Abstract
Vascular access stenosis is a major complication in hemodialysis patients. We prospectively observed 50 patients in whom 50 nitinol shape-memory alloy-recoverable technology (SMART) stents were used as salvage therapy for recurrent peripheral venous stenosis. Twenty-five stents each were deployed in native arteriovenous fistula (AVF) and synthetic arteriovenous polyurethane graft (AVG) cases. Vascular access patency rates were calculated by Kaplan-Meier analysis. The primary patency rates in AVF versus AVG at 3, 6, and 12 months were 80.3% versus 75.6%, 64.9% versus 28.3%, and 32.3% versus 18.9%, respectively. The secondary patency rates in AVF versus AVG at 3, 6, and 12 months were 88.5% versus 75.5%, 82.6% versus 61.8%, and 74.4% versus 61.8%, respectively. Although there were no statistically significant difference in patency between AVF and AVG, AVG showed poor tendency in primary and secondary patency. The usefulness of SMART stents was limited in a short period of time in hemodialysis patients with recurrent vascular access stenosis.
1. Introduction
Although percutaneous transluminal angioplasty (PTA) is the standard for treatment of vascular access venous stenosis and occlusion since the 1980s [1], it carries a high rate of restenosis, and repeated endovascular intervention is often necessary. Compared with native arteriovenous fistula (AVF), synthetic arteriovenous grafts (AVG) are associated with much higher rates of failure and intervention because of the increased rates of stenosis/thrombosis. The intervention rates for AVG are reported to be about five times higher than those for AVF, with patency rates less than 50% at 3 years [2]. To improve these statistics, various indications for the use of endovascular stents have been studied since 1989 [3], including elastic recoil [4–6], rapid recurrence [7], and venous rupture after PTA [8].
Although endovascular stent placement is one of the standard treatments in percutaneous coronary and peripheral artery disease, its role in the treatment of vascular access stenosis remains controversial. Early studies reported that routine use of metallic stents failed to provide any additional benefit when compared with PTA alone [3]. Multiple studies have compared endovascular stents to PTA in terms of patency, but most of references reported limited or no advantages to a stent placement for peripheral venous and graft stenoses [2, 4, 9–13]. Cohort study conducted by Vogel and Parise reported that nitinol shape-memory alloy-recoverable technology (SMART) stents improved primary and secondary graft patency in AVG cases [14]. Recent publications have reported that covered stents or stent-graft placement was not inferior in patency to PTA alone [15–17]. Therefore, the exact role and indications of stent placement in the treatment of stenotic lesions in AVF and AVG remain unclear.
In this study, we prospectively observed 50 patients in whom 50 SMART stents were used as salvage therapy for recurrent peripheral venous stenosis and compared patency between AVF and AVG in cases of post-PTA failure caused by elastic recoil and rapidly recurrent stenosis.
2. Patients and Methods
2.1. Study Population
This study was approved by the institutional ethical committee of Oyokyo Kidney Research Institute. Informed consent was obtained from all patients. From June 2009 to September 2010, 548 patients underwent hemodialysis at the Oyokyo Kidney Research Institute in Hirosaki, Japan. During that period, total 453 PTAs were performed and all endovascular procedures were performed by the well-trained interventional urologists in this institute. We followed up consecutive 50 patients who underwent SMART stent placement as salvage therapy for recurrent peripheral venous stenosis. Twenty-five stents each were deployed in native AVF and synthetic AVG.
