Abstract
Aim
To estimate the general and racial-ethnic specific cumulative probability of remission from nicotine alcohol cannabis or cocaine dependence, and to identify predictors of remission across substances.
Design
Data were collected from structured diagnostic interviews using the Alcohol Use Disorder and Associated Disabilities Interview Schedule – DSM-IV version.
Setting
The 2001–2002 NESARC surveyed a nationally representative sample from USA adults (n=43,093) selected in a three-stage sampling design.
Participants
The subsamples of individuals with lifetime DSM-IV diagnosis of dependence on nicotine (n=6,937), alcohol (n=4,781), cannabis (n=530) and cocaine (n=408).
Measurements
Cumulative probability estimates of dependence remission for the general population and across racial-ethnic groups. Hazard ratios for remission from dependence.
Findings
Lifetime cumulative probability estimates of dependence remission were 83.7% for nicotine, 90.6% for alcohol, 97.2% for cannabis, and 99.2% for cocaine. Half of the cases of nicotine, alcohol, cannabis and cocaine dependence remitted approximately 26, 14, 6 and 5 years after dependence onset, respectively. Males, Blacks and individuals with diagnosis of personality disorders and history of substance use comorbidity exhibited lower hazards of remission for at least two substances.
Conclusions
A significant proportion of individuals with dependence on nicotine, alcohol, cannabis or cocaine achieve remission at some point in their lifetime, although the probability and time to remission varies by substance and racial-ethnic group. Several predictors of remission are shared by at least two substances, suggesting that the processes of remission overlap. The lower rates of remission of individuals with comorbid personality or substance use disorders highlight the need of providing coordinated psychiatric and substance abuse interventions.
INTRODUCTION
Substance dependence is associated with increased risk of physical and mental illness, disability, lost work productivity, financial problems, perpetrating and suffering violence, accidents, and death [1]. In 2004, 0.5% of disability adjusted life years (DALY) and 0.2% of all deaths worldwide (approximately 91 million deaths) were attributed to substance use disorders (SUDs) [1]. However, a significant proportion of individuals with these disorders achieve remission at some point in their lives [2–8]. Therefore, recognizing the patterns and predictors of remission from substance dependence is essential for increasing our understanding of its natural history, and developing timely prevention and treatment strategies.
Results from clinical and population-based studies have shown higher remission rates for dependence on cannabis [7–9] and alcohol [4, 5, 10], followed by cocaine [2, 6, 11] and nicotine [3, 12]. These studies have also identified several factors associated with remission, including being female, older, White, married, with higher educational attainment, and a later onset of substance use [5, 7, 10, 13–15]. Despite this extensive body of research, important questions remain. To date, comparisons of remission rates across all racial/ethnic groups in the US are not available, due to limited sample size for minority groups in most datasets [3, 5]. Scarce information exists regarding the commonality of remission predictors across substances. Few studies have examined the role of psychiatric comorbidity on the probability of remission, and even fewer have assessed time-varying predictors of remission [5, 14, 16, 17].
The goal of this study was to advance knowledge in these areas. Specifically, we sought to: 1) estimate the time from onset of nicotine, alcohol, cannabis, or cocaine dependence until remission of all dependence criteria (herein remission) for each substance; 2) examine the cumulative probability of remission from dependence across racial/ethnic groups; and, 3) identify predictors of remission across substances.
METHODS
SAMPLE
The 2001–2002 National Epidemiological Survey of Alcohol and Related Conditions (NESARC) surveyed a representative sample of the U.S. population [18]. The target population was the civilian non-institutionalized population 18 years and older residing in households and group quarters (e.g., college quarters, group homes, boarding houses, and non-transient hotels). The survey included residents of the continental United States, District of Columbia, Alaska and Hawaii. Face-to-face computer-assisted interviews were conducted among a multistage cluster sample of 43,093 respondents. Theoverall survey response rate was 81%. Blacks, Hispanics, and adults aged 18–24 were oversampled, with data adjusted for oversampling, household- and person-level non-response [18]. This report examined data of the subsample of respondents with lifetime history of dependence on nicotine (n=6,937), alcohol (n=4,781), cannabis (n=530) or cocaine (n=408).
Interviews were conducted by professional interviewers from the US Census Bureau. On average, the interviewers had 5 years of experience working on census and other health-related national surveys. Training was standardized under the direction of the National Institute on Alcohol Abuse and Alcoholism Alcohol (NIAAA). All procedures, including informed consent, received full ethical review and approval from the U.S. Census Bureau and U.S. Office of Management and Budget.
MEASURES
Socio-demographics
Self-reported race/ethnicity was recoded into 5 groups: Whites, Blacks, Hispanics, Native Hawaiians or other Pacific Islanders (NH/PI) and American Indians or Alaskan Natives (AI/AN). Other socio-demographic variables included gender, age, nativity, level of education, individual income and marital status.
Substance dependence and remission
All diagnoses were made according to the DSM-IV criteria using the NIAAA Alcohol Use Disorder and Associated Disabilities Interview Schedule – DSM-IV version (AUDADIS) [19], which allows measurement of substance use and mental disorders in large-scale surveys. Detailed information on survey methods is described elsewhere [18]. Dependence diagnoses require the existence of 3 or more of the 7 DSM-IV diagnostic criteria within a 12 month period [20]. Age of dependence onset for each substance was defined as the age at which the respondent first met dependence diagnostic criteria for that particular substance.
The outcome variables remission and age at remission were determined by asking individuals with a lifetime diagnosis of dependence “about how old were you when you finally stopped having any of these experiences (dependence criteria) with (name of drug)? By finally stopped, I mean they never started happening again.” Time to remission for each specific substance was defined as the time interval between age of dependence onset and age of remission.
Early substance use onset (defined as before age 14) and family history of SUDs (any alcohol or drug use disorder among first degree relatives) were also included as substance use-related covariates. The good to excellent (κ=0.54–0.91) test-retest reliability and validity of AUDADIS-IV SUD diagnoses is well documented in clinical and general population samples [21–24].
Psychiatric disorders
Mood disorders included DSM-IV primary major depressive disorder (MDD), dysthymia, and bipolar disorders. Anxiety disorders included DSM-IV primary panic disorder (with and without agoraphobia), social anxiety disorder, specific phobias and generalized anxiety disorder. Consistent with DSM-IV, “primary” AUDADIS-IV diagnoses excluded disorders that are substance-induced or due to general medical conditions. Diagnoses of MDD ruled out bereavement [20]. AUDADIS-IV diagnostic methods and advantages over other survey interviews are described in detail elsewhere.[19] Personality disorders (PDs) and conduct disorder diagnoses have been described in detail elsewhere [20]. Personality disorders included avoidant, dependent, obsessive-compulsive, paranoid, schizoid, histrionic, and antisocial PDs. Personality disorder diagnoses require long-term patterns of social and occupational impairment [20].
Test-retest reliabilities for AUDADIS-IV mood, anxiety and personality disorders diagnoses in the general population and clinical settings were fair to good (κ=0.40–0.77) [21, 23]. Convergent validity was good to excellent for all affective, anxiety, and personality disorders diagnoses [25, 26], and selected diagnoses showed good agreement (κ=0.64–0.68) with psychiatrist reappraisals [27].
