Abstract
Background
To evaluate how smoking affects the time to disease quiescence and time to disease recurrence in patients with ocular inflammation.
Methods
A retrospective cohort study of patients with ocular inflammation who were followed longitudinally and had smoking information available in the Systemic Immunosuppressive Therapy for Eye Diseases Cohort Study database.
Results
Among 2676 patients with active ocular inflammation, smokers were more likely to have bilateral ocular disease and poorer visual acuity on presentation compared with non-smokers and previous smokers. In a multivariate analysis, there was no statistically significant difference in the time to disease quiescence between groups. However, the median time to recurrence of ocular inflammation was statistically significantly longer for non-smokers (9.4 months) and for previous smokers (10.7 months) than for current smokers (7.8 months) (p=0.02). The RR of ocular inflammation recurrence was higher for smokers than for non-smokers (adjusted HR=1.19, 95% CI 1.03 to 1.37) and tended towards significance in previous smokers (adjusted HR=1.11, 95% CI 0.93 to 1.35).
Conclusions
Smoking was associated with an increased likelihood of bilateral ocular inflammation and reduced vision upon presentation, and an increased risk of recurrence compared with not smoking. These results suggest that patients with ocular inflammation should be counselled to stop smoking as part of routine management.
INTRODUCTION
Ocular inflammatory disease encompasses a heterogeneous group of disorders that may affect one or more anatomic locations of the eye, including uveitis (anterior, intermediate, posterior and panuveitis), scleritis and inflammation of the ocular surface (eg, mucous membrane pemphigoid). Ocular inflammation may affect any age group,1 from young children2 to older adults.3,4 Women are affected more often than men.1,4,5
While some patients with non-infectious ocular inflammation respond well to topical corticosteroids and oral non-steroidal anti-inflammatory agents, in the tertiary setting many require oral corticosteroids and immunosuppressive drugs to control their disease.1,5 Because such treatment is not always effective and has potential risks, it would be desirable to identify modifiable risk factors which potentially could reduce the need for treatment to control ocular inflammation.
Smoking has previously been implicated as delaying treatment response among patients with episcleritis and scleritis,6 and has been associated with an increased frequency of cystoid macular oedema at presentation among patients with intermediate uveitis.7 To assess whether smoking affects responsiveness to treatment and/or increases the risk of recurrence of ocular inflammation, we evaluated the relationship between smoking and the kinetics of ocular inflammation in a large cohort of patients with ocular inflammation.
METHODS
Study population
The methods of the Systemic Immunosuppressive Therapy for Eye Diseases (SITE) Cohort Study have been described previously.1 In brief, all patients with non-infectious ocular inflammatory diseases seen since the inception of the centre at four subspeciality ocular inflammation centres, and an ~40% random sample of such patients at a fifth centre, were eligible for inclusion in the SITE cohort. Patients with known HIV infection and those with infectious ocular inflammation were excluded. For this analysis, data from one of the first four centres were excluded, because the centre’s co-management approach substantially overestimated ascertainment of the time to quiescence and recurrence, because many follow-up visits were conducted elsewhere. Among the remaining patients (n=6325), those for whom smoking status was known and who were observed to have active inflammation at some point during follow-up and had one or more follow-up visits after that point were included in this analysis. A total of 3649 patients were therefore excluded because they (1) had no smoking status information (n=1021); (2) had no active disease during observation (n=1790); or (3) had no follow-up visits after active inflammation was observed (n=838), leaving a total of 2676 patients who were ‘at risk’ for events of interest. While several differences in baseline demographic information were observed between patients included in and excluded from the study, these differences were not large and reached statistical significance only due to the large number of patients in the study. A clinically significant difference in the prevalence of cataract surgery was noted as patients included in the study were more likely to have undergone cataract extraction compared with patients excluded from the study (table 1).
Table 1.