2.2. Inclusion and Exclusion Criteria
Inclusion criteria were (1) age of 18 to 90 yr and a hemodialysis access consisting of an AVF or an AVG located in the arm, (2) stable hemodialysis sessions were performed, (3) color Doppler ultrasonography or angiographic evidence of 1 or more stenotic lesion, 7 cm or less in length, and 50% or more stenosis compared with previous evaluation, (4) difficult vascular access; percutaneous endovascular therapy was thought to be the best treatment choice for the identified lesion because it is difficult to develop new vascular accesses in other lesions, (5) recoil and/or kinked venous stenosis within the past 3 months, (6) more than 3 time of PTA history, (7) Eastern Cooperative Oncology Group Performance Status (ECOG PS) [18] grade 0 to 4. In this study, poor general health (ECOG PS > 2), major concomitant disease (e.g., terminal cancer), or other medical condition likely to result in death within 6 months after the time of implantation were not in exclusion criteria. Exclusion criteria were (1) recurrent stenosis with a corresponding thrombosis treated within 7 days before enrollment, (2) a blood coagulation disorder or sepsis, (3) a contraindication to the use of contrast medium, (4) infected arteriovenous access graft, (5) presence of an alternate stent, (6) stenotic lesion which needed more than 6 mm diameter stent in upper-arm outflow vein or subclavian vein, and (7) occluded vascular access.
2.3. Technical Description
AVF and AVG were initially cannulated with a 16–18-gauge puncture needle, and a 6-French catheter sheath was inserted over a guide wire into the lumen of AVF or AVG. Diluted contrast media was injected for digital subtraction angiography to localize stenotic lesions. An angioplasty balloon (Conquest, 6 mm in diameter, 25–30 atmospheres in pressure; BARD Inc., Murray Hill, NJ, USA or LUMEFA, 6 mm in diameter, 18 atmospheres in pressure; Toray Medical Co., Chiba, Japan) was placed and inflated at the level of the stenotic site. If severe elastic recoil or significant residual stenosis was observed or if the stenosis had recurred shortly after a previous intervention, a SMART stent (SMART Control; Cordis/Johnson & Johnson, Warren, NJ, USA) that was 6 mm in diameter and 40–80 mm in length was deployed. All patients received 3000–4000 U of heparin during the procedure. Antiplatelet agents remained unchanged in all patients before and after the intervention.
2.4. Follow-Up Protocol and Intervention Indications
For blood access followup, peripheral vascular stenosis was monitored by color Doppler ultrasonography every 1–3 months. Catheter-based interventions were performed in patients who met the clinical criteria for vascular access dysfunction and had stenosis of more than 50%.
2.5. Evaluation
Primary (unassisted) and secondary (assisted) patency was calculated from the date of stent placement to the first subsequent intervention and permanent blood access failure. Primary and secondary patency rates were calculated using the Kaplan-Meier method and log-rank test.
2.6. Statistical Analysis
Statistical analyses were performed using SPSS (SPSS Inc., ver. 12.0, Chicago, IL, USA) and Microsoft Excel (Microsoft Co., Redmond, WA, USA) programs. All values included in the figures and text are expressed as means ± SD. Datasets were compared using the Mann-Whitney's U test or a paired t test. A P value of less than 0.05 was considered to be significant.
3. Results
Fifty SMART stents were deployed in cases of dysfunctional blood access for salvage therapy in 50 patients. The median number of follow-up days after SMART stent deployment was 290. Patient characteristics are shown in Table 1. Twenty-five SMART stents each were deployed in AVF and AVG cases. No difference was observed in patient backgrounds. Stent location was significantly different between AVF and AVG cases because major stenotic lesions were located in the lower arm in AVF cases and in the upper arm in AVG cases.
Table 1.
Patient characteristics. Twenty-five SMART stents each were deployed in AVF and AVG cases. There was no significant difference, other than stent location, between the backgrounds of the patients in the two groups.
| ALL | AVF | AVG | P value | |
|---|---|---|---|---|
| Number of patients | 50 | 25 | 25 | |
| Number of stents | 50 | 25 | 25 | |
| Age | 71.6 ± 11.3 | 71.2 ± 11.3 | 72.2 ± 11.6 | n.s. |
| Gender (M/F) | 24/27 | 13/12 | 10/15 | n.s. |
| Primary renal disease | ||||
| DM | 24 | 12 | 12 | n.s. |
| non DM | 26 | 13 | 13 | |
| Hemodialysis history (years) | 7.3 ± 6.1 | 7.8 ± 6.2 | 6.6 ± 5.9 | n.s. |
| PTA history (times) | 4.6 ± 3.9 | 4.5 ± 3.5 | 4.8 ± 4.3 | n.s. |
| Difficult vascular access* | 50 | 25 | 25 | |
| Poor general health** (%) | 35 (70%) | 18 (72%) | 17 (68%) | n.s. |
| Use of antiplatelet agents | 49 | 24 | 25 | n.s. |
| Stent location | ||||
| Upper arm | 22 | 4 | 18 | 0.0002 |
| Lower arm | 28 | 21 | 7 |
*Difficult vascular access; percutaneous endovascular therapy thought to have been the best treatment choice for the identified lesion because it is difficult to develop new vascular accesses in other lesions. **Poor general health; Eastern Cooperative Oncology Group Performance Status grade 3 or 4.
