Synopsis
The protein components of living cells in the hair follicle are amenable to study by standard molecular biological techniques, but identifying those in the hair shaft has been problematic until recently. Most of the protein, primarily keratins and keratin associated proteins, can be extracted under denaturing conditions, but 15–20% is intractable due to transglutaminase-mediated cross-linking. Shotgun proteomics now permits identifying >300 constituents of the isopeptide cross-linked proteome and even certain post-translational modifications. The proteins originate from all the intracellular compartments, indicating that the cross-linking process makes effective use of available resources to produce structures with great mechanical stability. Knowing this proteome provides a foundation for correlating defects in hair shaft structure with protein deficiencies. Such investigations can be extended to mouse models of aberrant pelage hair. Thus, inbred mouse strains can be distinguished by their hair proteomes, raising the possibility of similar variation in the human population. The nail plate is also amenable to this shotgun proteomic approach. Providing discrete and noninvasive sampling of the human proteome, these epidermal appendages could have diagnostic utility for certain disease states.
Background
Mature corneocytes in hair shaft, nail plate and epidermal callus are designed by nature to resist external physical stress and chemical exposures. They are comprised largely of keratin and keratin-associated proteins surrounded by an envelope of cross-linked protein. While the majority of protein is extractable from these corneocytes under strongly denaturing conditions, a substantial fraction (15–20% in the case of hair) resists solubilization due to considerable transglutaminase-mediated isopeptide bonding. An inability to separate constituent proteins has prevented their identification until very recently. Microscopy of the structures has revealed important physical features, and immunohistochemical studies of developing regions bordering the mature ones has permitted identification of major components. The powerful approach of targeted gene ablation provides complementary information on structural and developmental defects. Now that mass spectrometry, coupled with database searching of peptide masses, permits identification of proteolytic fragments of complex protein mixtures, the identities of the components of the cross-linked material are being revealed.
Studies by pioneering dermatologists a half century ago pointed to a particularly resistant structure at the outer boundary of corneocytes in the callus layer of the skin [1, 2]. Similar features are seen in the nail plate, where the interlocking borders of adjacent cells provide great cohesiveness to the overall structure [3]. The native hair shaft presents difficulties in transmission electron microscopy due to poor penetration by embedding media without treatment to permit their diffusion into cellular interiors. A simple such treatment (Figure 1) is to incubate the hair in sodium dodecyl sulfate (SDS) under reducing conditions for an hour or two at room temperature, inducing swelling and softening of the fiber and permitting ultrastructural visualization of bundles of intermediate filaments in the cortex [4]. More extensive detergent extraction at elevated temperature yields nearly empty cortical cells with their outer boundaries clearly delineated. By contrast, cuticle cells appear largely intact, with clear demarcation of the endocuticle, exocuticle and marginal band. Cells of the medulla contain remnant nuclei and amorphous deposits as well as areas without electron dense material. The cross-linking process in hair resembles that in the analogous cornified appendages of bird feather and hagfish teeth, and thus occurs generally among vertebrates [5].
