Abstract
Background
Proximity to roadways increases the risk of asthma in developed countries; however, relatively little is known about this relationship in developing countries, where rapid and uncontrolled growth of cities has resulted in urban sprawl and heavy traffic volumes.
Objective
Determine the effect of distance from a heavily transited avenue on asthma symptoms and quantitative respiratory outcome measures in a peri-urban shanty town in Lima, Peru.
Methods
We enrolled 725 adolescents aged 13–15 years, administered a survey on asthma symptoms and measured spirometry, response to allergy skin testing and eNO. We calculated distances from the main avenue for all households and measured indoor PM in 100 households. We used multivariable regression to model the risk of asthma symptoms, risk of atopy, eNO and FEV1/FVC as a function of distance.
Results
Compared against 384 meters, the odds of current asthma symptoms in households living within 100 meters increased by a factor of 2 (p<0.05). The odds of atopy increased by a factor of 1.07 for every 100 meters difference in the distance from the avenue (p=0.03). We found an inverse relationship in pre-bronchodilator FEV1/FVC and distance to the avenue in females (p=0.01) but not in males. We did not find an association between eNO or household PM levels and distance.
Conclusion
Living in close proximity to a high traffic-density avenue in a peri-urban community in Peru was associated with a greater risk of asthma symptoms and atopy. Regulation of mobile source pollutants in peri-urban areas of developing countries may help reduce the burden of asthma symptoms and atopy.
Keywords: Asthma symptoms, atopy, distance, traffic, particulate matter, spirometry
INTRODUCTION
There is increasing evidence that living within close proximity to a major roadway increases the prevalence and severity of asthma. However, nearly all research into the effects of traffic-related pollution exposure on asthma risk and severity has been investigated in developed countries (1–11) where high traffic-density is observed from multiple large, intersecting roadways. Relatively little is known about the association between traffic exposure and risk of asthma in developing countries (12, 13), where rapid and uncontrolled growth of cities has resulted in urban sprawl and heavy traffic volumes over the last two decades.
The majority of studies look at self- or parent-reported asthma symptoms and their association with distance to major roadways. Venn and colleagues documented an increase in wheeze for patients living within 150 meters of a major roadway in the United Kingdom (1). More recent studies, also in developed countries, found similar increases in parent-reported lifetime asthma, current asthma, and current wheeze for those living within 50 meters or 75 meters of the road compared to greater distances (2–5). These findings, however, have not been consistently replicated (6, 7). Studies evaluating the proximity to roadways and asthma in developed countries are complicated by the complex nature of urban transportation networks where it is difficult to isolate the impact of individual roads. Moreover, there are only limited data on quantitative respiratory outcome measures and traffic exposure, all of which has been predominantly collected in developed countries. In the Netherlands, investigators did not find an association between traffic and spirometry outcomes despite having reported an association between high-density truck traffic and self-reported wheeze (8). A similar trend was seen in a large study from the United Kingdom where a smaller forced expiratory volume in one second (FEV1) was seen for children living within 150 meters to main roadways, but these findings did not achieve statistical significance (9).
Furthermore, mobile source-related pollutant exposures such as particulate matter 2.5 µm in size (PM2.5), nitrogen dioxide (NO2), and sulfur dioxide (SO2) may directly increase the risk of asthma symptoms (14, 15). In asthmatic children, ambient PM2.5 levels were directly associated with an increase in exhaled nitric oxide (eNO) values for up to 12 hours (16). Epidemiologic studies have shown higher atopy rates in urban areas with higher pollution levels (17). However, little is known about the direct relationship between mobile source-related pollutants exposures and risk of atopy.
With a 26 percent prevalence, the International Study on Asthma and Allergies in Childhood (ISAAC) found Peru to have the highest prevalence of childhood asthma symptoms in Latin America (18), ranking it among the top quintile worldwide. Lima, the capital of Peru, is a rapidly growing city with high population density, peri-urban sprawl, and heavy, unregulated, automotive traffic. We sought to determine the effect of distance from a heavily transited avenue on asthma symptoms, lung function, atopy, airways inflammation, and household PM concentrations in a cohort of adolescents living in a peri-urban shanty town in Lima, Peru. In contrast to previous research conducted in developed countries, our community only has one major roadway with high traffic-density as the source of exposure, which simplifies the evaluation of the effect of traffic. Since our community is relatively homogenous in sociodemographic composition, it provides a naturally-controlled environment to study the effects of distance from a major roadway on the risk of asthma and atopy. The results of this study were presented in part previously in an abstract(19).
METHODS
Study Setting
We conducted our study in Pampas de San Juan de Miraflores, a peri-urban shanty-town located 25 kilometers south of the center of Lima (20, 21). This community has grown rapidly (population 60,000) as a result of urban sprawl, and homes are tightly packed with a mixture of paved and unpaved roads. It is cut in half by a highly trafficked avenue that serves as a main commuter route, travelled mainly by unregulated commuter buses. There are no point sources of industrial pollution nearby. Our research team has participated in several community-based research projects at Pampas for more than two decades. As a result, we have conducted community-wide censuses and updates thereof over the years in which we visited every house in the community and asked about the number of people per household, and the age and sex of each household member.
Study Design
We recruited an age and sex stratified random sample of 725 adolescents aged 13 to 15 years by home visitation. Adolescents were eligible to participate if they were capable of understanding or performing procedures, if their parents or guardians were capable of providing written informed consent and they were capable of providing assent; if they had no ocular, abdominal, or thoracic surgery or hospitalized for cardiac reasons in the last three months; and, were ineligible if they had a chronic respiratory condition other than asthma such as cystic fibrosis or chronic lung disease of prematurity, if they were pregnant, or if they had pulmonary tuberculosis or were currently receiving treatment for pulmonary tuberculosis. We recruited only one adolescent per household. After obtaining written informed consent, we asked participants to complete a survey on asthma symptoms, sociodemographic and exposure data. We used a Spanish version of the ISAAC questionnaire previously validated in Peru (22).
During a second home visit, we asked participants to undergo eNO testing, allergy skin testing, and spirometry before and after bronchodilators. We measured eNO levels using the NIOX MINO portable eNO monitor (Aerocrine, New Providence, NJ). We applied allergy skin prick tests for cockroach, dust mite mix, cat hair, dog epithelium, mouse epithelium, and mixed molds, along with positive histamine control and negative glycerol control in the inner forearm using the Multi-Test II allergen applicator (Lincoln Diagnostics, Decatur, IL). An allergy skin test was considered positive if the sum of the vertical and horizontal dimensions of the induration was >3 mm than the negative control and if the sum of the vertical and horizontal dimensions of erythema was >5 mm larger than the negative control.
