Abstract
Background
A head-to-head comparison of the 72-week and 48-week anti-HCV therapies in slow responders with genotype 1 infection has been performed in several randomized clinical trials (RCTs).
Objectives
This review aimed at summarizing and pooling the results of these studies.
Materials and Methods
RCTs that had evaluated the 72-week vs. 48-week anti-HCV therapy (peginterferon and ribavirin) in slow responders with HCV genotype 1 infection were systematically identified. A meta-analysis was performed using the random effects model. Heterogeneity in results was assessed on the basis of the Q statistics, and publication bias was evaluated by using Harbord’s modified test. The end point was set as a sustained virological response (SVR).
Results
Data of 1206 subjects were retrieved from 7 studies. A total of 631 patients had received extended therapy. Slow virological responders who received the 72-week therapy had a significantly higher probability of achieving SVR than their counterpartswho received the 48-week therapy [RR = 1.44 (95% CI, 1.20–1.73)]. With regard to publication biases, the heterogeneity in funnel plots was not significant (P = 0.19, I2 = 30%, PHarbord = 0.1).
Conclusion
Our meta-analysis showed that the 72-week therapy with peginterferon and ibavirin is significantly superior to the standard 48-week therapy in slow responders th HCV genotype 1 infection.
Keywords: Hepatitis C virus, Genotype, Treatments
1. Background
Hepatitis C virus (HCV) infection is a major cause of chronic liver disease, cirrhosis, and hepatocellular carcinoma (HCC) worldwide, and may necessitate liver transplant in the patients with these diseases [1][2][3]. In 80% of the cases with HCV infection, the infection progresses to a chronic state, and in approximately 20% of the cases, chronic HCV infection may lead to cirrhosis [4]. Achievement of a sustained virological response (SVR) to antiviral therapy prevents the progression of fibrosis and cirrhosis and decreases the risk of HCC, thereby improving the survival rate of patients. Therefore, antiviral therapy is an important treatment option in the clinical management of these patients [5][6][7][8][9].
Currently, the standard therapy for HCV genotype 1 and genotype 2/3 is administration of regimens of peginterferon alpha and ribavirin for 48 weeks and 24 weeks, respectively. Patients with rapid virological response (RVR; undetectable HCV-RNA level at week 4 of therapy) have 80–100% likelihood of achieving SVR, whereas those who do not achieve an early virological response (EVR; undetectable HCV-RNA level at week 12 of therapy or less than 2-log decrease in RNA level compared to a pretreatment RNA level) have only an 8% chance of achieving SVR [10][11]. Since viral kinetics play an important role during therapy, several studies have attempted to evaluate individualized anti-HCV therapy on the basis of the patients’ virological response instead of HCV genotype alone. In rapid virological responders with HCV genotype 2/3 infection, therapy for a duration shorter than that of the standard therapy was as effective as the standard therapy; however, in cases of HCV genotype 1 infection, different results have been obtained [12][13]. Several studies have evaluated the effects of extending the therapy in slow responders.
2. Objectives
In this review, we aimed to summarize and pool the results of these studies to determine the optimal duration of treatment for HCV genotype 1 infection in slow responders.
3. Materials and Methods
3.1. Search Methods for Identification of Studies
We performed an electronic search of Medline, EMBASE, Scopus, Cochrane Central Register of Controlled Trials, and ISI Web of Knowledge (SV Tabatabaei, B Behnava). The keywords we used were different combinations of “hepatitis C virus” or “HCV” with the following terms: “slow responders”; “72-week”; “extended therapy”; “rapid virological response” or “RVR”; “early virological response” or “EVR”; “peginterferon alpha-2a”; “peginterferon alpha- 2b”; and “ribavirin” or “RVB” or “RBV”. In addition, terms such as “Pegasys,” “Pegintron,” “Rebetol,” and “Copegus” were used. We examined the references cited in the reviewed papers to find other relevant studies. Temporal limits were not applied in our search strategy.
3.2. Data Collection and Analysis
All citations were imported to an EndNote library. Further, the titles and abstracts were screened by 2 investigators (SV Tabatabaei, B Behnava) who were unaware of each other’s study selection. Full texts of all the selected reports were retrieved and assessed according to our predefined inclusion and exclusion criteria. Data from studies that met our criteria were extracted by 2 investigators separately and rechecked by a third investigator. Data of the outcomes of treatment were tabulated according to the treatment regimen in Excel spreadsheets. Predefined assumptions and the decision to include or exclude a study were made and agreed by all authors before the meta-analysis. Data on the characteristics of patients and studies were summarized by using standard questionnaires that included the first author’s name, name of journal, methodology of randomization, allocation concealment, publication year, and sample size in each treatment arm as well as viral loads, liver histologies, frequencies of genotypes, and SVR.
3.3. End Point of Interest
The end point for comparison of efficacy was SVR, defined as undetectable HCV-RNA level 6 months after treatment cessation.
3.4. Inclusion and Exclusion Criteria
Randomized clinical trials (RCTs) on patients with chronic HCV infection who were seronegative for HIV and hepatitis B virus (HBV) infection were included if the patients in the trials [1] were randomized to receive either peginterferon alpha-2a 180 μg/kg per week or peginterferon alpha-2b 1.5 μg/kg per week plus weightbased ribavirin during either the standard (48 weeks) or extended therapy (72 weeks), [2] had detectable HCVRNA level at week 4 of the treatment, [3] achieved at least a 2-log decrease in HCV-RNA levels from the baseline to 12 weeks or undetectable HCV-RNA at 24 weeks, [4] had genotype 1 infection, and [5] were diagnosed with chronic HCV infection on the basis of detectable HCV-RNA level and infection for at least 6 months. We included articles from all languages that met these criteria. Furthermore, our meta-analysis included studies that had patients with a history of treatment and for whom study dose modification and administration of growth factors and antidepressants were performed. Even the studies that included patients with infections caused by other HCV genotypes or both rapid and slow responders were included if they provided enough data for reanalysis of the data for the subset of slow responders with HCV genotype 1 infection. Studies were excluded if the patients: [1] had decompensated liver disease, [2] were seropositive for HIV or HBV markers and, [3] had significant co-morbidities such as decompensated liver disease, autoimmune diseases, hemoglobinopathies, and chronic kidney disease, [4] received low-dose ribavirin or peginterferon during some parts of their treatment period, and [5] had negative PCR findings at week 4 of treatment.