Baseline demographic information and eye-specific characteristics in patients included in and excluded from this study
| Included | Excluded | p Value | |
|---|---|---|---|
| Patient characteristic | |||
| Number | 2676 | 3649 | |
| Gender, male (%) | 935 (35%) | 1358 (37%) | 0.12 |
| Race White (%) | 1949 (73%) | 2705 (74%) | <0.001 |
| Black (%) | 97 (4%) | 621 (17%) | |
| Other (%) | 547 (20%) | 182 (5%) | |
| Asian (%) | 83 (3%) | 141 (4%) | |
| Age, years±SD | 45±19 | 44±19 | 0.08 |
| Time from diagnosis to first visit, months±SD |
38±70 | 51±83 | <0.001 |
| Eye, bilateral disease | 1791 (67%) | 2555 (70%) | 0.008 |
| Eye-specific characteristic* | |||
| Inflammation type | |||
| Anterior uveitis (%) | 1018 (38%) | 1614 (44%) | <0.001 |
| Intermediate uveitis (%) | 348 (13%) | 478 (13%) | |
| Posterior or panuveitis (%) | 568 (21%) | 885 (24%) | |
| Scleritis (%) | 386 (14%) | 365 (10%) | |
| MMP (%) | 251 (9%) | 203 (6%) | |
| Other (%) | 105 (4%) | 103 (3%) | |
| Snellen-equivalent visual acuity 20/50 or worse (%) |
1336 (50%) | 1679 (46%) | 0.002 |
| Snellen-equivalent visual acuity 20/200 or worse (%) |
722 (27%) | 938 (26%) | 0.26 |
| History of cataract surgery (%) |
956 (36%) | 804 (22%) | <0.001 |
Eye-specific characteristics were considered present if either eye had the listed finding. MMP, mucous membrane pemphigoid.
The Institutional Review Boards of all institutions reviewed and approved this study, which was conducted in accordance with the principles of the Declaration of Helsinki.
Data collection
All data were obtained via a retrospective chart review and entered by expert reviewers into a standardised computerised database prepared specifically for the SITE Cohort Study, using a suite of quality control mechanisms designed to minimise errors.1 The data utilised for this analysis included age, gender and race; centre where treatment was administered; laterality of inflammation; type of ocular inflammation; visual acuity (equal to or worse than 20/50 and 20/200) as obtained by Snellen visual acuity charts; history of cataract surgery; smoking status (current smoker (smoking at the present time), previous smoker (smoked previously but not smoking at present) or non-smoker (never smoked)); and inflammatory status (inactive, active or slightly active). Inflammatory status was categorised for every eye at every visit, based on the clinician’s notations at the time of each visit. The study defined ‘slightly active’ inflammation as ‘activity that is minimally present, described also by terms such as mild, few or trace cells, etc.’, whereas inflammation was scored as inactive when described by terms such as ‘quiet’, ‘quiescent’, ‘no cells’ and ‘no active inflammation’. For the purposes of the analysis, success in controlling inflammation was considered to have been achieved when a patient improved from ‘active’ to either ‘slightly active’ or ‘inactive’, whereas recurrence or relapse of disease was considered to have occurred when the reverse took place.
Main outcome measures
The main outcome measures were time to ocular inflammation quiescence (defined as the time from active disease to inactive disease) and time to ocular inflammation recurrence (defined as time from inactive disease to active disease)—outcome measures which directly reflect the underlying inflammatory condition. Furthermore, previous studies have demonstrated that smokers with scleritis and episcleritis have a delayed response to treatment.6
Statistical analysis
All statistical analyses were performed using the SPSS statistical package (SPSS, Cary, North Carolina, USA). All p values are two-sided and nominal. Continuous variables were compared between the groups using analysis of variance (ANOVA) and categorical variables were compared using the χ2 test. Time to disease quiescence and to relapse were compared using Kaplan–Meier survival curves and the log rank test. Univariate and multivariate Cox proportional hazards modelling was used to evaluate prognostic factors associated with disease control and relapse. Analyses were conducted by patient rather than by eye; patients were considered to have active disease if either eye had active ocular inflammation, and were considered quiescent when both eyes were inactive. Univariant analyses were not corrected for multiplicity (Bonferroni), and therefore all p values should be considered as ‘nominal p values.’
RESULTS
Study population
Initial demographic and clinical characteristics of these patients are summarised in table 2.
Table 2.