ANALYSES
Cumulative probability of remission, defined as the proportion of individuals that achieve remission by a specified time point or interval [28], was obtained for the general population and across racial-ethnic groups for the first year after substance dependence onset, a decade later, and lifetime. The log-rank test was used to determine whether survival curves differed statistically across racial-ethnic groups. These projections were obtained by the standard actuarial method [29] as implemented in SAS (version 9.1.3), (SAS Institute, Cary, N.C.).
Weighted frequencies and their respective 95% confidence intervals (95% CI) were computed. Univariate and multivariate discrete-time time survival analyses (with person-year as the unit of analysis) [29] were implemented to assess the association between covariates and the probability of remission among individuals with lifetime substance dependence. Associations are expressed as hazard ratios measuring relative risk. The person-year variable was defined as the number of years from dependence onset to the age of remission or age at interview (for censored cases). DSM-IV mood, anxiety and comorbid substance use disorders, level of education and marital status were included as time-varying covariates. Taylor series linearization methods implemented in SUDAAN (version 9.1) (Research Triangle Institute, Research Triangle Park, N.C.) were used to estimate standard errors and significance and accommodate for complex survey design. Predictors reaching statistical significance at the 0.2 level in the univariate analyses were included in the multivariate model. Descriptive and discrete-time survival models were implemented using SUDAAN.
RESULTS
CHARACTERISTICS OF THE STUDY POPULATION
Socio-demographic, and psychiatric and substance use-related characteristics of the study population are presented in tables 1 and table 2. Approximately 80% of individuals with dependence on nicotine and alcohol and almost all individuals with cannabis or cocaine dependence had a lifetime diagnosis of another psychiatric disorder (Table 2). About one-third of individuals with nicotine or alcohol dependence and almost two-thirds of those with cannabis or cocaine dependence had a lifetime diagnosis of a mood, anxiety or PD. Approximately two-thirds of individuals with dependence on any of the substances assessed had a family history of substance use. Rates of early substance use onset were highest among individuals with dependence on nicotine, followed by cannabis, alcohol and cocaine.
Table 1.
Socio-demographic characteristics of individuals who reported substance use dependence by type of substance in the NESARC
| Characteristic | Nicotine Dependence (N=6,937) % (95%CI) |
Alcohol Dependence (N=4,781) % (95%CI) |
Cannabis Dependence (N=530) % (95%CI) |
Cocaine Dependence (N=408) % (95%CI) |
|---|---|---|---|---|
| Gender (Male) | 54.18 (52.79–55.57) | 66.67 (64.98–68.32) | 64.55 (59.37–69.41) | 60.28 (54.66–65.63) |
| Age group | ||||
| 18–29 | 25.72 (24.25–27.26) | 31.57 (29.64–33.57) | 44.93 (39.65–50.34) | 21.7 (16.96–27.32) |
| 30–44 | 37.28 (35.66–38.94) | 39.72 (38.05–41.42) | 40.22 (34.79–45.91) | 53.61 (47.39–59.71) |
| ≥45 | 36.99 (35.55–38.46) | 28.71 (27.09–30.38) | 14.84 (11.28–19.28) | 24.7 (19.83–30.31) |
| Race/ethnicity | ||||
| Whites | 80.53 (78.72–82.23) | 78.25 (75.38–80.87) | 73.78 (69.33–77.8) | 69.06 (62.71–74.77) |
| Blacks | 8.18 (7.11–9.39) | 7.43 (6.44–8.56) | 9.97 (7.48–13.17) | 13.64 (10.25–17.92) |
| Hispanics | 5.65 (4.72–6.75) | 8.81 (6.8–11.34) | 8.79 (6.37–12) | 8.65 (5.98–12.36) |
| NH/PI | 2.01 (1.47–2.72) | 2.1 (1.45–3.05) | 2.34 (1.19–4.54) | 2.08 (0.88–4.8) |
| AI/AN | 3.63 (3.02–4.36) | 3.41 (2.7–4.3) | 5.12 (3.24–7.99) | 6.57 (3.57–11.78) |
| US Born | 94.04 (92.75–95.11) | 93.56 (91.75–95) | 94.5 (90–97.04) | 95.43 (92.35–97.31) |
| Education | ||||
| < High school | 16.95 (15.81–18.16) | 12.77 (11.37–14.31) | 17.05 (13.61–21.14) | 17.12 (12.8–22.53) |
| High school | 32.46 (30.83–34.14) | 28.03 (26.15–29.99) | 28.28 (23.79–33.25) | 32.58 (26.83–38.91) |
| ≥ College | 50.58 (48.75–52.42) | 59.20 (56.96–61.41) | 54.67 (49.49–59.75) | 50.30 (43.83–56.75) |
| Individual Income | ||||
| $0–$19,999 | 48.31 (46.70–49.91) | 42.80 (40.87–44.74) | 55.82 (50.53–60.97) | 49.90 (43.77–56.03) |
| $20,000–$34,999 | 23.97 (22.74–25.25) | 24.94 (23.43–26.52) | 21.92 (17.65–26.89) | 22.57 (18.03–27.87) |
| $35,000–$69,999 | 21.19 (19.88–22.57) | 24.80 (23.18–26.50) | 17.46 (13.87–21.75) | 21.01 (16.71–26.08) |
| ≥ $70,000 | 6.53 (5.59–7.61) | 7.46 (6.43–8.63) | 4.80 (2.93–7.78) | 6.52 (3.97–10.54) |
| Marital Status | ||||
| Married/living with someone | 58.08 (56.46–59.68) | 53.93 (52.1–55.74) | 44.36 (38.63–50.24) | 54.85 (49.17–60.4) |
| Never married | 21.16 (19.77–22.63) | 28.86 (27.09–30.7) | 41.70 (36.2–47.42) | 22.37 (17.68–27.88) |
| Divorded/Separated/Widowed | 20.76 (19.65–21.91) | 17.21 (15.84–18.68) | 13.94(10.9–17.66) | 22.78(18.87–27.23) |
| Employed in past 12 months | 68.62(67.17–70.03) | 73.68(72.06–75.24) | 72.48 (67.56–76.9) | 69.23 (63.25–74.63) |
Note: NH/PI=Native Hawaiians or other Pacific Islanders and AI/AN=American Indians or Alaskan Natives
Table 2.