We performed spirometry with a handheld spirometer (SpiroPro, Jaeger, Hochberg, Germany). SpiroPro uses disposable, single-use factory-calibrated pneumotachometer tubes. We asked each participant to perform up to a maximum of eight pre-bronchodilator tests to attain three acceptable and reproducible tests as per standard quality criteria (23). We then administered four doses of inhaled salbutamol (100µg each) and repeated spirometry 15 minutes later. All maneuvers were performed seated upright and with a nose clip. Participants who did not meet quality criteria were revisited up to two more times on a different day and asked to repeat spirometry. We asked participants to withhold any short-acting bronchodilators within 8 hours and long-acting bronchodilators for 24–48 hours of testing unless clinically necessary; however, we did not have instances where this occurred. We revisited participants who reported having a respiratory infection in the last two weeks on a later date.
We assessed indoor PM concentrations in 100 homes that were randomly selected from our study sample according to 11 geographic zones determined by distance from the main avenue. We measured PM concentrations over 48-hour periods on weekdays only using the personal DataRam (pDR)-1000 (Thermo Scientific, Franklin, MA). We also measured relative humidity (RH) concurrently using a HOBO Data Logger (Onset Corp., Bourne, MA). We adjusted PM concentrations to RH levels as previously specified (24). Outdoor pollution data were provided by DIGESA (www.digesa.sld.pe). We obtained all 24-hour ambient measurements of PM2.5 and PM10 for the years 2008 and 2009, collected from a governmental post located within 3 kilometers of Pampas. Global positioning system coordinates were determined for every participating household and the perpendicular distance from the avenue was calculated using ArcGis 9.3 (ESRI Corp., Redlands, CA).
This study was approved by the Institutional Review Boards of the Johns Hopkins Bloomberg School of Public Health in Baltimore, MD, USA, and A.B. PRISMA in Lima, Peru. We obtained written informed consent from parents or guardians and assent from adolescent participants.
Definitions
We defined current asthma symptoms as those who reported wheezing or used any asthma medications in the past 12 months. We defined atopy as a positive test to one or more skin allergen. We defined bronchodilator-induced reversibility by a change ≥12% in FEV1 between the pre and the post measurement (23).
Biostatistical methods
We used a multivariable logistic additive model to model the effect of distance to the main avenue on the risk of current asthma symptoms and atopy (25). We regressed the log odds of current asthma on a smooth (spline) function of distance from the main avenue, age, sex, maternal education and household income. Fewer participants had data on body-mass index (BMI) or smoking status; however, adding BMI and current smoking in subset analysis did not alter findings (data not shown). We obtained a 95% confidence interval using a percentile-based bootstrap approach (26). Since only 84 participants had current asthma symptoms, we did not have sufficient sample size to adequately explore for differences by sex.
In exploratory analysis, we found that the relationship between the log odds of atopy and distance from the main avenue was approximately linear. We regressed the log odds of atopy on distance from the avenue, sex, age, height, BMI, smoking, passive tobacco exposure, maternal education, and household income. We did not find a significant interaction between distance from the avenue and sex (data not shown). To model lung function, we used linear regression stratified by sex. We regressed FEV1/FVC on quartile of distance from the avenue, calendar quarter, age, BMI, history of personal smoking, household income and maternal education. We regressed FEV1 on quartile of distance from the avenue, height, BMI, personal history of smoking, household income and maternal education.
To model eNO levels and indoor PM concentration, we used a generalized linear model with a log-normal distribution. We regressed eNO on distance from the avenue, gender, age, height, BMI, wheeze in the past 12 months, lifetime diagnosis of asthma diagnosis, use of asthma medication in the past 12 months, atopy, calendar quarter, personal history of smoking, passive tobacco exposure, maternal education, and household income. We regressed indoor PM on distance from the road, calendar quarter, outdoor PM2.5 and PM10, personal history of smoking and household tobacco smoke exposure.
We conducted our analyses in R (www.r-project.org) and STATA (StataCorp, College Station, Texas).
RESULTS
Baseline characteristics