Baseline demographic information and eye-specific characteristics by smoking status in patients at the first visit when active inflammation was observed
| Non-smoker | Previous smoker |
Current smoker |
p Value | |
|---|---|---|---|---|
| Patient characteristic | ||||
| Number | 1752 | 401 | 523 | |
| Gender, male (%) | 589 (34%) | 161 (40%) | 185 (35%) | 0.05 |
| Race White | 1280 (73%) | 286 (71%) | 383 (73%) | <0.001 |
| Black | 330 (19%) | 101 (25%) | 116 (22%) | |
| Other | 72 (4%) | 10 (3%) | 15 (3%) | |
| Asian | 70 (4%) | 4 (1%) | 9 (2%) | |
| Age, years±SD | 45±21 | 55±16 | 42±13 | <0.001 |
| Time from diagnosis to first visit, months±SD |
36±70 | 43±75 | 40±65 | 0.19 |
| Eye, bilateral disease |
1147 (65%) | 269 (67%) | 375 (72%) | 0.03 |
| Eye -specific characteristic* | ||||
| Inflammation type | ||||
| Anterior uveitis | 512 (29%) | 115 (29%) | 141 (27%) | |
| Intermediate uveitis | 457 (26%) | 76 (19%) | 129 (25%) | |
| Posterior or panuveitis | 337 (19%) | 83 (21%) | 107 (21%) | 0.003 |
| Scleritis | 214 (12%) | 56 (14%) | 85 (16%) | |
| MMP | 163 (9%) | 55 (14%) | 53 (10%) | |
| Other | 68 (4%) | 16 (4%) | 8 (2%) | |
| Snellen-equivalent visual acuity 20/50 or worse |
806 (46%) | 188 (47%) | 301 (58%) | <0.001 |
| Snellen-equivalent visual acuity 20/200 or worse |
397 (23%) | 93 (23%) | 156 (30%) | 0.003 |
| History of cataract surgery | 601 (34%) | 160 (40%) | 154 (29%) | 0.004 |
Eye-specific characteristics were considered present if either eye had the listed finding. MMP, mucous membrane pemphigoid.
Overall, 20% of patients in our cohort were current smokers, a figure that is similar to the National Health and Nutrition Examination Survey estimate that 21% of the US population age ≥18 currently smokes.8 Males were over-represented among former smokers (40%) with respect to non-smokers (34%) and current smokers (35%) (overall p=0.05). The proportion of non-smokers who were Asian was relatively higher than the proportion of former smokers and current smokers who were Asian (4%, vs 1% and 2%, respectively), whereas the proportion of non-smokers who were African-American was relatively low (19%, vs 25% and 22%) (overall p<0.001). Current smokers were also significantly more likely to have bilateral ocular inflammation (64%; 95% CI 60% to 68%) than either former smokers (55%; 95% CI 50% to 60%) or non-smokers (56%; 95% CI 54% to 58%) (overall p=0.03). Eye-specific characteristics differed in that more eyes of current smokers (58%; 95% CI 54% to 62%) had a Snellen-equivalent visual acuity of 20/50 or worse compared with eyes of non-smokers (46%; CI 44% to 48%) and previous smokers (47%; 95% CI 42% to 52%) (overall p<0.001). A contributing factor to the poorer vision may have been the finding that fewer smokers had a history of cataract extraction in one or both eyes at the beginning of follow-up (29%; 95% CI 25% to 33%) compared with non-smokers (34%; 95% CI 31% to 36%) and previous smokers (40%; 95% CI 35% to 45%) (overall p=0.004).
Time to control of ocular inflammation
During observation, disease quiescence was achieved in 1567 non-smokers (89%), 361 previous smokers (90%) and 444 current smokers (85%). Smokers experienced a longer time to control of ocular inflammation compared with non-smokers (p=0.02) and previous smokers (p=0.04) (overall log rank p=0.05). The median time to resolution of ocular inflammation was 1.4 months for non-smokers (95% CI 1.3 to 1.5), 1.3 months for previous smokers (95% CI 1.1 to 1.4) and 1.7 months for current smokers (95% CI 1.5 to 1.9). Cox regression analysis (table 3) demonstrated that non-smokers and previous smokers achieved disease quiescence faster than smokers in the crude analysis, but the association was no longer statistically significant after adjusting for confounding factors (non-smokers vs current smokers HR=1.11, 95% CI 1.00 to 1.24, adjusted HR=1.09, 95% CI 0.98 to 1.22; previous smokers vs current smokers HR=1.18, 95% CI 1.03 to 1.36, adjusted HR=1.06, 95% CI 0.92 to 1.23).