Psychopathologic and substance use-related characteristics of individuals with substance use dependence by type of substance in the NESARC
| Characteristic | Nicotine Dependence (N=6,937) % (95%CI) |
Alcohol Dependence (N=4,781) % (95%CI) |
Cannabis Dependence (N=530) % (95%CI) |
Cocaine Dependence (N=408) % (95%CI) |
|---|---|---|---|---|
| Any lifetime psychiatric disorder | 81.49 (80.23–82.69) | 79.52 (78.1–80.88) | 98.95 (97.79–99.5) | 98.92 (96.73–99.65) |
| Any lifetime mood disorder | 34.28 (32.75–35.85) | 37.12 (35.31–38.97) | 56.95 (51.19–62.52) | 60.31 (54.08–66.22) |
| Any lifetime anxiety disorder | 36.35 (34.9–37.82) | 37.07 (35.49–38.68) | 56.07 (51.03–60.99) | 55.55 (49.9–61.06) |
| Any lifetime conduct disorder | 1.60 (1.24–2.06) | 1.55 (1.16–2.08) | 2.74 (1.42–5.25) | 0.79 (0.24–2.59) |
| Any lifetime personality disorder | 35.85 (34.29–37.44) | 40.71 (38.73–42.71) | 65.38 (60.4–70.05) | 62.97 (57.53–68.1) |
| Nicotine Dependence | 48.88 (46.97–50.8) | 69.64 (64.26–74.53) | 71.67 (66.09–76.65) | |
| Alcohol Dependence | 34.45 (32.95–35.97) | 72.15 (67.09–76.7) | 77.97 (73.01–82.25) | |
| Cannabis Dependence | 5.09 (4.41–5.88) | 7.49 (6.65–8.43) | 30.55 (25.66–35.92) | |
| Cocaine Dependence | 3.97 (3.41–4.62) | 6.13 (5.37–7) | 23.14 (19.46–27.27) | |
| Family history of SUD | 56.88 (55.41–58.34) | 59.04 (57.18–60.88) | 70.75 (65.64–75.39) | 77.1 (72.07–81.45) |
| Substance use onset of specific substance before age 14a | 43.44 (41.82–45.07) | 18.84 (17.34–20.43) | 35.35 (30.57–40.44) | 4.84 (2.93–7.9) |
Use onset for the substance of interest described in the column (i.e. nicotine, alcohol, cannabis or cocaine/crack)
CUMULATIVE LIFETIME PROBABILITY OF SUBSTANCE DEPENDENCE REMISSION
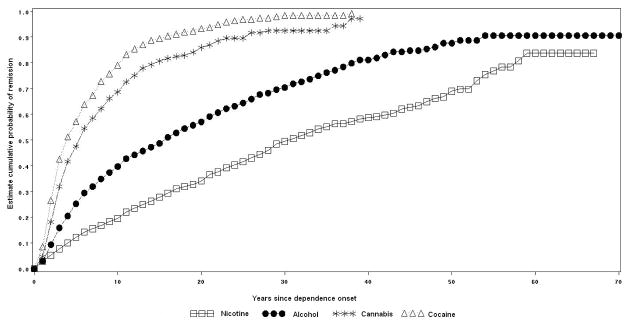
The cumulative probability estimates of dependence remission within the first year of dependence onset were 3.0% for nicotine and alcohol, 4.7% for cannabis, and 8.6% for cocaine. The cumulative probability of remission a decade after onset of dependence was 18.4% for nicotine, 37.4% for alcohol, 66.2% for cannabis, and 75.8% for cocaine. Lifetime cumulative probability estimates of dependence remission were 83.7% for nicotine, 90.6% for alcohol, 97.2% for cannabis, and 99.2% for cocaine. Half of the cases of nicotine, alcohol, cannabis and cocaine dependence remitted approximately 26, 14, 6 and 5 years after dependence onset, respectively (Figure 1).
Figure 1.
Cumulative probability of remission from lifetime dependence on nicotine, alcohol, cannabis and cocaine.
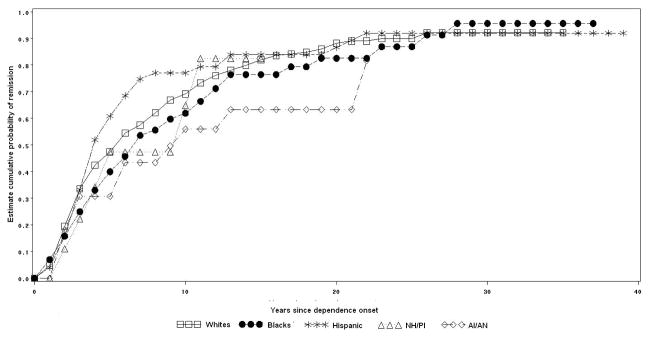
Variations in the probability and time to remission for each specific substance by racial/ethnic group are presented in figures 2 to 5.
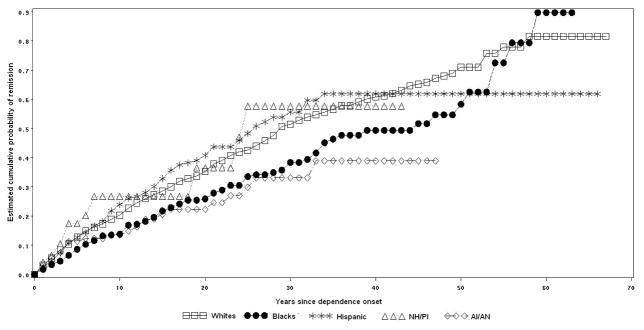
Figure 2.
Cumulative probability of remission from lifetime nicotine dependence by racial-ethnic group.
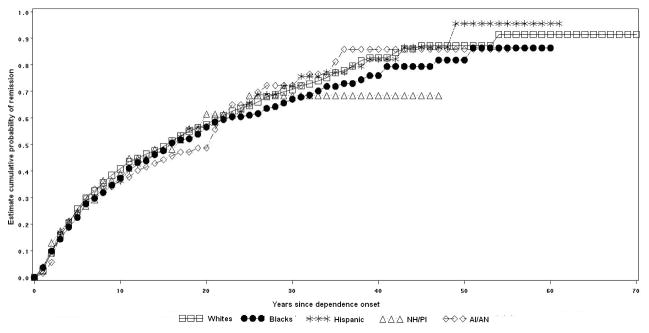
Figure 5.
Cumulative probability of remission from lifetime cocaine dependence by racial-ethnic group.
Nicotine dependence remission by racial-ethnic group
Among individuals with nicotine dependence, 81.6% of Whites, 89.7% of Blacks, 61.9% of Hispanics, 57.7% of NH/PI, and 38.9% of AI/AN remitted at some time in their lifetime (log rank test= 23.7, p<0.01). Half of nicotine dependence remissions occurred approximately 24 years after dependence onset among Whites, 35 years among Blacks, 16 years among Hispanics, 8 years among NH/PI, and 13 years among AI/AN (Figure 2).
Alcohol dependence remission by racial-ethnic group
Among individuals with alcohol dependence, 91.5% of Whites, 86.4% of Blacks, 86.6% of Hispanics, 68.5% of NH/PI and 85.9% of AI/AN remitted at some time in their lifetime (log rank test= 1.9, p=0.8). Half of alcohol dependence remissions occurred approximately 14 years after dependence onset among Whites, 13 years among Blacks and Hispanics, 8 years after among NH/PI, and 15 years among AI/AN (Figure 3).
Figure 3.
Cumulative probability of remission from lifetime alcohol dependence by racial-ethnic group.
