, whereas the median number of days to reach 3,000 mg was 52.2, 53.2, 58.7, and >70 days, respectively. By day 70, 60% to 70% of the mice in the control, SSA, and Camptosar treatment groups reached the maximum tumor weight (4,000 mg). By comparison, only 20% of mice in the SSA plus Camptosar treatment group reached maximum tumor weight.
Discussion
Preclinical, clinical, and epidemiologic studies have shown promising evidence of antineoplastic efficacy with a number of NSAIDs and COX-2 inhibitors. However, toxicity resulting from COX-1 and/or COX-2 inhibition limits their clinical use for cancer chemoprevention, which is generally believed to require chronic administration and appreciably higher dosages compared with those required for their intended anti-inflammatory indications. Sulindac is considered to be one of the most efficacious NSAIDs based on its ability to cause the regression of colonic adenomas in patients with familial adenomatous polyposis (3–6). Unfortunately, its benefits are limited to a 60% to 70% reduction in the number and size of polyps. In addition, case reports have described familial adenomatous polyposis patients who developed colorectal cancer despite long term treatment with sulindac (6, 38).
Some investigators have concluded that the mechanism responsible for the tumor cell growth inhibitory activity of NSAIDs may, in part or fully, be COX independent, although alternative molecular target(s) have not yet been well defined and used to discover new drug candidates for chemoprevention. Our results support the possibility that the mechanism responsible for the tumor cell growth inhibitory activity of sulindac is COX independent, which suggests the feasibility of developing safer and more efficacious drugs by chemically modifying sulindac to selectively design out its COX inhibitory activity. For example, appreciably higher concentrations of SS were required to inhibit colon tumor cell growth compared with its potency to inhibit COX-1 or COX-2. In addition, the non-COX inhibitory sulfoxide and sulfone forms of sulindac inhibited tumor cell growth, albeit with low potency. Finally, the HCT116 tumor cell line displayed comparable sensitivity to SS as other colon tumor cell lines, although previous reports indicate that this tumor cell line lacks COX-2 expression (39, 40).
To identify specific chemical properties of SS that are required for COX binding, we performed molecular modeling studies using crystal structures of COX-1 and COX-2 available from X-ray diffraction studies. In silico docking analysis showed that the carboxylic acid moiety is necessary for binding COX-1 and COX-2 and suggested that modifications to the carboxylic acid moiety could effectively block COX inhibitory activity. These studies prompted the synthesis of a series of amide derivatives that were screened for tumor cell growth and COX inhibitory activity. SSA was selected as a prototypic derivative, which displayed potent growth inhibitory activity but had no detectable COX-1 inhibitory activity or pharmacologically significant COX-2 inhibitory activity. The formation of the neutral amide and addition of a nearby positively charged moiety in SSA is suspected to block formation of a salt bridge with positively charged amino acids within the COX-1 and COX-2 binding domain. Although chemical properties other than the carboxylic acid likely also contribute to the binding (41), modifying the carboxylic acid with a positively charged moiety effectively blocked binding to both isozymes. It is noteworthy that this strategy is different from the approach described by Kalgutkar and colleagues (42–44) who synthesized a series of NSAID derivatives that had bulky substitutions to the carboxylic acid. Unlike our approach, these derivatives displayed improved COX-2 selectivity by taking advantage of a smaller binding pocket in the COX-1 isozyme compared with COX-2.
As in the case of SS, the growth inhibitory activity of SSA was associated with its ability to suppress DNA synthesis and induce apoptosis. Both compounds suppressed growth and induced caspase-dependent apoptosis with similar kinetics, which suggests that SSA and SS share a similar mechanism of action. However, further studies are necessary to identify the molecular target of SSA, to determine if SSA shares a common target with SS and other NSAIDs, and the potential role of this target in tumor cell proliferation or survival.
