Abstract
Restorative and endodontic procedures have been recently developed in an attempt to preserve the vitality of dental pulp after exposure to external stimuli, such as caries infection or traumatic injury. When damage to dental pulp is reversible, pulp wound healing can proceed, whereas irreversible damage induces pathological changes in dental pulp, eventually requiring its removal. Nonvital teeth lose their defensive abilities and become severely damaged, resulting in extraction. Development of regeneration therapy for the dentin-pulp complex is important to overcome limitations with presently available therapies. Three strategies to regenerate the dentin-pulp complex have been proposed; regeneration of the entire tooth, local regeneration of the dentin-pulp complex from amputated dental pulp, and regeneration of dental pulp from apical dental pulp or periapical tissues. In this paper, we focus on the local regeneration of the dentin-pulp complex by application of exogenous growth factors and scaffolds to amputated dental pulp.
1. Limitations of Conventional Therapy for Preservation of Dental Pulp
Dental pulp is sometimes affected by external stimuli such as caries infection or traumatic injury. Preservation of dental pulp and maintenance of its viability are essential to avoid tooth loss, and dentists carry out restorative procedures with pulp capping to regulate inflammatory responses of dental pulp, or cement lining on a cavity floor to block external stimuli. Reversible damage induces pulp wound healing, and direct pulp capping and pulpotomy with calcium hydroxide are known to be effective to induce pulp wound healing mechanisms.
After external stimuli such as cavity preparation, apoptosis of pulp cells including odontoblasts is induced [1–5], followed by pulp wound healing including reactionary and reparative dentinogenesis. Reactionary dentin is formed by surviving odontoblasts, whereas reparative dentin is formed by odontoblast-like cells that are differentiated from pulp cells of residual dental pulp, resulting in a reduction in dental pulp size and vitality [6–8].
When the external damage to dental pulp induces irreversible changes of the pulp, dentists carry out pulpectomy. Generally, a root canal after pulpectomy is tightly filled with biomaterials such as gutta-percha to prevent reinfection by bacteria. However, a tooth without vital dental pulp has lost its defensive ability, which is often followed by the severe damage such as the progression of deep radicular caries or tooth facture, resulting in extraction of the tooth. Furthermore, a treated tooth is often reinfected by bacteria because of its complicated anatomical structure or inadequate treatment by a dentist, resulting in formation of a lesion around the root apex with bone resorption. The success rate of the endodontic retreatment is lower than that of pulpectomy [9–12]. To overcome these limitations of the present endodontic treatment, the preservation of dentin-pulp complex is the clear strategy. However, when a dentin defect and the resultant exposure of dental pulp tissue reach a critical size, no treatments available are able to preserve and maintain the vitality of dental pulp. It is considered important to develop regeneration therapy for dental pulp or the dentin-pulp complex.
2. Regeneration of the Dentin-Pulp Complex
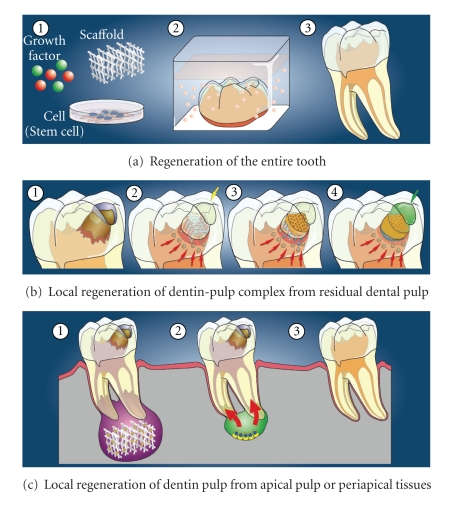
It is well known that growth factors, such as bone morphogenetic proteins (BMPs) and fibroblast growth factors (FGFs), stem cells, and scaffolds, are essential for tissue engineering to regenerate tissues [13]. During regeneration processes, stem cells differentiate into specific cells for tissue defects, growth factors such as BMPs and FGFs induce proliferation and differentiation of stem cells, and scaffolds with properties of extracellular matrix temporally support structures for cell growth, differentiation, and tissue formation. In studies to develop the regeneration therapy of the dentin-pulp complex, three strategies that utilize these essential three factors have been proposed; regeneration of the entire tooth, local regeneration of the dentin-pulp complex in dentin defect area from residual dental pulp, and regeneration of dental pulp from apical dental pulp or periapical tissues including the periodontal ligament and bone (Figure 1).
Figure 1.
Strategies for regeneration of the dentin-pulp complex with three factors for tissue regeneration; growth factors, scaffolds, and cells (stem cells or progenitor cells). (a) Regeneration of the entire tooth. (b) Local regeneration of the dentin-pulp complex in the dentin defect area from residual dental pulp. (c) Local regeneration of dental pulp from apical dental pulp or periapical tissues.
2.1. Regeneration of Entire Tooth
Regeneration of the entire tooth is accepted as a model of organ replacement and regeneration therapy. Recently, it was reported that tooth germs can be bioengineered using a three-dimensional organ-germ culture method, in which dental epithelial and mesenchymal cells isolated from tooth germs were cultured in three-dimensional scaffolds for the replacement therapy. Scaffolds consisted of synthetic polymers such as poly (lactide-co-glycolide) (PLGA) and bioceramics such as hydroxyapatite, tricalcium phosphate and calcium carbonate hydroxyapatite were examined in the three-dimensional organ-germ culture [14–21]. It was also reported that bioengineered teeth were generated from three-dimensionally arranged dental epithelial and mesenchymal cells in collagen gels by in vitro cell aggregate and manipulation method, and that the bioengineered tooth germ generated a structurally correct tooth showing penetration of blood vessels and nerve fibers in vivo transplantation into mouse maxilla, resulting in a successful fully functioning tooth replacement [22–25]. These bioengineered teeth, however, were reconstructed with dental epithelial and mesenchymal cells from genuine tooth germs. Further research will be needed to regenerate the entire tooth from other cell sources such as induced pluripotent stem (iPS) cells.
