Abstract
Klotho is a single-pass transmembrane protein that exerts its biological functions through multiple modes. Membrane-bound Klotho acts as coreceptor for the major phosphatonin fibroblast growth factor-23 (FGF23), while soluble Klotho functions as an endocrine substance. In addition to in the distal nephron where it is abundantly expressed, Klotho is present in the proximal tubule lumen where it inhibits renal Pi excretion by modulating Na-coupled Pi transporters via enzymatic glycan modification of the transporter proteins – an effect completely independent of its role as the FGF23 coreceptor. Acute kidney injury (AKI) and chronic kidney disease (CKD) are states of systemic Klotho deficiency, making Klotho a very sensitive biomarker of impaired renal function. In addition to its role as a marker, Klotho also plays pathogenic roles in renal disease. Klotho deficiency exacerbates decreases in, while Klotho repletion or excess preserves, glomerular filtration rate in both AKI and CKD. Soft tissue calcification, and especially vascular calcification, is a dire complication in CKD, associated with high mortality. Klotho protects against soft tissue calcification via at least 3 mechanisms: phosphaturia, preservation of renal function and a direct effect on vascular smooth muscle cells by inhibiting phosphate uptake and dedifferentiation. In summary, Klotho is a critical molecule in a wide variety of renal diseases and bears great potential as a diagnostic and prognostic biomarker as well as for therapeutic replacement therapy.
Keywords: Acute kidney injury, Chronic kidney disease, Klotho, Phosphaturia, Vascular calcification
Introduction
Klotho was originally identified as an anti-aging protein (1). In addition to the transmembrane form, Klotho also exists in a soluble secreted form (2, 3), which can be derived from an alternatively spliced transcript or cleaved by the ADAM family of secretases (4, 5). Hence Klotho can circulate as a soluble protein in body fluids including blood, urine (6–8) and cerebrospinal fluid (6).
The highest expression of Klotho is in kidney and brain (1, 7, 8), but it is also expressed in parathyroid gland (9, 10) and heart (11) with less abundance. The multiorgan phenotype observed in Klotho-deficient (Kl−/−) mice, including many organs that do not normally express Klotho, is compatible with the notion that Klotho functions as a humoral factor exerting biologic function on remote organs. The fact that intravenous injection of Klotho regulates phosphate (Pi) (8) and potassium excretion (12) further supports its endocrine actions. We propose that Klotho may function as an endocrine, paracrine and autocrine substance.
The similarity of the phenotypes between Kl−/− mice (1) and Fgf23−/− mice is striking (13), which strongly suggests a common signaling pathway shared by these molecules (14, 15). Now it is well documented that membrane Klotho functions as coreceptor for fibroblast growth factor-23 (FGF23), which amplifies and confers specificity of FGF23 action (16–19). In contrast, soluble Klotho protein functions independently of FGF23 (8) and plays an important role in modulation of ion transporters or channels (8, 20), antioxidation (21) and antisenescence (22–25), in addition to simply supporting FGF23 action.
There are several comprehensive reviews addressing the anti-aging effects of Klotho (26, 27), Pi toxicity (26, 28, 29) and kidney ion channels (20). This manuscript will review recent data on Klotho as a phosphatonin and its role in renoprotection and prevention of soft tissue calcification.
Klotho: a novel phosphatonin
Hyperphosphatemia is a prominent feature in the Kl−/− mice (1). The restoration of Klotho levels via genetic manipulation (30), viral-based delivery (31) or recombinant protein administration (8) successfully normalizes blood Pi level. Kl−/− mice display increased activity of Na-coupled phosphate (NaPi) cotransport and elevation of NaPi-2a and NaPi-2c cotransporter proteins compared with wild-type (WT) mice (32). This suggests that the hyperphosphatemia at least in part is of renal origin.
Although abnormal mineral metabolism in Kl−/− mice is well documented, the mechanisms of these derangements are not well illustrated. Numerous studies described novel mechanisms whereby Klotho controls renal calcium homeostasis (33–35) and renal potassium channel ROMK1 (12), indicating that Klotho may have a broad function in ion channel regulation (20).
To better understand how Klotho affects Pi transport by the renal proximal tubule, Hu et al detected Klotho expression in the proximal convoluted tubule in addition to a stronger expression in the distal convoluted tubule (8). Klotho is found in the proximal tubule cell, brush border and urinary lumen where phosphate homeostasis resides (8), which provides direct accessibility to the Na-coupled transporters NaPi-2a, NaPi-2c and Pit2 (36, 37).
Transgenic Klotho overexpressing mice (Tg-Kl) have lower blood Pi, while renal fractional excretion of phosphorus (FEphos) is increased, indicating a renal leak of Pi (8). Injection of soluble Klotho significantly increased FEphos and decreased blood Pi in the normal rat. This phosphaturic action is FGF23-independent, as Klotho protein efficiently induces phosphaturia and leads to hypophosphatemia even in FGF23−/− mice (8). The high FEphos is proximal in origin as Pi flux is significantly reduced in Tg-Kl compared with WT mice, when a microdissected single proximal convoluted tubule was microperfused in vitro (8).
The direct action of Klotho was further demonstrated in a kidney proximal tubule cell line by addition of Klotho in vitro in the absence of FGF23 (8). Furthermore, Klotho inhibited NaPi cotransport activity in brush border membrane (BBM) vesicles, which is a cell-free system. NaPi-2a protein in OK cells and total amount of NaPi-2a protein in BBM are not appreciably decreased by Klotho in vitro in 2 hours (8), suggesting that early inhibition is not dependent upon modulation of NaPi-2a trafficking, which is the only known pathway of regulation of NaPi transporters to date (36–39). This represents a novel mechanism of regulation of NaPi activity. However, Klotho dramatically reduces NaPi-2a abundance in apical NaPi-2a protein in kidney and OK cells after 4 or more hours in vivo and in vitro, respectively, indicating that the more sustained effects of Klotho on NaPi involve the canonical pathway of NaPi-2a internalization (8).
The extracellular domain of Klotho contains 2 tandem repeats with 20%–40% amino acid identity with members of the glycosidase family including β-glucosidase and has β-glucuronidase-like enzymatic activity (1, 40). NaPi-2a is a glycosylated protein (41). The direct inhibition of NaPi transport by Klotho can be mimicked by recombinant β-glucuronidase but not by sialidase (8). The inhibitory effect of Klotho is blocked by the β-glucuronidase inhibitor, D-saccharic acid-1,4-lactone (DSAL) but not the sialidase inhibitor, deoxy-N-acetyl neuraminic acid (DANA). This puts glucoronate removal as a key mechanism and raises the question of how this glycan modification alters transport activity.
Klotho shifts NaPi-2a from the full-length form to smaller peptides, and NaPi-2a deglycosylation promotes its proteolysis (8). While protease inhibitors abolish the proteolysis, they do not reverse the Klotho-induced inhibition of transport. Those observations indicate that Klotho-induced deglycosylation is sufficient and that subsequent proteolysis is not required to suppress Na-dependent Pi transport. Identity of the resident protease(s) in BBM that mediate(s) this effect remains to be determined.