Consistent with the lack of COX inhibitory activity of SSA, appreciably higher dosages of SSA were tolerated in mice compared with sulindac. Pharmacokinetic studies showed that SSA was orally bioavailable and could generate plasma levels that exceeded its IC50 values for in vitro tumor cell growth inhibition, whereas sulindac generated plasma concentrations of SS that were appreciably less than its IC50 value for inhibiting colon tumor cell growth in vitro. These observations suggest that SSA has the potential for greater in vivo antitumor efficacy compared with sulindac, although more extensive studies are necessary to compare the two compounds in a side-by-side manner. Pharmacokinetic studies also showed that SSA is metabolized in a similar manner as sulindac by oxidation of the sulfonyl group and indicate that the amide bond is sufficiently metabolically stable.
The human HT-29 colon tumor xenograft mouse model was used to evaluate in vivo antitumor efficacy of SSA, which provided important continuity between in vitro and in vivo testing. Using a protocol where SSA treatment was initiated on the day of tumor implantation, a twice a day dosing schedule was well-tolerated and resulted in >60% inhibition of tumor growth. Given that xenograft models involve malignant cells, these data suggest SSA or a related analogue has the potential to protect from malignant disease, although additional studies, for example, using the APCmin mouse model or chemically induced models of colon tumorigenesis are required to fully assess its potential for colon cancer chemoprevention. SSA was, however, ineffective if treatment was delayed until after tumors were established. Nonetheless, the chemotherapeutic drug, Camptosar, was also only marginally effective under these conditions and likely reflects the overall resistance of colorectal cancer to single agent chemotherapy. Interestingly, combined treatment of SSA and Camptosar caused a more sustained suppression of tumor growth compared with Camptosar treatment alone. Together, these data suggest that SSA may have safety and efficacy benefits over sulindac and possibly other NSAIDs and COX-2 inhibitors for colon cancer chemoprevention as well as potential for the treatment of colorectal cancer in combination with standard chemotherapy.
Acknowledgments
Grant support: National Cancer Institute grants CA128021 (G.A. Piazza) and CA131378 (G.A. Piazza and R. Reynolds).
We thank Joyce Harwell, Angela Jones, and Crystal Able for providing technical assistance for cell culture studies; Larry Ross and Lucile White for COX assays; Karen Gilbert for supervision of in vivo antitumor efficacy studies; and Meghan Knight for carefully proofreading this manuscript.
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Thun MJ, Henley SJ, Patrono C. Nonsteroidal anti-inflammatory drugs as anticancer agents: mechanistic, pharmacologic, and clinical issues. J Natl Cancer Inst. 2002;94:252–256. doi: 10.1093/jnci/94.4.252. [DOI] [PubMed] [Google Scholar]
- 2.Chan TA. Nonsteroidal anti-inflammatory drugs, apoptosis, and colon-cancer chemoprevention. Lancet Oncol. 2002;3:166–174. doi: 10.1016/s1470-2045(02)00680-0. [DOI] [PubMed] [Google Scholar]
- 3.Giardiello FM, Hamilton SR, Krush AJ. Treatment of colonic and rectal adenomas with sulindac in familial adenomatous polyposis. N Engl J Med. 1993;328:1313–1316. doi: 10.1056/NEJM199305063281805. [DOI] [PubMed] [Google Scholar]