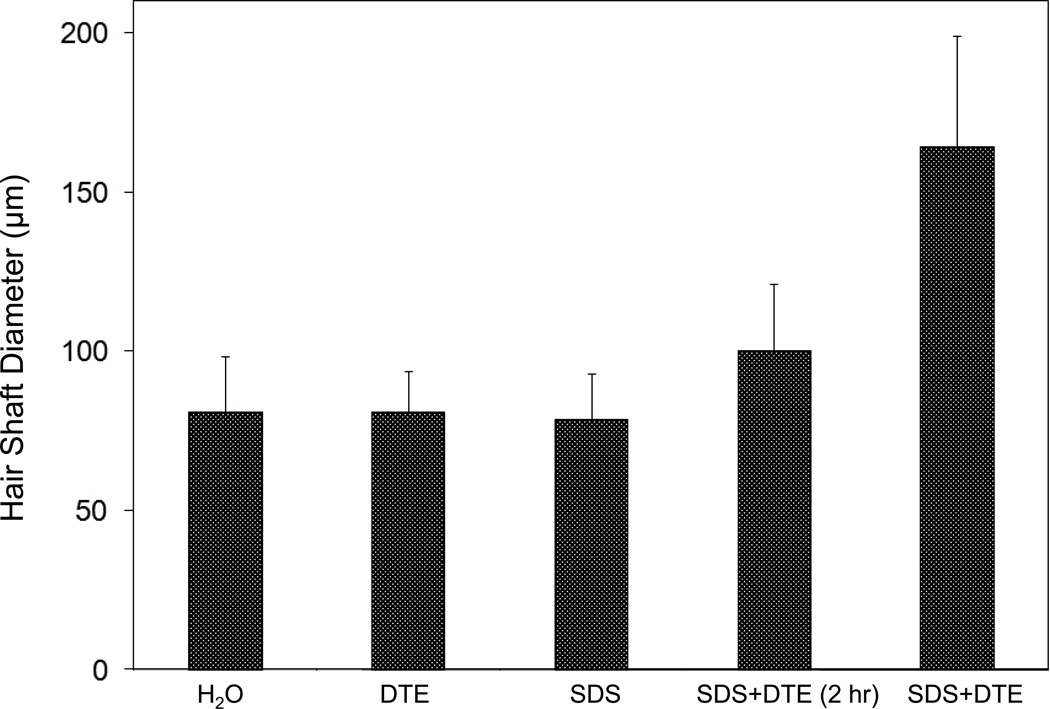
Figure 1.
Swelling of hair fibers at room temperature. Fibers from a Caucasian male were incubated in water or 0.1 M sodium phosphate (pH 7.8), in the latter case with 20 mM DTE and/or 2% SDS. Samples were examined after 6 hr except as indicated (2 hr). Shown are the means and standard deviations of diameters of 6–30 fibers for each condition measured microscopically (16×) using Slidebook 5.0 software. Swelling and increased flexibility are first noticeable visually after 1–2 hr, at which time hair fibers can be fixed and embedded.
Defects in the cross-linking process can be visualized microscopically in samples from epidermis and appendages after extraction of solubilizable protein with SDS and reducing agent. Individuals deficient in this process can exhibit lamellar ichthyosis and related skin conditions such as congenital ichthyosiform erythroderma characterized by high rates of transepidermal water loss and prominent scaling. Instead of displaying the normally observed empty cells with distinct boundaries, samples of extracted epidermal scale appear fragmented and disorganized. Small pieces of nail plate show analogous alterations in the diseased state with loss of the normally obvious cell borders [6, 7], while the hair cuticle shows considerably increased extraction or loss of features such as the marginal band [8, 9]. Structural alterations in the cortex and cuticle of uncertain basis are also seen in hair from individuals afflicted with trichothiodystrophy [8] and in the cuticle from mouse mutants such as matted and naked [10].
Based on original work on wool and hair in animal models, trapping of protein constituents in insoluble complexes was attributed to the action of transglutaminases found in the hair follicle [11, 12], analogous to the action of factor XIIIa in blood clotting [13]. Studying the biochemical basis for the cross-linking process was assisted by the discovery that it is manifested in cultures of human epidermal cells [14]. Requiring elevated calcium ions for their enzymatic activity, transglutaminases participate in the terminal stage of keratinocyte differentiation. A major form encoded by the gene TGM1, deficient in a large percentage of lamellar ichthyosis cases, is located on the inside of the plasma membrane and is thereby positioned to form the cross-linked envelope at the cell periphery [15]. Defects in the protein envelope are envisioned to perturb the attachment to it of the lipid barrier. Consequences of an improperly formed lipid barrier include entry of exogenous hydrophilic agents and excessive transepidermal water loss through the epidermis.