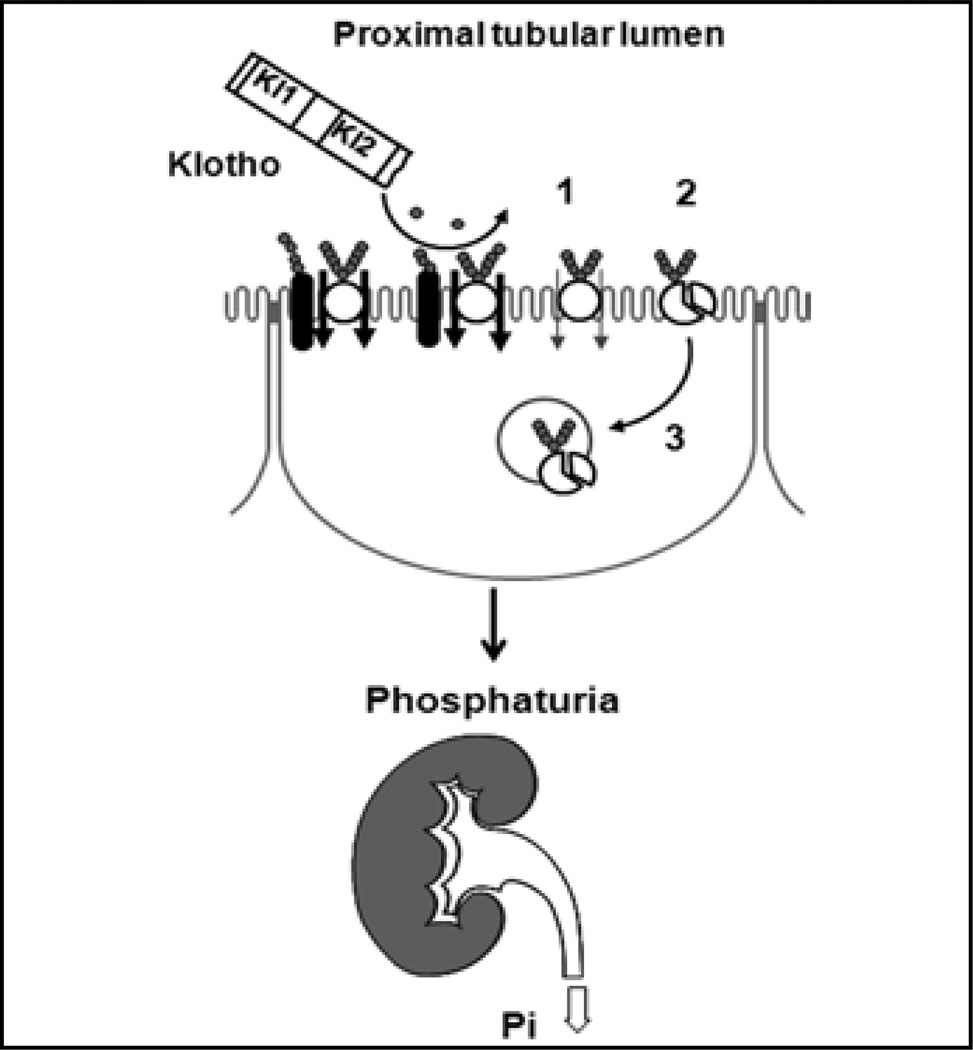
In summary, Klotho modulates NaPi-2a in a biphasic fashion with dual distinct mechanisms. It acutely (<4 hours) decreases its intrinsic transport activity via removal of glucuronate, followed by proteolytic cleavage, and in a second phase (>4 hours) induces changes in cell surface NaPi-2a (8) (Fig. 1).
Fig. 1.
Proposed model of how Klotho regulates NaPi-2a in the apical membrane of the renal proximal tubule. Klotho functions acutely as a direct extracellular enzyme deglycosylating NaPi-2a protein and/or a putative regulatory protein (black) to reduce transport activity (no. 1 in fgure). the deglycosylated NaPi-2a is sensitized to resident protease(s) in brush border membrane (BBM) and is proteolytically degraded (no. 2). Several hours later, deglycosylated NaPi-2a protein is endocytosed from BBM into the intracellular pool (no. 3).
Hyperphosphatemia is universally observed in chronic kidney disease (CKD) patients (42, 43) and is a potent predictor of cardiovascular morbidity and mortality (44). Controlling blood Pi by restriction of intake (45, 46), phosphate binder (47, 48) and more efficient dialysis (49) all improve clinical outcome in CKD patients. Undoubtedly, lack of the phosphaturic action of Klotho protein is an important pathogenic factor in CKD, and any means of restoring Klotho is of potential benefit. This will be addressed further below.
Kindey disease: a state of Klotho deficiency
Among its multiple effects, Klotho has been shown to be a cytoprotective protein that defends against oxidative stress and ischemia-reperfusion injury (IRI) (21). There is increasing evidence to suggest a relationship between oxidative stress and aging (50) and kidney disease (51–55).
Levels of Klotho protein and transcripts are decreased in kidneys or kidney cell lines by oxidative stress or IRI (56, 57), angiotensin II infusion (58, 59) and hypertension (60, 61). In addition, renal Klotho is decreased in CKD in humans (62) and several experimental animal models (61, 63, 64). Since the kidney is the major organ of Klotho expression, perhaps it is not too surprising to find decreased Klotho expression when this organ is severely diseased. The question is whether there is endocrine Klotho deficiency that can potentially have far-reaching effects. Until recently, there have been no data on blood Klotho in acute or/and chronic kidney disease. Two recent studies reported blood Klotho levels in rodents (65, 66). Rodents with acute kidney injury (AKI) have rapid and severe reduction of Klotho protein in blood, kidney and urine, and this reduction is fully reversible upon recovery of kidney function, indicating that AKI is a transient state of endocrine Klotho deficiency (65).
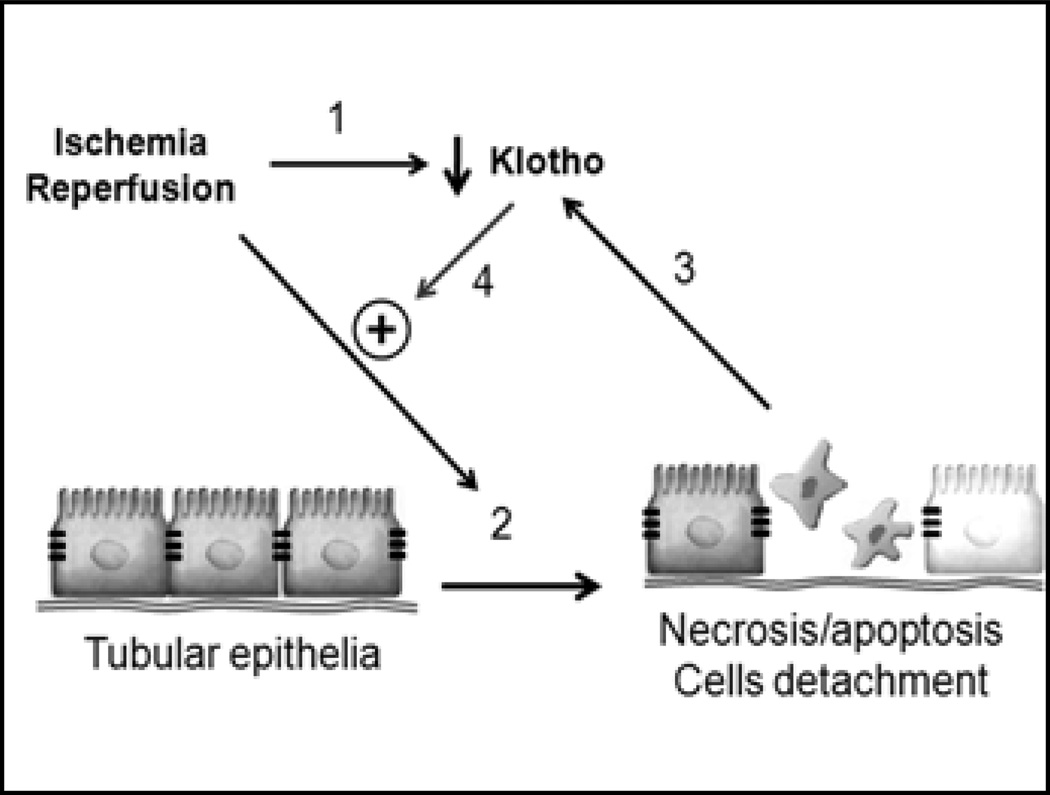
The mechanism of Klotho down-regulation in AKI is not known. The severe and rapid decrease may not stem only from reduction of Klotho mRNA, as Klotho transcripts are only down-regulated to 50% of baseline (65). In addition, Klotho down-regulation occurs before changes in other markers of kidney damage (Fig. 2) (65). Oxidative stress can decrease Klotho mRNA and protein in a cultured cell line (67). Tumor necrosis factor (TNF) and interferon-γ (IFN-γ) can reduce renal Klotho mRNA and protein (68). Whether increased TNF and IFN-γ in AKI (69) lead to Klotho down-regulation remains to be proven.