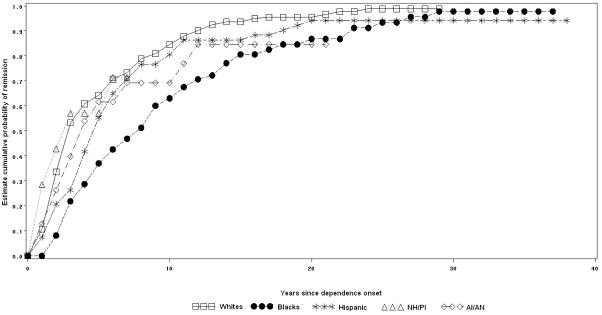
Cannabis dependence remission by racial-ethnic group
Among individuals with cannabis dependence, 92.2% of Whites, 95.6% of Blacks, 91.9% of Hispanics, 85.5% of NH/PI and 81.6% of AI/AN remitted at some time in their lifetime (log rank test= 3.9, p=0.4). Half of cannabis dependence remissions occurred approximately 6 years after dependence onset among Whites and Blacks, 5 years among Hispanics and NH/PI and 7 years among AI/AN (Figure 4).
Figure 4.
Cumulative probability of remission from lifetime cannabis dependence by racial-ethnic group.
Cocaine dependence remission by racial-ethnic group
Among individuals with cocaine dependence, 98.9% of Whites, 97.8% of Blacks, 94.2% of Hispanics, 71.4% of NH/PI and 84.6% of AI/AN remitted at some time in their lifetime (log rank test= 23.6, p<0.01). Half of cocaine dependence remissions occurred approximately 4 years after dependence onset among Whites, 9 years among Blacks, 8 years among Hispanics, 3 years among NH/PI and 5 years among AI/AN (Figure 5).
PREDICTORS OF REMISSION
In univariate and multivariate discrete-time survival models several socio-demographic, psychopathological and substance use-related variables (Tables 3 and 4) predicted dependence remission for most of the substances assessed.
Table 3.
Predictors of remission from dependence in NESARC. Results of univariate discrete-time survival analyses.
| Characteristics | Nicotine remission (N=1,300) HR (95%CI) |
Alcohol remission (N=2,008) HR (95%CI) |
Cannabis remission (N=344) HR (95%CI) |
Cocaine remission (N=336) HR (95%CI) |
|---|---|---|---|---|
| Gender (Male) | 0.9 (0.79–1.03) | 0.8 (0.72–0.89) | 0.76 (0.57–1.01) | 0.72 (0.56–0.92) |
| Age group | ||||
| 18–29 | 1.18 (0.91–1.54) | 0.77 (0.61–0.96) | 2.28 (1.56–3.34) | 4.57 (2.48–8.4) |
| 30–44 | 0.82 (0.69–0.96) | 1.04 (0.91–1.19) | 1.07 (0.72–1.58) | 1.49 (1–2.23) |
| ≥45* | 1.0 | 1.0 | 1.0 | 1.0 |
| Race/ethnicity | ||||
| Whites* | 1.0 | 1.0 | 1.0 | 1.0 |
| Blacks | 0.65 (0.52–0.82) | 0.9 (0.75–1.08) | 0.99 (0.65–1.52) | 0.46 (0.33–0.65) |
| Hispanics | 1.07 (0.84–1.38) | 0.94 (0.79–1.12) | 1.2 (0.78–1.86) | 0.71 (0.46–1.08) |
| NH/PI | 1.39 (0.89–2.16) | 0.75 (0.37–1.54) | 1.16 (0.51–2.63) | 1.63 (0.52–5.17) |
| AI/AN | 0.69 (0.45–1.05) | 1.05 (0.78–1.41) | 0.64 (0.29–1.39) | 1 (0.54–1.85) |
| US Born | 0.87 (0.66–1.14) | 1.3 (1.04–1.63) | 1.28 (0.35–4.65) | 0.86 (0.42–1.75) |
| Education (years) | 1.11 (1.09–1.13) | 1.01 (0.99–1.03) | 1.07 (1.01–1.14) | 1.06 (0.98–1.14) |
| Individual Income | ||||
| $0–$19,999 | 0.51 (0.41–0.64) | 1.13 (0.91–1.41) | 0.85 (0.45–1.61) | 0.79 (0.49–1.27) |
| $20,000–$34,999 | 0.65 (0.52–0.82) | 1.13 (0.89–1.42) | 1.28 (0.67–2.44) | 0.70 (0.41–1.19) |
| $35,000–$69,999 | 0.81 (0.65–1.01) | 1.32 (1.06–1.64) | 1.20 (0.64–2.26) | 1.06 (0.65–1.73) |
| ≥ $70,000 | 1.0 | 1.0 | 1.0 | 1.0 |
| Marital Status | ||||
| Married/living with someone* | 1.0 | 1.0 | 1.0 | 1.0 |
| Never married | 1.03 (0.84–1.26) | 0.87 (0.74–1.01) | 1.29 (0.82–2.04) | 1.21 (0.85–1.72) |
| Divorced/Separated/Widowed | 0.75 (0.64–0.88) | 1.07 (0.94–1.23) | 0.86 (0.49–1.52) | 1.13 (0.71–1.79) |
| Diagnosed with a mood disorder | 0.78 (0.59–1.03) | 0.97 (0.79–1.2) | 0.85 (0.52–1.4) | 0.95 (0.59–1.55) |
| Diagnosed with an anxiety disorder | 1.34 (0.96–1.88) | 0.86 (0.65–1.14) | 0.72 (0.34–1.51) | 0.7 (0.32–1.56) |
| Any lifetime conduct disorder | 1.03 (0.52–2.04) | 0.62 (0.42–0.94) | 3.38 (1.9–6.02) | 4.32 (1.95–9.56) |
| Any lifetime personality disorder | 0.73 (0.63–0.85) | 0.85 (0.76–0.96) | 0.59 (0.46–0.76) | 1.09 (0.84–1.42) |
| Diagnosed with nicotine dependence | 0.85 (0.74–0.98) | 0.82 (0.59–1.12) | 1.17 (0.81–1.68) | |
| Diagnosed with alcohol dependence | 0.49 (0.37–0.63) | 0.58 (0.41–0.82) | 0.8 (0.57–1.14) | |
| Diagnosed with cannabis dependence | 0.38 (0.15–0.93) | 0.55 (0.32–0.94) | 0.17 (0.07–0.44) | |
| Diagnosed with cocaine dependence | 0.08 (0.02–0.33) | 0.26 (0.14–0.51) | 0.1 (0.03–0.27) | |
| Family history of SUD | 0.83 (0.72–0.95) | 0.99 (0.88–1.12) | 0.97 (0.67–1.38) | 1.02 (0.77–1.36) |
| Specific SU onset (Early - before age 14) | 0.79 (0.69–0.9) | 0.91 (0.8–1.05) | 0.66 (0.48–0.92) | 0.83 (0.47–1.44) |
Reference categories; HR= hazard ratios; 95%CI= 95% confidence Interval; Note: NH/PI=Native Hawaiians or other Pacific Islanders and AI/AN=American Indians or Alaskan Natives
Table 4.