2.2. Local Regeneration from Residual Dental Pulp
Local regeneration of the dentin-pulp complex from residual dental pulp has been mainly delivered by researchers who are engaged in clinical practice. Several studies have reported the use of local applications of bioactive molecules such as BMPs and recombinant fusion ameloblastin to exposed pulp [26–28]. However, local application of bioactive molecules without scaffolds only induces reparative dentin formation toward residual dental pulp, which is the same result provided by conventional therapy such as pulp capping.
Induction of appropriate pulp wound healing and formation of new dentin in dentin defects are essential for the local regeneration of the dentin-pulp complex and vital pulp therapies to form new dentin in defects. Several papers demonstrated the local regeneration of dentin-pulp complex in different methods. It was reported that BMP-4 with dentin powder induced dentinogenesis in dentin cavity with pulp exposure [29]. In this research, stem or progenitor cells were induced from residual pulp through the exposure site at the bottom of the cavity. It was also reported that ultrasound-mediated gene delivery of growth factors such as growth/differentiation factor 11 (GDF-11)/BMP-11 into dental pulp stem cells by in vivo sonoporation induced reparative dentinogenesis [30–32], and that the ex vivo gene therapy by the transplantation of pulp stem/progenitor cells transfected with some growth factors such as GDF-11/BMP-11 stimulated reparative dentinogenesis [33–36].
FGF-2 is known to play a role in both physiological and pathological conditions [37–39]. It was previously demonstrated that a gradual and continual release of biologically active FGF-2 was achieved by in vivo biodegradation of gelatin hydrogels that incorporated FGF-2 [40–43] (Figure 2). Recently, we used FGF-2, gelatin hydrogels, and collagen sponge as a scaffold to induce local regeneration of rat dentin-pulp complex. We implanted free FGF-2 or gelatin hydrogels incorporating FGF-2 with collagen sponge into dentin defects above the amputated pulp of rat molars, and we found that a noncontrolled release of free FGF-2 only accelerated reparative dentin formation in the residual dental pulp, whereas a controlled release of FGF-2 from gelatin hydrogels induced formation of DMP-1-positive and nestin-negative osteodentin in the pulp proliferating in the dentin defects. Furthermore, the controlled release of an appropriate dosage of FGF-2 from gelatin hydrogels induced the formation of the dentinal bridge-like osteodentin on the surface of the regenerated pulp (Figure 3). These results suggest that our method inducing the regeneration of dentin and pulp into defect area from the amputated pulp is different from the conventional therapy that induces reparative dentinogenesis toward the amputated pulp [44, 45].
Figure 2.
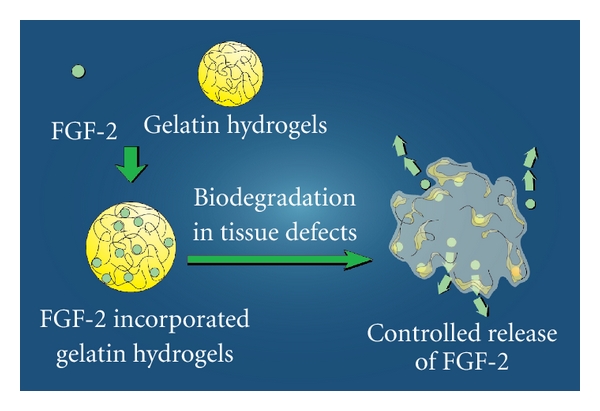
Controlled release of FGF-2. Gelatin hydrogels has an ability to incorporate growth factors such as FGF-2. After implantation of gelatin hydrogels incorporating FGF-2 with scaffolds, such as collagen sponge, FGF-2 is gradually released from gelatin hydrogels biodegraded by proteinase at tissue defect area. The controlled released FGF-2 can induce tissue regeneration.
Figure 3.
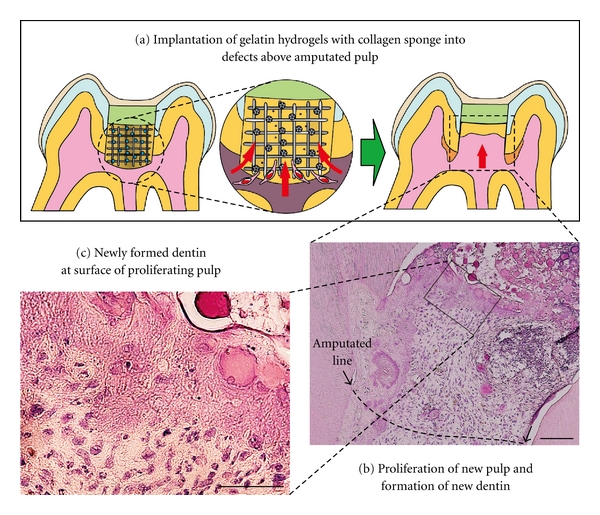
Local regeneration of the dentin-pulp complex in dentin defect area by implantation of gelatin hydrogels incorporating FGF-2. (a) Gelatin hydrogels incorporating FGF-2 with collagen sponge are implanted into dentin defect area. Controlled release of FGF-2 from biodegraded gelatin hydrogels can induce pulp stem cells or progenitor cells, as well as vessels, into collagen sponge at defect, resulting in the regeneration of pulp in the defect area and the formation of regenerative dentin on surface of the new pulp. (b) Histological photograph of proliferating pulp and newly regenerated dentin at surface of proliferating pulp. (c) High magnification of the regenerated dentin.