3.5. Methodological Quality Assessment of RCTs
The methodological quality, defined as the confidence that the design and reporting of a trail will restrict the possibility of a bias in intervention comparison, was evaluated as previously reported [14]. The only difference was that the blinding of patients or investigators was not feasible in the current study because of the different treatment durations. Allocation sequence generation and concealment were obtained as measures of bias control. The allocation sequence generation was considered adequate if it was obtained through a tableor computer-generated random numbers. The allocation concealment was considered adequate if the patients were randomized by a central independent unit or by using serially numberedopaque sealed envelopes.
3.6. Data Synthesis
All analyses were performed using the Mix 2.0 professional software for meta-analysis with Excel [15]. Data on all patients were included on the basis of intention-to-treat principle, irrespective of compliance or follow-up. To manage the missing data, we performed a worst case scenario analysis. However, since we had a positive outcome (virological response), all missing data were considered as data from the non-responders. The results are presented as relative risk ratio (RR) with 95% confidence interval (CI). The meta-analysis was performed using the DerSimonian and Laird random effects model. The random effects model provides a highly conservative estimate of significance. This model operates under the assumption that the included studies are only a random sample of all studies. Hence, heterogeneity between individual studies will result in a wider CI of the summary estimate. The summary estimate was calculated as an average of the individual study results weighted by the inverse of their variance by using the DerSimonian and Laird random effects model [16]. The estimate of heterogeneity was obtained from Q statistics. The study findings were considered heterogeneous if the resultant P-value was less than 0.1 [17]. Furthermore, I2 was used to provide a measure of the degree of inconsistency between the results of the studies. Its value describes the percentage of total variation across studies that is caused by heterogeneity rather than chance. The I2 value lies between 0% and 100%. A value of 0% indicates no observed heterogeneity, whereas larger values indicate increasing heterogeneity [18]. Publication bias was assessed by testing for funnel plot asymmetry by using Harbord’s modified test. Several statistical tests can be used to evaluate funnel plot asymmetry. However, among these tests, a regression-based approach is most appropriate for detecting significant publication bias. For analyzing binary outcomes, the Harbord’s modified test is preferred over Egger’s test because Harbord’s modified test has a lower likelihood of showing false-positive results due to the mathematical association between logRR and its standard error [19].
3.7. Transparency Declarations
The authors declare that there are no conflicts of interest, financial or otherwise regarding the contents of this review. Furthermore, this meta-analysis was not supported by any pharmaceutical company, government agency, or grants from other sources.
4. Results
4.1. Results of the Search
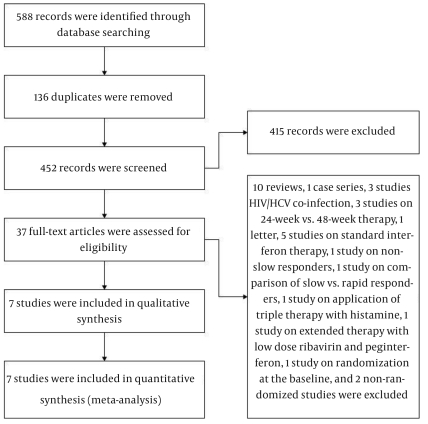
Our search strategy yielded 452 unique citations. Figure 1 shows our search analysis protocol. We discarded 415 nonrelevant records and retrieved full texts of 37 potentially relevant papers. We excluded: 10 reviews regarding the optimal treatment of infections caused by different HCV genotypes [20][21][22][23][24][25][26][27][28][29], 1 case series with 9 subjects [30], 3 studies that evaluated 24- vs. 48-week treatment of HCV genotype 1 infection in patients showing RVR [12][31][32], 1 study that compared rapid responders with slow responders [33]. Furthermore, we excluded 1 letter [34], 5 studies on long-term therapy with standard interferon [35][36][37][38][39], 3 studies on HCV/HIV co-infected patients [40][41][42], 2 non-randomized studies [43][44], 1 study in which patients in the extended therapy group were treated with low dose peginterferon or ribavirin [45]. We also excluded 1 study on slow responders with HCV genotype 2/3 infection, 1 study that treated the slow responders by administration of triple therapy with histamine as a part of or throughout the subjects’ treatment duration [46], and 1 study with patients randomized at the baseline but not according to RVR and EVR [47]. Ultimately, 7 studies that met our criteria were included [48][49][50][51][52][53][54].
Figure 1.