Table 3.
Crude and multivariate analysis evaluating prognostic factors to disease quiescence in patients with ocular inflammation
| Crude analysis |
Multivariate analysis |
|||||
|---|---|---|---|---|---|---|
| Prognostic factors | HR | 95% CI | p Value | HR | 95% CI | p Value |
| Smoking status: current smoker | 1.00 | |||||
| Non-smoker/current smoker | 1.11 | 1.00 to 1.24 | 0.05 | 1.09 | 0.98 to 1.22 | 0.11 |
| Previous smoker/current smoker | 1.18 | 1.03 to 1.36 | 0.02 | 1.06 | 0.92 to 1.23 | 0.39 |
| Gender, male/female | 0.97 | 0.89 to 1.06 | 0.51 | * | * | * |
| Race | ||||||
| Black/white | 0.98 | 0.89 to 1.09 | 0.76 | * | * | * |
| Other/white | 1.10 | 0.88 to 1.37 | 0.42 | * | * | * |
| Asian/white | 1.11 | 0.87 to 1.40 | 0.41 | * | * | * |
| Age, by decade | 1.04 | 1.02 to 1.06 | <0.001 | 1.04 | 1.03 to 1.05 | <0.001 |
| Eye | ||||||
| Bilateral/unilateral | 0.76 | 0.70 to 0.84 | <0.001 | 0.77 | 0.70 to 0.84 | <0.001 |
| Site of inflammation Intermediate/anterior uveitis | 0.86 | 0.77 to 0.96 | 0.009 | 0.90 | 0.80 to 1.02 | 0.09 |
| Posterior and pan/anterior uveitis | 0.94 | 0.84 to 1.06 | 0.31 | 0.94 | 0.83 to 1.06 | 0.32 |
| Scleritis/anterior uveitis | 0.89 | 0.78 to 1.02 | 0.09 | 0.90 | 0.78 to 1.02 | 0.11 |
| MMP/anterior uveitis | 0.99 | 0.85 to 1.14 | 0.85 | 0.97 | 0.84 to 1.13 | 0.70 |
| Other/anterior uveitis | 0.96 | 0.76 to 1.21 | 0.73 | 0.93 | 0.74 to 1.18 | 0.55 |
| Snellen-equivalent visual acuity 20/50 or worse | 0.93 | 0.86 to 1.02 | 0.10 | * | * | * |
| History of cataract surgery | 0.94 | 0.87 to 1.03 | 0.21 | * | * | * |
An HR >1 represents an increased likelihood of achieving disease quiescence.
Omitted from multivariate model because no crude association was observed. Multiple regression results also are adjusted for centre effects. MMP, mucous membrane pemphigoid.
Along with smoking status, several factors were significantly associated with the time to disease quiescence. The likelihood of achieving disease quiescence was lower in younger patients (vs older patients) and in patients with bilateral disease (vs unilateral disease). Patients with intermediate uveitis had a marginally significant delay in the time to disease quiescence, with respect to patients with anterior uveitis.