To determine the efficacy of SMART stent placement for AVF and AVG, primary patency rates were evaluated using the Kaplan-Meier method. The primary patency ratios for AVF versus AVG at 3, 6, and 12 months were 80.3% versus 75.6%, 64.9% versus 28.3%, and 32.3% versus 18.9%, respectively (Table 2). The 50% access patency times were 230 days in AVF cases and 133 days in AVG cases. There was no significant difference between the efficacy of SMART stent placement in AVF and AVG cases (P = 0.1010), but inferior in AVG at 6 and 12 months (64.9% versus 28.3% and 32.3% versus 18.9%, resp.,) (Table 2, Figure 1).
Table 2.
Primary patency for SMART stent placement in AVF and AVG cases. There was no significant difference in primary patency rates between AVF and AVG cases, but patency in AVG showed inferior to AVF.
| All | AVF | AVG | P value | |
|---|---|---|---|---|
| n | 50 | 25 | 25 | |
| Primary patency (Days) | 140 ± 105 | 168 ± 118 | 110 ± 78.9 | 0.0051 |
| (range) | (17–401) | (17–401) | (38–378) | |
| Primary patency (%) | ||||
| 3 months | 79 | 80.3 | 75.6 | 0.1010 |
| 6 months | 51.3 | 64.9 | 28.3 | |
| 12 months | 27.1 | 32.3 | 18.9 |
Figure 1.
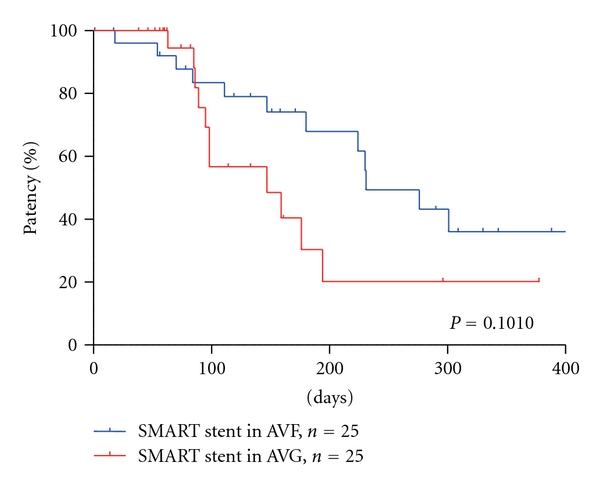
Primary patency rates in AVF and AVG. No significant difference was observed in the primary patency rates between AVF and AVG, but patency in AVG showed inferior to AVF.
The secondary patency rates for SMART stents at 3, 6, and 12 months were 83.4%, 74.2%, and 68.0%, respectively (Table 3). Restenosis after stent placement occurred in 23 patients (46%). The reasons for primary patency failure were in-stent stenosis (14/23, 61%), outflow stenosis (4/23, 17%), and stenosis unrelated to stent placement (5/23, 22%). The ratio of outflow stenosis onset was significantly higher in AVF cases (P = 0.045). The secondary patency ratios of patients with SMART stents did not significantly differ between AVF and AVG (P = 0.1299), but inferior in AVG at 3, 6, and 12 month (88.5% versus 75.5%, 82.6% versus 61.8%, and 74.4% versus 61.8%, resp.,) (Table 3, Figure 2).
Table 3.