Search Result Analysis
4.2. Included Studies
One study was from Japan, 2 from Spain, and one from each of Austria, the US, Italy, and Germany. All studies were published as full texts in peer-reviewed journals from 2005 to 2010. One study, by Sanchez-Tapias et al., was partially randomized. Methodological quality factorsincluding random sequence generation and allocation concealment were not provided in the reports by Ferenci et al., Miyase et al., and Berg et al. One report by Miyase et al. was in Japanese. We excluded some patients from the relevant studies. From the study reported by Ferenci et al., 262 subjects were excluded because they showed RVR or because of the lack of randomization in the study for the duration of therapy, and 28 subjects were excluded because of infection with HCV genotype 4. 35 subjects with non-genotype 1 infection and 184 with RVR were excluded from the Sanchez-Tapias study and 185 subjects with RVR and 128 patients who were not slow responders were excluded from the study reported by Mangia et al. From the study reported by Berg et al. 81 subjects were excluded because they showed RVR and 168 were excluded because of lack of information about whether they were slow responders. Ultimately, 1206 subjects, 631 of whom received extended therapy, were included in the meta-analysis. SVR was similarly defined across all studies as the lack of RVR along with achieving an undetectable PCR or at least a 2-log decrement in the HCV-RNA levels from baseline after 12 weeks or undetectable HCV-RNA levels after 24 weeks. Table 1 shows some characteristics of the included studies. All trials had similar inclusion criteria. Chronic hepatitis C was diagnosed on the basis of the presence of HCV-RNA in the blood, elevated plasma transaminase levels for at least 6 months, and evidence of chronic viral hepatitis on performing a pretreatment liver biopsy. The exclusion criteria were also very similar in all the trials and consisted of decompensated liver disease; autoimmune mediated diseases; chronic hepatitis B; significant co-morbidities such as HIV, kidney disease, cardiovascular disease, psychiatric illnesses, or history of hospitalization for major depression; poorly controlled diabetes; prior organ transplantation; seizures or brain injury requiring medication for stabilization; or hematological diseases with anemia, low platelet count, or neutropenia. Pregnant or breast-feeding women were also excluded from the study.
Table 1. Characteristics and Results of Methodological Quality Assessment of the Included Studies.
| Authors | Origin ofSamples | Randomization | Allocation Concealment | Naive | Type of Peginterferon |
| Mangia et al., 2008, [48] | Italy | Adequate | Adequate | Yes | 2a/2b |
| Berg et al., 2006, [49] | Germany | Unclear | Unclear | Yes | 2a |
| Ferenci et al., 2010, [50] | Austria | Unclear | Unclear | Yes | 2a |
| Pearlman et al., 2007, [51] | US | Adequate | Adequate | Yes | 2b |
| Sanchez-Tapias et al., 2006, [52] | Spain | partially | Inadequate | Yes | 2a |
| Miyase et al., 2010, [53] | Japan | Unclear | unclear | NR a | 2b |
| Buti et al., 2010, [54] | Spain | Adequate | Adequate | Yes | 2b |
a Abbreviation: NR, not reported
4.3. Characteristics of the Patients
Patients’ characteristics are presented in Table 2. Miyase et al. reported their patients’ baseline data according to the virological responses but not according to the treatment groups. Sanchez-Tapias et al. did not report the percentages of patients with extensive fibrosis (stage F3/F4), and Pearlman did not report the patients’ baseline levels of liver enzymes. In the study by Mangia et al. 44% of the total studied patients were excluded, so the data provided by the authors could not be considered representative of the characteristics of the included patients.
Table 2. Characteristics of the Patients in the Included Studiesa.
| Authors | Men, % | Age, y | ALT b, IU/L | Viral load, IU/mL | Cirrhosis, F3/F4, % | |||||
| 72 wk | 48 wk | 72 wk | 48 wk | 72 wk | 48 wk | 72 wk | 48 wk | 72 wk | 48 wk | |
| Ferenci et al. | 65 | 64 | 44.3 ± 10.2 c | 45.1 ± 10.6 c | 93.3 ± 62.7 c | 91.9 ± 74.7 c | 700 (K d) | 650 (K) | 19 | 20 |
| Pearlman et al. | 65 | 67 | 54 | 56 | NR b | NR | 5400 (K) | 5300 (K) | 25 | 27 |
| Nagaki et al. | 67 | 62 | 54 | 62 | 52 | 64 | ≥ 1500 (K) 67% | ≥ 1500 (K) 77% | 77 | 62 |
| Sanchez-Tapias et al. | 63 | 69 | 43.2 ± 10.2 c | 42.8 ± 9.9 c | 2.7 ± 1.6 c ULN b | 2.4 ±1.3 c ULN | 1110 ± 1333 c (K) | 963 ± 1153 c (K) | NR | NR |
| Buti et al. | 63 | 60 | 46.5 ± 11.6 c | 44.5 ± 9.9 c | 85 ± 71 c | 76 ± 48 c | > 800 (K) 93.2% | > 800 (K) 87.2% | NR | NR |
| Miyase et al. | 33 | 58 | 83 | 2243 (K) | NR | |||||
a In the study by Mangia et al. 44% of the patients were excluded, so the data provided by authors could not be considered representative for the characteristics of the included patients.
b Abbreviations: ALT, Antiretroviral therapy; NR, Not reported; ULN, Upper limit of normal
c Value ± SD
d K: 103
4.4. Comparison of SVR Achieved during the 72-week Therapy with that Achieved with the Standard 48-week Therapy
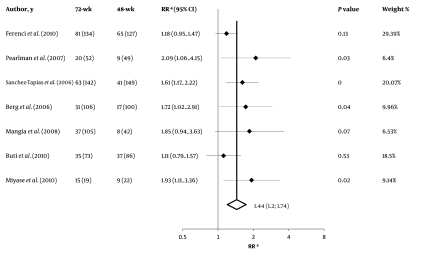
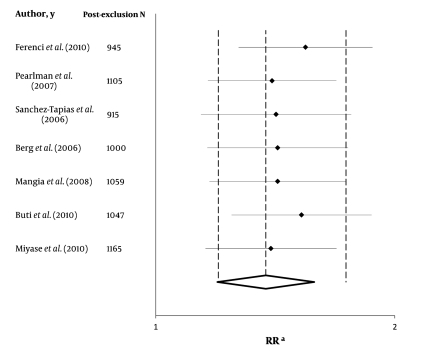
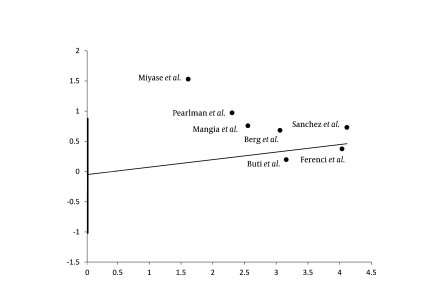
The slow virological responders who received the 72-week anti-HCV therapy had a significantly higher likelihood of achieving SVR than their counterparts who received the standard 48-week anti-HCV therapy [RR = 1.44 (95% CI 1.20–1.74)]. The heterogeneity in funnel plots was not significant (P = 0.19), and the actual heterogeneity between study results that could not be justified by chance alone was 30% (I2). Furthermore, Tau-squared value was 0.01 (95% CI, 0–0.09). Figure 2 shows summary estimates with their 95% CI for each study along with the weighted-pooled estimate. Figure 3 shows results of the exclusion sensitivity analysis. The pooled result was evidently robust and did not rely on any single study result. The assessment of publication biases by using Harbord’s modified test showed a non-significant P value (P = 0.11). Moreover, the P value for Egger’s test was 0.59. Figure 4 shows Harbord’s regression line.