Fig. 2.
Proposed model of Klotho effect on acute kidney injury. Ischemia-reperfusion injury (IRI) down-regulates Klotho by 3 hours after injury (no. 1). IRI causes kidney damage (no. 2). Damaged tubules further decrease Klotho expression (no. 3). Reduced Klotho renders kidney more prone to further damage (no. 4).
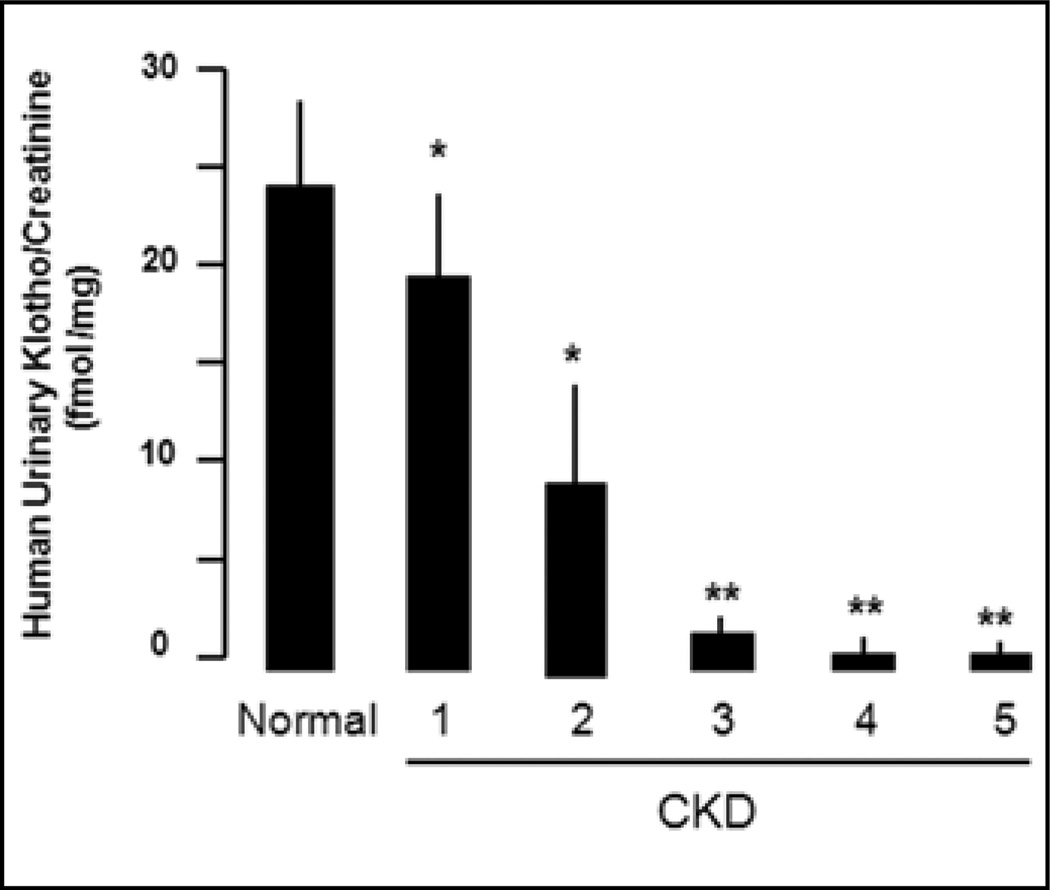
The connection between AKI and CKD is complex, and several hypotheses have been proposed to explain this slow and largely irreversible process. Interventions that retard or halt progression will be most valuable. End-stage CKD patients (62) and animals (58, 63, 70, 71) have reduced Klotho in kidneys, but there were no data on blood or urine Klotho until a recent study which showed very low blood, kidney and urine Klotho in CKD mice and postulated that CKD is a state of “pan-deficiency” of Klotho (66). This study measured urinary Klotho in CKD patients as a surrogate and found that human CKD patients have reduced urinary Klotho levels (Fig. 3). More importantly, Klotho deficiency occurs as early as stage 1 and 2 CKD in patients, and the magnitude of decrease remarkably correlates with the severity of declined estimated glomerular filtration rate in both rodent and human CKD. Therefore, urinary Klotho is an extremely sensitive and early marker in CKD, and its decline parallels loss of kidney function (66). One needs to determine the mechanism(s) of Klotho down-regulation in AKI and CKD, which may generate treatment modalities to restore endogenous Klotho expression.
Fig. 3.
Urinary Klotho protein in chronic kidney disease (CKD) patients normalized to creatinine in spot samples from 13 normal volunteers and 40 CKD patients. For measurement of urinary Klotho protein, 4-ml fresh urine was concentrated to 0.2 ml through Amicon ultra-4 filters with 100-kDa cutoff. Concentrated urines (with identical urine creatinine) along with recombinant murine Klotho (rMKl) protein of known concentration were subject to immunoblot. Klotho protein concentrations in urine samples were quantifed using the rMKl as a standard curve (66). *p<0.05; **p<0.01, vs. normal subjects by 1-way ANOVA followed by Student-Newman-Keuls test.
Klotho: a renoprotective protein
Given the extensive animal and some human data, there is no doubt that Klotho levels closely parallel kidney function, rendering Klotho a useful novel biomarker for presence of renal disease. However, a much more fundamental question of enormous biologic and clinical significance is whether Klotho deficiency contributes to the pathogenesis and complications of kidney disease. If this is true, it will take the utility from the diagnostic and prognostic straight to therapeutic realms. To this end, Hu and colleagues induced AKI or CKD in mice with different Klotho levels: lower (heterozygous Klotho haplodeficiency, Kl+/−), normal (WT) and higher levels (transgenic overexpression of Klotho, Tg-Kl) (65, 66). In the AKI model, Kl+/− mice have lower Klotho protein levels in plasma, kidney and urine at baseline, which become undetectable after AKI, as they develop more severe renal dysfunction. Conversely, Tg-Kl mice have higher renal, plasma and urinary Klotho levels at baseline, and are more resistant to IRI insult compared with WT AKI mice (65). These results indicate that Klotho deficiency accentuates and Klotho overexpression attenuates rodent AKI, rendering it more than a mere biomarker. The protective effect of Klotho on AKI was also shown by Sugiura using adenovirus delivery of the Klotho gene before IRI (57). In contrast, Hu et al gave recombinant Klotho protein 30–60 minutes after the IRI and demonstrated attenuation of histologic and functional damage (65). This is of more practical value because practitioners rarely have the luxury of interfering with AKI prior to the insult.