2.3. Local Regeneration from Periapical Tissues
Studies on regeneration of dental pulp from the apical area began from the identification of stem cells in the apical areas of developing teeth in which root formation is incomplete. It is suggested the existence of a new population of mesenchymal stem cells residing in the apical papilla (SCAPs) of incompletely developed teeth, and these cells have the ability to differentiate into odontoblast-like cells [46–48]. SCAPs play important roles in continued root formation, and they have been suggested to participate in pulp wound healing and regeneration. It is also known that bone-marrow-derived mesenchymal stem cells (BMMSCs) have multipotent abilities to differentiate into several cell types and undergo osteogenic differentiation. Periapical tissues include periodontal ligament, and bone marrow, which is the source of BMMSCs [49–54]. Localization of SCAPs and BMMSCs in the apical area indicate the possibility of the induction of these stem cells for the regeneration of the dentin-pulp complex.
3. Scaffolds for Regeneration of Dentin-Pulp Complex
It is important to select appropriate scaffolds for successful tissue regeneration. It is well known that essential properties of scaffolds are mechanical properties such as porous three-dimension structure, and mechanical strength, as well as biological properties such as biocompatibility and biodegradation [55]. In recent research and clinical approach, the following biomaterials are utilized for tissue regeneration therapy; polyethylene terephthalate, poly(L-lactide-co-D, L lactide), polylactic acid, polyglycolic acid, PLGA, polyvinyl alcohol, collagen, hyaluronic acid, hydroxyapatite, tricalcium phosphate, silk fibroin, bioactive glasses, and ceramic materials [56]. Of the variety of biomaterials tested, collagen sponge has been found to be well suited for the regeneration of bone defects, as collagen is a major component of the extracellular matrix. Also in the research field of tooth regeneration therapy, several lines of studies analyzed and discussed which three-dimensional scaffolds were suitable for the regeneration of dentin-pulp complex [57–60].
Recently, we have been focusing on the application of hyaluronic acid for local regeneration of the dentin-pulp complex. Hyaluronic acid is one of the glycosaminoglycans present in the extracellular matrix and plays important roles in maintaining morphologic organization by preserving extracellular spaces, and it has been reported to have excellent potential for tissue engineering [61–65]. The roles of hyaluronic acid in some biological processes, including inhibition of inflammation and pain, and differentiation of osteoblastic and osteoclastic cells, were recently studied [66–68]. In addition, some researchers have reported that intra-articular hyaluronic acid treatment for patients with osteoarthritic knees reduced painful symptoms and improved joint mobility [69, 70].
Dental pulp is a type of connective tissue derived from the dental papilla, and contains large amounts of glycosaminoglycans [71, 72]. Previously, the contribution of hyaluronic acid to the initial development of dentin matrix and dental pulp [73], in vivo application of hyaluronic acid gels on the wound healing processes of dental pulp, and the application of gelatin-chondroitin-hyaluronan tri-copolymer scaffold to dental bud cells were reported [74, 75].
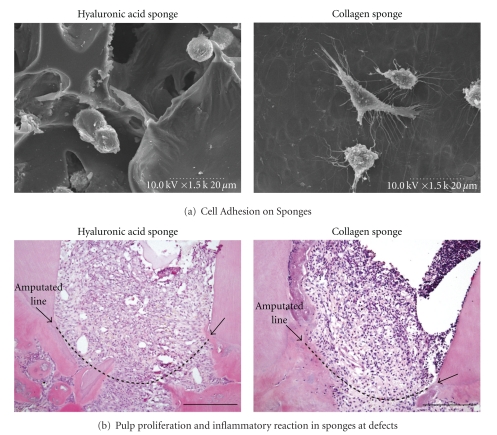
To clarify whether hyaluronic acid sponge (molecular weight 800 kDa) is useful as a scaffold for wound healing and regeneration of dental pulp, we compared in vitro and in vivo effects of hyaluronic acid sponge and collagen sponge on KN-3 odontoblast-like cell line and amputated dental pulp of rat molars. KN-3 cells, which were established from dental pulp of rat incisors, have odontoblastic properties such as high alkaline phosphatase activity and calcification ability [76]. We found that KN-3 cells adhered to both hyaluronic acid and collagen sponges during the culture period. In vivo results following implantation of both sponges in dentin defect areas above the amputated pulp showed that dental pulp proliferation and invasion of vessels into the hyaluronic acid and collagen sponges were well induced from the amputated dental pulp. These results suggest that hyaluronic acid sponge has an ability to induce and sustain dental pulp tissue regenerated from residual amputated dental pulp. In addition, we found that the inflammatory responses of KN-3 cells and the amputated dental pulp to hyaluronic acid sponge were lower than those against collagen sponge, suggesting that hyaluronic acid sponge has biocompatibility and biodegradation characteristics as well as an appropriate structure to make it suitable as a scaffold for dental pulp regeneration [77] (Figure 4).
Figure 4.
Application of hyaluronic acid sponge for local regeneration of the dentin-pulp complex. (a) KN-3 cells, odontoblastic progenitor cells, adhered to hyaluronic acid sponge, as well as collagen sponge. (b) Histological changes of amputated dental pulp after implantation of hyaluronic acid sponge in vivo. Amputated dental pulp well proliferated into hyaluronic acid and collagen sponges. Compared with collagen sponge, hyaluronic acid sponge significantly suppressed inflammatory reaction of dental pulp.