Of 1056 adolescents who were identified from census data, we enrolled 725 (69%) into our study. During the first home visit, all 725 children completed the survey. Of those recruited, 646 (89%) completed at least one or more of the clinical tests. 4 (1%) participants did not complete testing because they moved out of the community, 3 (<1%) became pregnant and thus ineligible to continue in the study, and 60 (8%) declined to continue with the study. 625 (86%) of participants completed spirometry, 604 (83%) completed eNO testing, and 614 (85%) completed an allergy skin test. We did find not differences in the distribution of sex, age, demographics, or socioeconomic status by categories of asthma symptoms and atopy (Table I). 698 (96%) participants lived in Lima since birth, and >99% have lived in the study community for 5 years or longer. 716 (99%) participants reported that propane gas was the predominant type of fuel used for cooking at home.
Table I.
Personal characteristics and sociodemographics according to the presence or absence asthma symptoms and atopy; Lima, Peru, 2009–2010.
| Asthma and Atopy |
Asthma and No Atopy |
No asthma and atopy |
No asthma and no atopy |
p-value | ||
|---|---|---|---|---|---|---|
| Number of adolescents | 57 | 17 | 285 | 255 | ||
| Number of boys (%) | 35 (61%) | 7 (41%) | 144 (51%) | 120 (47%) | 0.22 | |
| Average age (SD) | 14.7 (1.0) | 14.8 (0.9) | 14.8 (0.9) | 14.9 (0.8) | 0.07 | |
| Born in Lima (%) | 56 (98%) | 16 (94%) | 272 (95%) | 249 (98%) | 0.33 | |
| Parents from Lima (%) | 20 (35%) | 9 (53%) | 99 (35%) | 102 (40%) | 0.31 | |
| Parents from highlands (%) | 45 (79%) | 14 (82%) | 221 (78%) | 194 (76%) | 0.95 | |
| Parents from rainforest (%) | 2 (4%) | 0 (0%) | 10 (4%) | 4 (2%) | 0.42 | |
| Income < 175 USD (%) | 15 (26%) | 4 (24%) | 68 (24%) | 54 (21%) | 0.80 | |
| Maternal education years, Mean (SD) | 8.5 (4.0) | 8.3 (4.2) | 8.4 (3.8) | 8.0 (3.6) | 0.76 | |
| Paternal education years, Mean (SD) | 8.9 (3.2) | 9.6 (4.0) | 9.6 (3.2) | 9.9 (2.8) | 0.21 | |
| Electricity 24 hours (%) | 57 (100%) | 17 (100%) | 282 (99%) | 254 (100%) | 0.78 | |
| Water 24 hours (%) | 53 (93%) | 16 (94%) | 259 (91%) | 238 (93%) | 0.77 | |
| Hygienic services in home (%) | 55 (96%) | 17 (100%) | 274 (96%) | 246 (96%) | 1.00 | |
| Rooms in household (SD) | 4.9 (1.4) | 4.7 (1.8) | 5.0 (1.8) | 4.8 (1.7) | 0.74 | |
| People per room (SD) | 1.3 (0.7) | 1.3 (1.0) | 1.3 (0.7) | 1.4 (0.8) | 0.81 | |
| Concrete floor (%) | 18 (32%) | 6 (35%) | 113 (40%) | 92 (36%) | 0.65 | |
| Unprocessed concrete floor (%) | 26 (46%) | 7 (41%) | 106 (37%) | 106 (42%) | 0.58 | |
| Computer (%) | 11 (19%) | 6 (35%) | 75 (26%) | 62 (24%) | 0.52 | |
| Smoking in home (%) | 7 (12%) | 3 (18%) | 51 (18%) | 37 (15%) | 0.60 | |
| Landline (%) | 34 (60%) | 11 (65%) | 95 (33%) | 107 (42%) | 0.21 | |
| Cellular telephone (%) | 50 (88%) | 15 (88%) | 246 (86%) | 224 (88%) | 0.96 | |
| Owns dogs (%) v | 32 (56%) | 11 (65%) | 159 (56%) | 156 (61%) | 0.57 | |
| Owns cats (%) | 22 (39%) | 8 (47%) | 122 (43%) | 125 (49%) | 0.36 | |
| Cockroaches present in home in past month (%) | 32 (56%) | 11 (65%) | 174 (61%) | 161 (63%) | 0.76 | |
| Time spent in house (%) | ||||||
| 0–6 | 0 (0%) | 0 (0%) | 0 (0%) | 2 (1%) | 0.40 | |
| 7–10 | 0 (0%) | 0 (0%) | 7 (2%) | 8 (3%) | 0.70 | |
| 11–15 | 9 (16%) | 5 (29%) | 88 (31%) | 68 (27%) | 0.12 | |
| 16+ | 48 (84%) | 12 (71%) | 190 (67%) | 177 (69%) | 0.08 | |
| Height (SD) | ||||||
| Male | 162.5 (6.8) | 165.0 (6.7) | 162.6 (7.4) | 161.3 (7.5) | 0.36 | |
| Female | 151.3 (5.0) | 152.3 (6.1) | 152.9 (5.7) | 153.1 (5.4) | 0.54 | |
| Weight (SD) | ||||||
| Male | 60.6 (13.5) | 59.8 (10.8) | 56.7 (10.7) | 55.1 (11.4) | 0.07 | |
| Female | 52.8 (12.4) | 56.6 (6.5) | 52.5 (9.3) | 51.2 (8.5) | 0.25 | |
| BMI (SD) | ||||||
| Male | 22.8 (3.9) | 21.9 (3.4) | 21.3 (3.2) | 21.0 (3.2) | 0.05 | |
| Female | 23.0 (4.7) | 24.4 (2.9) | 22.4 (3.4) | 21.8 (3.3) | 0.07 | |
Our study community is divided in half by the main avenue (Figure I). Participating houses were located adjacent to the road up to a maximum distance of 1063 meters. 196 (27%) participants lived within 200 meters of the avenue. We did not find differences in the distribution of sex, age, or demographics according to distance from the avenue, and only minor differences in household characteristics (Table II).
Figure I. Distribution of the 725 study households in Pampas de San Juan around the main avenue, Lima, Peru.
The thick, black line represents the main avenue (Avenida Miguel Iglesias) that intersects our study community. The black circles represent households in our study. The thin, white lines represent distances from the avenue in 100 meter intervals.
Table II.
Personal characteristics and sociodemographics according to distance from the main avenue; Lima, Peru, 2009–2010.
| Distance from road (meters) | ||||||
|---|---|---|---|---|---|---|
| 0 – 199.99 (n=196) |
200 – 399.99 (n=176) |
400 – 649.99 (n=171) |
≥650 (n=182) |
Trend p-value |
||
| Number of adolescents | 196 | 176 | 171 | 182 | ||
| Number of boys (%) | 95 (48%) | 88 (50%) | 76 (44%) | 98 (54%) | 0.511 | |
| Average age (SD) | 14.8 (0.9) | 14.9 (0.9) | 14.9 (0.9) | 14.8 (0.8) | 0.586 | |
| Born in Lima (%) | 192 (98%) | 170 (97%) | 167 (98%) | 169 (93%) | 0.021 | |
| Parents from Lima (%) | 87 (44%) | 66 (38%) | 63 (37%) | 53 (29%) | 0.003 | |
| Parents from highlands (%) | 143 (73%) | 134 (76%) | 132 (77%) | 147 (81%) | 0.07 | |
| Parents from rainforest (%) | 5 (3%) | 7 (4%) | 3 (2%) | 6 (3%) | 0.97 | |
| Income < 175 USD % | 56 (29%) | 38 (22%) | 27 (16%) | 52 (29%) | 0.703 | |