Currently, supportive renal replacement therapy remains the core of clinical management in AKI (72). Replacement therapy is unlikely to significantly affect the disease course of AKI per se, and intensive therapy appears not to consistently or dramatically increase survival rate or improve long-term outcome (73, 74). The prospect of using Klotho therapeutically in reducing kidney damage and promoting kidney recovery could potentially be a seminal discovery. The mechanisms whereby Klotho protects kidney from injury are not known but potentially include antioxidation (21, 63), antiapoptosis (23, 56, 57), antisenescence (23, 25) and angiogenesis (75).
In addition to AKI, one also needs to examine the vast number of patients with chronic Klotho deficiency in CKD. Again, one poses the question, is Klotho a mere biomarker, or is it pathogenic? Results from several laboratories using different CKD animal models support a beneficial effect of Klotho on CKD. Viral delivery of the Klotho gene leads to better maintenance of kidney function, a decrease in urinary protein and amelioration of tubulointerstitial changes induced by chronic angiotensin II infusion (58). Restoration of Klotho in immune-mediated glomerulonephritis through an overexpressing Klotho gene could suppress oxidation, decrease kidney damage and increase survival (63). Furthermore, viral delivery of Klotho decreases blood pressure, improves kidney histology and inhibits oxidation in spontaneous hypertensive rats (60).
Another study used a CKD model generated by uninephrectomy plus contralateral IRI and showed that Kl+/− CKD mice have hypertension, anemia, decreased creatinine clearance, increased proteinuria and much more severe interstitial fibrosis and glomerular sclerosis compared with WT CKD mice. Conversely, all changes are much attenuated in the Tg-Kl CKD mice (8). It is clear in animals that Klotho is a renoprotective agent
Klotho: guardian against soft tissue calcification in CKD
Hyperphosphatemia can accelerate CKD complications such as hyperparathyroidism, osteodystrophy and cardiovascular calcification (42, 76–78), and control of blood Pi ameliorates these dire conditions (79–82). The fact that CKD-associated vascular calcification in Kl−/− and WT mice is almost eliminated in Tg-Kl mice supports the paradigm that Klotho suppresses the ectopic calcification (30). Klotho has been shown to modulate the vascular endothelium and induce relaxing of vasculature (61, 83–86).
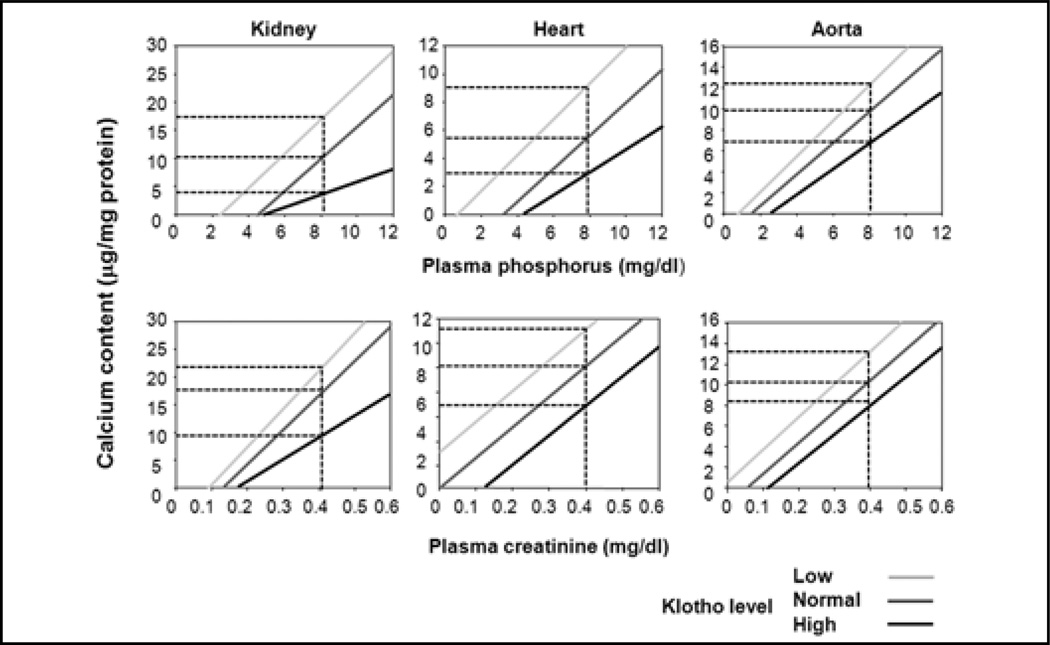
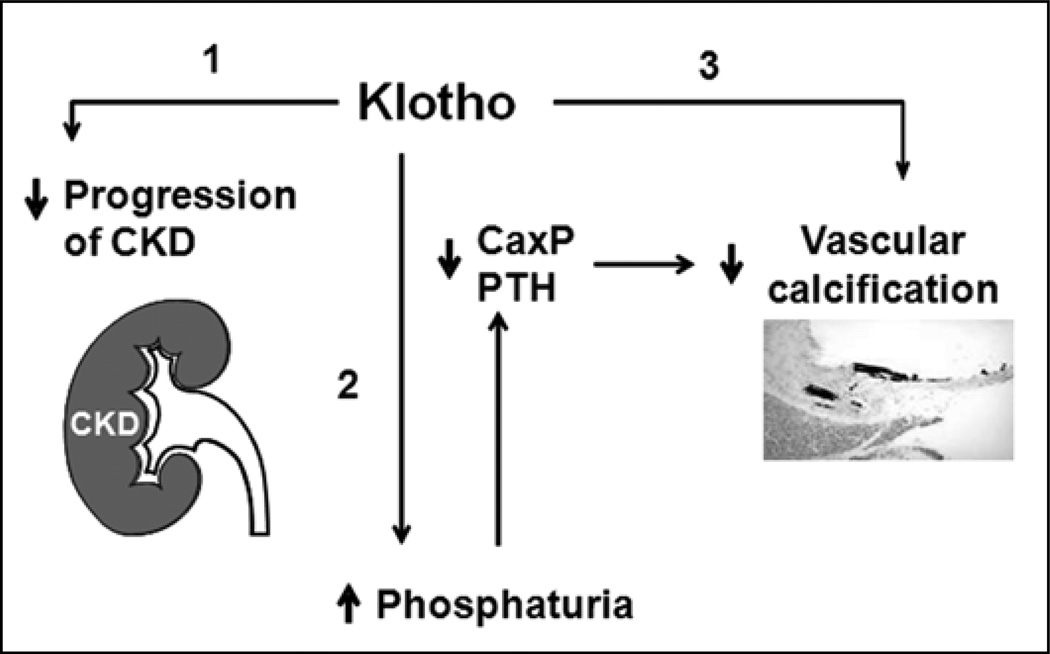
While there is abundant calcification in the multiple organs of both WT and Kl+/− CKD mice, Tg-Kl CKD animals have very few or no calcification (66). Calcium content is higher in the aortas and kidneys of CKD than sham, in both WT and Kl+/− mice. This increase is ameliorated by overexpression of Klotho (66). When soft tissue calcium content is analyzed as a function of plasma creatinine (Cr) and Pi levels, the calcium content is positively correlated with plasma Pi and Cr, which is not a surprise (Fig. 4). As mentioned above, Tg-Kl CKD mice have better, Kl+/− mice worse, kidney function compared with WT CKD mice. Elevation of parathyroid hormone (PTH) in WT CKD mice is blunted by Klotho overexpression and worsened by Klotho deficiency. Hence, amelioration of CKD per se and with milder secondary hyperparathyroidism can be a potential factor for less severe soft tissue calcification when Klotho levels are maintained (Fig. 5).