It is also important to clarify neuronal differentiation and neurite outgrowth during regeneration of the dentin-pulp complex. We examined the effects of hyaluronic acid gel on neuronal differentiation of PC12 pheochromocytoma cells, which respond to nerve growth factor (NGF) by extending neurites and exhibit a variety of properties of adrenal medullary chromaffin cells. We applied diluted solutions of 800 kDa hyaluronic acid to NGF-exposed PC12 cells, and noted inhibition of NGF-induced neuronal differentiation of PC12 cells via inhibition of ERK and p38 MAPK activation, caused by the interaction of hyaluronic acid to its receptor, RHAMM [78].
Our results demonstrated that hyaluronic acid sponge is useful for local regeneration of the dentin-pulp complex, whereas hyaluronic acid gel inhibits the differentiation or neurite outgrowth of neurons. In vivo, our results showed that hyaluronic acid sponge is gradually biodegraded during the regeneration processes, leaving soluble hyaluronic acid in the regenerated dental pulp. Next, we intend to clarify the biological and physiological behaviors of hyaluronic acid throughout the regeneration the of dentin-pulp complex.
4. Future Challenges to Achieve Local Regeneration of the Dentin-Pulp Complex
In our strategy, growth factors and scaffolds are exogenously supplied as bioactive materials, while the source of stem cells that are able to differentiate into odontoblast-like cells and pulp cells is dependent on the residual dental pulp. The vitality of the residual dental pulp is a critical point to achieve local regeneration of the dentin-pulp complex. It is generally accepted that the pulp wound healing proceeds well under conditions of low inflammatory responses by the dental pulp. In addition, regulation of dental pulp infection is another critical point regarding the success of such regeneration therapy. The resin bonding system is commonly used as one of materials showing favorable adhesion to enamel and dentin, and composite resin system with antimicrobial ability was reported [79–81]. These restorative materials may inhibit further bacterial invasion after tissue regeneration of dentin-pulp complex. Furthermore, when we try to induce revascularization and SCAPs and BMMSCs from the apical area into scaffolds at the root canal to regenerate dentin-pulp complex, disinfection of infected root canal systems, as well as proper apical enlargement to permit the induction from periapical tissues, should be successfully established [82]. Local regeneration of the dentin-pulp complex will be accomplished when the regulation mechanisms of pulp inflammation and infection, as well as pulp wound healing and regeneration mechanisms, are clarified.
References
- 1.Kitamura C, Kimura K, Nakayama T, Toyoshima K, Terashita M. Primary and secondary induction of apoptosis in odontoblasts after cavity preparation of rat molars. Journal of Dental Research. 2001;80(6):1530–1534. doi: 10.1177/00220345010800061001. [DOI] [PubMed] [Google Scholar]
- 2.Kitamura C, Ogawa Y, Nishihara T, Morotomi T, Terashita M. Transient co-localization of c-Jun N-terminal kinase and c-Jun with heat shock protein 70 in pulp cells during apoptosis. Journal of Dental Research. 2003;82(2):91–95. doi: 10.1177/154405910308200203. [DOI] [PubMed] [Google Scholar]
- 3.Kitamura C, Nishihara T, Ueno Y, Nagayoshi M, Kasugai S, Terashita M. Thermotolerance of pulp cells and phagocytosis of apoptotic pulp cells by surviving pulp cells following heat stress. Journal of Cellular Biochemistry. 2005;94(4):826–834. doi: 10.1002/jcb.20340. [DOI] [PubMed] [Google Scholar]
- 4.Kitamura C, Nishihara T, Ueno Y, et al. Effects of sequential exposure to lipopolysaccharide and heat stress on dental pulp cells. Journal of Cellular Biochemistry. 2006;99(3):797–806. doi: 10.1002/jcb.20967. [DOI] [PubMed] [Google Scholar]
- 5.Ueno Y, Kitamura C, Terashita M, Nishihara T. Re-oxygenation improves hypoxia-induced pulp cell arrest. Journal of Dental Research. 2006;85(9):824–828. doi: 10.1177/154405910608500909. [DOI] [PubMed] [Google Scholar]
- 6.Smith AJ, Cassidy N, Perry H, Bègue-Kirn C, Ruch JV, Lesot H. Reactionary dentinogenesis. International Journal of Developmental Biology. 1995;39(1):273–280. [PubMed] [Google Scholar]
- 7.Mitsiadis TA, Fried K, Goridis C. Reactivation of Delta-Notch signaling after injury: complementary expression patterns of ligand and receptor in dental pulp. Experimental Cell Research. 1999;246(2):312–318. doi: 10.1006/excr.1998.4285. [DOI] [PubMed] [Google Scholar]
- 8.Kawagishi E, Nakakura-Ohshima K, Nomura S, Ohshima H. Pulpal responses to cavity preparation in aged rat molars. Cell and Tissue Research. 2006;326(1):111–122. doi: 10.1007/s00441-006-0230-4. [DOI] [PubMed] [Google Scholar]