| Maternal education years, Mean (SD) | 8.6 (3.6) | 8.2 (3.8) | 8.5 (3.7) | 7.5 (3.6) | 0.013 | |
| Paternal education years, Mean (SD) | 9.8 (3.1) | 9.9 (3.1) | 10.1 (3.0) | 9.5 (3.0) | 0.601 | |
| Electricity 24 hours % | 195 (99%) | 175 (99%) | 169 (99%) | 182 (100%) | 0.812 | |
| Water 24 hours (%) | 179 (91%) | 175 (99%) | 160 (94%) | 153 (84%) | 0.063 | |
| Hygienic services in home (%) | 185 (94%) | 176 (100%) | 167 (98%) | 170 (93%) | 0.451 | |
| Rooms in household (SD) | 4.8 (1.7) | 5.2 (1.9) | 4.8 (1.7) | 4.6 (1.5) | 0.054 | |
| People per room (SD) | 1.3 (0.9) | 1.3 (0.7) | 1.4 (0.9) | 1.4 (0.9) | 0.491 | |
| Concrete floor (%) | 77 (39%) | 70 (40%) | 63 (37%) | 62 (34%) | 0.246 | |
| Unprocessed concrete floor (%) | 81 (42%) | 61 (35%) | 73 (43%) | 71 (39%) | 0.986 | |
| Computer (%) | 51 (26%) | 49 (28%) | 53 (31%) | 27 (15%) | 0.033 | |
| Smoking in home (%) | 29 (15%) | 28 (16%) | 29 (17%) | 24 (13%) | 0.296 | |
| Landline (%) | 130 (66%) | 107 (61%) | 116 (68%) | 93 (51%) | 0.014 | |
| Cellular telephone (%) | 168 (86%) | 149 (85%) | 154 (90%) | 157 (86%) | 0.542 | |
| Owns dogs (%) | 112 (57%) | 104 (59%) | 93 (54%) | 104 (57%) | 0.786 | |
| Owns cats (%) | 87 (44%) | 64 (36%) | 73 (43%) | 96 (53%) | 0.062 | |
| Cockroaches present in home in past month (%) | 117 (60%) | 98 (56%) | 110 (64%) | 121 (66%) | 0.086 | |
| Time spent in house (%) | ||||||
| 0–6 | 0 (0%) | 0 (0%) | 1 (1%) | 1 (1%) | 0.199 | |
| 7–10 | 2 (1%) | 2 (1%) | 7 (4%) | 7 (4%) | 0.026 | |
| 11–15 | 46 (23%) | 47 (27%) | 49 (29%) | 56 (31%) | 0.1 | |
| 16+ | 148 (76%) | 127 (72%) | 114 (67%) | 118 (65%) | 0.13 | |
| Height (SD) | ||||||
| Male | 162.9 (6.9) | 161.4 (7.6) | 163.5 (7.7) | 160.5 (7.1) | 0.077 | |
| Female | 152.5 (5.9) | 152.7 (5.0) | 153.3 (5.8) | 152.8 (5.1) | 0.463 | |
| Weight (SD) | ||||||
| Male | 58.3 (13.1) | 55.9 (9.3) | 57.3 (11.4) | 55.4 (10.9) | 0.179 | |
| Female | 51.5 (8.5) | 53.1 (10.3) | 52.6 (11.1) | 51.1 (7.0) | 0.916 | |
| BMI (SD) | ||||||
| Male | 21.8 (3.6) | 21.1 (2.8) | 21.3 (3.0) | 21.4 (3.6) | 0.555 | |
| Female | 22.1 (3.4) | 22.7 (3.8) | 22.3 (4.1) | 21.9 (2.8) | 0.672 | |
Asthma symptoms
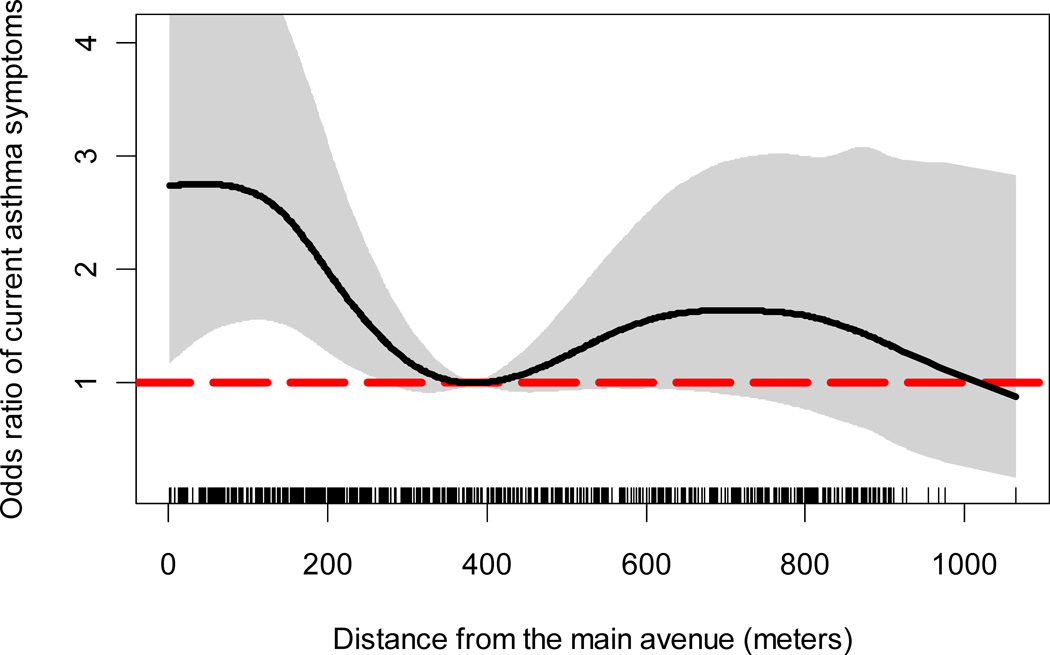
The prevalence of current asthma symptoms was 12% (84/725). 94 (13%) participants reported ever having a physician-diagnosis of asthma, and 44 (6%) had used inhaled or oral corticosteroids or bronchodilators (β-agonists) for asthma in the past year. The odds of current asthma symptoms increased with closer proximity to the main avenue (Figure II). Compared against 384 meters, the odds of current asthma symptoms in households living within 100 meters increased by a factor of 2 and remained significantly greater until about 250 meters (p<0.05). We did not find a difference in the odds of current asthma symptoms at distances beyond 384 meters.
Figure II. Odds ratio of current asthma symptoms and distance from the main avenue (reference distance = 384 meters); Lima, Peru 2009–2010.
The thick black line represents the odds ratio of current asthma symptoms using 384 meters as the reference distance. The grey area represents the 95% bootstrap confidence intervals. The vertical segments in the x-axis represents the distribution of household distances from the main avenue.
Spirometry
Of the 625 participants who completed spirometry, 24 (3%) demonstrated post-bronchodilator reversibility. The proportion of cases with post-bronchodilator reversibility appeared to be greater with closer proximity to the main avenue; however, this increase was not statistically significant (Table III). We observed a decrease in pre-bronchodilator FEV1/FVC with closer proximity to the avenue in girls (p=0.01) but not in boys. We also observed a marginally significant decrease in post-bronchodilator FEV1/FVC with closer proximity to the avenue in girls. We did not find important differences in pre or post FEV1 and distance from the main avenue (Table III).
Table III.
Spirometry outcomes according to distance from the main avenue; Lima, Peru, 2009–2010.
| Distance from road (meters) | |||||
|---|---|---|---|---|---|
| 0 – 199.99 (n=172) |
200 – 399.99 (n=143) |
400 – 649.99 (n=154) |
≥650 (n=156) |
p-value | |
| Post-bronchodilator reversibility (%) | 9 (5.2%) | 6 (4.2%) | 4 (2.6%) | 5 (3.2%) | 0.25 |
| Mean pre-bronchodilator FEV1 (SD) Male Female |
3.87 (0.6) 3.05 (0.4) |
3.84 (0.7) 3.08 (0.4) |
3.92 (0.6) 3.11(0.4) |
3.72 (0.6) 3.10(0.4) |
0.80¥ 0.20¥ |
| Mean pre-bronchodilator FEV1/FVC % (SD) Male Female |
88.2 (5.7) 88.0(7.1) |
88.8 (6.4) 89.5 (5.3) |
88.0 (6.3) 90.4 (5.8) |
87.9(6.5) 89.8(6.3) |
0.74* 0.01* |
| Mean post-bronchodilator FEV1 (SD) Male Female |
4.02 (0.6) 3.16(0.4) |
3.96 (0.7) 3.18(0.4) |
4.04 (0.6) 3.20 (0.4) |
3.80(0.6) 3.18(0.4) |
0.46¥ 0.67¥ |
| Mean post-bronchodilator FEV1/FVC (SD) Male Female |
90.7 (5.3) 91.1(5.3) |
90.6 (6.0) 91.6(5.5) |
89.8 (5.9) 92.2 (5.0) |
89.8(6.9) 92.0(5.8) |
0.44* 0.08* |
adjusted for calendar quarter, age, household income, maternal education, personal history of current smoking, and BMI.
adjusted by calendar quarter, age, height, BMI, personal history of current smoking, household income, and maternal education.
