Abstract
Vitamin K is a cofactor required for gamma-glutamyl carboxylation of several proteins regulating blood clotting, bone formation and soft tissue mineralization. Vitamin K3 is an important intermediate during conversion of the dietary vitamin K1 to the most abundant vitamin K2 form. It has been suggested that ABCC6 may have a role in transporting Vitamin K or its derivatives from the liver to the periphery. This activity is missing in pseudoxanthoma elasticum, a genetic disorder caused by mutations in ABCC6 characterized by abnormal soft tissue mineralization. Here we examined the efflux of the glutathione conjugate of vitamin K3 (VK3GS) from the liver in wild type and Abcc6−/− mice, and in transport assays in vitro. We found in liver perfusion experiments that VK3GS is secreted into the inferior vena cava, but we observed no significant difference between wild type and Abcc6−/− animals. We overexpressed the human ABCC6 transporter in Sf9 insect and MDCKII cells and assayed its Vitamin K3-conjugate transport activity in vitro. We found no measurable transport of VK3GS by ABCC6, whereas ABCC1 transported this compound at high rate in these assays. These results show that VK3GS is not the essential metabolite transported by ABCC6 from the liver and preventing the symptoms of pseudoxanthoma elasticum.
Keywords: Vitamin K metabolism, liver perfusion, conjugate transport, soft tissue calcification, pseudoxanthoma elasticum
INTRODUCTION
Mutations in the ABCC6 gene are responsible for the development of pseudoxanthoma elasticum (PXE, OMIM 26480) [1; 2; 3]. PXE is a multisystem disease characterized by ectopic mineralization of dermal, cardiovascular and ocular tissues (for recent reviews, see refs [4; 5; 6]). Carriers of one loss-of-function mutant ABCC6 allele have an increased risk of developing coronary artery disease [7; 8]. A fraction of beta-thalassemic patients develops PXE-like phenotype [9; 10] and this is most probably due to the down-regulation of ABCC6 [11]. A PXE-like phenotype can also develop in ABCC6 mutation carriers when they are carriers of mutations in the GGCX gene as well [12].
ABCC6 is an organic anion transporter[13; 14] predominantly present in the basolateral membrane of hepatocytes. Evidence that liver ABCC6 is essential and sufficient to prevent PXE came from transplantation studies in which skin grafts were exchanged between Abcc6−/− and Abcc6+/+ mice [15] as well as from a parabiotic mouse model [16]. These experiments show that PXE is a metabolic disease due to the absence of a substance that needs to be secreted from the liver by ABCC6 to prevent the development of mineral deposits in the peripheral soft tissues of PXE patients [5]. Unfortunately, the identity of this substance remains unknown.
An autosomal recessive syndrome with PXE-like cutaneous features is also associated with multiple coagulation factor deficiency caused by mutations in the GGCX gene [17; 18]. Since Vitamin K is an essential cofactor of gamma-glutamyl carboxylation, insufficient supply of peripheral Vitamin K may prevent the gamma-carboxylation of proteins, which are known to be required for counteracting calcification of connective tissue. Based on these observations, Borst and coworkers proposed that the ABCC6 substrate is Vitamin K or one of its derivatives [19]. An obvious candidate is VK3GS, since this vitamin K derivate is formed in many cell types including hepatocytes, and since ABCC6 is known to transport glutathione conjugates [13].
We have now tested VK3GS in several independent ABCC6-dependent transporter systems and find that it is not substrate of ABCC6.
MATERIALS AND METHODS
Liver perfusion with Vitamin K3 (VK3)
The Abcc6−/− mouse was developed by targeted inactivation of the Abcc6 gene and maintained as described [20]. Twelve month-old mice were used for in situ non-recirculating (ex vivo) liver perfusion experiments as described [21]. Mice were starved overnight, anaesthetized with an i.p. injection of ketamine/xylazine (2mg/0.3mg per g body weight) and fixed to an operation table. After laparatomy, the portal vein and the inferior vena cava were cannulated with 22-gauge and 24-gauge Teflon cannula, respectively. The blood in the liver was flushed out with 3 ml of 0.3% BSA/PBS at 37°C through the portal vein (in) and inferior vena cava (out), and the perfusate consisting of 0.5 μM VK3 in 0.3% BSA/PBS was infused into the liver at a constant flow rate of 3 ml/min. Three ml of perfusate was held in the liver after the inferior vena cava and the portal veins were ligated. Five minutes later, the efflux was collected via the inferior vena cava. Samples were kept at −80°C. For analysis 9 μl of 60% trichloroacetic acid was added to 100 μl of the efflux fluid for deproteination. After vigorous vortexing, the samples were centrifuged (13,000 g, 3 min), the supernatants were transferred into dark glass vials and kept at −20 °C for HPLC-MS analysis.