Mature corneocytes consist largely of hydrophobic keratin intermediate filaments and keratin associated proteins held together by disulfide bonds. These components are further stabilized by proteins cross-linked to them by transglutaminase activity. In addition to the TGM1 gene product at the cell periphery, which incorporates membrane-bound and junctional proteins, a transglutaminase in the cytoplasm encoded by the TGM3 gene is available to incorporate soluble proteins [16]. Our recent proteomic efforts analyzing the corneocyte have focused on these disulfide- and isopeptide-linked components. Thus, samples of hair, nail or callus are first rinsed in detergent (2% SDS) to remove soluble protein, generally negligible in amount, and adherent adventitious material to yield the sample to be analyzed.
Methodological Approach
Our strategy has been to separate the material solubilized in SDS and dithioerythritol (DTE) from the remainder, resistant to solubilization due to isopeptide bonding. To facilitate release and removal of the solubilized protein, the hair was extracted 5 times, as shown in Figure 2. Each time, it was incubated overnight at 70°C in 2% SDS - 0.1 M sodium phosphate (pH 7.8) - 20 mM DTE and then pulverized with a magnetic stirring bar for several hours. Aliquots of the solubilized and insoluble fractions were alkylated with iodoacetamide, and the solubilized protein was recovered as a flocculent precipitate by addition of ethanol to 70%. The two fractions were rinsed in 70% ethanol and fresh 0.1 M ammonium bicarbonate and then digested with stabilized trypsin (≈1% by weight) in the bicarbonate buffer adjusted to 10% in acetonitrile. For this purpose, TPCK-treated bovine trypsin was stabilized by reductive methylation [17]. Yields were improved by digestion for 2–3 days at room temperature with daily additions of trypsin. At the conclusion of digestion, the samples were clarified, taken to dryness, submitted for mass spectrometric analysis and the data presented using Scaffold Proteomics Software [18]. The reports permit ready access to many details of the protein identification including degree of peptide coverage, spectral characteristics, unique peptides and statistical description.
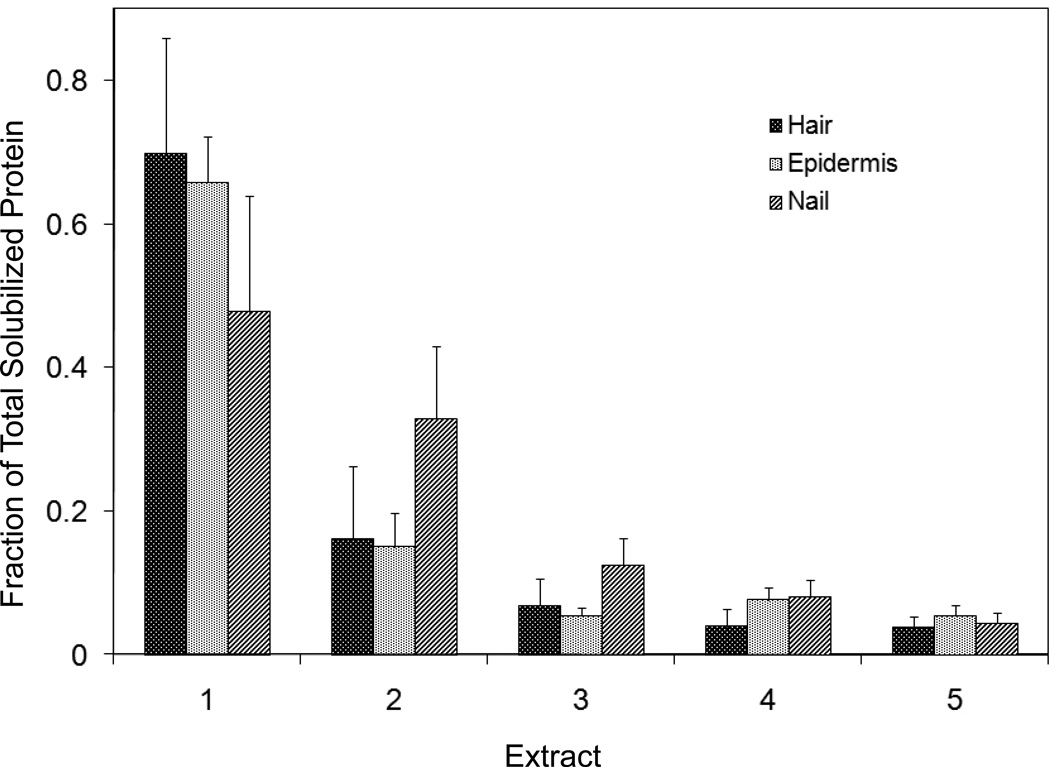
Figure 2.