Secondary patency for SMART stent placement in AVF and AVG cases.There was no significant difference in secondary patency rates between AVF and AVG cases, but patency in AVG showed inferior to AVF. The rate of outflow stenosis onset was significantly higher in AVF cases (P = 0.045).
| All | AVF | AVG | P value | |
|---|---|---|---|---|
| n | 50 | 25 | 25 | |
| Secondary patency (Days) | 189 ± 129 | 224 ± 129 | 151 ± 121 | n.s. |
| (range) | (18–259) | (18–241) | (63–259) | |
| Secondary patency (%) | ||||
| 3 months | 83.4 | 88.5 | 75.5 | |
| 6 months | 74.2 | 82.6 | 61.8 | 0.1299 |
| 12 months | 68 | 74.4 | 61.8 | |
| Reasons of primary patency failure | ||||
| In-stent stenosis (%) | 14 (28) | 6 (24) | 8 (32) | n.s. |
| Out-flow stenosis (%) | 4 (8) | 4 (16) | 0 (0) | 0.045 |
| Others (%) | 5 (10) | 2 (8) | 3 (12) | n.s. |
Figure 2.
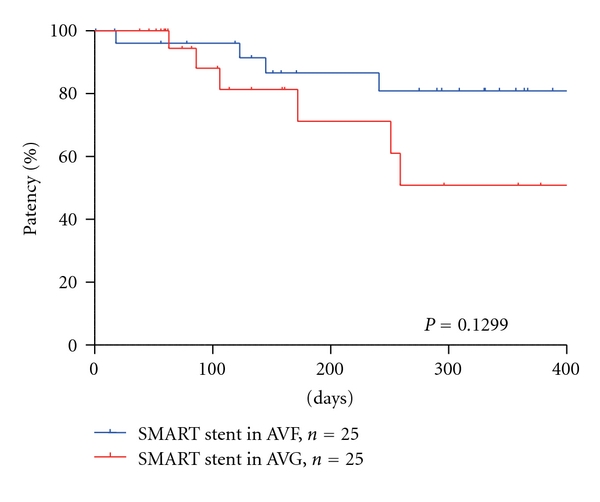
Secondary patency rates of SMART stent placement in AVF and AVG. There was no significant difference in the secondary patency rates between AVF and AVG, but patency in AVG showed inferior to AVF.
Complications associated with SMART stent deployment were not observed. Episodes of symptomatic arterial embolization of thrombus, signs of pulmonary embolism, or stent infection during followup were not observed.
4. Discussion
The maintenance of vascular access in hemodialysis patients is critical to the quality of life and survival. However, high rates of restenosis and repeat endovascular intervention associated with PTA remain problematic issues. Multiple devices and techniques such as cutting balloon angioplasty [19], metallic stents [12, 14], and more recently stent grafts or covered stents [15] have been used. Metallic stents have been used for blood access for more than 20 years [20], but the effect of metallic stents in peripheral vascular access is still controversial. Although there is some evidence that metallic stents have the potential to alleviate rapidly recurring peripheral venous stenosis [7], use of metallic stents (Wallstent) was not recommended because a prospective, randomized trial showed no advantage of its use over conventional angioplasty [11].
On the other hand, some studies supported the efficacy of metallic stent (SMART) placement for salvage angioplasty. A SMART stent is composed of nitinol, a nickel-titanium metallic alloy with shape memory function. The advantages of a SMART stent over conventional metallic stents (Wallstent) include a high degree of strength and superelasticity. Nitinol possesses a high degree of flexibility and kink and fatigue resistance; these properties are not affected by repeated interventions. In addition, its superelasticity ensures equal distribution of wall contact and confers the ability to adapt to native vessel contours more successfully than conventional stents. Vogel and Parise reported improved performance using the nitinol stent, demonstrating the efficacy of SMART stent placement in retrospective [12] and prospective, nonrandomized studies [14] for dysfunctional AVG salvage therapy. In previous reports, the 3-, 6-, and 12-month patency rates of metallic stents were 77–88%, 51–67%, and 20–41%, respectively (Table 4).
Table 4.