HPLC-MS determination of Vitamin K3 glutathione conjugate
Mass spectrometric measurements were run on an AB Sciex 3200 QTrap tandem mass spectrometer. The components were ionized in positive electrospray ionization (ESI) conditions. The instrument was operated in multiple reactions monitoring (MRM) mode. The MRM transition was 478/331 for VK3GS and 173/105 for VK3. The samples were separated prior to mass spectrometric analysis using a Perkin Elmer HPLC system. Mobile phases were: 10 mM ammonium-formate in water and acetonitrile with a flow rate of 0.3 ml/min. A Merck Purosphere Star column (C18, 55 × 2 mm, 3 μm particle size) was used for the separation. The oven temperature was kept at 40°C. Calibration curves were used for concentration determination; known amounts of VK3GS were added to 0.3% BSA/PBS prior extraction. Each quantitative determination was done in duplicates; the difference between the two parallels was always < 5%. The detection limit was 5 fmol (2.3 × 10−12 g), with a signal-to-noise ratio of > 5, which allowed us to analyze samples with concentrations higher than 0.5 nM.
Synthesis and purification of radiolabeled VK3GS conjugate the synthesis protocol described [22] was modified for micro-synthetic conditions. A 30 μl solution of 280 μM reduced glutathione (GSH) was prepared by mixing [3H]GSH (36.6 Ci/mmol) and GSH (Sigma) and rapidly added to 0.25 ml of 0.1M VK3, dissolved in ethanol. The reaction was incubated at 4°C overnight in silanized glass tubes. Crystals were vacuum dried and dissolved in 5% trifluoric acetate (TFA). [3H]VK3GS was purified by HPLC on a Teknokroma TR-011349 column (Nucleosil C18, 250x4.6 mm, pore size: 100Å, particle size: 5 μm) eluted with a water – acetonitrile gradient in 0.1% TFA. Crystals, recovered after lyophylization, were dissolved in 5%TFA and the chemical identity of the product was verified by HPLC-MS. Unlabeled VK3GS was synthesized as described previously [22], purified by crystallization and chloroform-washing, and its chemical identity was verified by HPLC-MS. VK3GS solutions were stored at −20°C in dark glass vials and used within 30 days.
Expression of human ABC proteins in Sf9 insect cells, membrane preparation, immunoblotting and vesicular transport
Expression of ABCC1 and ABCC6 in Sf9 insect cells [13; 23] and the isolation of the membrane fraction were done as described [24]. Protein content of the samples was determined with the modified Lowry method. Transport measurements were performed using the rapid filtration method and the transport activity was verified using [3H]LTC4 as substrate. ATP-dependent VK3GS uptake of the vesicles was calculated from rates measured in the presence of 4 mM MgAMP or 4 mM MgATP. Data represent the results of transport experiments performed with vesicles originating from two independent membrane preparations. The amount of inside-out vesicles in the different membrane preparation-samples was estimated by measuring the 45Ca2+ uptake mediated by endogenous Ca2+ transporters.
Cell cultures and vectorial transepithelial transport assays
MDCKII-control, MDCKII-ABCC1 [25] and MDCKII-ABCC6 [26; 27] cells were cultured as described. Transepithelial vectorial transport assays were performed essentially as described previously [27], with minor modifications. Three days before the start of the experiment 5 × 105 cells in 0.5 ml were seeded on microporous polycarbonate filters (Transwell 3415, Corning, Lowell, MA). Cells were grown for 3 days with medium replacements every day. Prior to the start of the experiment the complete cell culture medium was replaced with Hank’s buffered saline. The experiment was started by replacing Hank’s buffered saline on either the apical or basolateral side with 0.5 ml Hank’s buffered saline containing [3H]VK3 (0.09 μCi, 35 nM). After 5, 10, 30, 60 and 120 minutes 25 μl was taken from each compartment and the radioactivity was measured by liquid scintillation counting. Any radioactivity crossing the monolayer and appearing in the opposite compartment was noted as the fraction of total radioactivity added at the beginning of the experiment.