Predictors of remission from dependence upon nicotine, alcohol, cannabis and cocaine in the NESARC. Results of multivariable discrete-time survival analyses.
| Characteristics | Nicotine remission (N=1300) HR(95%CI) |
Alcohol remission (N=2008) HR(95%CI) |
Cannabis remission (N=344) HR(95%CI) |
Cocaine remission (N=336) HR(95%CI) |
|---|---|---|---|---|
| Gender (Male) | 0.81 (0.70–0.95) | 0.80 (0.71–0.90) | 0.64 (0.48–0.86) | 0.65 (0.49–0.84) |
| Age group | ||||
| 18–29 | 1.30 (0.99–1.70) | 0.78 (0.62–0.99) | 2.40 (1.56–3.69) | 4.61 (2.41–8.81) |
| 30–44 | 0.87 (0.74–1.03) | 1.05 (0.92–1.21) | 1.07 (0.74–1.56) | 1.36 (0.88–2.10) |
| >45* | 1.0 | 1.0 | 1.0 | 1.0 |
| Race/ethnicity | ||||
| Whites* | 1.0 | 1.0 | ||
| Blacks | 0.75 (0.59–0.94) | 0.48 (0.34–0.68) | ||
| Hispanics | 1.21 (0.88–1.66) | 0.78 (0.49–1.26) | ||
| NH/PI | 1.28 (0.78–2.08) | 1.27 (0.38–4.24) | ||
| AI/AN | 0.74 (0.46–1.20) | 0.96 (0.50–1.86) | ||
| Education (years) | 1.10 (1.07–1.13) | |||
| Individual Income | ||||
| $0–$19,999 | 0.68 (0.52–0.90) | 1.08 (0.86–1.36) | 0.78 (0.39–1.55) | 0.73 (0.42–1.26) |
| $20,000–$34,999 | 0.79 (0.59–1.05) | 1.12 (0.87–1.43) | 1.36 (0.73–2.53) | 0.65 (0.36–1.15) |
| $35,000–$69,999 | 0.94 (0.72–1.21) | 1.24 (0.99–1.57) | 1.35 (0.69–2.64) | 1.06 (0.60–1.88) |
| ≥ $70,000* | 1.0 | 1.0 | 1.0 | 1.0 |
| Marital Status | ||||
| Married/living with someone* | 1.0 | 1.0 | ||
| Never married | 1.05 (0.85–1.29) | 0.88 (0.75–1.03) | ||
| Divorced/Separated/Widowed | 0.77 (0.64–0.93) | 1.07 (0.92–1.24) | ||
| Any lifetime conduct disorder | 1.95 (1.02–3.73) | |||
| Any lifetime personality disorder | 0.87 (0.77–0.99) | 0.66 (0.50–0.87) | ||
| Diagnosed with nicotine dependence | 1.71 (1.20–2.43) | |||
| Diagnosed with alcohol dependence | 0.51 (0.39–0.67) | 0.64 (0.44–0.94) | ||
| Diagnosed with cannabis dependence | 0.64 (0.37–1.13) | 0.12 (0.04–0.38) | ||
| Diagnosed with cocaine dependence | 0.28 (0.14–0.54) | 0.11 (0.04–0.33) | ||
| Early specific substance use onset | 0.75 (0.56–1.00) | |||
Reference categories; HR= hazard ratios; 95%CI= 95% confidence Interval; Note: NH/PI=Native Hawaiians or other Pacific Islanders and AI/AN=American Indians or Alaskan Natives
Socio-Demographic Predictors
Males were less likely than females to remit from dependence on all the substances assessed (Table 4). Individuals in the youngest age group (18–29 years old) were less likely to remit from alcohol dependence and more likely to remit from cannabis dependence or cocaine dependence than individuals in the oldest age group (≥ 45 years old). Unadjusted and adjusted models indicated that Blacks with nicotine dependence or cocaine dependence were less likely to remit than Whites with dependence of those substances. Each additional year of education attained increased the probability of nicotine dependence remission by 10% in the adjusted model. Individuals with nicotine dependence who reported an income lower than $20,000 were less likely to remit than individuals with incomes of $70,000 or more. Individuals with nicotine dependence who were divorced, separated, widowed or who stopped living with someone were less likely to remit than those married or living with someone.
Psychopathological Predictors and Drug-Use Related Predictors
Having a previous diagnosis of a conduct disorder increased the probability of remission from cannabis dependence and cocaine dependence, and decreased the probability of remission from alcohol dependence in the univariate models (Table 3). However, after controlling for the effect of other covariates this association remained significant only for cannabis dependence (Table 4). A diagnosis of a PD decreased the probability of remission from alcohol or cannabis dependence in the adjusted models (Tables 4). No association was observed between mood and anxiety disorders and dependence remission for any of the substances assessed.
According to the adjusted models (table 4), a diagnosis of nicotine dependence increased the likelihood of remission from cocaine dependence. A previous diagnosis of alcohol dependence decreased the probability of remission from nicotine or cannabis dependence. A past diagnosis of cannabis dependence predicted decreased likelihood of remission from cocaine dependence. Individuals with a past diagnosis of cocaine dependence were less likely to remit from alcohol or cannabis dependence.
DISCUSSION
In a large, nationally representative sample of US adults, we found that: 1) the vast majority of individuals with lifetime dependence on nicotine, alcohol, cannabis or cocaine would remit at some point in their lives; 2) remission from cannabis or cocaine dependence occurred faster than remission from nicotine or alcohol dependence; 3) significant racial-ethnic differences were observed in the cumulative probability of remission from nicotine dependence and cocaine dependence; and, 4) several socio-demographic, psychopathological and drug use-related predictors of remission were shared by at least two substances.
Cumulative probability estimates of remission were high for all the substances assessed, however these estimates should be interpreted with caution given the irregular course of addictions punctuated by remissions and relapses. Social factors, such as the progressive adoption of adult roles and responsibilities [30] and increased contact with environments in which substance dependence has lower acceptability may exert a powerful influence on the likelihood of experiencing remission. Age-related decreases in impulsivity and other neurodevelopmental changes [31], increased self-efficacy to abstain, and awareness of the health-related, social, and judicial [32–34] consequences of substance use are also likely contributors of remission.
More than two thirds remissions from cannabis and cocaine dependence occurred within the first decade after onset of dependence, whereas only one-fifth of remissions from nicotine dependence and one-third of remissions from alcohol dependence occurred within that period. The differences in the rate of remission across substances may be explained, at least in part, by the speed at which physical, psychological and social adverse consequences manifest after the onset of dependence. For instance, the risk of early cardiovascular adverse consequences is much higher among individuals with cocaine dependence than among those with nicotine or alcohol dependence [33]. The behavioral disturbances resulting from cannabis or cocaine dependence and their illegal status impose stronger social pressures to remit [15]. The high availability of alcohol and nicotine environmental cues for their consumption may also contribute to explain the difficulty stopping the use of these substances [35]. Particularly for nicotine, the immediate perceived benefits from its use, including anxiety and stress reduction, improved performance on a variety of cognitive tasks, and decreased food consumption and metabolism [35], may initially outweigh the potential perceived harms produced by its chronic use.