Time to disease recurrence in eyes with ocular inflammation
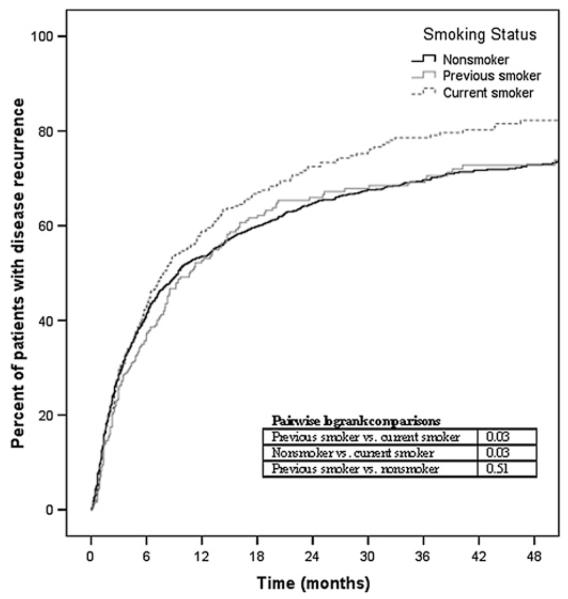
Among patients at risk (ie, patients who achieved disease quiescence), disease reactivation occurred in 845 (54%) non-smokers, 195 previous smokers (54%) and 266 current smokers (60%). Kaplan–Meier curves depicting time to recurrence for the three groups are given in figure 1. The survival curves demonstrate that smokers had a shorter time to disease recurrence compared with non-smokers (p=0.03) and previous smokers (p=0.03) (overall p=0.05). The median time to ocular mation inflam-recurrence was 9.4 months for non-smokers (95% CI 7.7 to 11.2), 10.7 months for previous smokers (95% CI 7.8 to 13.5) and 7.8 months for current smokers (95% CI 5.8 to 9.9). Crude and multivariate Cox regression analysis (table 4) demonstrated that non-smokers were ~15% less likely to experience a recurrence of ocular inflammation compared with smokers (non-smokers vs current smokers HR=0.86, 95% CI 0.75 to 0.98, adjusted HR=0.84, 95% CI 0.73 to 0.97).
Figure 1.
Kaplan–Meier survival analysis demonstrating time to recurrence of inflammation among smokers, previous smokers and non-smokers (overall log rank p=0.05).
Table 4.
Univariate and multivariate analysis evaluating prognostic factors to disease recurrence in patients with ocular inflammation
| Crude analysis |
Multivariate analysis |
|||||
|---|---|---|---|---|---|---|
| Prognostic factors | HR | 95% CI | p Value | HR | 95% CI | p Value |
| Smoking status | ||||||
| Non-smoker/current smoker | 0.86 | 0.75 to 0.98 | 0.03 | 0.84 | 0.73 to 0.97 | 0.02 |
| Previous smoker/current smoker | 0.81 | 0.68 to 0.98 | 0.03 | 0.90 | 0.74 to 1.08 | 0.26 |
| Gender, male/female | 1.06 | 0.94 to 1.19 | 0.34 | * | * | * |
| Race | ||||||
| Black/white | 1.08 | 0.95 to 1.23 | 0.54 | * | * | * |
| Other/white | 0.97 | 0.69 to 1.37 | 0.87 | |||
| Asian/white | 0.97 | 0.69 to 1.36 | 0.85 | |||
| Age, by decade | 0.93 | 0.91 to 0.95 | <0.001 | 0.90 | 0.88 to 0.91 | <0.001 |
| Eye | ||||||
| Bilateral/unilateral | 1.09 | 0.97 to 1.23 | 0.16 | * | * | * |
| Inflammation type | ||||||
| Intermediate/anterior uveitis | 1.05 | 0.91 to 1.22 | 0.53 | |||
| Posterior and pan/anterior uveitis | 0.98 | 0.83 to 1.14 | 0.75 | * | * | * |
| Scleritis/anterior uveitis | 1.06 | 0.89 to 1.27 | 0.52 | |||
| MMP/anterior uveitis | 0.91 | 0.74 to 1.11 | 0.35 | |||
| Other/anterior uveitis | 1.00 | 0.72 to 1.39 | 0.99 | |||
| Snellen-equivalent visual acuity 20/50 or worse | 1.20 | 1.09 to 1.35 | 0.001 | 1.14 | 1.01 to 1.29 | 0.03 |
| History of cataract surgery | 1.23 | 1.11 to 1.39 | <0.001 | 1.37 | 1.21 to 1.55 | <0.001 |
An HR>1 represents an increased likelihood of disease recurrence.
Omitted from multivariate model because no crude association was observed. Multiple regression results also are adjusted for centre effects. MMP, mucous membrane pemphigoid.