RESULTS AND DISCUSSION
Liver perfusion
First, we characterized glutathione conjugation of Vitamin K3 (VK3) in mouse liver. Livers of wild type (WT) and Abcc6−/− mice were perfused with 0.5 μM VK3 through the portal vein, and the outflow was collected via the inferior vena cava (corresponding to secretion toward the blood through the basolateral membranes of hepatocytes) and analyzed by HPLC-MS. We detected the glutathione-monoconjugate form (VK3GS) of VK3 in the outflow fluid only when VK3 was added to the perfusing fluid. Figure 1 shows that there was no significant difference between wt and Abcc6−/− mice in the VK3GS concentration of the inferior vena cava outflow. These experiments show that VK3GS is formed in the liver and secreted towards the blood circulation via basolateral transport, but the results suggest that ABCC6 does not play a role in this transport.
Figure 1. Liver perfusion of wild type and Abcc6−/− mice with Vitamin K3.
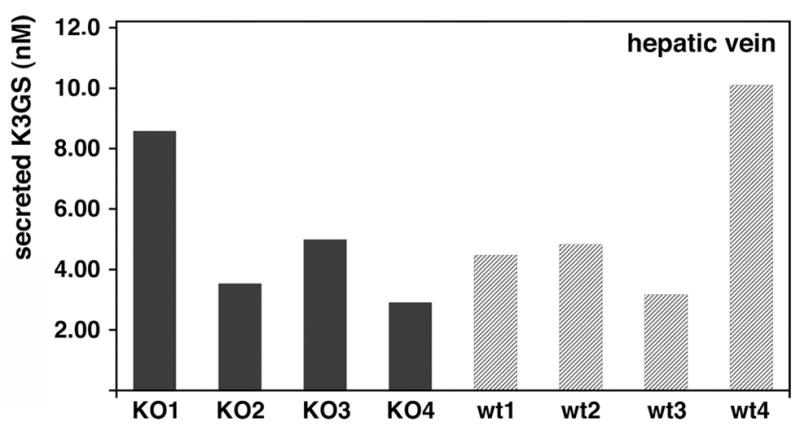
Livers were perfused through the portal vein with 0.5 μM VK3. The flow was stopped for 5 minutes, and after restoring the flow the outflow fluid from the inferior vena cava was collected, deproteinated and analyzed by HPLC-MS. Quantitative determination was done in duplicates; values are the concentration of the outflow fluid; the difference between two parallels was always < 5%. Each bar represents an individual animal. Wt refers to wild type, KO refers to Abcc6−/− mouse. There was no significant difference between the VK3GS concentrations of wt and Abcc6−/− mice (p=0.758; n=4).
Lack of transport of VK3GS by human ABCC6 expressed in Sf9 insect cells
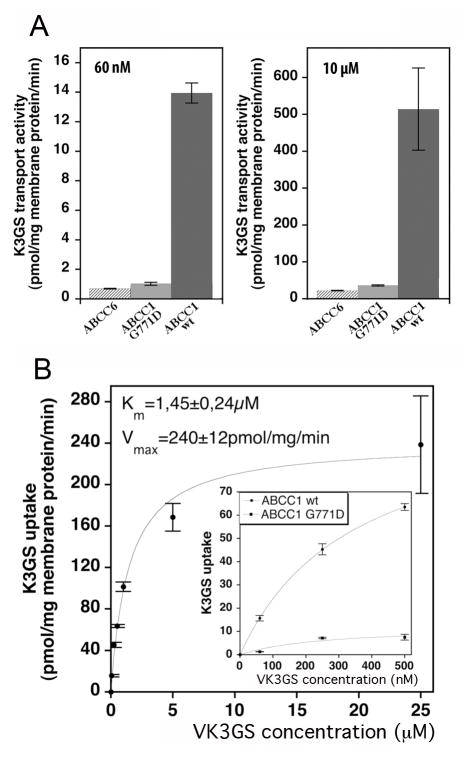
To investigate ABCC6-mediated VK3GS vesicular transport, we used the baculovirus/insect cell expression system, and radioactively labeled VK3GS generated by chemical conjugation of VK3 and [3H]GSH or [3H]VK3 and GSH (see Methods). Figure 2A shows the transport results obtained in the presence of 60 nM and 10 μM VK3GS. As controls we tested Sf9 vesicles overexpressing the human ABCC1 or an inactive mutant form of ABCC1 (G771D, see [28]). Whereas ABCC1 transported VK3GS at high rate, we were unable to detect uptake of radioactively labeled VK3GS into vesicles containing ABCC6 (even at 370C) at several concentrations and under multiple conditions (Fig. 2A). In addition, no inhibition of the LTC4 transport by ABCC6 was observed in the presence of VK3GS (up to 10 μM, not shown).