Solubilized protein from human hair shaft, nail plate or epidermis extracted in 2% SDS - 20 mM DTE - 0.1 M sodium phosphate (pH 7.8). Each extract of hair and nail consisted of daily incubation for ≈ 22 hr at 70°C and then magnetic stirring for ≈ 2 hr at room temperature. For epidermis, isolated from skin at 55°C [29], extractions were performed by heating in the above SDS - DTE - phosphate buffer solution for 5–10 min in a boiling water bath followed by vigorous vortexing. Solubilized protein was recovered by centrifugation between extractions. The slower rate of protein extraction from nail plate reflects the slower rate of pulverization during magnetic stirring. Illustrated are the means and standard deviations of 4–6 samples of each type.
The original findings from our analyses [19] were that the solubilized protein fraction of hair shaft was comprised of a score of keratin and keratin-associated proteins known to be present, while the insoluble fraction was much more complex. Using two chromatographic steps of peptide separation permitted identification of some 350 proteins in the latter fraction originating from all regions of the cell. Careful examination of the peptide fragmentation patterns permitted identification of methylated lysines in a dozen proteins and evidence of ubiquitination as a glycylglycine posttranslational lysine modification of ubiquitin itself. For these analyses, the peptide digest was fractionated by ion exchange chromatography, and each fraction was then submitted to online reverse phase chromatography and mass spectrometry (LC-MS/MS). In the interest of higher throughput in later work, the first step of ion exchange chromatography was omitted subsequently. Although not as comprehensive, the results typically identify >100 proteins in a given sample, including novel components, a benefit of this discovery approach.
Quantitating protein amounts in the shotgun approach is a challenge in general due to the unknown yields of peptides in mass spectrometry. Contributing further to the uncertainty are the possibly incomplete yields from digestion of cross-linked material and the inability to identify peptides derived from protein cross-linkage sites. Since only a minority of lysines are involved in isopeptide linkages, estimated as ≈18% for epidermal callus [20], sufficient peptides are generated from the proteins to permit successful identification. With these caveats, estimates have been made of relative amounts based on the empirical relation between the protein abundance and the number of unique peptides (i.e., not counting overlaps in sequence coverage) detected compared to those predicted [21]. Although only semi-quantitative, estimates derived by this relation, called an exponentially modified protein abundance index, offer a convenient way to rank proteins in relative abundance [22].
Applications of Shotgun Proteomics
This approach to human hair syndromes exhibiting structural abnormalities plausibly will permit identifying protein deficiencies, although it is not guaranteed to do so. In the case of a family displaying autosomal recessive woolly hair, samples from afflicted offspring did not show any deficiencies in the prominent proteins identified [23]. In this instance, the lipase H gene was found to be defective by genetic testing. The availability of numerous mouse strains with anomalous structures suggests proteomic analysis could be useful in characterizing bases for the defects. AKR mice, for example, display hair with an “interior defect” consisting of haphazard patterns of medulla cell arrangement due to mutations in steroyl O-acetyltransferase 1 [24]. In strains without this defect, where medulla cells are typically in an orderly alignment with regular spacing, electron microscopy of mature hair shafts reveals projections from the cortex into the middle of each medulla cell. The AKR strain lacks these indentations, evidently permitting irregular or disorderly spacing of the medulla cells. Proteomic analysis revealed only a low level of trichohyalin, a major component of the projections, in the cortical cells of AKR mice compared to two other strains not showing this phenotype [4]. In the course of this work, it was noted that all three mouse strains could be distinguished by their proteomic profiles, raising the question whether, by analogy, human hair from individuals of different ethnic origin could be distinguished by protein profiling.