Previous smokers also had a longer time to relapse of inflammation than current smokers (HR=0.81, 95% CI 0.68 to 0.96, adjusted HR=0.90, 95% CI 0.74 to 1.08), although the association was no longer statistically significant after adjusting for other factors. The risk of disease recurrence was also increased in younger patients (vs older patients). Recurrence risk did not differ by site of ocular inflammation.
A total of 213 patients under the age of 18 were included in the analysis, most of them non-smokers. In a secondary analysis excluding those 213 patients, the Kaplan–Meier survival curves for time to disease quiescence and time to disease recurrence were unchanged.
Smoking and mortality in the SITE cohort
In the published mortality analysis of the SITE cohort, in which 7957 patients with ocular inflammation were followed over 66 802 person-years for mortality during 1979–2005,1 current smokers were ~60% more likely to die of all causes and of cancer specifically (p<0.001 in all analyses), after adjusting for age, race, sex, site of ocular inflammation, systemic inflammatory disease diagnoses, bilaterality of ocular inflammation, other co-morbidities and use of systemic immunosuppressive drugs using Cox regression (data not shown). In contrast, overall mortality and cancer mortality were estimated to be ~20% higher among former smokers than among non-smokers, a difference which in most analyses was not statistically significant.
DISCUSSION
Our results suggest that smoking has adverse effects on patients with ocular inflammation, including a 17–27% higher risk of relapses of inflammation and an ~60% higher risk of mortality, whereas differences in these outcomes between former smokers and non-smokers were small and usually not statistically significantly different. Smoking was also associated with lower visual acuity at the time of presentation, and a greater likelihood of bilateral inflammation. While this effect is not large, it represents a potential opportunity for clinically meaningful improvement in the course of ocular inflammation using an intervention with no adverse side effects (smoking cessation). Furthermore, smoking cessation has a wide variety of other benefits as smoking is associated with a number of other ocular and systemic diseases.9–12 Smoking cessation has been proposed as one of the single most valuable steps available to increase health and survival.13 These results suggest that patients with ocular inflammation should be counselled to stop smoking as part of routine management.
Few studies have examined the effect of smoking on inflammatory eye disease. A prospective study of patients with Graves disease treated with corticosteroids and orbital irradiation demonstrated that clinical activity scores improved faster and to a greater extent in non-smokers compared with smokers.14 Two retrospective studies examined the effect of smoking on macular oedema in patients with intermediate uveitis; one found an association between smoking and cystoid macular oedema (CME) at presentation7 and another found an association between smoking and reduced vision due to CME (OR 3.2; 95% CI 1.3 to 7.8).15 Of note, patients included in the first macular oedema study were also included in our study as this patient population is part of the SITE database. In a retrospective study examining the effect of smoking in 103 patients with ocular inflammation, Boonman et al found that smokers were more likely to have poor vision at presentation and to experience a delay in treatment response compared with non-smokers.6 The effect of smoking on the time to disease quiescence was much larger in this study than in ours (OR 5.4, 95% CI 1.9 to 15.5 by logistic regression analysis vs HR ~1.15). However, comparisons are difficult given that different definitions were used to assess treatment response, and delay in clinical resolution was defined as a binary outcome (resolution >4 weeks after starting treatment) rather than as a continuous variable. As in our study, Boonman et al found that previous smokers (patients who had not smoked for >1 year) had a clinical course similar to that of non-smokers.
The possible mechanism(s) behind the effects of smoking on treatment response and disease relapse is unclear. Tobacco may act by decreasing the effectiveness of anti-inflammatory medication,16 altering immune responses,17,18 or the many components of tobacco may cause disease directly.19 Clinically, smoking has been linked with the development of several autoimmune disease including rheumatoid arthritis, systemic lupus erythematosus and Graves disease.18,20 Other potential explanations for the noted effect of smoking may include surface irritation due to tobacco smoke, differences in treatment adherence between smokers and non-smokers or former smokers, and smoking-associated co-morbidities.