Atopy
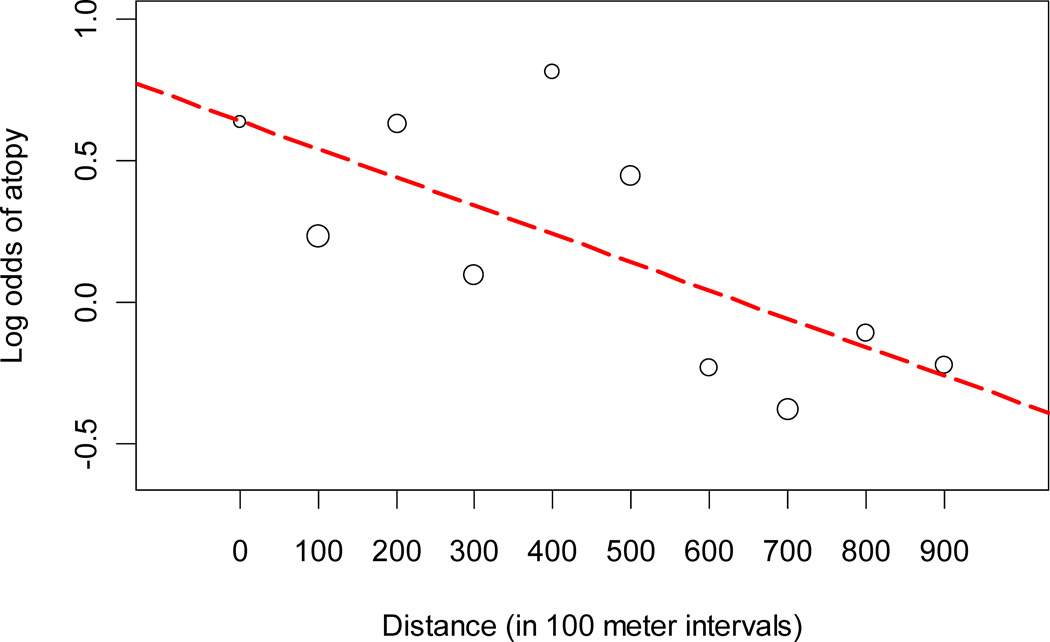
Of 725 total participants, 614 (85%) underwent skin allergy testing and 342 (56%) were atopic. 108 (18%) were positive to one allergen, 90 (15%) were positive to two, and 144 (23%) were positive to three or more allergens. The log odds of atopy increased approximately linearly with closer proximity to the main avenue (Figure III). The odds of atopy increased multiplicatively by a factor of 1.07 for every 100 meters difference in the distance from the main avenue (Table IV). We did not find an interaction between distance from the main avenue and sex on risk of atopy (p=0.42).
Figure III. Log odds of atopy and distance from the main avenue, Lima, Peru 2009–2010.
The circles represent the log odds of atopy calculated by 100-meter intervals of distance from the main avenue. The size of the circles is proportional the square root of the number of participants at each interval. The dashed red line represents a fitted line of the log odds of atopy by 100-meter intervals of distance from the main avenue.
Table IV.
Multivariable logistic regression of odds of atopy on distance from the main avenue; Lima, Peru, 2009–2010.
| Predictor | Coefficient (as log odds ratio) |
Standard error |
p- value |
|---|---|---|---|
| (Intercept) | 0.87 | 2.47 | 0.73 |
| Distance (per 100m) | −0.07 | 0.03 | 0.03 |
| Gender | −0.16 | 0.22 | 0.47 |
| Age | −0.09 | 0.10 | 0.34 |
| Height | 0.01 | 0.01 | 0.62 |
| Body mass index (BMI) | 0.00 | 0.00 | 0.83 |
| Current smoker | −0.11 | 0.38 | 0.77 |
| Passive tobacco exposure | 0.33 | 0.25 | 0.18 |
| Maternal education (6–8 years completed) | 0.08 | 0.26 | 0.75 |
| Maternal education (9–10 years completed) | −0.46 | 0.31 | 0.14 |
| Maternal education (≥11 years completed) | 0.18 | 0.24 | 0.45 |
| Income 175–280 USD | −0.16 | 0.23 | 0.48 |
| Income 281–400 USD | −0.18 | 0.24 | 0.45 |
| Income >400 USD | 0.68 | 0.44 | 0.12 |
Exhaled nitric oxide
604 (83%) participants completed eNO testing. Values ranged from <5 ppb to 269 ppb, with a mean of 21.7 ppb (SD = 19.6). We did not find an association between proximity to the main avenue and eNO levels (Table V). Females had a lower mean eNO value than males. Neither a personal history nor passive exposure to tobacco smoke affected eNO level. We found higher eNO values in participants with current asthma, a history of physician-diagnosed asthma, and atopy.
Table V.
Multivariable regression of eNO on distance from the main avenue; Lima, Peru, 2009–2010.
| Predictor | Coefficient (in log scale) |
Standard Error |
p-value |
|---|---|---|---|
| (Intercept) | −0.83 | 1.16 | 0.48 |
| Distance (per 100m) | 0.00 | 0.01 | 0.97 |
| Gender | −0.23 | 0.11 | 0.04 |
| Age | 0.20 | 0.04 | <0.001 |
| Height | 0.00 | 0.01 | 0.89 |
| Body mass index (BMI) | 0.00 | 0.00 | 0.57 |
| Current smoker | 0.07 | 0.14 | 0.63 |
| Passive tobacco exposure | −0.13 | 0.11 | 0.23 |
| Maternal education (≥11 years completed) | 0.30 | 0.08 | <0.001 |
| Income ≤175 USD | −0.19 | 0.11 | 0.08 |
| Apr-Jun | 0.01 | 0.17 | 0.93 |
| Jul–Sep | 0.16 | 0.12 | 0.17 |
| Oct–Dec | 0.15 | 0.12 | 0.21 |
| Current asthma | 0.70 | 0.09 | <0.001 |
| Asthma diagnosis | 0.29 | 0.09 | <0.01 |
| Atopy | 0.68 | 0.11 | <0.001 |
Indoor air pollution
The mean 24-hour PM concentration was 43.4 µg/m3 (SD = 24.3), and the median was 30.9 µg/m3 (IQR = 16.9). 24-hour PM concentrations ranged from 9.0 µg/m3 to 159.1 µg/m3. In 2009, average 24-hour outdoor concentrations were 37.5 µg/m3 (SD = 31.6) and 75.1 µg/m3 (SD = 27.6) for PM2.5 and PM10, respectively. We did not find an association between indoor PM levels and distance to the main avenue (p=0.48). However, indoor PM was positively correlated with outdoor PM2.5.
DISCUSSION
The risk of both asthma symptoms and atopy increased with closer proximity to a high traffic-density avenue in a developing country. Measures of airflow limitation (27) decreased with closer proximity to the avenue in girls but not in boys. We did not find higher levels of airways inflammation or higher levels of indoor particulate matter with closer proximity to the main avenue.