Contrasting with results from the National Comorbidity Survey [3], a lower rate of remission from nicotine dependence was observed in Blacks. Compared to White smokers, Black smokers start smoking later in life, have lower daily nicotine consumption, tend to smoke cigarettes with longer rod length and higher tar and nicotine content (i.e., menthols), and have slower clearance of cotinine and higher intake of nicotine per cigarette.[36, 37] Furthermore, the reduced access of Blacks and Hispanics to tobacco preventive services [38], as well as the intensive targeting of these minority groups by tobacco companies [39], could also help explain the observed racial-ethnic differences in the probability of nicotine dependence remission. Consistent with previous studies [40], we also found that Blacks with a lifetime diagnosis of cocaine dependence report lower rates of remission than their White counterparts. Psychosocial factors that commonly affect Black populations, including discrimination and lower levels of social capital, have been recognized as established barriers to dependence remission and triggers to use or relapse [41]. Furthermore, an array of genetic factors appear to increase the vulnerability to develop cocaine dependence among Blacks [42].
Men were less likely than women to remit from dependence on all the substances assessed. Women tend to experience worse physical, mental, and social consequences of substance use than males [43], which may lead to an increased motivation to stop using drugs and help explain women’s higher rates of remission. Feelings of guilt and concerns regarding the effects of using substances during pregnancy and child-rearing can lead to decrease drug use among women [44]. Gender differences in the neural response to cue-induced craving and stress [45], as well as in the activity of the lateral and medial regions of the orbitofrontal cortex (OFC), which modulates impulsivity and decision making [46] can also help explain the different course of cocaine dependence among males and females.
Substance use comorbidity and PD predicted remission across most substances assessed. Individuals with a diagnosis of a PD had a lower probability of remission from alcohol or cannabis dependence. Converging evidence supports the commonalities between PD and SUDs [47]. For instance, there are high rates of comorbidity between PD with high impulsivity traits (e.g., borderline PD) and SUD. Individuals with PD with high levels of stress reactivity, neuroticism and anxiety sensitivity can engage use substances to relieve their feelings. Novelty-seeking, reward-seeking, extraversion and gregariousness can also motivate drug use experimentation and sustained drug intake [47]. Associations between polymorphisms at candidate genes and personality dimensions correlated with the liability to SUD have also been documented [48]. A temporal relation in this association has been noted, with PD anteceding the onset of SUD [47]. The chronic course of PD [49] may increase the risk for relapse and interfere with psychological and social factors that help motivate remission [47]. The lack of association between previous diagnosis of mood and anxiety disorders and dependence remission observed in this study has been documented before [50].
Dependence on one substance tended to decrease the probability of remission from dependence on another substance. Chronic substance users may have difficulty overcoming the effect of drug-associated environmental cues and associative learning related to their drug-seeking behavior [51, 52]. Vulnerability to relapse and drug use maintenance have been also associated with the development of molecular adaptations resulting from chronic drug use, including the elevation of the GluR1 glutamate receptor subunit in the ventral tegmental area, alterations in the content and function of proteins such as the tyrosine hydroxylase, dopamine transporters, RGS9-2, and D2 autoreceptors and the D1-receptor- mediated stimulation of ΔFosB, a transcriptional regulator that modulates the synthesis of certain AMPA glutamate receptor subunits and cell-signaling enzymes [53, 54]. The existence of shared molecular pathways as well as the potential of certain drugs of abuse to induce cross-tolerance and cross-sensitization to one another can also contribute to the lower probability of remission among individuals with dependence on other drugs [55]. Genetic studies also suggest a significant overlap across substances in the genetic liability to dependence. For example, nicotine and alcohol dependence may share over 60% of their genetic vulnerabilities and alleles of several genes have been associated with alcohol, nicotine and polysubstance dependence [56]. The existence of comorbid SUDs poses a therapeutic challenge for clinicians, given the mixed evidence supporting sequential treatment over simultaneous treatment [57]. The findings of this study also emphasize the need to understand common mechanisms underlying the rewarding effects of drugs and the sequential neuropharmacological neuroadaptations once an addiction has developed.[54].
This study has the limitations common to most large-scale mental health surveys. First, self-report of substance use and psychiatric disorders are prone to social desirability bias leading to underreport of substance use and SUD and overreport of remission. Second, diagnoses of SUD, individual SUD criteria endorsement, or age of remission reported may be subject to recall bias (the longer the time interval between the event and assessment, the higher the probability of incorrect recalls) and to cognitive impairment resulting from the use of drugs. Third, institutionalized individuals or those who experienced fatal or severe consequences due to their SUD may have not been sampled, leading to overestimation of the cumulative probability of remission. Fourth, lack of a uniform operational definition of dependence remission across population-based studies limits our ability to compare our results with estimates from previous reports [3–5, 8, 17]. Fifth, lack of information on the number and duration of dependence remission episodes experienced over the individual’s lifetime limits our understanding of the role of these factors on dependence remission and relapse. Sixth, factors that may help explain the racial-ethnic differences, such as socio-economic status could not be included in the models as a time-dependent covariate since the NESARC, as most large scale surveys, does not include information on changes in income over time.
Despite these limitations, the current study expands the knowledge gained from previous investigations. The results of this study indicate that the majority of individuals with nicotine, alcohol, cannabis and cocaine dependence achieve remission at some point in their lives, although the probability and time to remission vary by drug and racial/ethnic group. Although there are no common predictors of remission across all four substances, several of these predictors were shared by at least two substances, suggesting that the processes of remission overlap, but are not identical. The lower rates of remission among individuals with comorbid PD or dependence on other drugs identified in this study highlight the need to recognize biological and social mechanisms that interfere with recovery from substance dependence, strengthen preventive efforts, particularly among vulnerable population subgroups, and provide coordinated psychiatric and substance abuse interventions.
Acknowledgments
Funding/Support: The National Epidemiologic Survey on Alcohol and Related Conditions was sponsored by the National Institute on Alcohol Abuse and Alcoholism with supplemental support from the National Institute on Drug Abuse. Work on this manuscript was supported by NIH grants DA019606, DA020783, DA023200, DA023973, MH076051, MH082773 and CA133050 (Dr. Blanco) and AA014223, DA018652, and AA018111 (Dr. Hasin), a grant from the American Foundation for Suicide Prevention (Dr. Blanco), and the New York State Psychiatric Institute (Drs. Blanco and Hasin).
Footnotes
Disclaimer: The views and opinions expressed in this report are those of the authors and should not be construed to represent the views of any of the sponsoring organizations, agencies, or the U.S. government.