Figure 2. Transport of VK3GS by human ABCC6 and ABCC1 overexpressed in Sf9 insect cells.
ABCC1, ABCC1 G771D mutant and ABCC6 were expressed in Sf9 insect cells. Expression levels of the investigated transporters were validated by immunoblot and Coomassie Blue staining. Panel A: Results of transport experiments performed on vesicles from two independent membrane preparations are shown. Two concentrations, 60 nM and 10 μM, of VK3GS were used. Panel B: Concentration dependence of VK3GS uptake by ABCC1 expressing Sf9 insect vesicles. The rate of transport was measured at 23°C for 0.5 min. The transport rate of the G771D inactive mutant (◆) compared to wild type (●) at 60, 250 or 500 nM concentrations is shown in the insert. The kinetic constants of transport are also indicated.
The VK3GS transport of ABCC1 was essentially linear up to 1 min (not shown), and this allowed us to determine the kinetic parameters of transport at 0.5 min. The Michaelis constant was about 1500 nM and the maximal transport rate about 240 pmol VK3GS/mg of membrane protein/min at 23°C using our standard membrane preparations (Figure 2B). The results with the inactive ABCC1 mutant are representative of the background activity (Figure 2B inset).
Transepithelial transport using MDCKII cell monolayers
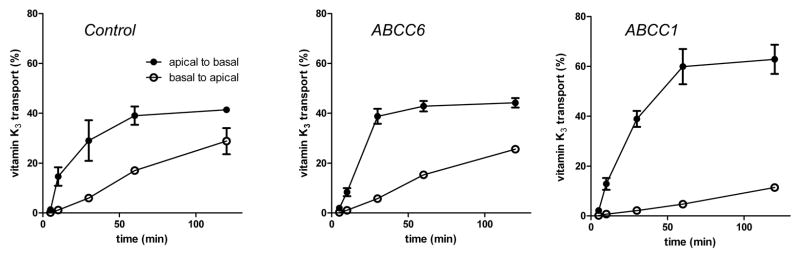
Cells were loaded with radiolabeled VK3 either from the apical or from the basal side and the radioactivity effluxed was recorded on the opposite side (Figure 3). The efflux from the MDCKII-ABCC6 cells was similar to that of the MDCKII control cells indicating no contribution of ABCC6 to the transport of this glutathione conjugate. In contrast, MDCKII – ABCC1 cells showed the expected increase in apical-to-basal efflux relative to MDCKII control cells. The control cells effluxed more radioactivity towards the basolateral compartment as well (Figure 3). We attribute this to the presence of endogenous canine ABCC1.
Figure 3. Vectorial transport of Vitamin K3 derivatives from MDCKII cells overexpressing human ABCC6 or ABCC1.
MDCKII cells were loaded with radiolabeled VK3 either from the apical or from the basal side and the radioactivity due to efflux was recorded on the opposite side and plotted as percent of the total radioactivity included into the experiment.
Our conclusion that ABCC6 is not a VK3GS transporter is in line with our recent data, showing that intravenous administration of VK3GS does not alter the degree of mineralization in the PXE (Abcc6−/−) mouse [29].