- 9.van Nieuwenhuysen JP, Aouar M, D’Hoore W. Retreatment or radiographic monitoring in endodontics. International Endodontic Journal. 1994;27(2):75–81. doi: 10.1111/j.1365-2591.1994.tb00234.x. [DOI] [PubMed] [Google Scholar]
- 10.Rotstein I, Salehrabi R, Forrest JL. Endodontic treatment outcome: survey of oral health care professionals. Journal of Endodontics. 2006;32(5):399–403. doi: 10.1016/j.joen.2005.10.056. [DOI] [PubMed] [Google Scholar]
- 11.Imura N, Pinheiro ET, Gomes BPFA, Zaia AA, Ferraz CCR, Souza-Filho FJ. The outcome of endodontic treatment: a retrospective study of 2000 cases performed by a specialist. Journal of Endodontics. 2007;33(11):1278–1282. doi: 10.1016/j.joen.2007.07.018. [DOI] [PubMed] [Google Scholar]
- 12.Ng YL, Mann V, Gulabivala K. Outcome of secondary root canal treatment: a systematic review of the literature. International Endodontic Journal. 2008;41(12):1026–1046. doi: 10.1111/j.1365-2591.2008.01484.x. [DOI] [PubMed] [Google Scholar]
- 13.Discher DE, Mooney DJ, Zandstra PW. Growth factors, matrices, and forces combine and control stem cells. Science. 2009;324(5935):1673–1677. doi: 10.1126/science.1171643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yelick PC, Vacanti JP. Bioengineered teeth from tooth bud cells. Dental Clinics of North America. 2006;50(2):191–203. doi: 10.1016/j.cden.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 15.Duailibi SE, Duailibi MT, Zhang W, Asrican R, Vacanti JP, Yelick PC. Bioengineered dental tissues grown in the rat jaw. Journal of Dental Research. 2008;87(8):745–750. doi: 10.1177/154405910808700811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Duailibi MT, Duailibi SE, Young CS, Bartlett JD, Vacanti JP, Yelick PC. Bioengineered teeth from cultured rat tooth bud cells. Journal of Dental Research. 2004;83(7):523–528. doi: 10.1177/154405910408300703. [DOI] [PubMed] [Google Scholar]
- 17.Honda MJ, Sumita Y, Kagami H, Ueda M. Histological and immunohistochemical studies of tissue engineered odontogenesis. Archives of Histology and Cytology. 2005;68(2):89–101. doi: 10.1679/aohc.68.89. [DOI] [PubMed] [Google Scholar]
- 18.Sumita Y, Honda MJ, Ohara T, et al. Performance of collagen sponge as a 3-D scaffold for tooth-tissue engineering. Biomaterials. 2006;27(17):3238–3248. doi: 10.1016/j.biomaterials.2006.01.055. [DOI] [PubMed] [Google Scholar]
- 19.Honda M, Morikawa N, Hata K, et al. Rat costochondral cell characteristics on poly (L-lactide-co-ε-caprolactone) scaffolds. Biomaterials. 2003;24(20):3511–3519. doi: 10.1016/s0142-9612(03)00210-2. [DOI] [PubMed] [Google Scholar]
- 20.Zheng L, Yang F, Shen H, et al. The effect of composition of calcium phosphate composite scaffolds on the formation of tooth tissue from human dental pulp stem cells. Biomaterials. 2011;32(29):7053–7059. doi: 10.1016/j.biomaterials.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 21.Yuan Z, Nie H, Wang S, et al. Biomaterial selection for tooth regeneration. Tissue Engineering Part B. 2011;17(5):373–388. doi: 10.1089/ten.teb.2011.0041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakao K, Morita R, Saji Y, et al. The development of a bioengineered organ germ method. Nature Methods. 2007;4(3):227–230. doi: 10.1038/nmeth1012. [DOI] [PubMed] [Google Scholar]
- 23.Ishida K, Murofushi M, Nakao K, Morita R, Ogawa M, Tsuji T. The regulation of tooth morphogenesis is associated with epithelial cell proliferation and the expression of Sonic hedgehog through epithelial-mesenchymal interactions. Biochemical and Biophysical Research Communications. 2011;405(3):455–461. doi: 10.1016/j.bbrc.2011.01.052. [DOI] [PubMed] [Google Scholar]
- 24.Ikeda E, Morita R, Nakao K, et al. Fully functional bioengineered tooth replacement as an organ replacement therapy. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(32):13475–13480. doi: 10.1073/pnas.0902944106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ikeda E, Tsuji T. Growing bioengineered teeth from single cells: potential for dental regenerative medicine. Expert Opinion on Biological Therapy. 2008;8(6):735–744. doi: 10.1517/14712598.8.6.735. [DOI] [PubMed] [Google Scholar]
- 26.Rutherford RB, Gu K. Treatment of inflamed ferret dental pulps with recombinant bone morphogenetic protein-7. European Journal of Oral Sciences. 2000;108(3):202–206. doi: 10.1034/j.1600-0722.2000.108003202.x. [DOI] [PubMed] [Google Scholar]
- 27.Six N, Lasfargues JJ, Goldberg M. Differential repair responses in the coronal and radicular areas of the exposed rat molar pulp induced by recombinant human bone morphogenetic protein 7 (osteogenic protein 1) Archives of Oral Biology. 2002;47(3):177–187. doi: 10.1016/s0003-9969(01)00100-5. [DOI] [PubMed] [Google Scholar]