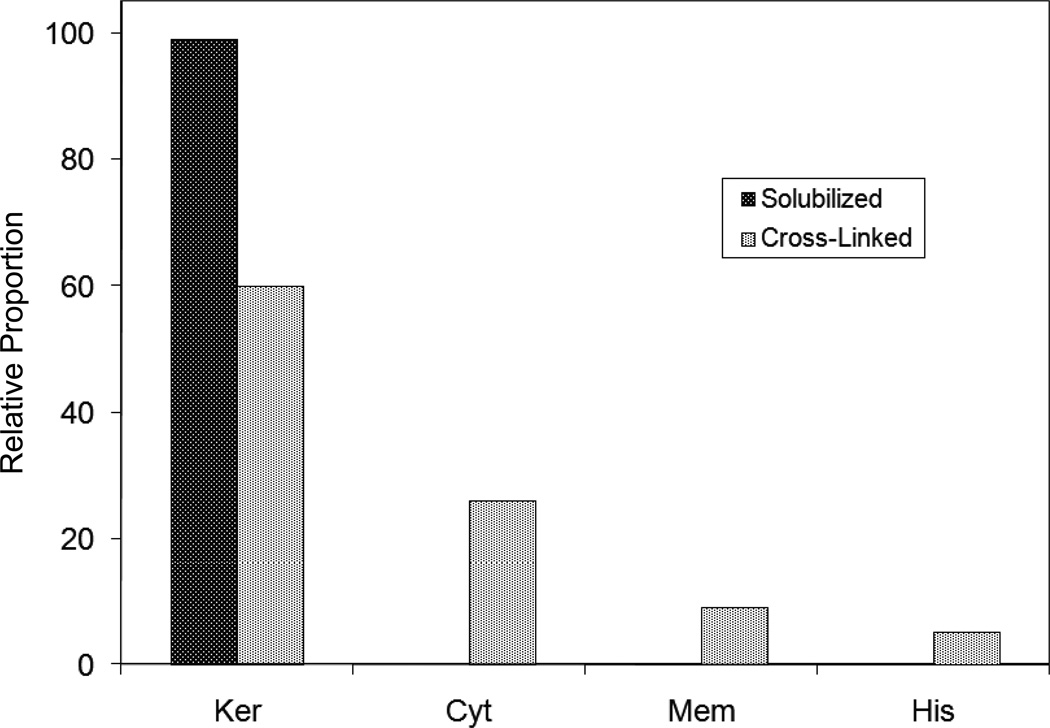
This approach to analysis of hair is easily adaptable to other keratinized structures such as nail plate. The nail plate proteome resembles that of the hair shaft in its high content of keratin proteins and the more complex profile of the insoluble fraction compared to the solubilized proteins [25]. Nearly all the 30 proteins in the solubilized fraction were keratins and keratin-associated proteins, while the insoluble fraction was comprised of, in addition to these, cytoplasmic proteins, membrane and junctional proteins and histones (Figure 3). Many of the proteins overlapped considerably with those detected in hair shaft, notably some abundant keratins (K31, K33B, K34, K39, K85, K86), junctional proteins (DSP, JUP, DSG4, PKP1) and numerous intracellular proteins (SELENBP1, SFN, ACTB, HSPA5). Some proteins were detected only in nail plate, including certain keratins (K5, K6A, K14, K17), junctional proteins (DSG1, EPPK1) and intracellular proteins (KPRP, SERPINB12, CKB). Others seen only in hair shaft included several keratins (K40, K82) and intracelluar proteins (CTTNB1, ATP5B). The finding of keratins (and KPRP) found only in nail, which are also found in epidermis, supports the established view that the nail unit expresses features of both hair and epidermis [26].
Figure 3.