We were surprised to find that the risk of atopy increased linearly with closer proximity to the main avenue. We have not found any other epidemiological studies that report a significant association between distance from a major roadway and risk of atopy. The study in Jimma, Ethiopia, found a trend on the relationship between distance from major roadways and skin sensitization to dust mites, but this increase did not achieve statistical significance (12). Evidence of this relationship can be supported with data from animal experiments. For example, guinea pigs exposed to high concentrations of NO2 after immunization and antigen challenge showed significantly higher levels of specific IgG and IgE than controls (28). Increased levels of total IgG have been shown when ovalbumin was coupled SO2 exposure in rats (29). In human populations, increased levels of CD4+ and CD8+ lymphocytes have been documented with increased exposure to PM2.5 and PM10, and increases in total IgG were linked with higher levels of PM2.5 (30), demonstrating that particulate matter activates immunologic response (31, 32). The increased risk of atopy with closer proximity to major roadways provides mechanistic insights into the increased risk of asthma symptoms.
Our findings on asthma symptoms and proximity to high traffic-density roads are similar to those observed by other studies conducted in developed countries (1–5, 11, 12). It is widely accepted that environmental pollutants have an adverse effect on asthma symptoms (14, 15, 33), which supports findings that areas of increased exposure such as the roadside lead to increased symptoms. The majority of studies have found these effects to be strongest within 150 meters from the road (1–5, 11, 12), substantiated by research that shows ambient PM and NO2 concentrations significantly decline around 100 to 200 meters from a traffic source (34–36). Many studies have also found these effects to be stronger in girls than boys, showing higher odds of wheeze and asthma symptoms for girls living in close proximity to high traffic densities compared to their male counterparts (1, 37, 38).
Despite the strong evidence of an association between traffic exposures and asthma symptoms in the developed world, very little is known about this relationship in developing countries. In the developing world, many cities are experiencing fast population growth with relatively unregulated sprawl, providing a setting with high traffic flow and pollution in highly crowded communities. A study conducted on the United States-Mexico border represents one of only two studies we identified outside of a developed country (11). In this study, investigators found that asthmatic children living within 200 meters of the road had a lower FEV1 associated with increasing traffic density, and a positive association between eNO values and closer distances. The other study was conducted in Jimma, Ethiopia, in which investigators found that the risk of wheeze increased inversely with distance within 150 meters of a main road (12). Jimma is a town of about 80,000 people with no major industry and very little motorized transport, and main roads were defined as any roadway with a traffic density of ≥55 vehicles per hour (12, 13). However, asthma rates in both Mexico and Ethiopia are relatively low when compared to other developing countries with large urban cities like Peru or Brazil (18). The prevalence of wheeze in the past 12 months in 13–14 year olds is approximately 7% in Mexico (18), and in Jimma, the reported prevalence of asthma symptoms is less than 4% (13). The prevalence of asthma symptoms in the last 12 months in our study community was 12%. This estimate is lower than that found by ISAAC in Peru (18), and the reasons for this difference needs to be explored further. Pollution rates were also much lower in the US-Mexico border community than in Lima, with a mean PM2.5 of 17.5 µg/m3 during the study (11).
Statistical modeling of environmental exposures and disease susceptibility can be very complex. Therefore, we explored alternative models to establish the robustness of our findings. Specifically, we found similar results when we used a probit link instead of a logit link to model the effects of distance from the road on either risk of asthma symptoms or atopy. When we used quantile regression to model effects on median FEV1/FVC, we found that both the pre-bronchodilator and post-bronchodilator ratios in girls were inversely associated with distance from the main avenue at the 0.05 level of significance. One limitation of our study is that we did not collect quantitative measures of allergen exposure as there appear to be some slight differences in household characteristics and distance from the road. However, we collected qualitative data on exposure to cockroaches, dogs and cats and did not observe any differences by distance from the main avenue. We were also not able to collect other pollutants associated with traffic such as NO2 and O3. Another limitation is that about 70% of the adolescents agreed to participate in our study. While this may limit generazability of the study findings to the population, this participate rate is similar to that of other studies. Furthermore, we did not offer any incentives or payments for participation in our study and our study sample was similar in age and sex to the overall eligible population. Another potential shortcoming is that passive sampling of particulate matter does not include a specific particle size-selection inlet; however, passive PM measurements with the pDR-1000 have been shown to be a good proxy for PM2.5 concentrations (24). Furthermore, in our study, we found that outdoor PM2.5 concentrations were significantly associated with indoor PM using our particulate matter monitor. Additionally, we did not assess bronchial hyper-responsiveness, which is an additional quantitative marker of asthma. However, this would have been unfeasible since all physical testing was done by home visitation. Finally, the cross-sectional nature of our study design, particularly in the case of the effect particulate matter on asthma symptoms, is a limitation. A strength of our study is that it is a population-based of a random selection of participants from a high-density peri-urban community. Moreover, there is only one main source of heavy traffic in our community; however, there are roads with lower traffic density that may nonetheless contribute to traffic pollution for which we not account in our analysis.
Lima is undergoing strong economic development in some areas of the city, while other areas are experiencing rapid, uncontrolled urban sprawl. Peri-urban communities located in the outskirts of the city and are home to poor populations living in communities with high population density and are exposed to heavy pollution traffic that is largely unregulated. Indoor and outdoor levels of particulate matter in our community exceed current safety recommendations of the World Health Organization by a factor of four(39). As the city continues to grow, policy changes will be necessary to regulate mobile source emissions and control traffic volumes. Policy makers in Lima may use Santiago, Chile as a lead to follow, where air pollution has significantly decreased in recent years as a result of successful traffic control campaigns. Over the period of 1989–2000, PM2.5 concentrations decreased 52% in Santiago, attributable to the removal of old buses, the addition of catalytic converters to vehicles, and cleaning and paving of streets (40).
In summary, we found a link between asthma symptoms and proximity to traffic, and our findings add new evidence towards associations of living near a major road and risk of atopy. Policy to regulate traffic control in peri-urban communities with high-traffic density will have a direct health benefit for the population.
Key Messages.
Proximity to multiple, intersecting roadways has been shown to increase the risk of asthma in developed countries; however, relatively little is known about the association between traffic exposure and risk of asthma in developing countries, where rapid and uncontrolled growth of cities has resulted in urban sprawl and heavy traffic volumes.
Living in close proximity to a high traffic-density avenue in a peri-urban community in Peru is associated with an increased risk of asthma symptoms. We also show for the first time that proximity to the main avenue is associated with an increased risk of atopy.
Acknowledgements
All contributing authors were involved in the study design and writing of the manuscript. Lauren Baumann directly contributed to the design and conduct of the study; collection of data in both Tumbes and Lima, is responsible for data management and analysis, and writing of this manuscript. Colin Robinson contributed equally to the study design, administration of field work, and data management and analysis. Robert Gilman is the main investigator at the Lima site and provided technical support during the conduct of the study. Karina Romero, Juan Combe and Alfonso Gomez were the on-site study physicians who performed most physical testing and were extensively involved in administering field work. Lilia Cabrera is the field work supervisor at the Lima site, provided census data and technical support. Nadia Hansel and Robert Wise contributed to the study design and provided technical support for the study. Kathleen Barnes contributed to the study design and provided logistical support for the study. Patrick Breysse contributed to study design of the environmental measurements, and provided both technical support and equipment for our study. Juan E Hernandez did geographical analysis of the data. William Checkley had ultimate oversight over study design and administration, and was equally responsible for the analysis and writing of the manuscript. He had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. He also served as a mentor to Lauren Baumann and Colin Robinson throughout the conduct of the study.