As with all retrospective studies, our results must be interpreted considering the strengths and limitations of the study. A strength of this study is its large sample size, and ascertainment of smoking status prior to resolution or relapse of inflammation; limitations include the inaccuracy of assessing smoking status retrospectively (eg, some patients may have changed smoking habits after being diagnosed with uveitis but prior to presentation) and the exclusion of 28% of patients at risk of the event due to a lack of smoking information. Furthermore, as smoking has been associated with poorer healthcare utilisation and treatment compliance,21–23 the more severe disease manifestations seen at baseline and the faster time to disease relapse in the smoking group may in part be due to these factors. Given the retrospective nature of the study, it is difficult to evaluate the effect of such factors on the outcome measures. With these limitations in mind, we found that smoking was cross-sectionally associated with an increased risk of having bilateral ocular inflammation and of having reduced visual acuity, and was associated with a modest but clinically relevant shortening of the time between inflammatory relapses among patients with ocular inflammation. In addition, our study confirms that smoking greatly reduces overall survival, specifically in the context of patients with ocular inflammation. These data suggest that cessation of smoking may be beneficial in the management of patients with ocular inflammation.
Acknowledgments
Funding This study was supported primarily by National Eye Institute Grant EY014943 (to JHK). Data analysis was supported by core center grant P30 EY014801 (Bascom Palmer Eye Institute), from the National Eye Institute, unrestricted funds from Research to Prevent Blindness, and the Paul and Evanina Mackall Foundation. JHK is a Research to Prevent Blindness James S. Adams Special Scholar Award recipient. DAJ and JTR are Research to Prevent Blindness Senior Scientific Investigator Award recipients. JET is a Research to Prevent Blindness Harrington Special Scholar Award recipient. GAL-C was previously supported by, and RBN continues to be supported by, intramural funds of the National Eye Institute. AG and EBS receive support from the US Department of Veterans’ Affairs.
Footnotes
Competing interests None.
Ethics approval The Institutional Review Boards of all institutions reviewed and approved this study, which was conducted in accordance with the principles of the Declaration of Helsinki.
Provenance and peer review Not commissioned; externally peer reviewed.
REFERENCES
- 1.Kempen JH, Daniel E, Gangaputra S, et al. Methods for identifying long-term adverse effects of treatment in patients with eye diseases: the systemic immunosuppressive therapy for eye diseases (SITE) cohort study. Ophthalmic Epidemiol. 2008;15:47–55. doi: 10.1080/09286580701585892. [DOI] [PubMed] [Google Scholar]
- 2.Woreta F, Thorne JE, Jabs DA, et al. Risk factors for ocular complications and poor visual acuity at presentation among patients with uveitis associated with juvenile idiopathic arthritis. Am J Ophthalmol. 2007;143:647–55. doi: 10.1016/j.ajo.2006.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thorne JE, Woreta FA, Jabs DA, et al. Treatment of ocular mucous membrane pemphigoid with immunosuppressive drug therapy. Ophthalmology. 2008;115:2146–52. e1. doi: 10.1016/j.ophtha.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 4.Gritz DC, Wong IG. Incidence and prevalence of uveitis in Northern California; the Northern California Epidemiology of Uveitis Study. Ophthalmology. 2004;111:491–500. doi: 10.1016/j.ophtha.2003.06.014. discussion 500. [DOI] [PubMed] [Google Scholar]
- 5.Jabs DA, Mudun A, Dunn JP, et al. Episcleritis and scleritis: clinical features and treatment results. Am J Ophthalmol. 2000;130:469–76. doi: 10.1016/s0002-9394(00)00710-8. [DOI] [PubMed] [Google Scholar]
- 6.Boonman ZF, de Keizer RJ, Watson PG. Smoking delays the response to treatment in episcleritis and scleritis. Eye. 2005;19:949–55. doi: 10.1038/sj.eye.6701731. [DOI] [PubMed] [Google Scholar]
- 7.Thorne JE, Daniel E, Jabs DA, et al. Smoking as a risk factor for cystoid macular edema complicating intermediate uveitis. Am J Ophthalmol. 2008;145:841–6. doi: 10.1016/j.ajo.2007.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention [accessed 6 July 2009];National center for health statistics, US smoking statistics from 2006. http://www.cdc.gov/nchs/fastats/smoking.htm.