Fig. 4.
Correlation of calcium content in the kidneys, hearts and aortas in sham and chronic kidney disease (CKD) mice. Calcium content was assayed using o-cresolphthalein complexone (OCPC) in the kidney, heart and aortas of sham and CKD mice at different genetic Klotho levels: Kl+/− (light gray) and Tg-Kl (black gray) and their wild-type (WT) littermates (dark gray). For given concentration of blood creatinine (Cr) or phosphate (Pi) (vertical dotted line) Kl+/− (light gray) mice have the highest, and Tg-Kl (black) the lowest and their WT littermates (dark gray) intermediate levels of Ca content in soft tissues.
Fig. 5.
Proposed model of potential effects of Klotho on the kidney progression and vascular calcification in chronic kidney disease (CKD). Klotho protects the vasculature against calcification in CKD probably by 3 actions: slowing progression of CKD (no. 1); maintenance of normophosphatemia through induction of phosphaturia (no. 2); direct inhibition of phosphate (Pi) infulx into vascular smooth muscle cells (VSMCs), which in turn suppresses the dedifferentiation of VSMCs (no. 3). Ca × P = calcium × phosphorus product; PtH = parathyroid hormone.
One determinant of soft tissue calcification is plasma Pi concentration. Both Kl+/− and WT animals with CKD have higher levels of blood Pi. In contrast, Tg-Kl CKD mice do not show much hyperphosphatemia (66). Since Klotho is a potent phosphaturic substance, a second mechanism by which Klotho decreases soft tissue calcification is by lowering plasma phosphate levels through promotion of phosphaturia (Fig. 5).
Figure 4 clearly shows that for a given plasma Pi and Cr concentration, Tg-Kl mice have the lowest soft tissue calcium, Kl+/− the highest, with WT somewhere in between (66). Therefore, differences in plasma Pi or Cr, although important, are not sufficient to explain the different levels of ectopic calcification in the various Klotho backgrounds. This pattern of change strongly suggests that Klotho has a direct protective effect on soft tissue calcification above and beyond that of the renal effects of phosphaturia and preservation of glomerular filtration discussed previously. How does Klotho act on the vasculature?
The Na-coupled Pi transporters Pit1 and Pit2 are key modulators for Pi influx into vascular smooth muscle cells (VSMCs) and play a pathogenic role in vascular calcification (87–90). Up-regulation of Runx2, a marker of osteoblast-like phenotype, and down-regulation of SM22, a marker of contractile smooth muscle cell, are typically seen in vascular calcification (87–92). Pit1, Pit2 and Runx2 mRNA are increased and SM22 is decreased in Kl−/−, while overexpression of Klotho has the opposite effect. Klotho may control the balance between differentiation and dedifferentiation of VSMCs (66). CKD induces a similar pattern as Klotho deficiency, and Klotho overexpression completely blocked the changes induced by CKD. One limitation of in vivo studies is that they do not provide evidence of direct Klotho effect on Pit expression and VSMC differentiation.
When VSMCs are grown in vitro, Klotho inhibits Na-dependent Pi infux and minimizes the mineralization induced by high ambient Pi (66). The up-regulation of Runx2 and down-regulation of SM22 by high Pi are reversed by recombinant Klotho, suggesting that Klotho directly blocks Pi-induced dedifferentiation of A10 (66). Taken together, the data indicate that Klotho bestows its anticalcification effect via at least 3 potential mechanisms: phosphaturia, preservation of kidney function and a direct effect on the vascular smooth muscle (Fig. 5).
Conclusion
AKI induces transient but severe renal and endocrine Klotho deficiency, while CKD is a sustained state of systemic Klotho deficiency. Klotho is not merely a sensitive and early biomarker of kidney disease, but also plays a pathogenic role in kidney disease progression, disturbed mineral metabolism and vascular calcification in CKD. Early administration of exogenous Klotho protein or enhancement of endogenous Klotho could improve kidney function in both AKI and CKD. The potential utility of Klotho in clinical practice is at least twofold. First, Klotho could serve as an early and sensitive biomarker of kidney diseases. Second, Klotho supplementation may provide a novel therapy to treat AKI by limiting damage and promoting recovery and to treat CKD by slowing progression as well as preventing and reversing complications.
Acknowledgements
The authors are grateful to collaborators (Michel Baum, Kevin Rosenblatt, Henry Quinones) and our clinical and technical staff (Mingjun Shi, Jianning Zhang, Carolyn Griffith, Becky Aricheta, Jan Koska) whose contributions have been invaluable in the completion of the first series of studies of Klotho in phosphate metabolism and kidney disease.
Financial support: The work cited in this review from authors’ laboratories was primarily supported by the Simmons Family Foundation, National Institutes of Health (AG19712, AG25326, DK067158, DK41612, DK081423, DK081523, and DK20543), the O’Brien Center of Kidney Research (DK-079328), American Heart Association Western Affiliate (0865235F), Eisai Research Fund, Ellison Medical Foundation, Ted Nash Long Life Foundation, and the Charles and Jane Pak Center of Mineral Metabolism.
Footnotes
Conflict of interest statement: None declared.