- 28.Nakamura Y, Slaby I, Spahr A, Pezeshki G, Matsumoto K, Lyngstadaas SP. Ameloblastin fusion protein enhances pulpal healing and dentin formation in porcine teeth. Calcified Tissue International. 2006;78(5):278–284. doi: 10.1007/s00223-005-0144-2. [DOI] [PubMed] [Google Scholar]
- 29.Nakashima M. Induction of dentin formation on canine amputated pulp by recombinant human bone morphogenetic proteins (BMP)-2 and -4. Journal of Dental Research. 1994;73(9):1515–1522. doi: 10.1177/00220345940730090601. [DOI] [PubMed] [Google Scholar]
- 30.Nakashima M, Tachibana K, Iohara K, Ito M, Ishikawa M, Akamine A. Induction of reparative dentin formation by ultrasound-mediated gene delivery of growth/differentiation factor 11. Human Gene Therapy. 2003;14(6):591–597. doi: 10.1089/104303403764539369. [DOI] [PubMed] [Google Scholar]
- 31.Nakashima M, Iohara K, Ishikawa M, et al. Stimulation of reparative dentin formation by ex vivo gene therapy using dental pulp stem cells electrotransfected with growth/differentiation factor 11 (Gdf11) Human Gene Therapy. 2004;15(11):1045–1053. doi: 10.1089/hum.2004.15.1045. [DOI] [PubMed] [Google Scholar]
- 32.Nakashima M, Mizunuma K, Murakami T, Akamine A. Induction of dental pulp stem cell differentiation into odontoblasts by electroporation-mediated gene delivery of growth/differentiation factor 11 (Gdf11) Gene Therapy. 2002;9(12):814–818. doi: 10.1038/sj.gt.3301692. [DOI] [PubMed] [Google Scholar]
- 33.Iohara K, Nakashima M, Ito M, Ishikawa M, Nakasima A, Akamine A. Dentin regeneration by dental pulp stem cell therapy with recombinant human bone morphogenetic protein 2. Journal of Dental Research. 2004;83(8):590–595. doi: 10.1177/154405910408300802. [DOI] [PubMed] [Google Scholar]
- 34.Nakashima M, Iohara K, Zheng L. Gene therapy for dentin regeneration with bone morphogenetic proteins. Current Gene Therapy. 2006;6(5):551–560. doi: 10.2174/156652306778520665. [DOI] [PubMed] [Google Scholar]
- 35.Iohara K, Zheng L, Ito M, et al. Regeneration of dental pulp after pulpotomy by transplantation of CD31−/CD146− side population cells from a canine tooth. Regenerative Medicine. 2009;4(3):377–385. doi: 10.2217/rme.09.5. [DOI] [PubMed] [Google Scholar]
- 36.Nakashima M. Tissue engineering in endodontics. Australian Endodontic Journal. 2005;31(3):111–113. doi: 10.1111/j.1747-4477.2005.tb00317.x. [DOI] [PubMed] [Google Scholar]
- 37.Tsuboi T, Mizutani S, Nakano M, Hirukawa K, Togari A. FGF-2 regulates enamel and dentine formation in mouse tooth germ. Calcified Tissue International. 2003;73(5):496–501. doi: 10.1007/s00223-002-4070-2. [DOI] [PubMed] [Google Scholar]
- 38.Madan AK, Kramer B. Immunolocalization of fibroblast growth factor-2 (FGF-2) in the developing root and supporting structures of the murine tooth. Journal of Molecular Histology. 2005;36(3):171–178. doi: 10.1007/s10735-005-2684-1. [DOI] [PubMed] [Google Scholar]
- 39.Tran-Hung L, Mathieu S, About I. Role of human pulp fibroblasts in angiogenesis. Journal of Dental Research. 2006;85(9):819–823. doi: 10.1177/154405910608500908. [DOI] [PubMed] [Google Scholar]
- 40.Tabata Y, Ikada Y. Vascularization effect of basic fibroblast growth factor released from gelatin hydrogels with different biodegradabilities. Biomaterials. 1999;20(22):2169–2175. doi: 10.1016/s0142-9612(99)00121-0. [DOI] [PubMed] [Google Scholar]
- 41.Tabata Y, Miyao M, Inamoto T, et al. De novo formation of adipose tissue by controlled release of basic fibroblast growth factor. Tissue Engineering. 2000;6(3):279–289. doi: 10.1089/10763270050044452. [DOI] [PubMed] [Google Scholar]
- 42.Tabata Y, Nagano A, Ikada Y, Ikada Y. Biodegradation of hydrogel carrier incorporating fibroblast growth factor. Tissue Engineering. 1999;5(2):127–138. doi: 10.1089/ten.1999.5.127. [DOI] [PubMed] [Google Scholar]
- 43.Yamamoto M, Ikada Y, Tabata Y. Controlled release of growth factors based on biodegradation of gelatin hydrogel. Journal of Biomaterials Science, Polymer Edition. 2001;12(1):77–88. doi: 10.1163/156856201744461. [DOI] [PubMed] [Google Scholar]
- 44.Kikuchi N, Kitamura C, Morotomi T, et al. Formation of dentin-like particles in dentin defects above exposed pulp by controlled release of fibroblast growth factor 2 from gelatin hydrogels. Journal of Endodontics. 2007;33(10):1198–1202. doi: 10.1016/j.joen.2007.07.025. [DOI] [PubMed] [Google Scholar]
- 45.Ishimatsu H, Kitamura C, Morotomi T, et al. Formation of dentinal bridge on surface of regenerated dental pulp in dentin defects by controlled release of fibroblast growth factor-2 from gelatin hydrogels. Journal of Endodontics. 2009;35(6):858–865. doi: 10.1016/j.joen.2009.03.049. [DOI] [PubMed] [Google Scholar]