Cellular locations of identified proteins in nail plate sorted into the categories keratin and keratin-associated proteins (Ker), other cytoplasmic proteins (Cyt), membrane and junctional proteins (Mem) and histones (His). Relative amounts were estimated based on unique peptides by the exponentially modified protein abundance index [21, 25].
Interpretation and Prospects
The nail unit and hair follicle produce structures that are remarkably tough and cohesive by virtue of their protein components, an abundance of intramolecular and intermolecular disulfide bonds and translgutaminase-mediated isopeptide bonding connecting the intermediate filament matrix to the cell surface. The final stage of the intricate keratinocyte differentiation program involves transglutaminase activation by increasing concentration of intracellular calcium ions, thereby connecting membrane and junctional proteins to the cytoskeleton. The enzymes encoded by the TGM1 and TGM3 genes appear effective in that most of the proteins found in the solubilized fraction are also found in the insoluble (cross-linked) fraction of both hair shaft and nail plate. That so many proteins from all the compartments of the cell were identified in the insoluble fraction is evidence that the corneocyte incorporates available protein rather than a few specific proteins. A future goal for hair is to analyze the proteome of cuticle, cortex and medulla cells separately. This has now been accomplished for wool cuticle, where >100 proteins were identified representing a variety of cellular processes [27].
Hair shaft, nail plate and epidermal callus, the latter through sampling with tape strips, provide essentially noninvasive sources of discrete protein subsets of the total organismal proteome. The shotgun approach to analysis is even anticipated to permit distinguishing the proteomes of epidermis at various anatomic sites, including glabrous surfaces or those influenced by adjoining abnormal conditions (e.g., acne). For analysis of disease states, it has the advantage of surveying many gene products in parallel, permitting discovery of single components that may be deficient. While homozygous protein loss may be readily detectable, the approach does have obvious limitations for identifying the basis for any given disease or adverse condition, since only the most prominent proteins are surveyed. Even if a given protein is identified in the sample examined, a point mutation could easily be overlooked if the protein coverage did not include the peptide in which the mutation occurs, or the affected peptide, one of many unique peptides, is not specifically monitored. Nevertheless, downstream effects of a given defect might still be visible in altered levels of other proteins that may be important for the phenotype. Detection of a heterozygous defective allele in a carrier of a recessive condition is also problematic without better quantitation.
Complementing the above shotgun (or discovery) approach, targeting specific peptides has potential utility. For example, quantitating only a small number of proteotypic peptides from a given protein, those unique to that protein and obtained reproducibly in high yield with suitable fragmentation patterns, can provide improved relative quantitation [28]. This approach (multiple reaction monitoring) would be attractive if it would permit detection of heterozygous gene loss. For those diseases where a limited number of mutations were anticipated, and they occurred in suitable locations in a detectable protein, expected mutant peptides could be monitored. Additionally, focusing on specific peptides has the advantage of permitting much greater sensitivity. Ultimately, a desirable outcome would be to use a panel of specific peptides to distinguish alternative diagnoses for given diseases or conditions. While this would not replace the gold standard of genetic testing, it could serve as a useful screen in a comprehensive diagnostic paradigm.
Acknowledgments
This work was supported by USPHS grant P42 ES04699 from the National Institute of Environmental Health Sciences. I thank Drs. Young Jin Lee, Rich Eigenheer and Brett Phinney of the Proteomics Core, University of California, Davis for mass spectrometry guidance and data collection and Dr. Ai Hayashi for expert technical assistance with measuring hair shaft diameters.