Summary of recent reports of outcome using metallic stents. The 3-, 6-, and 12-month patency rates were 77–88%, 51–67%, and 20–41%, respectively.
| Investigators | Year | n | Study design | Stent type | AVF or AVG | Primary patency (%) | (months) | ||
|---|---|---|---|---|---|---|---|---|---|
| 3 M | 6 M | 12 M | |||||||
| Vogel and Parise [12] | 2004 | 53 | Retrospective | SMART | AVG | 77 (61–93) | 51 (34–67) | 20 (12–27) | mean 8.9 |
| Vogel and Parise [14] | 2005 | 25 | Prospective, Non-randomized | SMART | AVG | 88 (75–100) | 67 (48–86) | 41 (21–61) | mean 8.2 |
| Pan et al. [21] | 2005 | 12 | Retrospective | Wallstent, Jostent | AVF | 92 ± 8 | 81 ± 12 | 31 ± 17 | n/a |
| Liang et al. [22] | 2006 | 23 | Observational | Wallstent, nitinol | AVG | 69 ± 9 | 41 ± 10 | 30 ± 10 | n/a |
| Maya and Allon [23] | 2006 | 14 | Prospective, Non-randomized | Wallstent, SMART, Protégé, Fluency | AVG | 48 | 19 | n/a | median 2.8 |
| Chan, M.R. et al. [24] | 2008 | 211 | Retrospective | SMART | AVG | 69 | 25 | n/a | median 4.4 |
| Current study | 2011 | 50 | Prospective, Observational | SMART | Both | 79 ± 9 | 51 ± 15 | 27 ± 16 | median 3.8 |
| 25 | AVF | 80 ± 10 | 65 ± 16 | 32 ± 21 | median 5.2 | ||||
| 25 | AVG | 76 ± 15 | 28 ± 22 | 19 ± 17 | median 2.9 | ||||
Our results showed that there was no statistical different in patency of AVF and AVG, but AVG showed poor tendency in primary and secondary patency. Incidents of out-flow stenosis were significantly higher in AVF, and in-stent stenosis were significantly higher in AVG. Out-flow stenoses in AVF might be caused by longer out-flow vein of forearm AVF, secondary to hemodynamic change or selection bias because of the small number of patients. In-stent stenoses in AVG were mainly caused by ingrowth of neointimal hyperplastic tissue through the mesh of the metallic stent at the venous-graft anastomotic site. These data suggest a prevention of in-growth tissue in SMART stent has potential to improve poor patency in AVG. To further improve patency and reduce the incidence of luminal hyperplasia, several authors have explored the use of the stent-graft, which is a self-expanding nitinol stent covered in carbon-impregnated expanded polytetrafluoroethylene. The use of stent-grafts or covered stents appears to be logical to prevent ingrowth of neointimal hyperplastic tissue. Haskal et al. [15] performed a randomized, prospective, multicenter trial involving 190 patients and clearly indicated noninferiority extending the patency of AVG cases at 6 months. Further studies are necessary to determine appropriate indications for the use of stents in AVG with rapid recurrence.
In this present study, salvage SMART stent placement provides similar primary patency in previous reports [12, 14, 21–24] (Table 4). The main reason of insufficient patency was that the patients in this study were selected because they were basically PTA failures with either elastic lesions or rapid recurrences. Therefore, a use of SMART stent is only in selective patients, in whom without stent placement, the vascular access will be restenotic or abandoned immediately or in a very short time. But after stent placement, the patency may be the same or only slightly better than that of regular cases [4, 10, 11]. These results suggest that there were no or limited clinical advantage of routine use of bare metallic stent over angioplasty only, although stent graft had revealed some clinical benefits. However, our results have certain limitations such as small sample size, a nonrandomized trail performed at a single institute, without appropriate control group. Large randomized, comparative studies are necessary to confirm the usefulness and appropriate indications stent placement in hemodialysis patients.
5. Conclusion
SMART stent placement was a safe for salvage angioplasty in treating recurrent peripheral vascular stenosis, but the usefulness of SMART stents was limited in a short period of time despite our efforts to improve patency. Larger studies are required to determine appropriate indications.