- 46.Sonoyama W, Liu Y, Fang D, et al. Mesenchymal stem cell-mediated functional tooth regeneration in Swine. PLoS ONE. 2006;1(1):p. e79. doi: 10.1371/journal.pone.0000079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sonoyama W, Liu Y, Yamaza T, et al. Characterization of the apical papilla and its residing stem cells from human immature permanent teeth: a pilot study. Journal of Endodontics. 2008;34(2):166–171. doi: 10.1016/j.joen.2007.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huang GTJ, Sonoyama W, Liu Y, Liu H, Wang S, Shi S. The hidden treasure in apical papilla: the potential role in pulp/dentin regeneration and bioroot engineering. Journal of Endodontics. 2008;34(6):645–651. doi: 10.1016/j.joen.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ebert R, Zeck S, Krug R, et al. Pulse treatment with zoledronic acid causes sustained commitment of bone marrow derived mesenchymal stem cells for osteogenic differentiation. Bone. 2009;44(5):858–864. doi: 10.1016/j.bone.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 50.Abdallah BM, Kassem M. The use of mesenchymal (skeletal) stem cells for treatment of degenerative diseases: current status and future perspectives. Journal of Cellular Physiology. 2009;218(1):9–12. doi: 10.1002/jcp.21572. [DOI] [PubMed] [Google Scholar]
- 51.Lim TY, Wang W, Shi Z, Poh CK, Neoh KG. Human bone marrow-derived mesenchymal stem cells and osteoblast differentiation on titanium with surface-grafted chitosan and immobilized bone morphogenetic protein-2. Journal of Materials Science: Materials in Medicine. 2009;20(1):1–10. doi: 10.1007/s10856-008-3528-9. [DOI] [PubMed] [Google Scholar]
- 52.Wan XC, Liu CP, Li M, et al. Staphylococcal enterotoxin C injection in combination with ascorbic acid promotes the differentiation of bone marrow-derived mesenchymal stem cells into osteoblasts in vitro. Biochemical and Biophysical Research Communications. 2008;373(4):488–492. doi: 10.1016/j.bbrc.2008.06.037. [DOI] [PubMed] [Google Scholar]
- 53.Post S, Abdallah BM, Bentzon JF, Kassem M. Demonstration of the presence of independent pre-osteoblastic and pre-adipocytic cell populations in bone marrow-derived mesenchymal stem cells. Bone. 2008;43(1):32–39. doi: 10.1016/j.bone.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 54.Lavery K, Swain P, Falb D, Alaoui-Ismaili MH. BMP-2/4 and BMP-6/7 differentially utilize cell surface receptors to induce osteoblastic differentiation of human bone marrow-derived mesenchymal stem cells. Journal of Biological Chemistry. 2008;283(30):20948–20958. doi: 10.1074/jbc.M800850200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Horst M, Madduri S, Gobet R, Sulser T, Hall H, Eberli D. Scaffold characteristics for functional hollow organ regeneration. Materials. 2010;3(1):241–263. [Google Scholar]
- 56.Sundelacruz S, Kaplan DL. Stem cell- and scaffold-based tissue engineering approaches to osteochondral regenerative medicine. Seminars in Cell and Developmental Biology. 2009;20(6):646–655. doi: 10.1016/j.semcdb.2009.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cordeiro MM, Dong Z, Kaneko T, et al. Dental pulp tissue engineering with stem cells from exfoliated deciduous teeth. Journal of Endodontics. 2008;34(8):962–969. doi: 10.1016/j.joen.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 58.Kim JY, Xin X, Moioli EK, et al. Regeneration of dental-pulp-like tissue by chemotaxis-induced cell homing. Tissue Engineering Part A. 2010;16(10):3023–3031. doi: 10.1089/ten.tea.2010.0181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chandrahasa S, Murray PE, Namerow KN. Proliferation of mature ex vivo human dental pulp using tissue engineering scaffolds. Journal of Endodontics. 2011;37(9):1236–1239. doi: 10.1016/j.joen.2011.05.030. [DOI] [PubMed] [Google Scholar]
- 60.Garcia-Godoy F, Murray PE. Recommendations for using regenerative endodontic procedures in permanent immature traumatized teeth. doi: 10.1111/j.1600-9657.2011.01044.x. Dental Traumatology. In press. [DOI] [PubMed] [Google Scholar]
- 61.Angele P, Kujat R, Nerlich M, Yoo J, Goldberg V, Johnstone B. Engineering of osteochondral tissue with bone marrow mesenchymal progenitor cells in a derivatized hyaluronan-gelatin composite sponge. Tissue Engineering. 1999;5(6):545–554. doi: 10.1089/ten.1999.5.545. [DOI] [PubMed] [Google Scholar]
- 62.Ramamurthi A, Vesely I. Smooth muscle cell adhesion on crosslinked hyaluronan gels. Journal of Biomedical Materials Research. 2002;60(1):196–205. doi: 10.1002/jbm.10061. [DOI] [PubMed] [Google Scholar]
- 63.Liu Y, Shu XZ, Gray SD, Prestwich GD. Disulfide-crosslinked hyaluronan-gelatin sponge: growth of fibrous tissue in vivo . Journal of Biomedical Materials Research Part A. 2004;68(1):142–149. doi: 10.1002/jbm.a.10142. [DOI] [PubMed] [Google Scholar]
- 64.Kim HD, Valentini RF. Retention and activity of BMP-2 in hyaluronic acid-based scaffolds in vitro . Journal of Biomedical Materials Research. 2002;59(3):573–584. doi: 10.1002/jbm.10011. [DOI] [PubMed] [Google Scholar]