References
- 1.Rehm J, Taylor B, Room R. Global burden of disease from alcohol, illicit drugs and tobacco. Drug Alcohol Rev. 2006;25:503–513. doi: 10.1080/09595230600944453. [DOI] [PubMed] [Google Scholar]
- 2.Newcomb MD, Galaif ER, Locke TF. Substance use diagnosis within a community sample of adults: Distinction, comorbidity, and progression over time. Prof Psychol-Res Pr. 2001;32:239–247. [Google Scholar]
- 3.Breslau N, Johnson EO, Hiripi E, Kessler R. Nicotine dependence in the United States: prevalence, trends, and smoking persistence. Arch Gen Psychiatry. 2001;58:810–816. doi: 10.1001/archpsyc.58.9.810. [DOI] [PubMed] [Google Scholar]
- 4.de Bruijn C, van den Brink W, de Graaf R, Vollebergh WA. The three year course of alcohol use disorders in the general population: DSM-IV, ICD-10 and the Craving Withdrawal Model. Addiction. 2006;101:385–392. doi: 10.1111/j.1360-0443.2006.01327.x. [DOI] [PubMed] [Google Scholar]
- 5.Kalaydjian A, Swendsen J, Chiu WT, Dierker L, Degenhardt L, Glantz M, et al. Sociodemographic predictors of transitions across stages of alcohol use, disorders, and remission in the National Comorbidity Survey Replication. Compr Psychiatry. 2009;50:299–306. doi: 10.1016/j.comppsych.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hser YI, Stark ME, Paredes A, Huang D, Anglin MD, Rawson R. A 12-year follow-up of a treated cocaine-dependent sample. J Subst Abuse Treat. 2006;30:219–226. doi: 10.1016/j.jsat.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 7.Gilder DA, Lau P, Corey L, Ehlers CL. Factors associated with remission from cannabis dependence in southwest California Indians. J Addict Dis. 2007;26:23–30. doi: 10.1300/J069v26n04_04. [DOI] [PubMed] [Google Scholar]
- 8.Calabria B, Degenhardt L, Briegleb C, Vos T, Hall W, Lynskey M, et al. Systematic review of prospective studies investigating “remission” from amphetamine, cannabis, cocaine or opioid dependence. Addict Behav. 35:741–749. doi: 10.1016/j.addbeh.2010.03.019. [DOI] [PubMed] [Google Scholar]
- 9.Perkonigg A, Goodwin RD, Fiedler A, Behrendt S, Beesdo K, Lieb R, et al. The natural course of cannabis use, abuse and dependence during the first decades of life. Addiction. 2008;103:439–449. doi: 10.1111/j.1360-0443.2007.02064.x. discussion 450–431. [DOI] [PubMed] [Google Scholar]
- 10.Dawson DA. Correlates of past-year status among treated and untreated persons with former alcohol dependence: United States, 1992. Alcohol Clin Exp Res. 1996;20:771–779. doi: 10.1111/j.1530-0277.1996.tb01685.x. [DOI] [PubMed] [Google Scholar]
- 11.Warner LA, Alegria M, Canino G. Remission from drug dependence symptoms and drug use cessation among women drug users in puerto rico. Arch Gen Psychiatry. 2004;61:1034–1041. doi: 10.1001/archpsyc.61.10.1034. [DOI] [PubMed] [Google Scholar]
- 12.West R, McEwen A, Bolling K, Owen L. Smoking cessation and smoking patterns in the general population: a 1-year follow-up. Addiction. 2001;96:891–902. doi: 10.1046/j.1360-0443.2001.96689110.x. [DOI] [PubMed] [Google Scholar]
- 13.Chen K, Kandel DB. Predictors of cessation of marijuana use: an event history analysis. Drug Alcohol Depend. 1998;50:109–121. doi: 10.1016/s0376-8716(98)00021-0. [DOI] [PubMed] [Google Scholar]
- 14.Hahn LP, Folsom AR, Sprafka JM, Norsted SW. Cigarette smoking and cessation behaviors among urban blacks and whites. Public Health Rep. 1990;105:290–295. [PMC free article] [PubMed] [Google Scholar]
- 15.von Sydow K, Lieb R, Pfister H, Hofler M, Sonntag H, Wittchen HU. The natural course of cannabis use, abuse and dependence over four years: a longitudinal community study of adolescents and young adults. Drug Alcohol Depend. 2001;64:347–361. doi: 10.1016/s0376-8716(01)00137-5. [DOI] [PubMed] [Google Scholar]
- 16.Cunningham JA. Untreated remissions from drug use: the predominant pathway. Addict Behav. 1999;24:267–270. doi: 10.1016/s0306-4603(98)00045-8. [DOI] [PubMed] [Google Scholar]
- 17.Price RK, Risk NK, Spitznagel EL. Remission from drug abuse over a 25-year period: patterns of remission and treatment use. Am J Public Health. 2001;91:1107–1113. doi: 10.2105/ajph.91.7.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grant B, Moore T, Shepard J, Kaplan K. Source and Accuracy Statement: Wave 1 of the 2001–2002 National Epidemiologic Survey on Alcohol and Related Conditions (NESARC) Bethesda, MD: National Institute on Alcohol Abuse and Alcoholism; 2003. [Google Scholar]
- 19.Grant B, Dawson D, Hasin D. The Alcohol Use Disorders and Associated Disabilities Interview Schedule—Version for DSM-IV (AUDADIS-IV) Bethesda, MD: National Institute on Alcohol Abuse and Alcoholism; 2001. [Google Scholar]
- 20.American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM- IV. 4. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 21.Grant BF, Dawson DA, Stinson FS, Chou PS, Kay W, Pickering R. The Alcohol Use Disorder and Associated Disabilities Interview Schedule-IV (AUDADIS-IV): reliability of alcohol consumption, tobacco use, family history of depression and psychiatric diagnostic modules in a general population sample. Drug Alcohol Depend. 2003;71:7–16. doi: 10.1016/s0376-8716(03)00070-x. [DOI] [PubMed] [Google Scholar]
- 22.Hasin D, Carpenter KM, McCloud S, Smith M, Grant BF. The alcohol use disorder and associated disabilities interview schedule (AUDADIS): reliability of alcohol and drug modules in a clinical sample. Drug Alcohol Depend. 1997;44:133–141. doi: 10.1016/s0376-8716(97)01332-x. [DOI] [PubMed] [Google Scholar]
- 23.Ruan WJ, Goldstein RB, Chou SP, Smith SM, Saha TD, Pickering RP, et al. The alcohol use disorder and associated disabilities interview schedule-IV (AUDADIS-IV): reliability of new psychiatric diagnostic modules and risk factors in a general population sample. Drug Alcohol Depend. 2008;92:27–36. doi: 10.1016/j.drugalcdep.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hasin DS, Schuckit MA, Martin CS, Grant BF, Bucholz KK, Helzer JE. The validity of DSM-IV alcohol dependence: what do we know and what do we need to know? Alcohol Clin Exp Res. 2003;27:244–252. doi: 10.1097/01.ALC.0000060878.61384.ED. [DOI] [PubMed] [Google Scholar]
- 25.Hasin DS, Goodwin RD, Stinson FS, Grant BF. Epidemiology of major depressive disorder: results from the National Epidemiologic Survey on Alcoholism and Related Conditions. Arch Gen Psychiatry. 2005;62:1097–1106. doi: 10.1001/archpsyc.62.10.1097. [DOI] [PubMed] [Google Scholar]
- 26.Grant BF, Hasin DS, Stinson FS, Dawson DA, Chou SP, Ruan WJ, et al. Prevalence, correlates, and disability of personality disorders in the United States: results from the national epidemiologic survey on alcohol and related conditions. J Clin Psychiatry. 2004;65:948–958. doi: 10.4088/jcp.v65n0711. [DOI] [PubMed] [Google Scholar]