Figure 2.
Summary Estimate with 95% Confidence Interval for Relative Risk (RR) of Sustained Virological Responses (SVR) Rates Achieved with 72- vs. 48-week Treatment of Hepatitis C Virus (HCV) Infection with Peginterferon and Weight-Based Ribavirin
Figure 3.
Exclusion Sensitivity Plot
Figure 4.
Harbord’s Regression Line for Publication Bias Assessment
4.5. Comparison of the Safety of the 72-week Therapy with that of the Standard 48-week Therapy
The information about the safety profiles of the 72week therapy vs. the standard 48-week therapy was not well elucidated. Table 3 shows the pooled estimate of comparative safety and number of patients included in the analyses. Due to the low number of patients and lack of data on adequate reporting of safety, the evidence on the comparative safety of the 72-week and 48-week therapies is not robust. Although none of the differences in the individual adverse effects reached statistical significance, the number of patients who discontinued the therapy due to safety concerns or voluntarily was considerably greater among the patients who received the 72-week therapy.
5. Discussion
In this systematic review, we assessed the comparative efficacy of anti-HCV therapy with peginterferon alpha and weight-based ribavirin for durations of 72 weeks and 48 weeks in slow virological responders with HCV genotype 1 infection who were seronegative for HIV and HBV co-infections. Our results suggest that the 72-week treatment helped in achieving a significantly higher SVR rate than the 48-week treatment in slow virological responders with HCV genotype 1 infection. Because of the high mean viral load of the included patients, our findings can easily be extrapolated to the slow virological responders with high viral load of HCV genotype 1 or hard to treat HCV infections. Although the differences among any individual adverse effects were not statistically significant, voluntary treatment discontinuation or discontinuation because of safety reasons was significantly greater in the 72-week treatment group. This finding can be attributed to longer or severer adverse effects in the extended therapy group. The SVR rate achieved with the 72-week therapy can be increased by encouraging the patients to complete the 72-week therapy, closely following-up the patients’ condition, and reducing the treatment discontinuations by managing the adverse effects.
We are highly confident of the effectiveness of our finding for the following reasons: [1] we considered nonsignificant heterogeneity test results despite clinical heterogeneity across studies including (a) different treatment protocols for ribavirin therapy, (b) inclusion of some patients with previous history of treatment, (c) different peginterferon type, and (d) different ethnicity of the subjects; [2] we found robust results that were confirmed by exclusion sensitivity analysis, which showed that the final estimate was not dependent on any single study results (Figure 4); and [3] we performed non-significant publication assessment. The empirical evidence suggests that trials that fail to refute the null hypothesis have low odds of being published, especially those that are not funded by the industry [55][56]. Our publication bias assessment did not revealed such finding. Trim-and-fill method used to account for the missing data showed results similar to the findings of our original pooled estimate [RR = 1.29 (95% CI 1.13–1.47)] [4]. Of seven included studies, 6 studies had complete and 1 had partial randomized design. Moreover, the robust results of our sensitivity analysis suggest that both naive and treated patients can benefit from the extended therapy.
The greatest limitation in this review was insufficient reporting of safety data and methodological qualities of the RCTs. Therefore, we recommend that investigators of future trials adhere to the Consolidated Standards for Reporting of Trials (CONSORT) to improve the quality of trial reports [57]. Another limitation of our work was that the long-term clinical advantage of extended anti- HCV treatment for HCC, decompensated liver disease, and cirrhosis could not be clarified. Furthermore, there are some concerns about the validity of our findings outside of a clinical trial setting. Our analysis showed that the 72-week treatment course is difficult to tolerate for most patients and there is a high likelihood of premature withdrawal. The odds ratio of 2.7 for voluntary therapy discontinuation in Table 3 supports this perspective. Therefore, in routine clinical practice and outside of a clinical trial setting, this rate of treatment discontinuation can become even higher to an extent that can reduce the rate of SVR. Low external validity of the findings of this review cannot be attributed to the reliability of our findings, but to the nature of the clinical trials included in the review. We suggest that making decision to extend the treatment course should be made on an individually basis and according to the patients’ tolerance during the first 48 weeks of therapy. Although the number of patients who discontinued the treatment because of safety reasons or personal causes was high in the extended therapy group, our meta-analysis showed that the 72-week therapy with peginterferon and ribavirin is significantly superior to the standard 48-week therapy for slow responders with genotype 1 infection.