Conflict of Interests
The authors declare no financial conflict of interests.
Acknowledgments
The authors thank Mrs. Saori Araya for the invaluable assistance provided for the original data. They are grateful to the entire staff of the Oyokyo Kidney Research Institute for their support. This study was supported by the grants-in-aid for Scientific Research 23791737 from the Japan Society for the Promotion of Science.
Abbreviation
- PTA:
Percutaneous transluminal angioplasty,
- SMART:
Shape memory alloy recoverable technology
- AVF:
Arteriovenous fistula
- AVG:
Arteriovenous graft
- DM:
Diabetes mellitus.
References
- 1.Noveline RA. Percutaneous transluminal angioplasty: newer applications. American Journal of Roentgenology. 1980;135(5):983–988. doi: 10.2214/ajr.135.5.983. [DOI] [PubMed] [Google Scholar]
- 2.White JJ, Bander SJ, Schwab SJ, et al. Is percutaneous transluminal angioplasty an effective intervention for arteriovenous graft stenosis? Seminars in Dialysis. 2005;18(3):190–202. doi: 10.1111/j.1525-139X.2005.18307.x. [DOI] [PubMed] [Google Scholar]
- 3.Gunther RW, Vorwerk D, Klose KC, et al. Self-expanding stents for the treatment of a long venous stenosis in a dialysis shunt: case report. CardioVascular and Interventional Radiology. 1989;12(1):29–31. doi: 10.1007/BF02577122. [DOI] [PubMed] [Google Scholar]
- 4.Patel RI, Peck SH, Cooper SG, et al. Patency of Wallstents placed across the venous anastomosis of hemodialysis grafts after percutaneous recanalization. Radiology. 1998;209(2):365–370. doi: 10.1148/radiology.209.2.9807560. [DOI] [PubMed] [Google Scholar]
- 5.Kolakowski S, Dougherty MJ, Calligaro KD. Salvaging prosthetic dialysis fistulas with stents: forearm versus upper arm grafts. Journal of Vascular Surgery. 2003;38(4):719–723. doi: 10.1016/s0741-5214(03)00947-9. [DOI] [PubMed] [Google Scholar]
- 6.Gray RJ, Horton KM, Dolmatch BL, et al. Use of Wallstents for hemodialysis access-related venous stenoses and occlusions untreatable with balloon angioplasty. Radiology. 1995;195(2):479–484. doi: 10.1148/radiology.195.2.7724770. [DOI] [PubMed] [Google Scholar]
- 7.Turmel-Rodrigues LA, Blanchard D, Pengloan J, et al. Wallstents and Craggstents in hemodialysis grafts and fistulas: results for selective indications. Journal of Vascular and Interventional Radiology. 1997;8(6):975–982. doi: 10.1016/s1051-0443(97)70697-3. [DOI] [PubMed] [Google Scholar]
- 8.Rajan DK, Clark TWI. Patency of Wallstents placed at the venous anastomosis of dialysis grafts for salvage of angioplasty-induced rupture. CardioVascular and Interventional Radiology. 2003;26(3):242–245. doi: 10.1007/s00270-003-2706-x. [DOI] [PubMed] [Google Scholar]
- 9.Beathard GA. Gianturco self-expanding stent in the treatment of stenosis in dialysis access grafts. Kidney International. 1993;43(4):872–877. doi: 10.1038/ki.1993.122. [DOI] [PubMed] [Google Scholar]
- 10.Quinn SF, Schuman ES, Demlow TA, et al. Percutaneous transluminal angioplasty versus endovascular stent placement in the treatment of venous stenoses in patients undergoing hemodialysis: intermediate results. Journal of Vascular and Interventional Radiology. 1995;6(6):851–855. doi: 10.1016/s1051-0443(95)71200-3. [DOI] [PubMed] [Google Scholar]
- 11.Hoffer EK, Sultan S, Herskowitz MM, Daniels ID, Sclafani SJA. Prospective randomized trial of a metallic intravascular stent in hemodialysis graft maintenance. Journal of Vascular and Interventional Radiology. 1997;8(6):965–973. doi: 10.1016/s1051-0443(97)70695-x. [DOI] [PubMed] [Google Scholar]