We found no indication that ABCC6 can transport VK3GS, but this compound is readily transported by ABCC1. Whether ABCC1 is physiologically responsible for the basolateral efflux of VK3GS from the liver remains to be determined, as the level of ABCC1 in the liver is low [30] and ABCC1 appears to be undetectable in normal hepatocytes [31]. We have tested other VK-related compounds as potential substrates for ABCC6, but without success (unpublished results). We have therefore started to look for compounds missing in plasma of PXE patients and Abcc6−/− mice by an untargeted metabolomics approach.
Highlights.
The substrate of ABCC6 preventing soft tissue calcification is not known.
It was proposed that Vitamin K3 glutathione conjugate is the physiological substrate.
We tested VK3GS transport by several assay systems.
We found that this conjugate is not a substrate of ABCC6.
Vitamin K3 glutathione conjugate may not prevent pseudoxanthoma-related symptoms.
Acknowledgments
This study was supported by NIH/NIAMS grants R01 AR28450, R01 AR55225, and K08 AR57099, by the Hungarian research grants (from the National Research Foundation, OTKA) OTKA CK 80135, OTKA NK 81204, OTKA PD 79183, by PXE International Inc., and by a Top grant (40-00812-98-07-028) of ZonMw to KvdW and PB. T. A. is a recipient of Bolyai Fellowship of the Hungarian Academy of Sciences.
ABBREVIATIONS
- ABC
ATP Binding Cassette
- GGCX
gamma-glutamyl carboxylase enzyme
- LTC4
Leukotriene C4
- MRM
multiple reaction monitoring
- PXE
pseudoxanthoma elasticum
- VK1, VK2 and VK3
Vitamin K1, 2 and 3, respectively
- VK3GS
glutathione-mono-conjugate of Vitamin K3
- WT
wild type
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bergen AA, Plomp AS, Schuurman EJ, Terry S, Breuning M, Dauwerse H, Swart J, Kool M, van Soest S, Baas F, ten Brink JB, de Jong PT. Mutations in ABCC6 cause pseudoxanthoma elasticum. Nat Genet. 2000;25:228–31. doi: 10.1038/76109. [DOI] [PubMed] [Google Scholar]
- 2.Le Saux O, Urban Z, Tschuch C, Csiszar K, Bacchelli B, Quaglino D, Pasquali-Ronchetti I, Pope FM, Richards A, Terry S, Bercovitch L, de Paepe A, Boyd CD. Mutations in a gene encoding an ABC transporter cause pseudoxanthoma elasticum. Nat Genet. 2000;25:223–7. doi: 10.1038/76102. [DOI] [PubMed] [Google Scholar]
- 3.Ringpfeil F, Lebwohl MG, Christiano AM, Uitto J. Pseudoxanthoma elasticum: mutations in the MRP6 gene encoding a transmembrane ATP-binding cassette (ABC) transporter. Proc Natl Acad Sci U S A. 2000;97:6001–6. doi: 10.1073/pnas.100041297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li Q, Jiang Q, Pfendner E, Varadi A, Uitto J. Pseudoxanthoma elasticum: clinical phenotypes, molecular genetics and putative pathomechanisms. Exp Dermatol. 2009;18:1–11. doi: 10.1111/j.1600-0625.2008.00795.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Uitto J, Li Q, Jiang Q. Pseudoxanthoma elasticum: molecular genetics and putative pathomechanisms. J Invest Dermatol. 2010;130:661–70. doi: 10.1038/jid.2009.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Varadi A, Szabo Z, Pomozi V, de Boussac H, Fulop K, Aranyi T. ABCC6 as a target in pseudoxanthoma elasticum. Curr Drug Targets. 2011;12:671–82. doi: 10.2174/138945011795378612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koblos G, Andrikovics H, Prohaszka Z, Tordai A, Varadi A, Aranyi T. The R1141X loss-of-function mutation of the ABCC6 gene is a strong genetic risk factor for coronary artery disease. Genet Test Mol Biomarkers. 2010;14:75–8. doi: 10.1089/gtmb.2009.0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Trip MD, Smulders YM, Wegman JJ, Hu X, Boer JM, ten Brink JB, Zwinderman AH, Kastelein JJ, Feskens EJ, Bergen AA. Frequent mutation in the ABCC6 gene (R1141X) is associated with a strong increase in the prevalence of coronary artery disease. Circulation. 2002;106:773–5. doi: 10.1161/01.cir.0000028420.27813.c0. [DOI] [PubMed] [Google Scholar]