Table 3. Comparative Safety Profile of the 72-Week and 48-Week Anti-HCV Therapies with Peginterferon and Ribavirin.
| Adverse event | Patients, No. | OR (95% CI) | Heterogeneity Assessment | ||
| Q (K) | P | I 2 | |||
| Neutropenia/leukopenia | 1571 | 1 (0.71–1.41) | 1.34 (5) | 0.85 | 0% |
| Thrombocytopenia | 326 | 0.61 (0.29–1.25) | |||
| Fever | 485 | 1.03 (0.69–1.54) | 0 (1) | 0.96 | 0% |
| Asthenia | 485 | 0.96 (0.66–1.38) | 0.06 (1) | 0.81 | 0% |
| Headache | 485 | 1.06 (0.72–1.56) | 0.21 (1) | 0.65 | 0% |
| Flu-like symptoms | 485 | 0.89 (0.51–1.56) | 1.85 (1) | 0.17 | 46% |
| Anemia | 1412 | 1.06 (0.72–1.56) | 2.7 (3) | 0.44 | 0% |
| Depression | 615 | 0.95 (0.12–7.39) | 2.14 (1) | 0.14 | 53% |
| Voluntary therapy discontinuation | 1571 | 2.70 (1.60–4.56) | 4.15 (4) | 0.39 | 4% |
| Therapy discontinuation due to safety reasons | 1470 | 1.54 (1.11–2.14) | 0.22 (3) | 0.97 | 0% |
Acknowledgments
None declared.
Footnotes
Financial Disclosures: Financial Disclosures
Funding/Support: None declared.
Implication for health policy/practice/research/medical education:: Treatment of hepatitis C is associated with eradication of 50% of the cases. Introduction of new methods for treatment of disease by increasing the duration of therapy can rise the chance of recovery. This article can be useful for all researchers in the field of gastroenterology, infectious disease and virology.
Please cite this paper as:: Alavian SM, Tabatabaei SV, Behnava B, Mahboobi N. Optimal Duration of Treatment for HCV Genotype 1 Infection in Slow Responders: A Meta-Analysis. Hepat Mon. 2011;11(8):612-9. [DOI: 10.5812/kowsar.1735143X.721]
References
- 1.Ahmadipour MH, Alavian SM, Amini S, Azadmanesh K. Hepatitis C Virus Genotypes. Hepat Mon. 2005;5(3):77–82. [Google Scholar]
- 2.Arens M. Clinically relevant sequence-based genotyping of HBV, HCV, CMV, and HIV. J Clin Virol. 2001;22(1):11–29. doi: 10.1016/s1386-6532(01)00156-1. [DOI] [PubMed] [Google Scholar]
- 3.Lavanchy D. The global burden of hepatitis C. Liver Int. 2009;29(Suppl 1):74–81. doi: 10.1111/j.1478-3231.2008.01934.x. [DOI] [PubMed] [Google Scholar]
- 4.Seeff LB. Natural history of chronic hepatitis C. Hepatology. 2002;36(5 Suppl 1):S35–S46. doi: 10.1053/jhep.2002.36806. [DOI] [PubMed] [Google Scholar]
- 5.Berenguer J, Alvarez-Pellicer J, Martín PM, López-Aldeguer J, Von-Wichmann MA, Quereda C, Mallolas J, Sanz J, Tural C, Bellón JM, González-García J, GESIDA3603/5607 Study Group Sustained virological response to interferon plus ribavirin reduces liver-related complications and mortality in patients coinfected with human immunodeficiency virus and hepatitis C virus. Hepatology. 2009;50(2):407–13. doi: 10.1002/hep.23020. [DOI] [PubMed] [Google Scholar]
- 6.Singal AK, Singh A, Jaganmohan S, Guturu P, Mummadi R, Kuo YF, Sood GK. Antiviral therapy reduces risk of hepatocellular carcinoma in patients with hepatitis C virus-related cirrhosis. Clin Gastroenterol Hepatol. 2010;8(2):192–9. doi: 10.1016/j.cgh.2009.10.026. [DOI] [PubMed] [Google Scholar]
- 7.Breitenstein S, Dimitroulis D, Petrowsky H, Puhan MA, Mullhaupt B, Clavien PA. Systematic review and meta-analysis of interferon after curative treatment of hepatocellular carcinoma in patients with viral hepatitis. Br J Surg. 2009;96(9):975–81. doi: 10.1002/bjs.6731. [DOI] [PubMed] [Google Scholar]
- 8.Poynard T, McHutchison J, Manns M, Trepo C, Lindsay K, Goodman Z, Ling MH, Albrecht J. Impact of pegylated interferon alfa-2b and ribavirin on liver fibrosis in patients with chronic hepatitis C. Gastroenterology. 2002;122(5):1303–13. doi: 10.1053/gast.2002.33023. [DOI] [PubMed] [Google Scholar]
- 9.Miyake Y, Takaki A, Iwasaki Y, Yamamoto K. Meta-analysis: interferon-alpha prevents the recurrence after curative treatment of hepatitis C virus-related hepatocellular carcinoma. J Viral Hepat. 2010;17(4):287–92. doi: 10.1111/j.1365-2893.2009.01181.x. [DOI] [PubMed] [Google Scholar]
- 10.Fried MW, Hadziyannis SJ, Shiffman ML, Messinger D, Zeuzem S. Rapid virological response is the most important predictor of sustained virological response across genotypes in patients with chronic hepatitis C virus infection. J Hepatol. 2011;55(1):69–75. doi: 10.1016/j.jhep.2010.10.032. [DOI] [PubMed] [Google Scholar]
- 11.Reau N, Satoskar R, Te H, DeVoss A, Elsen C, Reddy G, Mohanty S, Jensen D. Evaluation of early null response to pegylated interferon and ribavirin as a predictor of therapeutic nonresponse in patients undergoing treatment for chronic hepatitis C. Am J Gastroenterol. 2011;106(3):452–8. doi: 10.1038/ajg.2010.424. [DOI] [PubMed] [Google Scholar]