Footnotes
Presented at the TRI Fourth International Conference on Applied Hair Science, October 5-6, 2010 in Princeton, NJ
References
- 1.Matoltsy G, Balsamo CA. A study of the components of the cornified epithelium of human skin. J Biophys Biochem Cytol. 1955;1:339–361. doi: 10.1083/jcb.1.4.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Matoltsy AG. The membrane of horny cells. In: Seiji M, Bernstein IA, editors. Biochemistry of Cutaneous Epidermal Differentiation. Tokyo: University of Tokyo Press; 1977. pp. 93–109. [Google Scholar]
- 3.Rice RH, Mehrpouyan M, Qin Q, Phillips MA. Transglutaminases in keratinocytes. In: Leigh IM, Lane B, Watt FM, editors. The Keratinocyte Handbook. Cambridge University Press; 1994. pp. 259–274. [Google Scholar]
- 4.Rice RH, Rocke DM, Tsai H-S, Lee YJ, Silva KA, Sundberg JP. Distinguishing mouse strains by proteomic analysis of pelage hair. J Invest Dermatol. 2009;129:2120–2125. doi: 10.1038/jid.2009.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rice RH, Wong VJ, Pinkerton KE. Ultrastructural visualization of cross-linked protein features in epidermal appendages. J Cell Sci. 1994;107:1985–1992. doi: 10.1242/jcs.107.7.1985. [DOI] [PubMed] [Google Scholar]
- 6.Rice RH, Crumrine D, Hohl D, Munro CS, Elias PM. Cross-linked envelopes in nail plate in lamellar ichthyosis. Br J Dermatol. 2003;149:1050–1054. doi: 10.1111/j.1365-2133.2003.05510.x. [DOI] [PubMed] [Google Scholar]
- 7.Rice RH, Crumrine D, Uchida Y, Gruber R, Elias PM. Structural changes in epidermal scale and appendages as indicators of defective TGM1 activity. Arch Dermatol Res. 2005;297:127–133. doi: 10.1007/s00403-005-0591-7. [DOI] [PubMed] [Google Scholar]
- 8.Rice RH, Wong VJ, Price VH, Hohl D, Pinkerton KE. Cuticle cell defects in lamellar ichthyosis hair and anomalous hair shaft syndromes visualized after detergent extraction. Anatomic Rec. 1996;246:433–440. doi: 10.1002/(SICI)1097-0185(199612)246:4<433::AID-AR2>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 9.Rice RH, Wong VJ, Williams ML, Price VH, Hohl D, Sundberg JP, et al. Hair shaft defects visualized after detergent extraction. Exp Dermatol. 1999;8:308–310. [PubMed] [Google Scholar]
- 10.Rice RH, Wong VJ, Pinkerton KE, Sundberg JP. Cross-linked features of mouse pelage hair resistant to detergent extraction. Anatomic Rec. 1999;254:231–237. doi: 10.1002/(SICI)1097-0185(19990201)254:2<231::AID-AR9>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 11.Chung SI, Folk JE. Transglutaminase from hair follicle of guinea pig. Proc Natl Acad Sci USA. 1972;69:303–307. doi: 10.1073/pnas.69.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harding HWJ, Rogers GE. Formation of the ε-(γ-glutamyl) lysine cross-link in hair proteins. Investigation of transamidases in hair follicles. Biochemistry. 1972;11:2858–2863. doi: 10.1021/bi00765a019. [DOI] [PubMed] [Google Scholar]
- 13.Lorand L. Fibrinoligase: the fibrin-stabilizing factor system of blood plasma. Annals NY Acad Sci. 1972;202:6–30. doi: 10.1111/j.1749-6632.1972.tb16319.x. [DOI] [PubMed] [Google Scholar]
- 14.Sun T-T, Green H. Differentiation of the epidermal keratinocyte in cell culture: Formation of the cornified envelope. Cell. 1976;9:511–521. doi: 10.1016/0092-8674(76)90033-7. [DOI] [PubMed] [Google Scholar]
- 15.Thacher SM, Rice RH. Keratinocyte-specific transglutaminase of cultured human epidermal cells: Relation to cross-linked envelope formation and terminal differentiation. Cell. 1985;40:685–695. doi: 10.1016/0092-8674(85)90217-x. [DOI] [PubMed] [Google Scholar]