Additional support came from A.B. PRISMA and collaborators at JHU. Lincoln Diagnostics (Decatur, IL) and ALK-Abello (Round Rock, TX) generously donated all skin prick atopy kits and antigens, respectively, used in this in this study. Aerocrine (New Providence, NJ) provided us, at discount, materials for eNO testing.
Funding sources: This study was supported by a Johns Hopkins Center for Global Health Award (PI: Hansel) and the Fogarty International Center Training Grant (Grant R24 TW007988). William Checkley was supported by a Clinician Scientist Award from the Johns Hopkins University, a K99/R00 Pathway to Independence Award (K99HL096955) from the National Heart, Lung and Blood Institute, National Institutes of Health and by a contract (HHSN268200900033C) with the National Heart, Lung and Blood Institute, National Institutes of Health. Kathleen Barnes was supported in part by the Mary Beryl Patch Turnbull Scholar Program. Colin Robinson was a Fogarty International Clinical Research Scholar during the time of this work and was further supported by Tufts University School of Medicine. Lauren Baumann was supported by a pre-doctoral NIH T35 Training Grant (T35AI065385). Support for exposure measurements were provided by National Institute for Environmental Health Sciences Grant numbers (ES015903 and ES03819).
Abbreviations
- BMI
Body mass index
- eNO
Exhaled nitric oxide
- FVC
Forced vital capacity
- FEV1
Forced expiratory volume in one second
- PM
Particulate matter
- PM2.5
Particulate matter 2.5µm in diameter
- PM10
Particulate matter 10µm in diameter
- RH
Relative humidity
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
All contributing authors have no conflicts of interest to report.
REFERENCES
- 1.Venn AJ, Lewis SA, Cooper M, Hubbard R, Britton J. Living near a main road and the risk of wheezing illness in children. Am J Respir Crit Care Med. 2001 Dec 15;164(12):2177–2180. doi: 10.1164/ajrccm.164.12.2106126. [DOI] [PubMed] [Google Scholar]
- 2.McConnell R, Berhane K, Yao L, Jerrett M, Lurmann F, Gilliland F, et al. Traffic, susceptibility, and childhood asthma. Environ Health Perspect. 2006 May;114(5):766–772. doi: 10.1289/ehp.8594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim JJ, Huen K, Adams S, Smorodinsky S, Hoats A, Malig B, et al. Residential traffic and children's respiratory health. Environ Health Perspect. 2008 Sep;116(9):1274–1279. doi: 10.1289/ehp.10735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morgenstern V, Zutavern A, Cyrys J, Brockow I, Koletzko S, Kramer U, et al. Atopic diseases, allergic sensitization, and exposure to traffic-related air pollution in children. Am J Respir Crit Care Med. 2008 Jun 15;177(12):1331–1337. doi: 10.1164/rccm.200701-036OC. [DOI] [PubMed] [Google Scholar]
- 5.Ryan PH, LeMasters G, Biagini J, Bernstein D, Grinshpun SA, Shukla R, et al. Is it traffic type, volume, or distance? Wheezing in infants living near truck and bus traffic. J Allergy Clin Immunol. 2005 Aug;116(2):279–284. doi: 10.1016/j.jaci.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 6.Lewis SA, Antoniak M, Venn AJ, Davies L, Goodwin A, Salfield N, et al. Secondhand smoke, dietary fruit intake, road traffic exposures, and the prevalence of asthma: a cross-sectional study in young children. Am J Epidemiol. 2005 Mar 1;161(5):406–411. doi: 10.1093/aje/kwi059. [DOI] [PubMed] [Google Scholar]
- 7.English P, Neutra R, Scalf R, Sullivan M, Waller L, Zhu L. Examining associations between childhood asthma and traffic flow using a geographic information system. Environ Health Perspect. 1999 Sep;107(9):761–767. doi: 10.1289/ehp.99107761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Janssen NA, Brunekreef B, van Vliet P, Aarts F, Meliefste K, Harssema H, et al. The relationship between air pollution from heavy traffic and allergic sensitization, bronchial hyperresponsiveness, and respiratory symptoms in Dutch schoolchildren. Environ Health Perspect. 2003 Sep;111(12):1512–1518. doi: 10.1289/ehp.6243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pujades-Rodriguez M, Lewis S, McKeever T, Britton J, Venn A. Effect of living close to a main road on asthma, allergy, lung function and chronic obstructive pulmonary disease. Occup Environ Med. 2009 Oct;66(10):679–684. doi: 10.1136/oem.2008.043885. [DOI] [PubMed] [Google Scholar]
- 10.Wjst M, Reitmeir P, Dold S, Wulff A, Nicolai T, von Loeffelholz-Colberg EF, et al. Road traffic and adverse effects on respiratory health in children. BMJ. 1993 Sep 4;307(6904):596–600. doi: 10.1136/bmj.307.6904.596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holguin F, Flores S, Ross Z, Cortez M, Molina M, Molina L, et al. Traffic-related exposures, airway function, inflammation, and respiratory symptoms in children. Am J Respir Crit Care Med. 2007 Dec 15;176(12):1236–1242. doi: 10.1164/rccm.200611-1616OC. [DOI] [PubMed] [Google Scholar]
- 12.Venn A, Yemaneberhan H, Lewis S, Parry E, Britton J. Proximity of the home to roads and the risk of wheeze in an Ethiopian population. Occup Environ Med. 2005 Jun;62(6):376–380. doi: 10.1136/oem.2004.017228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yemaneberhan H, Bekele Z, Venn A, Lewis S, Parry E, Britton J. Prevalence of wheeze and asthma and relation to atopy in urban and rural Ethiopia. Lancet. 1997 Jul 12;350(9071):85–90. doi: 10.1016/S0140-6736(97)01151-3. [DOI] [PubMed] [Google Scholar]
- 14.Brauer M, Hoek G, Van Vliet P, Meliefste K, Fischer PH, Wijga A, et al. Air pollution from traffic and the development of respiratory infections and asthmatic and allergic symptoms in children. Am J Respir Crit Care Med. 2002 Oct 15;166(8):1092–1098. doi: 10.1164/rccm.200108-007OC. [DOI] [PubMed] [Google Scholar]
- 15.Stone V. Environmental air pollution. Am J Respir Crit Care Med. 2000 Aug;162(2 Pt 2):S44–S47. doi: 10.1164/ajrccm.162.supplement_1.maic-12. [DOI] [PubMed] [Google Scholar]