- 12.Yu ML, Dai CY, Huang JF, Chiu CF, Yang YH, Hou NJ, Lee LP, Hsieh MY, Lin ZY, Chen SC, Hsieh MY, Wang LY, Chang WY, Chuang WL. Rapid virological response and treatment duration for chronic hepatitis C genotype 1 patients: a randomized trial. Hepatology. 2008;47(6):1884–93. doi: 10.1002/hep.22319. [DOI] [PubMed] [Google Scholar]
- 13.Slavenburg S, Weggelaar I, van Oijen MG, Drenth JP. Optimal length of antiviral therapy in patients with hepatitis C virus genotypes 2 and 3: a meta-analysis. Antivir Ther. 2009;14(8):1139–48. doi: 10.3851/IMP1464. [DOI] [PubMed] [Google Scholar]
- 14.Gluud LL. Bias in clinical intervention research. Am J Epidemiol. 2006;163(6):493–501. doi: 10.1093/aje/kwj069. [DOI] [PubMed] [Google Scholar]
- 15.Bax L, Yu LM, Ikeda N, Tsuruta H, Moons KG. Development and validation of MIX: comprehensive free software for meta-analysis of causal research data. BMC Med Res Methodol. 2006;6:50. doi: 10.1186/1471-2288-6-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Borenstein M, Hedges LV. Introduction to Meta-Analysis. UK: John Wiley and Sons, Ltd; 2009. [Google Scholar]
- 17.Petitti DB. Meta-Analysis, Decision Analysis, and Cost-Effectiveness Analysis: Methods for Quantitative Synthesis in Medicine. New York: Oxford University Press; 2000. [Google Scholar]
- 18.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harbord RM, Harris RJ, Sterne JAC. Updated tests for small-study effects in meta-analyses. Stata J. 2009;9(2):197–210. [Google Scholar]
- 20.Heathcote J. Retreatment of chronic hepatitis C: who and how? Liver Int. 2009;29(Suppl 1):49–56. doi: 10.1111/j.1478-3231.2008.01932.x. [DOI] [PubMed] [Google Scholar]
- 21.Dieterich DT, Rizzetto M, Manns MP. Management of chronic hepatitis C patients who have relapsed or not responded to pegylated interferon alfa plus ribavirin. J Viral Hepat. 2009;16(12):833–43. doi: 10.1111/j.1365-2893.2009.01218.x. [DOI] [PubMed] [Google Scholar]
- 22.Nakamura J, Toyabe SI, Aoyagi Y, Akazawa K. Economic impact of extended treatment with peginterferon alpha-2a and ribavirin for slow hepatitis C virologic responders. J Viral Hepat. 2008;15(4):293–9. doi: 10.1111/j.1365-2893.2007.00943.x. [DOI] [PubMed] [Google Scholar]
- 23.Pearlman BL. Extended-therapy duration for chronic hepatitis C, genotype 1: the long and the short of it. World J Gastroenterol. 2008;14(23):3621–7. doi: 10.3748/wjg.14.3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tarantino G, Craxi A. Optimizing the treatment of chronic hepatitis due to hepatitis C virus genotypes 2 and 3: a review. Liver Int. 2009;29(Suppl 1):31–8. doi: 10.1111/j.1478-3231.2008.01924.x. [DOI] [PubMed] [Google Scholar]
- 25.Poordad FF. Review article: the role of rapid virologic response in determining treatment duration for chronic hepatitis C. Aliment Pharmacol Ther. 2010;31(12):1251–67. doi: 10.1111/j.1365-2036.2010.04300.x. [DOI] [PubMed] [Google Scholar]
- 26.Poordad F, Reddy KR, Martin P. Rapid virologic response: a new milestone in the management of chronic hepatitis C. Clin Infect Dis. 2008;46(1):78–84. doi: 10.1086/523585. [DOI] [PubMed] [Google Scholar]
- 27.Pearlman BL. Chronic hepatitis C therapy: changing the rules of duration. Clin Gastroenterol Hepatol. 2006;4(8):963–71. doi: 10.1016/j.cgh.2006.05.022. [DOI] [PubMed] [Google Scholar]
- 28.Nguyen-Khac E, Capron D, Castelain S, Francois C, Braillon A. Personalized therapy for chronic viral hepatitis C in the naive patient: How can we optimize treatment duration as a function of viral genotype? Eur J Intern Med. 2007;18(7):510–5. doi: 10.1016/j.ejim.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 29.Marcellin P, Heathcote EJ, Craxi A. Which patients with genotype 1 chronic hepatitis C can benefit from prolonged treatment with the 'accordion' regimen? J Hepatol. 2007;47(4):580–7. doi: 10.1016/j.jhep.2007.07.015. [DOI] [PubMed] [Google Scholar]
- 30.Buti M, Valdes A, Sanchez-Avila F, Esteban R, Lurie Y. Extending combination therapy with peginterferon alfa-2b plus ribavirin for genotype 1 chronic hepatitis C late responders: a report of 9 cases. Hepatology. 2003;37(5):1226–7. doi: 10.1053/jhep.2003.50107. [DOI] [PubMed] [Google Scholar]
- 31.Liu CH, Liu CJ, Lin CL, Liang CC, Hsu SJ, Yang SS, Hsu CS, Tseng TC, Wang CC, Lai MY, Chen JH, Chen PJ, Chen DS, Kao JH. Pegylated interferon-alpha-2a plus ribavirin for treatment-naive Asian patients with hepatitis C virus genotype 1 infection: a multicenter, randomized controlled trial. Clin Infect Dis. 2008;47(10):1260–9. doi: 10.1086/592579. [DOI] [PubMed] [Google Scholar]
- 32.August-Jörg BS, Borovicka J, Dufour JF, Gonvers JJ, Henz S, Hermann R, Meyenberger C, Weitz M, Renner EL. Twenty-four vs. forty-eight weeks of re-therapy with interferon alpha 2b and ribavirin in interferon alpha monotherapy relapsers with chronic hepatitis C. Swiss Med Wkly. 2003;133(33-34):455–60. doi: 10.4414/smw.2003.10300. [DOI] [PubMed] [Google Scholar]