References
- 1.Kuro-o M, Matsumura Y, Aizawa H, et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997;390:45–51. doi: 10.1038/36285. [DOI] [PubMed] [Google Scholar]
- 2.Shiraki-Iida T, Aizawa H, Matsumura Y, et al. Structure of the mouse klotho gene and its two transcripts encoding membrane and secreted protein. FEBS Lett. 1998;424:6–10. doi: 10.1016/s0014-5793(98)00127-6. [DOI] [PubMed] [Google Scholar]
- 3.Matsumura Y, Aizawa H, Shiraki-Iida T, et al. Identification of the human klotho gene and its two transcripts encoding membrane and secreted klotho protein. Biochem Biophys Res Commun. 1998;242:626–630. doi: 10.1006/bbrc.1997.8019. [DOI] [PubMed] [Google Scholar]
- 4.Chen CD, Podvin S, Gillespie E, et al. Insulin stimulates the cleavage and release of the extracellular domain of Klotho by ADAM10 and ADAM17. Proc Natl Acad Sci U S A. 2007;104:19796–19801. doi: 10.1073/pnas.0709805104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bloch L, Sineshchekova O, Reichenbach D, et al. Klotho is a substrate for alpha-, beta- and gamma-secretase. FEBS Lett. 2009;583:3221–3224. doi: 10.1016/j.febslet.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Imura A, Iwano A, Tohyama O, et al. Secreted Klotho protein in sera and CSF: implication for post-translational cleavage in release of Klotho protein from cell membrane. FEBS Lett. 2004;565:143–147. doi: 10.1016/j.febslet.2004.03.090. [DOI] [PubMed] [Google Scholar]
- 7.Li SA, Watanabe M, Yamada H, et al. Immunohistochemical localization of Klotho protein in brain, kidney, and reproductive organs of mice. Cell Struct Funct. 2004;29:91–99. doi: 10.1247/csf.29.91. [DOI] [PubMed] [Google Scholar]
- 8.Hu MC, Shi M, Zhang J, et al. Klotho: a novel phosphaturic substance acting as an autocrine enzyme in the renal proximal tubule. FASEB J. 2010;24:3438–3450. doi: 10.1096/fj.10-154765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ben-Dov IZ, Galitzer H, Lavi-Moshayoff V, et al. The parathyroid is a target organ for FGF23 in rats. J Clin Invest. 2007;117:4003–4008. doi: 10.1172/JCI32409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hofman-Bang J, Martuseviciene G, Santini MA, Olgaard K, Lewin E. Increased parathyroid expression of klotho in uremic rats. Kidney Int. 2010 doi: 10.1038/ki.2010.215. (in press). [DOI] [PubMed] [Google Scholar]
- 11.Takeshita K, Fujimori T, Kurotaki Y, et al. Sinoatrial node dysfunction and early unexpected death of mice with a defect of klotho gene expression. Circulation. 2004;109:1776–1782. doi: 10.1161/01.CIR.0000124224.48962.32. [DOI] [PubMed] [Google Scholar]
- 12.Cha SK, Hu MC, Kurosu H, et al. Regulation of renal outer medullary potassium channel and renal K(+) excretion by Klotho. Mol Pharmacol. 2009;76:38–46. doi: 10.1124/mol.109.055780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Razzaque MS, Sitara D, Taguchi T, et al. Premature aging-like phenotype in fibroblast growth factor 23 null mice is a vitamin D-mediated process. FASEB J. 2006;20:720–722. doi: 10.1096/fj.05-5432fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Razzaque MS. The FGF23-Klotho axis: endocrine regulation of phosphate homeostasis. Nat Rev Endocrinol. 2009;5:611–619. doi: 10.1038/nrendo.2009.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Razzaque MS, Lanske B. Hypervitaminosis D and premature aging: lessons learned from Fgf23 and Klotho mutant mice. Trends Mol Med. 2006;12:298–305. doi: 10.1016/j.molmed.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 16.Urakawa I, Yamazaki Y, Shimada T, et al. Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature. 2006;444:770–774. doi: 10.1038/nature05315. [DOI] [PubMed] [Google Scholar]
- 17.Goetz R, Nakada Y, Hu MC, et al. Isolated C-terminal tail of FGF23 alleviates hypophosphatemia by inhibiting FGF23-FGFR-Klotho complex formation. Proc Natl Acad Sci U S A. 2010;107:407–412. doi: 10.1073/pnas.0902006107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kurosu H, Ogawa Y, Miyoshi M, et al. Regulation of fbro-blast growth factor-23 signaling by klotho. J Biol Chem. 2006;281:6120–6123. doi: 10.1074/jbc.C500457200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Razzaque MS, Lanske B. The emerging role of the fibroblast growth factor-23-klotho axis in renal regulation of phosphate homeostasis. J Endocrinol. 2007;194:1–10. doi: 10.1677/JOE-07-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang CL. Regulation of ion channels by secreted Klotho: mechanisms and implications. Kidney Int. 2010;77:855–860. doi: 10.1038/ki.2010.73. [DOI] [PubMed] [Google Scholar]
- 21.Rakugi H, Matsukawa N, Ishikawa K, et al. Anti-oxidative effect of Klotho on endothelial cells through cAMP activation. Endocrine. 2007;31:82–87. doi: 10.1007/s12020-007-0016-9. [DOI] [PubMed] [Google Scholar]
- 22.de Oliveira RM. Klotho RNAi induces premature senescence of human cells via a p53/p21 dependent pathway. FEBS Lett. 2006;580:5753–5758. doi: 10.1016/j.febslet.2006.09.036. [DOI] [PubMed] [Google Scholar]
- 23.Ikushima M, Rakugi H, Ishikawa K, et al. Anti-apoptotic and anti-senescence effects of Klotho on vascular endothelial cells. Biochem Biophys Res Commun. 2006;339:827–832. doi: 10.1016/j.bbrc.2005.11.094. [DOI] [PubMed] [Google Scholar]
- 24.Kuro-o M. Klotho as a regulator of oxidative stress and senescence. Biol Chem. 2008;389:233–241. doi: 10.1515/BC.2008.028. [DOI] [PubMed] [Google Scholar]
- 25.Liu H, Fergusson MM, Castilho RM, et al. Augmented Wnt signaling in a mammalian model of accelerated aging. Science. 2007;317:803–806. doi: 10.1126/science.1143578. [DOI] [PubMed] [Google Scholar]
- 26.Kuro-o M. A potential link between phosphate and aging-lessons from Klotho-deficient mice. Mech Ageing Dev. 2010;131:270–275. doi: 10.1016/j.mad.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kuro-o M. Klotho and aging. Biochim Biophys Acta. 2009;1790:1049–1058. doi: 10.1016/j.bbagen.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burke SK. Phosphate is a uremic toxin. J Ren Nutr. 2008;18:27–32. doi: 10.1053/j.jrn.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 29.Kuro-o M. Klotho as a regulator of fibroblast growth factor signaling and phosphate/calcium metabolism. Curr Opin Nephrol Hypertens. 2006;15:437–441. doi: 10.1097/01.mnh.0000232885.81142.83. [DOI] [PubMed] [Google Scholar]
- 30.Kurosu H, Yamamoto M, Clark JD, et al. Suppression of aging in mice by the hormone Klotho. Science. 2005;309:1829–1833. doi: 10.1126/science.1112766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shiraki-Iida T, Iida A, Nabeshima Y, et al. Improvement of multiple pathophysiological phenotypes of klotho (kl/kl) mice by adenovirus-mediated expression of the klotho gene. J Gene Med. 2000;2:233–242. doi: 10.1002/1521-2254(200007/08)2:4<233::AID-JGM110>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 32.Segawa H, Yamanaka S, Ohno Y, et al. Correlation between hyperphosphatemia and type II Na-Pi cotransporter activity in klotho mice. Am J Physiol Renal Physiol. 2007;292:F769–F779. doi: 10.1152/ajprenal.00248.2006. [DOI] [PubMed] [Google Scholar]