- 12.Vogel PM, Parise C. SMART stent for salvage of hemodialysis access grafts. Journal of Vascular and Interventional Radiology. 2004;15(10):1051–1060. doi: 10.1097/01.RVI.0000129915.48500.DC. [DOI] [PubMed] [Google Scholar]
- 13.Clark TWI. Nitinol stents in hemodialysis access. Journal of Vascular and Interventional Radiology. 2004;15(10):1037–1040. doi: 10.1097/01.RVI.0000136029.88286.48. [DOI] [PubMed] [Google Scholar]
- 14.Vogel PM, Parise C. Comparison of SMART stent placement for arteriovenous graft salvage versus successful graft PTA. Journal of Vascular and Interventional Radiology. 2005;16(12):1619–1626. doi: 10.1097/01.RVI.0000179792.23867.01. [DOI] [PubMed] [Google Scholar]
- 15.Haskal ZJ, Trerotola S, Dolmatch B, et al. Stent graft versus balloon angioplasty for failing dialysis-access grafts. New England Journal of Medicine. 2010;362(6):494–503. doi: 10.1056/NEJMoa0902045. [DOI] [PubMed] [Google Scholar]
- 16.Bent CL, Rajan DK, Tan K, et al. Effectiveness of Stent-graft Placement for Salvage of Dysfunctional Arteriovenous Hemodialysis Fistulas. Journal of Vascular and Interventional Radiology. 2010;21(4):496–502. doi: 10.1016/j.jvir.2009.12.395. [DOI] [PubMed] [Google Scholar]
- 17.Dolmatch BL. Stent graft versus balloon angioplasty for failing dialysis access grafts: a long-awaited advance in the treatment of permanent hemodialysis access. Journal of Vascular Access. 2010;11(2):89–91. doi: 10.1177/112972981001100201. [DOI] [PubMed] [Google Scholar]
- 18.Oken MM, Creech RH, Davis TE. Toxicology and response criteria of the Eastern Cooperative Oncology Group. American Journal of Clinical Oncology. 1982;5(6):649–655. [PubMed] [Google Scholar]
- 19.Vesely TM, Siegel JB. Use of the peripheral cutting balloon to treat hemodialysis-related stenoses. Journal of Vascular and Interventional Radiology. 2005;16(12):1593–1603. doi: 10.1097/01.RVI.0000190928.19701.DD. [DOI] [PubMed] [Google Scholar]
- 20.Vorwerk D, Guenther RW, Mann H, et al. Venous stenosis and occlusion in hemodialysis shunts: follow-up results of stent placement in 65 patients. Radiology. 1995;195(1):140–146. doi: 10.1148/radiology.195.1.7892456. [DOI] [PubMed] [Google Scholar]
- 21.Pan HB, Liang HL, Lin YH, et al. Metallic stent placement for treating peripheral outflow lesions in native arteriovenous fistula hemodialysis patients after insufficient balloon dilatation. American Journal of Roentgenology. 2005;184(2):403–409. doi: 10.2214/ajr.184.2.01840403. [DOI] [PubMed] [Google Scholar]
- 22.Liang HL, Pan HB, Lin YH, et al. Metallic stent placement in hemodialysis graft patients after insufficient balloon dilation. Korean Journal of Radiology. 2006;7(2):118–124. doi: 10.3348/kjr.2006.7.2.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maya ID, Allon M. Outcomes of thrombosed arteriovenous grafts: comparison of stents vs angioplasty. Kidney International. 2006;69(5):934–937. doi: 10.1038/sj.ki.5000214. [DOI] [PubMed] [Google Scholar]
- 24.Chan MR, Bedi S, Sanchez RJ, et al. Stent placement versus angioplasty improves patency of arteriovenous grafts and blood flow of arteriovenous fistulae. Clinical Journal of the American Society of Nephrology. 2008;3(3):699–705. doi: 10.2215/CJN.04831107. [DOI] [PMC free article] [PubMed] [Google Scholar]