- 33.Ikeda H, Suzuki M, Okuse C, Yamada N, Okamoto M, Kobayashi M, Nagase Y, Takahashi H, Matsunaga K, Matsumoto N, Itoh F, Yotsuyanagi H, Koitabashi Y, Yasuda K, Iino S. Short-term prolongation of pegylated interferon and ribavirin therapy for genotype 1b chronic hepatitis C patients with early viral response. Hepatol Res. 2009;39(8):753–9. doi: 10.1111/j.1872-034X.2009.00523.x. [DOI] [PubMed] [Google Scholar]
- 34.Hézode C. Treatment extension to 72 weeks of peginterferon and ribavirin in hepatitis C genotype 1-infected slow responders. Hepato Gastro. 2008;15(3):252–3. doi: 10.1002/hep.21919. [DOI] [PubMed] [Google Scholar]
- 35.Yao GB, Ji YY, Xu DZ, Gao J, Wu XH, Zhang QB, Hu DC. Long-term efficacy of recombinant interferon alpha 2a in the treatment of chronic hepatitis C: A randomized prospective study comparing two dose schedules in Chinese patients. Hepato Gastro. 1999;46(26):1059–64. [PubMed] [Google Scholar]
- 36.Yao G, Ji Y, Yang M, Xu D, Gao J, Wu X, Zhang Q, Hu D. Long-term efficacy of recombinant Interferon alpha 2a in the treatment of chronic hepatitis C : A randomized prospective study comparing two dose schedules in Chinese patients. Chinese Med J. 1998;111(10):922–6. [PubMed] [Google Scholar]
- 37.Piccinino F, Felaco FM, Sagnelli E, Aprea L, Messina V, Pasquale G, Filippini P, Scolastico C. Long-term lymphoblastoid interferon-alpha therapy for non-cirrhotic chronic hepatitis C: an Italian multicentre study on dose and duration of IFN alpha treatment. Res Virol. 1998;149(5):283–91. doi: 10.1016/s0923-2516(99)89007-4. [DOI] [PubMed] [Google Scholar]
- 38.Fattovich G, Zagni I, Fornaciari G, Minola E, Fabris P, Boccia S, Giusti M, Abbati G, Felder M, Rovere P, Redaelli A, Tonon A, Montanari R, Paternoster C, Distasi M, Castagnetti E, Tositti G, Rizzo C, Suppressa S, Pantalena M, Lomonaco L, Scattolini C, Tagger A. Efficacy of prolonged 5 million units of interferon in combination with ribavirin for relapser patients with chronic hepatitis C. J Viral Hepat. 2003;10(2):111–7. doi: 10.1046/j.1365-2893.2003.00409.x. [DOI] [PubMed] [Google Scholar]
- 39.Arase Y, Ikeda K, Tsubota A, Suzuki Y, Saitoh S, Kobayashi M, Kobayashi M, Suzuki F, Akuta N, Someya T, Kumada H. Efficacy of prolonged interferon therapy for patients with chronic hepatitis C with HCV-genotype 1b and high virus load. J Gastroenterol. 2003;38(2):158–63. doi: 10.1007/s005350300026. [DOI] [PubMed] [Google Scholar]
- 40.Núñez M, Miralles C, Berdún MA, Losada E, Aguirrebengoa K, Ocampo A, Arazo P, Cervantes M, de Los Santos I, San Joaquín I, Echeverría S, Galindo MJ, Asensi V, Barreiro P, Sola J, Hernandez-Burruezo JJ, Guardiola JM, Romero M, García-Samaniego J, Soriano V, PRESCO Study Group Role of weight-based ribavirin dosing and extended duration of therapy in chronic hepatitis C in HIV-infected patients: the PRESCO trial. AIDS Res Hum Retroviruses. 2007;23(8):972–82. doi: 10.1089/aid.2007.0011. [DOI] [PubMed] [Google Scholar]
- 41.Fuster D, Planas R, Gonzalez J, Force L, Cervantes M, Vilaró J, Roget M, García I, Pedrol E, Tor J, Ballesteros AL, Salas A, Sirera G, Videla S, Clotet B, Tural C. Results of a study of prolonging treatment with pegylated interferon-alpha2a plus ribavirin in HIV/HCV-coinfected patients with no early virological response. Antivir Ther. 2006;11(4):473–82. [PubMed] [Google Scholar]
- 42.Angeli E, Mainini A, Cargnel A, Uberti-Foppa C, Orani A, Carbone R, Andreoni M, Schiavini M, Giorgi R, Rizzardini G, Gubertini G, ICoS-2 Group Predictability of sustained virological response to pegylated interferon alpha-2b Plus ribavirin therapy by week-8 viral response in HIV-positive patients with chronic hepatitis C virus infection. Curr HIV Res. 2009;7(4):447–55. doi: 10.2174/157016209788680507. [DOI] [PubMed] [Google Scholar]
- 43.Akuta N, Suzuki F, Hirakawa M, Kawamura Y, Yatsuji H, Sezaki H, Suzuki Y, Hosaka T, Kobayashi M, Kobayashi M, Saitoh S, Arase Y, Ikeda K, Kumada H. A matched case-controlled study of 48 and 72 weeks of peginterferon plus ribavirin combination therapy in patients infected with HCV genotype 1b in Japan: amino acid substitutions in HCV core region as predictor of sustained virological response. J Med Virol. 2009;81(3):452–8. doi: 10.1002/jmv.21400. [DOI] [PubMed] [Google Scholar]
- 44.Watanabe S, Enomoto N, Koike K, Izumi N, Takikawa H, Hashimoto E, Moriyasu F, Kumada H, Imawari M, PERFECT Study Group Prolonged treatment with pegylated interferon alpha 2b plus ribavirin improves sustained virological response in chronic hepatitis C genotype 1 patients with late response in a clinical real-life setting in Japan. Hepatol Res. 2009;40(2):135–44. doi: 10.1111/j.1872-034X.2009.00567.x. [DOI] [PubMed] [Google Scholar]