- 4.Nugent KP, Farmer KC, Spigelman AD, Williams CB, Phillips RK. Randomized controlled trial of the effect of sulindac on duodenal and rectal polyposis and cell proliferation in patients with familial adenomatous polyposis. Br J Surg. 1993;80:1618–1619. doi: 10.1002/bjs.1800801244. [DOI] [PubMed] [Google Scholar]
- 5.Matsuhashi N, Nakajima A, Fukushima Y, Yazaki Y, Oka T. Effects of sulindac on sporadic colorectal adenomatous polyps. Gut. 1997;40:344–349. doi: 10.1136/gut.40.3.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matsuhashi N, Nakajima A, Shinohara K, Oka T, Yazaki Y. Rectal cancer after sulindac therapy for a sporadic adenomatous colonic polyp. Am J Gastroenterol. 1998;93:2261–2266. doi: 10.1111/j.1572-0241.1998.00630.x. [DOI] [PubMed] [Google Scholar]
- 7.Vane JR, Botting RM. Mechanism of action of antiinflammatory drugs. Int J Tissue React. 1998;20:3–15. [PubMed] [Google Scholar]
- 8.Vane JR, Bakhle YS, Botting RM. Cyclooxy-genases 1 and 2. Annu Rev Pharmacol Toxicol. 1998;38:97–120. doi: 10.1146/annurev.pharmtox.38.1.97. [DOI] [PubMed] [Google Scholar]
- 9.Mukherjee D. Selective cyclooxygenase-2 (COX-2) inhibitors and potential risk of cardiovascular events. Biochem Pharmacol. 2002;63:817–821. doi: 10.1016/s0006-2952(02)00842-0. [DOI] [PubMed] [Google Scholar]
- 10.Alberts DS, Hixson L, Ahnen D, et al. Do NSAIDs exert their colon cancer chemoprevention activities through the inhibition of mucosal prostaglandin synthetase? J Cell Biochem Suppl. 1995;22:18–23. doi: 10.1002/jcb.240590804. [DOI] [PubMed] [Google Scholar]
- 11.Piazza GA, Rahm AL, Krutzsch M, et al. Antineoplastic drugs sulindac sulfide and sulfone inhibit cell growth by inducing apoptosis. Cancer Res. 1995;55:3110–3116. [PubMed] [Google Scholar]
- 12.Hanif R, Pittas A, Feng Y, et al. Effects of nonsteroidal anti-inflammatory drugs on proliferation and on induction of apoptosis in colon cancer cells by a prostaglandin-independent pathway. Biochem Pharmacol. 1996;52:237–245. doi: 10.1016/0006-2952(96)00181-5. [DOI] [PubMed] [Google Scholar]
- 13.Elder DJ, Halton DE, Hague A, Paraskeva C. Induction of apoptotic cell death in human colorectal carcinoma cell lines by a cyclooxygenase-2 (COX-2)-selective nonsteroidal anti-inflammatory drug: independence from COX-2 protein expression. Clin Cancer Res. 1997;3 1679–8. [PubMed] [Google Scholar]
- 14.Piazza GA, Rahm AK, Finn TS, et al. Apoptosis primarily accounts for the growth-inhibitory properties of sulindac metabolites and involves a mechanism that is independent of cyclooxygenase inhibition, cell cycle arrest, and p53 induction. Cancer Res. 1997;57:2452–2459. [PubMed] [Google Scholar]
- 15.Rigas B, Shiff SJ. Is inhibition of cyclooxygenase required for the chemopreventive effect of NSAIDs in colon cancer? A model reconciling the current contradiction. Med Hypotheses. 2000;54:210–215. doi: 10.1054/mehy.1999.0023. [DOI] [PubMed] [Google Scholar]
- 16.Kashfi K, Rigas B. Non-COX-2 targets and cancer: expanding the molecular target repertoire of chemoprevention. Biochem Pharmacol. 2005;70:969–986. doi: 10.1016/j.bcp.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 17.Shureiqi I, Chen D, Lotan R, et al. 15-Lipoxygenase-1 mediates nonsteroidal anti-inflammatory drug-induced apoptosis independently of cyclooxygenase-2 in colon cancer cells. Cancer Res. 2000;60:6846–6850. [PubMed] [Google Scholar]