- 33.Chang Q, Hoefs S, van der Kemp AW, et al. The beta-glucuronidase klotho hydrolyzes and activates the TRPV5 channel. Science. 2005;310:490–493. doi: 10.1126/science.1114245. [DOI] [PubMed] [Google Scholar]
- 34.Cha SK, Ortega B, Kurosu H, et al. Removal of sialic acid involving Klotho causes cell-surface retention of TRPV5 channel via binding to galectin-1. Proc Natl Acad Sci U S A. 2008;105:9805–9810. doi: 10.1073/pnas.0803223105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Imura A, Tsuji Y, Murata M, et al. alpha-Klotho as a regulator of calcium homeostasis. Science. 2007;316:1615–1618. doi: 10.1126/science.1135901. [DOI] [PubMed] [Google Scholar]
- 36.Villa-Bellosta R, Ravera S, Sorribas V, et al. The Na+-Pi cotransporter PiT-2 (SLC20A2) is expressed in the apical membrane of rat renal proximal tubules and regulated by dietary Pi. Am J Physiol Renal Physiol. 2009;296:F691–F699. doi: 10.1152/ajprenal.90623.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moe OW. PiT-2 coming out of the pits. Am J Physiol Renal Physiol. 2009;296:F689–F690. doi: 10.1152/ajprenal.00007.2009. [DOI] [PubMed] [Google Scholar]
- 38.Breusegem SY, Takahashi H, Giral-Arnal H, et al. Differential regulation of the renal sodium-phosphate cotransporters Na-Pi-IIa, NaPi-IIc, and PiT-2 in dietary potassium deficiency. Am J Physiol Renal Physiol. 2009;297:F350–F361. doi: 10.1152/ajprenal.90765.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bacic D, Lehir M, Biber J, et al. The renal Na+/phosphate cotransporter NaPi-IIa is internalized via the receptor-mediated endocytic route in response to parathyroid hormone. Kidney Int. 2006;69:495–503. doi: 10.1038/sj.ki.5000148. [DOI] [PubMed] [Google Scholar]
- 40.Tohyama O, Imura A, Iwano A, et al. Klotho is a novel be-ta-glucuronidase capable of hydrolyzing steroid beta-glucuronides. J Biol Chem. 2004;279:9777–9784. doi: 10.1074/jbc.M312392200. [DOI] [PubMed] [Google Scholar]
- 41.Hayes G, Busch A, Lotscher M, et al. Role of N-linked glycosylation in rat renal Na/Pi-cotransport. J Biol Chem. 1994;269:24143–24149. [PubMed] [Google Scholar]
- 42.O’Neill WC, Sigrist MK, McIntyre CW. Plasma pyrophosphate and vascular calcification in chronic kidney disease. Nephrol Dial Transplant. 2010;25:187–191. doi: 10.1093/ndt/gfp362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ramos AM, Albalate M, Vázquez S, Caramelo C, Egido J, Ortiz A. Hyperphosphatemia and hyperparathyroidism in incident chronic kidney disease patients. Kidney Int Suppl. 2008;(111):S88–S93. doi: 10.1038/ki.2008.543. [DOI] [PubMed] [Google Scholar]
- 44.Kestenbaum B, Sampson JN, Rudser KD, et al. Serum phosphate levels and mortality risk among people with chronic kidney disease. J Am Soc Nephrol. 2005;16:520–528. doi: 10.1681/ASN.2004070602. [DOI] [PubMed] [Google Scholar]
- 45.Barsotti G, Morelli E, Giannoni A, et al. Restricted phosphorus and nitrogen intake to slow the progression of chronic renal failure: a controlled trial. Kidney Int Suppl. 1983;16:S278–S284. [PubMed] [Google Scholar]
- 46.Koizumi T, Murakami K, Nakayama H, et al. Role of dietary phosphorus in the progression of renal failure. Biochem Bio-phys Res Commun. 2002;295:917–921. doi: 10.1016/s0006-291x(02)00793-3. [DOI] [PubMed] [Google Scholar]
- 47.Suki WN, Zabaneh R, Cangiano JL, et al. Effects of sevelamer and calcium-based phosphate binders on mortality in hemo-dialysis patients. Kidney Int. 2007;72:1130–1137. doi: 10.1038/sj.ki.5002466. [DOI] [PubMed] [Google Scholar]
- 48.Isakova T, Gutierrez OM, Chang Y, et al. Phosphorus binders and survival on hemodialysis. J Am Soc Nephrol. 2009;20:388–396. doi: 10.1681/ASN.2008060609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tonelli M, Wang W, Hemmelgarn B, et al. Phosphate removal with several thrice-weekly dialysis methods in overweight he-modialysis patients. Am J Kidney Dis. 2009;54:1108–1115. doi: 10.1053/j.ajkd.2009.05.018. [DOI] [PubMed] [Google Scholar]
- 50.Papaconstantinou J. Insulin/IGF-1 and ROS signaling pathway cross-talk in aging and longevity determination. Mol Cell Endocrinol. 2009;299:89–100. doi: 10.1016/j.mce.2008.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Diepeveen SH, Verhoeven GH, van der Palen J, et al. Oxidative stress in patients with end-stage renal disease prior to the start of renal replacement therapy. Nephron Clin Pract. 2004;98:c3–c7. doi: 10.1159/000079921. [DOI] [PubMed] [Google Scholar]
- 52.Himmelfarb J, McMonagle E, Freedman S, et al. Oxidative stress is increased in critically ill patients with acute renal failure. J Am Soc Nephrol. 2004;15:2449–2456. doi: 10.1097/01.ASN.0000138232.68452.3B. [DOI] [PubMed] [Google Scholar]
- 53.Agarwal R. Chronic kidney disease is associated with oxi-dative stress independent of hypertension. Clin Nephrol. 2004;61:377–383. doi: 10.5414/cnp61377. [DOI] [PubMed] [Google Scholar]
- 54.Locatelli F, Canaud B, Eckardt KU, et al. Oxidative stress in end-stage renal disease: an emerging threat to patient outcome. Nephrol Dial Transplant. 2003;18:1272–1280. doi: 10.1093/ndt/gfg074. [DOI] [PubMed] [Google Scholar]
- 55.Tiwari AK, Prasad P, B KT, et al. Oxidative stress pathway genes and chronic renal insufficiency in Asian Indians with Type 2 diabetes. J Diabetes Complications. 2009;23:102–111. doi: 10.1016/j.jdiacomp.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 56.Sugiura H, Yoshida T, Tsuchiya K, et al. Klotho reduces apop-tosis in experimental ischaemic acute renal failure. Nephrol Dial Transplant. 2005;20:2636–2645. doi: 10.1093/ndt/gfi165. [DOI] [PubMed] [Google Scholar]
- 57.Sugiura H, Yoshida T, Mitobe M, et al. Klotho reduces apop-tosis in experimental ischaemic acute kidney injury via HSP-70. Nephrol Dial Transplant. 2010;25:60–68. doi: 10.1093/ndt/gfp451. [DOI] [PubMed] [Google Scholar]
- 58.Mitani H, Ishizaka N, Aizawa T, et al. In vivo klotho gene transfer ameliorates angiotensin II-induced renal damage. Hypertension. 2002;39:838–843. doi: 10.1161/01.hyp.0000013734.33441.ea. [DOI] [PubMed] [Google Scholar]
- 59.Saito K, Ishizaka N, Mitani H, et al. Iron chelation and a free radical scavenger suppress angiotensin II-induced down-regulation of klotho, an anti-aging gene, in rat. FEBS Lett. 2003;551:58–62. doi: 10.1016/s0014-5793(03)00894-9. [DOI] [PubMed] [Google Scholar]
- 60.Wang Y, Sun Z. Klotho gene delivery prevents the progression of spontaneous hypertension and renal damage. Hypertension. 2009;54:810–817. doi: 10.1161/HYPERTENSIONAHA.109.134320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Saito Y, Nakamura T, Ohyama Y, et al. In vivo klotho gene delivery protects against endothelial dysfunction in multiple risk factor syndrome. Biochem Biophys Res Commun. 2000;276:767–772. doi: 10.1006/bbrc.2000.3470. [DOI] [PubMed] [Google Scholar]
- 62.Koh N, Fujimori T, Nishiguchi S, et al. Severely reduced production of klotho in human chronic renal failure kidney. Bio-chem Biophys Res Commun. 2001;280:1015–1020. doi: 10.1006/bbrc.2000.4226. [DOI] [PubMed] [Google Scholar]