- 16.Thibaut S, Cavusoglu N, de Becker E, Zerbib F, Bednarczyk A, Schaeffer C, et al. Transglutaminase-3 enzyme: a putative actor in human hair shaft scaffolding? J Invest Dermatol. 2009;129:449–459. doi: 10.1038/jid.2008.231. [DOI] [PubMed] [Google Scholar]
- 17.Rice RH, Means GE, Brown WD. Stabilization of bovine trypsin by reductive methylation. Biochim Biophys Acta. 1977;492:316–321. doi: 10.1016/0005-2795(77)90082-4. [DOI] [PubMed] [Google Scholar]
- 18.Searle BC. Scaffold: a bioinformatic tool for validating MS/MS-based proteomic studies. Proteomics. 2010;10:1265–1269. doi: 10.1002/pmic.200900437. [DOI] [PubMed] [Google Scholar]
- 19.Lee YJ, Rice RH, Lee YM. Proteome analysis of human hair shaft: From protein identification to posttranslational modification. Molec Cell Proteom. 2006;5:789–800. doi: 10.1074/mcp.M500278-MCP200. [DOI] [PubMed] [Google Scholar]
- 20.Rice RH, Green H. The cornified envelope of terminally differentiated human epidermal keratinocytes consists of cross-linked protein. Cell. 1977;11:417–422. doi: 10.1016/0092-8674(77)90059-9. [DOI] [PubMed] [Google Scholar]
- 21.Ishihama Y, Oda Y, Tabata T, Sato T, Nagasu T, Rappsilber J, et al. Exponentially modified protein abundance index (emPAI) for estimation of absolute protein amount in proteomics by the number of sequenced peptides per protein. Molec Cell Proteom. 2005;4:1265–1272. doi: 10.1074/mcp.M500061-MCP200. [DOI] [PubMed] [Google Scholar]
- 22.Seibert C, Davidson BR, Fuller B, Patterson LH, Griffiths WJ, Wang Y. Multiple approaches to the identification and quantification of cytochromes P450 in human liver tissue by mass spectrometry. J Proteome Res. 2009;8:1672–1681. doi: 10.1021/pr800795r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shimomura Y, Wajid M, Zlotogorskic A, Lee YJ, Rice RH, Christiano AM. Founder mutations in the lipase H (LIPH) gene in families with autosomal recessive woolly hair/hypotrichosis. J Invest Dermatol. 2009;129:1927–1934. doi: 10.1038/jid.2009.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu B, Potter CS, Silva KA, Liang Y, Reinholdt LG, Alley LM, et al. Mutations in sterol O-acyltransferase 1 (Soat1) result in hair interior defects in AKR/J mice. J Invest Dermatol. 2010;130:2666–2668. doi: 10.1038/jid.2010.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rice RH, Xia Y, Alvarado RJ, Phinney BS. Proteomic analysis of human nail plate. J Proteome Res. 2010;9:6752–6758. doi: 10.1021/pr1009349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heid HW, Moll I, Franke WW. Patterns of expression of trichocytic and epithelial cytokeratins in mammalian tissues. II. Concomitant and mutually exclusive synthesis of trichocytic and epithelial cytokeratins in diverse human and bovine tissues (hair follicle, nail bed and matrix, lingual papilla, thymic reticulum) Differentiation. 1988;37:215–230. doi: 10.1111/j.1432-0436.1988.tb00724.x. [DOI] [PubMed] [Google Scholar]
- 27.Koehn H, Clerens S, Deb-Choudhury S, Morton J, Dyer JM, Plowman JE. The proteome of the wool cuticle. J Proteome Res. 2010;9:2920–2928. doi: 10.1021/pr901106m. [DOI] [PubMed] [Google Scholar]
- 28.James A, Jorgensen C. Basic design of MRM assays for peptide quantification. Meth Molec Biol. 2010;658:167–185. doi: 10.1007/978-1-60761-780-8_10. [DOI] [PubMed] [Google Scholar]
- 29.Macdiarmid J, Wilson JB. Separation of epidermal tissue from underlying dermis and primary keratinocyte culture. Meth Molec Biol. 2001;174:401–410. doi: 10.1385/1-59259-227-9:401. [DOI] [PubMed] [Google Scholar]