- 18.Herrmann C, Block C, Geisen C, et al. Sulindac sulfide inhibits Ras signaling. Oncogene. 1998;17:1769–1776. doi: 10.1038/sj.onc.1202085. [DOI] [PubMed] [Google Scholar]
- 19.He TC, Chan TA, Vogelstein B, Kinzler KW. PPARδ is an APC-regulated target of nonsteroidal anti-inflammatory drugs. Cell. 1999;99:335–345. doi: 10.1016/s0092-8674(00)81664-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yamamoto Y, Yin MJ, Lin KM, Gaynor RB. Sulindac inhibits activation of the NF-κB pathway. J Biol Chem. 1999;274:27307–27314. doi: 10.1074/jbc.274.38.27307. [DOI] [PubMed] [Google Scholar]
- 21.Zhu J, Huang JW, Tseng PH, et al. From the cyclooxygenase-2 inhibitor celecoxib to a novel class of 3-phosphoinositide-dependent protein kinase-1 inhibitors. Cancer Res. 2004;64:4309–4318. doi: 10.1158/0008-5472.CAN-03-4063. [DOI] [PubMed] [Google Scholar]
- 22.Thompson WJ, Piazza GA, Li H, et al. Exisulind induction of apoptosis involves guanosine 3′,5′-cyclic monophosphate phosphodiesterase inhibition, protein kinase G activation, and attenuated β-catenin. Cancer Res. 2000;60:3338–3342. [PubMed] [Google Scholar]
- 23.Zerbini LF, Czibere A, Wang Y, et al. A novel pathway involving melanoma differentiation associated gene-7/interleukin-24 mediates nonsteroidal anti-inflammatory drug-induced apoptosis and growth arrest of cancer cells. Cancer Res. 2006;66:11922–11931. doi: 10.1158/0008-5472.CAN-06-2068. [DOI] [PubMed] [Google Scholar]
- 24.Eling TE, Baek SJ, Shim M, Lee CH. NSAID activated gene (NAG-1), a modulator of tumorigenesis. J Biochem Mol Biol. 2006;39:649–655. doi: 10.5483/bmbrep.2006.39.6.649. [DOI] [PubMed] [Google Scholar]
- 25.Davies NM, Watson MS. Clinical pharmacokinetics of sulindac. A dynamic old drug. Clin Pharmacokinet. 1997;32:437–459. doi: 10.2165/00003088-199732060-00002. [DOI] [PubMed] [Google Scholar]
- 26.Brideau C, Kargman S, Liu S, et al. A human whole blood assay for clinical evaluation of biochemical efficacy of cyclooxygenase inhibitors. Inflamm Res. 1996;45:68–74. doi: 10.1007/BF02265118. [DOI] [PubMed] [Google Scholar]
- 27.Riendeau D, Charleson S, Cromlish W, Mancini JA, Wong E, Guay J. Comparison of the cyclooxygenase-1 inhibitory properties of nonsteroidal anti-inflammatory drugs (NSAIDs) and selective COX-2 inhibitors, using sensitive microsomal and platelet assays. Can J Physiol Pharmacol. 1997;75:1088–1095. [PubMed] [Google Scholar]
- 28.Duggan DE, Hooke KF, Noll RM, Hucker HB, Van Arman CG. Comparative disposition of sulindac and metabolites in five species. Biochem Pharmacol. 1978;27:2311–2320. doi: 10.1016/0006-2952(78)90137-5. [DOI] [PubMed] [Google Scholar]
- 29.Piazza GA, Alberts DS, Hixson LJ, et al. Sulindac sulfone inhibits azoxymethane-induced colon carcinogenesis in rats without reducing prostaglandin levels. Cancer Res. 1997;57:2909–2915. [PubMed] [Google Scholar]
- 30.Thompson HJ, Jiang C, Lu J, et al. Sulfone metabolite of sulindac inhibits mammary carcinogenesis. Cancer Res. 1997;57:267–271. [PubMed] [Google Scholar]
- 31.Thompson HJ, Briggs S, Paranka NS, et al. Inhibition of mammary carcinogenesis in rats by sulfone metabolite of sulindac. J Natl Cancer Inst. 1995;87:1259–1260. [PubMed] [Google Scholar]