- 63.Haruna Y, Kashihara N, Satoh M, et al. Amelioration of progressive renal injury by genetic manipulation of Klotho gene. Proc Natl Acad Sci U S A. 2007;104:2331–2336. doi: 10.1073/pnas.0611079104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yamagishi T, Saito Y, Nakamura T, et al. Troglitazone improves endothelial function and augments renal klotho mRNA expression in Otsuka Long-Evans Tokushima Fatty (OLETF) rats with multiple atherogenic risk factors. Hypertens Res. 2001;24:705–709. doi: 10.1291/hypres.24.705. [DOI] [PubMed] [Google Scholar]
- 65.Hu MC, Shi M, Zhang J, Quiñones H, Kuro-o M, Moe OM. Klotho deficiency is an early biomarker of renal ischemia-reperfusion injury and its replacement is protective. Kidney Int. 2010 doi: 10.1038/ki.2010.328. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hu MC, Shi M, Zhang J, et al. Klotho deficiency and vascular calcification in chronic kidney disease. J Am Soc Nephrol. 2010 doi: 10.1681/ASN.2009121311. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mitobe M, Yoshida T, Sugiura H, et al. Oxidative stress decreases klotho expression in a mouse kidney cell line. Nephron Exp Nephrol. 2005;101:e67–e74. doi: 10.1159/000086500. [DOI] [PubMed] [Google Scholar]
- 68.Thurston RD, Larmonier CB, Majewski PM, et al. Tumor necrosis factor and interferon-gamma down-regulate Klotho in mice with colitis. Gastroenterology. 2010;138:1384–1394. doi: 10.1053/j.gastro.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Goes N, Urmson J, Ramassar V, et al. Ischemic acute tubular necrosis induces an extensive local cytokine response: evidence for induction of interferon-gamma, transforming growth factor-beta 1, granulocyte-macrophage colony-stimulating factor, interleukin-2, and interleukin-10. Transplantation. 1995;59:565–572. [PubMed] [Google Scholar]
- 70.Yu J, Deng M, Zhao J, et al. Decreased expression of klotho gene in uremic atherosclerosis in apolipoprotein E-deficient mice. Biochem Biophys Res Commun. 2010;391:261–266. doi: 10.1016/j.bbrc.2009.11.046. [DOI] [PubMed] [Google Scholar]
- 71.Aizawa H, Saito Y, Nakamura T, et al. Downregulation of the Klotho gene in the kidney under sustained circulatory stress in rats. Biochem Biophys Res Commun. 1998;249:865–871. doi: 10.1006/bbrc.1998.9246. [DOI] [PubMed] [Google Scholar]
- 72.Schrier RW, Wang W, Poole B, et al. Acute renal failure: def-nitions, diagnosis, pathogenesis, and therapy. J Clin Invest. 2004;114:5–14. doi: 10.1172/JCI22353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Thakar CV, Quate-Operacz M, Leonard AC, et al. Outcomes of hemodialysis patients in a long-term care hospital setting: a single-center study. Am J Kidney Dis. 2010;55:300–306. doi: 10.1053/j.ajkd.2009.08.021. [DOI] [PubMed] [Google Scholar]
- 74.Macedo E, Bouchard J, Mehta RL. Renal recovery following acute kidney injury. Curr Opin Crit Care. 2008;14:660–665. doi: 10.1097/MCC.0b013e328317ee6e. [DOI] [PubMed] [Google Scholar]
- 75.Shimada T, Takeshita Y, Murohara T, et al. Angiogenesis and vasculogenesis are impaired in the precocious-aging klotho mouse. Circulation. 2004;110:1148–1155. doi: 10.1161/01.CIR.0000139854.74847.99. [DOI] [PubMed] [Google Scholar]
- 76.Voormolen N, Noordzij M, Grootendorst DC, et al. High plasma phosphate as a risk factor for decline in renal function and mortality in pre-dialysis patients. Nephrol Dial Transplant. 2007;22:2909–2916. doi: 10.1093/ndt/gfm286. [DOI] [PubMed] [Google Scholar]
- 77.Jean G, Terrat JC, Vanel T, et al. High levels of serum fbro-blast growth factor (FGF)-23 are associated with increased mortality in long haemodialysis patients. Nephrol Dial Transplant. 2009;24:2792–2796. doi: 10.1093/ndt/gfp191. [DOI] [PubMed] [Google Scholar]
- 78.Gordon PL, Frassetto LA. Management of osteoporosis in CKD Stages 3 to 5. Am J Kidney Dis. 2010;55:941–956. doi: 10.1053/j.ajkd.2010.02.338. [DOI] [PubMed] [Google Scholar]
- 79.Coladonato JA. Control of hyperphosphatemia among patients with ESRD. J Am Soc Nephrol. 2005 Suppl 2:S107–S114. doi: 10.1681/ASN.2005060663. [DOI] [PubMed] [Google Scholar]
- 80.Sprague SM, Abboud H, Qiu P, et al. Lanthanum carbonate reduces phosphorus burden in patients with CKD stages 3 and 4: a randomized trial. Clin J Am Soc Nephrol. 2009;4:178–185. doi: 10.2215/CJN.02830608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wolf M. Fibroblast growth factor 23 and the future of phosphorus management. Curr Opin Nephrol Hypertens. 2009;18:463–468. doi: 10.1097/MNH.0b013e328331a8c8. [DOI] [PubMed] [Google Scholar]
- 82.Navaneethan SD, Palmer SC, Craig JC, et al. Benefits and harms of phosphate binders in CKD: a systematic review of randomized controlled trials. Am J Kidney Dis. 2009;54:619–637. doi: 10.1053/j.ajkd.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 83.Maekawa Y, Ishikawa K, Yasuda O, et al. Klotho suppresses TNF-alpha-induced expression of adhesion molecules in the endothelium and attenuates NF-kappaB activation. Endocrine. 2009;35:341–346. doi: 10.1007/s12020-009-9181-3. [DOI] [PubMed] [Google Scholar]
- 84.Nagai R, Saito Y, Ohyama Y, et al. Endothelial dysfunction in the klotho mouse and downregulation of klotho gene expression in various animal models of vascular and metabolic diseases. Cell Mol Life Sci. 2000;57:738–746. doi: 10.1007/s000180050038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nakamura T, Saito Y, Ohyama Y, et al. Production of nitric oxide, but not prostacyclin, is reduced in klotho mice. Jpn J Pharmacol. 2002;89:149–156. doi: 10.1254/jjp.89.149. [DOI] [PubMed] [Google Scholar]
- 86.Saito Y, Yamagishi T, Nakamura T, et al. Klotho protein protects against endothelial dysfunction. Biochem Biophys Res Commun. 1998;248:324–329. doi: 10.1006/bbrc.1998.8943. [DOI] [PubMed] [Google Scholar]
- 87.Li X, Yang HY, Giachelli CM. Role of the sodium-dependent phosphate cotransporter, Pit-1, in vascular smooth muscle cell calcification. Circ Res. 2006;98:905–912. doi: 10.1161/01.RES.0000216409.20863.e7. [DOI] [PubMed] [Google Scholar]
- 88.Tomson C. Vascular calcification in chronic renal failure. Nephron Clin Pract. 2003;93:c124–c130. doi: 10.1159/000070231. [DOI] [PubMed] [Google Scholar]
- 89.Villa-Bellosta R, Bogaert YE, Levi M, et al. Characterization of phosphate transport in rat vascular smooth muscle cells: implications for vascular calcification. Arterioscler Thromb Vasc Biol. 2007;27:1030–1036. doi: 10.1161/ATVBAHA.106.132266. [DOI] [PubMed] [Google Scholar]
- 90.Wang CC, Sorribas V, Sharma G, et al. Insulin attenuates vascular smooth muscle calcification but increases vascular smooth muscle cell phosphate transport. Atherosclerosis. 2007;195:e65–e75. doi: 10.1016/j.atherosclerosis.2007.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Giachelli CM. The emerging role of phosphate in vascular cal-cifcation. Kidney Int. 2009;75:890–897. doi: 10.1038/ki.2008.644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Giachelli CM. Vascular calcification: in vitro evidence for the role of inorganic phosphate. J Am Soc Nephrol. 2003;14:S300–S304. doi: 10.1097/01.asn.0000081663.52165.66. [DOI] [PubMed] [Google Scholar]