- 16.Mar TF, Jansen K, Shepherd K, Lumley T, Larson TV, Koenig JQ. Exhaled nitric oxide in children with asthma and short-term PM2.5 exposure in Seattle. Environ Health Perspect. 2005 Dec;113(12):1791–1794. doi: 10.1289/ehp.7883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Corbo GM, Forastiere F, Dell'Orco V, Pistelli R, Agabiti N, De Stefanis B, et al. Effects of environment on atopic status and respiratory disorders in children. J Allergy Clin Immunol. 1993 Oct;92(4):616–623. doi: 10.1016/0091-6749(93)90086-u. [DOI] [PubMed] [Google Scholar]
- 18.Mallol J, Sole D, Asher I, Clayton T, Stein R, Soto-Quiroz M. Prevalence of asthma symptoms in Latin America: the International Study of Asthma and Allergies in Childhood (ISAAC) Pediatr Pulmonol. 2000 Dec;30(6):439–444. doi: 10.1002/1099-0496(200012)30:6<439::aid-ppul1>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 19.Baumann LM, Robinson CL, Gilman RH, Hansel NH, Cabrera L, Wise RA, et al. Association Between Distance From A Heavily Transited Avenue, Indoor Air Quality And Risk Of Asthma In A Poor Peri-urban Shanty-town In Lima, Peru. Am J Respir Crit Care Med. 2010;181:A2508. [Google Scholar]
- 20.Gilman RH, Marquis GS, Miranda E, Vestegui M, Martinez H. Rapid reinfection by Giardia lamblia after treatment in a hyperendemic Third World community. Lancet. 1988 Feb 13;1(8581):343–345. doi: 10.1016/s0140-6736(88)91131-2. [DOI] [PubMed] [Google Scholar]
- 21.Fernandez-Concha D, Gilman RH, Gilman JB. A home nutritional rehabilitation programme in a Peruvian peri-urban shanty town (pueblo joven) Trans R Soc Trop Med Hyg. 1991 Nov–Dec;85(6):809–813. doi: 10.1016/0035-9203(91)90465-b. [DOI] [PubMed] [Google Scholar]
- 22.Mata Fernandez C, Fernandez-Benitez M, Perez Miranda M, Guillen Grima F. Validation of the Spanish version of the Phase III ISAAC questionnaire on asthma. J Investig Allergol Clin Immunol. 2005;15(3):201–210. [PubMed] [Google Scholar]
- 23.Miller MRHJ, Brusasco V, et al. ATS/ERS Task Force: Standardisation of Lung Function Testing (No. 2: Standardisation of Spirometry) Eur Resp J. 2005;26:319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 24.Chakrabarti B, Fine PM, Delfino RJ, Sioutas C. Performance evaluation of the active-flow personal DataRAM PM2.5 mass monitor (Thermo Anderson pDR-1200) designed for continuous personal exposure measurements. Atmos Environ. 2004;38 3329-2240. [Google Scholar]
- 25.Hastie T, Tibshirani R. Generalized additive models. London: Chapman & Hall; 1990. [DOI] [PubMed] [Google Scholar]
- 26.Efron B, Tibshirani R. An Introduction to the Bootstrap. New York: Chapman & Hall; 1993. [Google Scholar]
- 27.Sears MR, Greene JM, Willan AR, Wiecek EM, Taylor DR, Flannery EM, et al. A longitudinal, population-based, cohort study of childhood asthma followed to adulthood. N Engl J Med. 2003 Oct 9;349(15):1414–1422. doi: 10.1056/NEJMoa022363. [DOI] [PubMed] [Google Scholar]
- 28.Gilmour MI, Park P, Selgrade MJ. Increased immune and inflammatory responses to dust mite antigen in rats exposed to 5 ppm NO2. Fundam Appl Toxicol. 1996 May;31(1):65–70. doi: 10.1006/faat.1996.0076. [DOI] [PubMed] [Google Scholar]
- 29.Riedel F, Kramer M, Scheibenbogen C, Rieger CH. Effects of SO2 exposure on allergic sensitization in the guinea pig. J Allergy Clin Immunol. 1988 Oct;82(4):527–534. doi: 10.1016/0091-6749(88)90961-x. [DOI] [PubMed] [Google Scholar]
- 30.Leonardi GS, Houthuijs D, Steerenberg PA, Fletcher T, Armstrong B, Antova T, et al. Immune biomarkers in relation to exposure to particulate matter: a cross-sectional survey in 17 cities of Central Europe. Inhal Toxicol. 2000;12 Suppl 4:1–14. [PubMed] [Google Scholar]
- 31.Samuelsen M, Nygaard UC, Lovik M. Allergy adjuvant effect of particles from wood smoke and road traffic. Toxicology. 2008 Apr 18;246(2–3):124–131. doi: 10.1016/j.tox.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 32.Viera L, Chen K, Nel A, Lloret M. The impact of air pollutants as an adjuvant for allergic sensitization and asthma. Curr Allergy Asthma Rep. 2009;9(4):327–333. doi: 10.1007/s11882-009-0046-x. [DOI] [PubMed] [Google Scholar]
- 33.Delfino RJ, Quintana PJ, Floro J, Gastanaga VM, Samimi BS, Kleinman MTX, et al. Association of FEV1 in asthmatic children with personal and microenvironmental exposure to airborne particulate matter. Environ Health Perspect. 2004 Jun;112(8):932–941. doi: 10.1289/ehp.6815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gilbert NL, Woodhouse S, Stieb DM, Brook JR. Ambient nitrogen dioxide and distance from a major highway. Sci Total Environ. 2003 Aug 1;312(1–3):43–46. doi: 10.1016/S0048-9697(03)00228-6. [DOI] [PubMed] [Google Scholar]
- 35.Roorda-Knape MC, Janssen NA, de Hartog J, Van Vliet PH, Harssema H, Brunekreef B. Traffic related air pollution in city districts near motorways. Sci Total Environ. 1999 Sep 1;235(1–3):339–341. doi: 10.1016/s0048-9697(99)00217-x. [DOI] [PubMed] [Google Scholar]
- 36.Zhu Y, Hinds WC, Kim S, Sioutas C. Concentration and size distribution of ultrafine particles near a major highway. J Air Waste Manag Assoc. 2002 Sep;52(9):1032–1042. doi: 10.1080/10473289.2002.10470842. [DOI] [PubMed] [Google Scholar]
- 37.van Vliet P, Knape M, de Hartog J, Janssen N, Harssema H, Brunekreef B. Motor vehicle exhaust and chronic respiratory symptoms in children living near freeways. Environ Res. 1997;74(2):122–132. doi: 10.1006/enrs.1997.3757. [DOI] [PubMed] [Google Scholar]
- 38.Oosterlee A, Drijver M, Lebret E, Brunekreef B. Chronic respiratory symptoms in children and adults living along streets with high traffic density. Occup Environ Med. 1996 Apr;53(4):241–247. doi: 10.1136/oem.53.4.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.WHO guidelines for air quality. Indian Pediatr. 1998 Aug;35(8):812–815. [PubMed] [Google Scholar]
- 40.Koutrakis P, Sax SN, Sarnat JA, Coull B, Demokritou P, Oyola P, et al. Analysis of PM10, PM2.5, and PM2 5-10 concentrations in Santiago, Chile, from 1989 to 2001. J Air Waste Manag Assoc. 2005 Mar;55(3):342–351. doi: 10.1080/10473289.2005.10464627. [DOI] [PubMed] [Google Scholar]