- 65.Peattie RA, Nayate AP, Firpo MA, Shelby J, Fisher RJ, Prestwich GD. Stimulation of in vivo angiogenesis by cytokine-loaded hyaluronic acid hydrogel implants. Biomaterials. 2004;25(14):2789–2798. doi: 10.1016/j.biomaterials.2003.09.054. [DOI] [PubMed] [Google Scholar]
- 66.Fraser JRE, Laurent TC, Laurent UBG. Hyaluronan: its nature, distribution, functions and turnover. Journal of Internal Medicine. 1997;242(1):27–33. doi: 10.1046/j.1365-2796.1997.00170.x. [DOI] [PubMed] [Google Scholar]
- 67.Dowthwaite GP, Edwards JCW, Pitsillides AA. An essential role for the interaction between hyaluronan and hyaluronan binding proteins during joint development. Journal of Histochemistry and Cytochemistry. 1998;46(5):641–651. doi: 10.1177/002215549804600509. [DOI] [PubMed] [Google Scholar]
- 68.Day AJ, Sheehan JK. Hyaluronan: polysaccharide chaos to protein organisation. Current Opinion in Structural Biology. 2001;11(5):617–622. doi: 10.1016/s0959-440x(00)00256-6. [DOI] [PubMed] [Google Scholar]
- 69.Liesegang TJ. Viscoelastic substances in ophthalmology. Survey of Ophthalmology. 1990;34(4):268–293. doi: 10.1016/0039-6257(90)90027-s. [DOI] [PubMed] [Google Scholar]
- 70.Fukuda K, Dan H, Takayama M, Kumano F, Saitoh M, Tanaka S. Hyaluronic acid increases proteoglycan synthesis in bovine articular cartilage in the presence of interleukin-1. Journal of Pharmacology and Experimental Therapeutics. 1996;277(3):1672–1675. [PubMed] [Google Scholar]
- 71.Linde A. A study of the dental pulp glycosaminoglycans from permanent human teeth and rat and rabbit incisors. Archives of Oral Biology. 1973;18(1):49–59. doi: 10.1016/0003-9969(73)90019-8. [DOI] [PubMed] [Google Scholar]
- 72.Sakamoto N, Okamoto H, Okuda K. Qualitative and quantitative analyses of bovine, rabbit and human dental pulp glycosaminoglycans. Journal of Dental Research. 1979;58(2):646–655. doi: 10.1177/00220345790580022001. [DOI] [PubMed] [Google Scholar]
- 73.Felszeghy S, Hyttinen M, Tammi R, Tammi M, Módis L. Quantitative image analysis of hyaluronan expression in human tooth germs. European Journal of Oral Sciences. 2000;108(4):320–326. doi: 10.1034/j.1600-0722.2000.108004311.x. [DOI] [PubMed] [Google Scholar]
- 74.Sasaki T, Kawamata-Kido H. Providing an environment for reparative dentine induction in amputated rat molar pulp by high molecular-weight hyaluronic acid. Archives of Oral Biology. 1995;40(3):209–219. doi: 10.1016/0003-9969(95)98810-l. [DOI] [PubMed] [Google Scholar]
- 75.Kuo TF, Huang AT, Chang HH, et al. Regeneration of dentin-pulp complex with cementum and periodontal ligament formation using dental bud cells in gelatin-chondroitin-hyaluronan tri-copolymer scaffold in swine. Journal of Biomedical Materials Research Part A. 2008;86(4):1062–1068. doi: 10.1002/jbm.a.31746. [DOI] [PubMed] [Google Scholar]
- 76.Nomiyama K, Kitamura C, Tsujisawa T, et al. Effects of lipopolysaccharide on newly established rat dental pulp-derived cell line with odontoblastic properties. Journal of Endodontics. 2007;33(10):1187–1191. doi: 10.1016/j.joen.2007.05.015. [DOI] [PubMed] [Google Scholar]
- 77.Inuyama Y, Kitamura C, Nishihara T, et al. Effects of hyaluronic acid sponge as a scaffold on odontoblastic cell line and amputated dental pulp. Journal of Biomedical Materials Research Part B. 2010;92(1):120–128. doi: 10.1002/jbm.b.31497. [DOI] [PubMed] [Google Scholar]
- 78.Washio A, Kitamura C, Jimi E, Terashita M, Nishihara T. Mechanisms involved in suppression of NGF-induced neuronal differentiation of PC12 cells by hyaluronic acid. Experimental Cell Research. 2009;315(17):3036–3043. doi: 10.1016/j.yexcr.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 79.Izutani N, Imazato S, Nakajo K, et al. Effects of the antibacterial monomer 12-methacryloyloxydodecylpyridinium bromide (MDPB) on bacterial viability and metabolism. European Journal of Oral Sciences. 2011;119(2):175–181. doi: 10.1111/j.1600-0722.2011.00817.x. [DOI] [PubMed] [Google Scholar]
- 80.Imazato S. Bio-active restorative materials with antibacterial effects: new dimension of innovation in restorative dentistry. Dental Materials Journal. 2009;28(1):11–19. doi: 10.4012/dmj.28.11. [DOI] [PubMed] [Google Scholar]
- 81.Imazato S, Tay FR, Kaneshiro AV, Takahashi Y, Ebisu S. An in vivo evaluation of bonding ability of comprehensive antibacterial adhesive system incorporating MDPB. Dental Materials. 2007;23(2):170–176. doi: 10.1016/j.dental.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 82.Murray PE, Garcia-Godoy F, Hargreaves KM. Regenerative endodontics: a review of current status and a call for action. Journal of Endodontics. 2007;33(11):p. 1277. doi: 10.1016/j.joen.2006.09.013. [DOI] [PubMed] [Google Scholar]