- 27.Canino G, Bravo M, Ramirez R, Febo VE, Rubio-Stipec M, Fernandez RL, et al. The Spanish Alcohol Use Disorder and Associated Disabilities Interview Schedule (AUDADIS): reliability and concordance with clinical diagnoses in a Hispanic population. J Stud Alcohol. 1999;60:790–799. doi: 10.15288/jsa.1999.60.790. [DOI] [PubMed] [Google Scholar]
- 28.Wagner FA, Anthony JC. From first drug use to drug dependence; developmental periods of risk for dependence upon marijuana, cocaine, and alcohol. Neuropsychopharmacology. 2002;26:479–488. doi: 10.1016/S0893-133X(01)00367-0. [DOI] [PubMed] [Google Scholar]
- 29.Machin D, Cheung YB, Parmar MKB, Parmar MKB. Survival analysis: a practical approach. 2. Chichester, England; Hoboken, NJ: Wiley; 2006. [Google Scholar]
- 30.Dawson DA, Grant BF, Stinson FS, Chou PS. Maturing out of alcohol dependence: the impact of transitional life events. J Stud Alcohol. 2006;67:195–203. doi: 10.15288/jsa.2006.67.195. [DOI] [PubMed] [Google Scholar]
- 31.Chambers RA, Taylor JR, Potenza MN. Developmental neurocircuitry of motivation in adolescence: a critical period of addiction vulnerability. Am J Psychiatry. 2003;160:1041–1052. doi: 10.1176/appi.ajp.160.6.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Benowitz NL. Neurobiology of nicotine addiction: implications for smoking cessation treatment. Am J Med. 2008;121:S3–10. doi: 10.1016/j.amjmed.2008.01.015. [DOI] [PubMed] [Google Scholar]
- 33.Chen CY, Lin KM. Health consequences of illegal drug use. Curr Opin Psychiatry. 2009;22:287–292. doi: 10.1097/yco.0b013e32832a2349. [DOI] [PubMed] [Google Scholar]
- 34.March JC, Oviedo-Joekes E, Romero M. Drugs and social exclusion in ten European cities. Eur Addict Res. 2006;12:33–41. doi: 10.1159/000088581. [DOI] [PubMed] [Google Scholar]
- 35.Hatsukami DK, Stead LF, Gupta PC. Tobacco addiction. Lancet. 2008;371:2027–2038. doi: 10.1016/S0140-6736(08)60871-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Perez-Stable EJ, Herrera B, Jacob P, 3rd, Benowitz NL. Nicotine metabolism and intake in black and white smokers. Jama. 1998;280:152–156. doi: 10.1001/jama.280.2.152. [DOI] [PubMed] [Google Scholar]
- 37.Signorello LB, Cai Q, Tarone RE, McLaughlin JK, Blot WJ. Racial differences in serum cotinine levels of smokers. Dis Markers. 2009;27:187–192. doi: 10.3233/DMA-2009-0661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lopez-Quintero C, Crum RM, Neumark YD. Racial/ethnic disparities in report of physician-provided smoking cessation advice: analysis of the 2000 National Health Interview Survey. Am J Public Health. 2006;96:2235–2239. doi: 10.2105/AJPH.2005.071035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.U.S. Department of Health and Human Services. Tobacco Use Among Racial/Ethnic Minority Groups African Americans, American Indians and Alaska Natives, Asian Americans and Pacific Islanders, and Hispanics: A Report of the Surgeon General. Atlanta, GA: US Department of Health and Human Services, Centers of Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 1998. [Google Scholar]
- 40.O’Brien MS, Anthony JC. Risk of becoming cocaine dependent: epidemiological estimates for the United States, 2000–2001. Neuropsychopharmacology. 2005;30:1006–1018. doi: 10.1038/sj.npp.1300681. [DOI] [PubMed] [Google Scholar]
- 41.Fothergill KE, Ensminger ME, Green KM, Robertson JA, Juon HS. Pathways to adult marijuana and cocaine use: a prospective study of African Americans from age 6 to 42. J Health Soc Behav. 2009;50:65–81. doi: 10.1177/002214650905000105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Farrer LA, Kranzler HR, Yu Y, Weiss RD, Brady KT, Anton R, et al. Association of variants in MANEA with cocaine-related behaviors. Arch Gen Psychiatry. 2009;66:267–274. doi: 10.1001/archgenpsychiatry.2008.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Diehl A, Croissant B, Batra A, Mundle G, Nakovics H, Mann K. Alcoholism in women: is it different in onset and outcome compared to men? Eur Arch Psychiatry Clin Neurosci. 2007;257:344–351. doi: 10.1007/s00406-007-0737-z. [DOI] [PubMed] [Google Scholar]
- 44.Ehrmin JT. Unresolved feelings of guilt and shame in the maternal role with substance- dependent African American women. J Nurs Scholarsh. 2001;33:47–52. doi: 10.1111/j.1547-5069.2001.00047.x. [DOI] [PubMed] [Google Scholar]
- 45.Li CS, Kemp K, Milivojevic V, Sinha R. Neuroimaging study of sex differences in the neuropathology of cocaine abuse. Gend Med. 2005;2:174–182. doi: 10.1016/s1550-8579(05)80046-4. [DOI] [PubMed] [Google Scholar]
- 46.Adinoff B, Williams MJ, Best SE, Harris TS, Chandler P, Devous MD., Sr Sex differences in medial and lateral orbitofrontal cortex hypoperfusion in cocaine-dependent men and women. Gend Med. 2006;3:206–222. doi: 10.1016/s1550-8579(06)80209-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Verheul R. Co-morbidity of personality disorders in individuals with substance use disorders. Eur Psychiatry. 2001;16:274–282. doi: 10.1016/s0924-9338(01)00578-8. [DOI] [PubMed] [Google Scholar]
- 48.Vanyukov MM, Tarter RE. Genetic studies of substance abuse. Drug Alcohol Depend. 2000;59:101–123. doi: 10.1016/s0376-8716(99)00109-x. [DOI] [PubMed] [Google Scholar]
- 49.Hampson SE, Goldberg LR. A first large cohort study of personality trait stability over the 40 years between elementary school and midlife. J Pers Soc Psychol. 2006;91:763–779. doi: 10.1037/0022-3514.91.4.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sellman JD, Joyce PR. Does depression predict relapse in the 6 months following treatment for men with alcohol dependence? Aust N Z J Psychiatry. 1996;30:573–578. doi: 10.3109/00048679609062652. [DOI] [PubMed] [Google Scholar]
- 51.Volkow ND, Fowler JS, Wang GJ. The addicted human brain: insights from imaging studies. J Clin Invest. 2003;111:1444–1451. doi: 10.1172/JCI18533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: the role of reward-related learning and memory. Annu Rev Neurosci. 2006;29:565–598. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- 53.Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry. 2005;162:1403–1413. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- 54.Koob GF, Volkow ND. Neurocircuitry of Addiction. Neuropsychopharmacology. 2009 doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nestler EJ. Is there a common molecular pathway for addiction? Nat Neurosci. 2005;8:1445–1449. doi: 10.1038/nn1578. [DOI] [PubMed] [Google Scholar]
- 56.Schlaepfer IR, Hoft NR, Ehringer MA. The genetic components of alcohol and nicotine co- addiction: From genes to behavior. Curr Drug Abuse Rev. 2008;1:124–134. doi: 10.2174/1874473710801020124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kodl M, Fu SS, Joseph AM. Tobacco cessation treatment for alcohol-dependent smokers: when is the best time? Alcohol Res Health. 2006;29:203–207. [PMC free article] [PubMed] [Google Scholar]