- 9.Hamlin N, Beck K, Bacchelli B, Cianciulli P, Pasquali-Ronchetti I, Le Saux O. Acquired Pseudoxanthoma elasticum-like syndrome in beta-thalassaemia patients. Br J Haematol. 2003;122:852–4. doi: 10.1046/j.1365-2141.2003.04484.x. [DOI] [PubMed] [Google Scholar]
- 10.Aessopos A, Floudas CS, Kati M, Tsironi M, Giakoumi X, Livir-Rallatos C, Farmakis D. Loss of vision associated with angioid streaks in beta-thalassemia intermedia. Int J Hematol. 2008;87:35–8. doi: 10.1007/s12185-007-0014-y. [DOI] [PubMed] [Google Scholar]
- 11.Martin L, Douet V, VanWart CM, Heller MB, Le Saux O. A mouse model of beta-thalassemia shows a liver-specific down-regulation of Abcc6 expression. Am J Pathol. 2011;178:774–83. doi: 10.1016/j.ajpath.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li Q, Grange DK, Armstrong NL, Whelan AJ, Hurley MY, Rishavy MA, Hallgren KW, Berkner KL, Schurgers LJ, Jiang Q, Uitto J. Mutations in the GGCX and ABCC6 genes in a family with pseudoxanthoma elasticum-like phenotypes. J Invest Dermatol. 2009;129:553–63. doi: 10.1038/jid.2008.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ilias A, Urban Z, Seidl TL, Le Saux O, Sinko E, Boyd CD, Sarkadi B, Varadi A. Loss of ATP-dependent transport activity in pseudoxanthoma elasticum-associated mutants of human ABCC6 (MRP6) J Biol Chem. 2002;277:16860–7. doi: 10.1074/jbc.M110918200. [DOI] [PubMed] [Google Scholar]
- 14.Belinsky MG, Chen ZS, Shchaveleva I, Zeng H, Kruh GD. Characterization of the drug resistance and transport properties of multidrug resistance protein 6 (MRP6, ABCC6) Cancer Res. 2002;62:6172–7. [PubMed] [Google Scholar]
- 15.Jiang Q, Endo M, Dibra F, Wang K, Uitto J. Pseudoxanthoma elasticum is a metabolic disease. J Invest Dermatol. 2009;129:348–54. doi: 10.1038/jid.2008.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jiang Q, Oldenburg R, Otsuru S, Grand-Pierre AE, Horwitz EM, Uitto J. Parabiotic heterogenetic pairing of Abcc6−/−/Rag1−/− mice and their wild-type counterparts halts ectopic mineralization in a murine model of pseudoxanthoma elasticum. Am J Pathol. 2010;176:1855–62. doi: 10.2353/ajpath.2010.090983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vanakker OM, Martin L, Gheduzzi D, Leroy BP, Loeys BL, Guerci VI, Matthys D, Terry SF, Coucke PJ, Pasquali-Ronchetti I, De Paepe A. Pseudoxanthoma elasticum-like phenotype with cutis laxa and multiple coagulation factor deficiency represents a separate genetic entity. J Invest Dermatol. 2007;127:581–7. doi: 10.1038/sj.jid.5700610. [DOI] [PubMed] [Google Scholar]
- 18.Li Q, Schurgers LJ, Smith AC, Tsokos M, Uitto J, Cowen EW. Coexistent pseudoxanthoma elasticum and vitamin K-dependent coagulation factor deficiency: compound heterozygosity for mutations in the GGCX gene. Am J Pathol. 2009;174:534–40. doi: 10.2353/ajpath.2009.080865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Borst P, van de Wetering K, Schlingemann R. Does the absence of ABCC6 (multidrug resistance protein 6) in patients with Pseudoxanthoma elasticum prevent the liver from providing sufficient vitamin K to the periphery? Cell Cycle. 2008;7:1575–9. doi: 10.4161/cc.7.11.6005. [DOI] [PubMed] [Google Scholar]