- 32.Malkinson AM, Koski KM, Dwyer-Nield LD, et al. Inhibition of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone-induced mouse lung tumor formation by FGN-1 (sulindac sulfone) Carcinogenesis. 1998;19:1353–1356. doi: 10.1093/carcin/19.8.1353. [DOI] [PubMed] [Google Scholar]
- 33.Piazza GA, Thompson WJ, Pamukcu R, et al. Exisulind, a novel proapoptotic drug, inhibits rat urinary bladder tumorigenesis. Cancer Res. 2001;61:3961–3968. [PubMed] [Google Scholar]
- 34.Stoner GD, Budd GT, Ganapathi R, et al. Sulindac sulfone induced regression of rectal polyps in patients with familial adenomatous polyposis. Adv Exp Med Biol. 1999;470:45–53. doi: 10.1007/978-1-4615-4149-3_5. [DOI] [PubMed] [Google Scholar]
- 35.Arber N, Kuwada S, Leshno M, Sjodahl R, Hultcrantz R, Rex D. Sporadic adenomatous polyp regression with exisulind is effective but toxic: a randomised, double blind, placebo controlled, dose-response study. Gut. 2006;55:367–373. doi: 10.1136/gut.2004.061432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Plowman J, Dykes DJ, Hollingshead M, Simpson-Herren L, Alley MC. Anticancer Drug Development Guide: Preclinical Screening, Clinical Trials, and Approval. Totowa (NJ): Humana Press Inc.; Human Tumor Xenograft Models in NCI Drug Development; pp. 101–125. [Google Scholar]
- 37.Hixson LJ, Alberts DS, Krutzsch M, et al. Antiproliferative effect of nonsteroidal antiinflammatory drugs against human colon cancer cells. Cancer Epidemiol Biomarkers Prev. 1994;3:433–438. [PubMed] [Google Scholar]
- 38.Niv Y, Fraser GM. Adenocarcinoma in the rectal segment in familial polyposis coli is not prevented by sulindac therapy. Gastroenterology. 1994;107:854–857. doi: 10.1016/0016-5085(94)90136-8. [DOI] [PubMed] [Google Scholar]
- 39.Agarwal B, Swaroop P, Protiva P, Raj SV, Shirin H, Holt PR. Cox-2 is needed but not sufficient for apoptosis induced by Cox-2 selective inhibitors in colon cancer cells. Apoptosis. 2003;8:649–654. doi: 10.1023/A:1026199929747. [DOI] [PubMed] [Google Scholar]
- 40.Pyo H, Choy H, Amorino GP, et al. A selective cyclooxygenase-2 inhibitor, NS-398, enhances the effect of radiation in vitro and in vivo preferentially on the cells that express cyclooxygenase-2. Clin Cancer Res. 2001;7:2998–3005. [PubMed] [Google Scholar]
- 41.Llorens O, Perez JJ, Palomer A, Mauleon D. Differential binding mode of diverse cyclooxygenase inhibitors. J Mol Graph Model. 2002;20:359–371. doi: 10.1016/s1093-3263(01)00135-8. [DOI] [PubMed] [Google Scholar]
- 42.Kalgutkar AS, Marnett AB, Crews BC, Remmel RP, Marnett LJ. Ester and amide derivatives of the nonsteroidal antiinflammatory drug, indomethacin, as selective cyclooxygenase-2 inhibitors. J Med Chem. 2000;43:2860–2870. doi: 10.1021/jm000004e. [DOI] [PubMed] [Google Scholar]
- 43.Kalgutkar AS, Crews BC, Rowlinson SW, et al. Biochemically based design of cyclooxygenase-2 (COX-2) inhibitors: facile conversion of nonsteroidal antiinflammatory drugs to potent and highly selective COX-2 inhibitors. Proc Natl Acad Sci U S A. 2000;97:925–930. doi: 10.1073/pnas.97.2.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kalgutkar AS, Rowlinson SW, Crews BC, Marnett LJ. Amide derivatives of meclofenamic acid as selective cyclooxygenase-2 inhibitors. Bioorg Med Chem Lett. 2002;12:521–524. doi: 10.1016/s0960-894x(01)00792-2. [DOI] [PubMed] [Google Scholar]