- 9.Seddon JM, Reynolds R, Maller J, et al. Prediction model for prevalence and incidence of advanced age-related macular degeneration based on genetic, demographic, and environmental variables. Invest Ophthalmol Vis Sci. 2009;50:2044–53. doi: 10.1167/iovs.08-3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Glynn RJ, Rosner B, Christen WG. Evaluation of risk factors for cataract types in a competing risks framework. Ophthalmic Epidemiol. 2009;16:98–106. doi: 10.1080/09286580902737532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thornton J, Kelly SP, Harrison RA, et al. Cigarette smoking and thyroid eye disease: a systematic review. Eye. 2007;21:1135–45. doi: 10.1038/sj.eye.6702603. [DOI] [PubMed] [Google Scholar]
- 12.Klein R, Knudtson MD, Lee KE, et al. The Wisconsin Epidemiologic Study of Diabetic Retinopathy XXIII: the twenty-five-year incidence of macular edema in persons with type 1 diabetes. Ophthalmology. 2009;116:497–503. doi: 10.1016/j.ophtha.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mokdad AH, Marks JS, Stroup DF, et al. Actual causes of death in the United States, 2000. JAMA. 2004;291:1238–45. doi: 10.1001/jama.291.10.1238. [DOI] [PubMed] [Google Scholar]
- 14.Eckstein A, Quadbeck B, Mueller G, et al. Impact of smoking on the response to treatment of thyroid associated ophthalmopathy. Br J Ophthalmol. 2003;87:773–6. doi: 10.1136/bjo.87.6.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lois N, Abdelkader E, Reglitz K, et al. Environmental tobacco smoke exposure and eye disease. Br J Ophthalmol. 2008;92:1304–10. doi: 10.1136/bjo.2008.141168. [DOI] [PubMed] [Google Scholar]
- 16.Kroon LA. Drug interactions with smoking. Am J Health Syst Pharm. 2007;64:1917–21. doi: 10.2146/ajhp060414. [DOI] [PubMed] [Google Scholar]
- 17.Zeidler R, Albermann K, Lang S. Nicotine and apoptosis. Apoptosis. 2007;12:1927–43. doi: 10.1007/s10495-007-0102-8. [DOI] [PubMed] [Google Scholar]
- 18.Costenbader KH, Karlson EW. Cigarette smoking and autoimmune disease: what can we learn from epidemiology? Lupus. 2006;15:737–45. doi: 10.1177/0961203306069344. [DOI] [PubMed] [Google Scholar]
- 19.Hoffmann D, Hoffmann I, El-Bayoumy K. The less harmful cigarette: a controversial issue. a tribute to Ernst L. Wynder. Chem Res Toxicol. 2001;14:767–90. doi: 10.1021/tx000260u. [DOI] [PubMed] [Google Scholar]
- 20.Harel-Meir M, Sherer Y, Shoenfeld Y. Tobacco smoking and autoimmune rheumatic diseases. Nat Clin Pract Rheumatol. 2007;3:707–15. doi: 10.1038/ncprheum0655. [DOI] [PubMed] [Google Scholar]
- 21.Sabit R, Griffiths TL, Watkins AJ, et al. Predictors of poor attendance at an outpatient pulmonary rehabilitation programme. Respir Med. 2008;102:819–24. doi: 10.1016/j.rmed.2008.01.019. [DOI] [PubMed] [Google Scholar]
- 22.Dobbels F, Vanhaecke J, Dupont L, et al. Pretransplant predictors of posttransplant adherence and clinical outcome: an evidence base for pretransplant psychosocial screening. Transplantation. 2009;87:1497–504. doi: 10.1097/TP.0b013e3181a440ae. [DOI] [PubMed] [Google Scholar]
- 23.Shuter J, Bernstein SL. Cigarette smoking is an independent predictor of nonadherence in HIV-infected individuals receiving highly active antiretroviral therapy. Nicotine Tob Res. 2008;10:731–6. doi: 10.1080/14622200801908190. [DOI] [PubMed] [Google Scholar]