- 20.Klement JF, Matsuzaki Y, Jiang QJ, Terlizzi J, Choi HY, Fujimoto N, Li K, Pulkkinen L, Birk DE, Sundberg JP, Uitto J. Targeted ablation of the abcc6 gene results in ectopic mineralization of connective tissues. Mol Cell Biol. 2005;25:8299–310. doi: 10.1128/MCB.25.18.8299-8310.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hartung T, Leist M, Tiegs G, Baschong W, Wendel A. Solcoseryl prevents inflammatory and hypoxic but not toxic liver damage in rodents. Inflammapharmacology. 1991;1:49–60. [Google Scholar]
- 22.Chang M, Shi M, Forman HJ. Exogenous glutathione protects endothelial cells from menadione toxicity. Am J Physiol. 1992;262:L637–43. doi: 10.1152/ajplung.1992.262.5.L637. [DOI] [PubMed] [Google Scholar]
- 23.Bakos E, Hegedus T, Hollo Z, Welker E, Tusnady GE, Zaman GJ, Flens MJ, Varadi A, Sarkadi B. Membrane topology and glycosylation of the human multidrug resistance-associated protein. J Biol Chem. 1996;271:12322–6. doi: 10.1074/jbc.271.21.12322. [DOI] [PubMed] [Google Scholar]
- 24.Sarkadi B, Price EM, Boucher RC, Germann UA, Scarborough GA. Expression of the human multidrug resistance cDNA in insect cells generates a high activity drug-stimulated membrane ATPase. J Biol Chem. 1992;267:4854–8. [PubMed] [Google Scholar]
- 25.Bakos E, Evers R, Szakacs G, Tusnady GE, Welker E, Szabo K, de Haas M, van Deemter L, Borst P, Varadi A, Sarkadi B. Functional multidrug resistance protein (MRP1) lacking the N-terminal transmembrane domain. J Biol Chem. 1998;273:32167–75. doi: 10.1074/jbc.273.48.32167. [DOI] [PubMed] [Google Scholar]
- 26.Sinko E, Ilias A, Ujhelly O, Homolya L, Scheffer GL, Bergen AA, Sarkadi B, Varadi A. Subcellular localization and N-glycosylation of human ABCC6, expressed in MDCKII cells. Biochem Biophys Res Commun. 2003;308:263–9. doi: 10.1016/s0006-291x(03)01349-4. [DOI] [PubMed] [Google Scholar]
- 27.Pavek P, Merino G, Wagenaar E, Bolscher E, Novotna M, Jonker JW, Schinkel AH. Human breast cancer resistance protein: interactions with steroid drugs, hormones, the dietary carcinogen 2-amino-1-methyl-6-phenylimidazo(4,5-b)pyridine, and transport of cimetidine. J Pharmacol Exp Ther. 2005;312:144–52. doi: 10.1124/jpet.104.073916. [DOI] [PubMed] [Google Scholar]
- 28.Szentpetery Z, Kern A, Liliom K, Sarkadi B, Varadi A, Bakos E. The role of the conserved glycines of ATP-binding cassette signature motifs of MRP1 in the communication between the substrate-binding site and the catalytic centers. J Biol Chem. 2004;279:41670–8. doi: 10.1074/jbc.M406484200. [DOI] [PubMed] [Google Scholar]
- 29.Jiang Q, Li Q, Grand-Pierre AE, Schurgers LJ, Uitto J. Administration of vitamin K does not counteract the ectopic mineralization of connective tissues in Abcc6 (−/−) mice, a model for pseudoxanthoma elasticum. Cell Cycle. 2011;10:701–7. doi: 10.4161/cc.10.4.14862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roelofsen H, Vos TA, Schippers IJ, Kuipers F, Koning H, Moshage H, Jansen PL, Muller M. Increased levels of the multidrug resistance protein in lateral membranes of proliferating hepatocyte-derived cells. Gastroenterology. 1997;112:511–21. doi: 10.1053/gast.1997.v112.pm9024305. [DOI] [PubMed] [Google Scholar]
- 31.Hannivoort RA, Dunning S, Vander Borght S, Schroyen B, Woudenberg J, Oakley F, Buist-Homan M, van den Heuvel FA, Geuken M, Geerts A, Roskams T, Faber KN, Moshage H. Multidrug resistance-associated proteins are crucial for the viability of activated rat hepatic stellate cells. Hepatology. 2008;48:624–34. doi: 10.1002/hep.22346. [DOI] [PubMed] [Google Scholar]