- 45.Nagaki M, Shimizu M, Sugihara JI, Tomita E, Sano C, Naiki T, Kimura K, Amano K, Sakai T, Ninomiya M, Kojima T, Katsumura N, Fujimoto M, Moriwaki H. Clinical trial: extended treatment duration of peginterferon-alpha2b plus ribavirin for 72 and 96 weeks in hepatitis C genotype 1-infected late responders. Aliment Pharmacol Ther. 2009;30(4):343–51. doi: 10.1111/j.1365-2036.2009.04048.x. [DOI] [PubMed] [Google Scholar]
- 46.Zeuzem S, Pawlotsky JM, Lukasiewicz E, von Wagner M, Goulis I, Lurie Y, Gianfranco E, Vrolijk JM, Esteban JI, Hezode C, Lagging M, Negro F, Soulier A, Verheij-Hart E, Hansen B, Tal R, Ferrari C, Schalm SW, Neumann AU, DITTO-HCV Study Group , editors. International, multicenter, randomized, controlled study comparing dynamically individualized versus standard treatment in patients with chronic hepatitis C. J Hepatol. 2005;43(2):250–7. doi: 10.1016/j.jhep.2005.05.016. [DOI] [PubMed] [Google Scholar]
- 47.Ide T, Hino T, Ogata K, Miyajima I, Kuwahara R, Kuhara K, Sata M. A randomized study of extended treatment with peginterferon alpha-2b plus ribavirin based on time to HCV RNA negative-status in patients with genotype 1b chronic hepatitis C. Am J Gastroenterol. 2009;104(1):70–5. doi: 10.1038/ajg.2008.60. [DOI] [PubMed] [Google Scholar]
- 48.Mangia A, Minerva N, Bacca D, Cozzolongo R, Ricci GL, Carretta V, Vinelli F, Scotto G, Montalto G, Romano M, Cristofaro G, Mottola L, Spirito F, Andriulli A. Individualized treatment duration for hepatitis C genotype 1 patients: A randomized controlled trial. Hepatology. 2008;47(1):43–50. doi: 10.1002/hep.22061. [DOI] [PubMed] [Google Scholar]
- 49.Berg T, von Wagner M, Nasser S, Sarrazin C, Heintges T, Gerlach T, Buggisch P, Goeser T, Rasenack J, Pape GR, Schmidt WE, Kallinowski B, Klinker H, Spengler U, Martus P, Alshuth U, Zeuzem S. Extended treatment duration for hepatitis C virus type 1: comparing 48 versus 72 weeks of peginterferon-alfa-2a plus ribavirin. Gastroenterology. 2006;130(4):1086–97. doi: 10.1053/j.gastro.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 50.Ferenci P, Laferl H, Scherzer TM, Maieron A, Hofer H, Stauber R, Gschwantler M, Brunner H, Wenisch C, Bischof M, Strasser M, Datz C, Vogel W, Löschenberger K, Steindl-Munda P, Austrian Hepatitis Study Group Peginterferon alfa-2a/ribavirin for 48 or 72 weeks in hepatitis C genotypes 1 and 4 patients with slow virologic response. Gastroenterology. 2010;138(2):503–12. doi: 10.1053/j.gastro.2009.10.058. [DOI] [PubMed] [Google Scholar]
- 51.Pearlman BL, Ehleben C, Saifee S. Treatment extension to 72 weeks of peginterferon and ribavirin in hepatitis c genotype 1-infected slow responders. Hepatology. 2007;46(6):1688–94. doi: 10.1002/hep.21919. [DOI] [PubMed] [Google Scholar]
- 52.Sánchez-Tapias JM, Diago M, Escartín P, Enríquez J, Romero-Gómez M, Bárcena R, Crespo J, Andrade R, Martínez-Bauer E, Pérez R, Testillano M, Planas R, Solá R, García-Bengoechea M, Garcia-Samaniego J, Muñoz-Sánchez M, Moreno-Otero R, TeraViC-4 Study Group Peginterferon-alfa2a plus ribavirin for 48 versus 72 weeks in patients with detectable hepatitis C virus RNA at week 4 of treatment. Gastroenterology. 2006;131(2):451–60. doi: 10.1053/j.gastro.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 53.Miyase S, Ogata K, Haraoka K, Morishita Y, Fujiyama S. The efficacy of prolonging treatment with peginterferon alfa-2b and ribavirin to 72 weeks in chronic hepatitis C genotype 1 patients HCV RNA positive at week 8 but negative at week 12. Acta Hepato Jpn. 2010;51(1):48–50. [Google Scholar]
- 54.Buti M, Lurie Y, Zakharova NG, Blokhina NP, Horban A, Teuber G, Sarrazin C, Balciuniene L, Feinman SV, Faruqi R, Pedicone LD, Esteban R, SUCCESS Study Investigators Randomized trial of peginterferon alfa-2b and ribavirin for 48 or 72 weeks in patients with hepatitis C virus genotype 1 and slow virologic response. Hepatology. 2010;52(4):1201–7. doi: 10.1002/hep.23816. [DOI] [PubMed] [Google Scholar]
- 55.Hopewell S, Loudon K, Clarke MJ, Oxman AD, Dickersin K. Publication bias in clinical trials due to statistical significance or direction of trial results. Cochrane Database Syst Rev. 2009;(1):MR000006. doi: 10.1002/14651858.MR000006.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Turner EH, Matthews AM, Linardatos E, Tell RA, Rosenthal R. Selective publication of antidepressant trials and its influence on apparent efficacy. N Engl J Med. 2008;385(3):252–60. doi: 10.1056/NEJMsa065779. [DOI] [PubMed] [Google Scholar]
- 57.Moher D, Jones A, Lepage L. Use of the CONSORT statement and quality of reports of randomized trials: a comparative before-and-after evaluation. JAMA. 2001;285(15):1992–5. doi: 10.1001/jama.285.15.1992. [DOI] [PubMed] [Google Scholar]