Abstract
The paired-click paradigm (PCP) is widely used to study sensory habituation or gating in a number of psychiatric and neurological conditions. The classic paradigm does not control for attentional factors. In order to assess the influences of incorporating attentional control measures we administered the auditory PCP (S1–S2) in three different attention (passive, auditory attention to S2, visual attention to a concurrent continuous performance task [CPT]) conditions to a group of chronic, medicated schizophrenia patients (N=12) and a group of healthy subjects (N=15) to evaluate the effects of attention on sensory gating measures. A significant effect of attention on S1 amplitudes was shown for P50 in both groups, and N100 or P200 in schizophrenia patients. Attention status had a significant effect on S2 amplitudes for N100 and P200, and N100 and P200 gating ratios. Despite the effect of attention on S1 P50 amplitudes there was no effect on the gating ratio. In terms of group differences, visual attention to the concurrent CPT during the paired-click sensory gating task significantly enhanced the detection of deficient gating of the N100 and P200 components in schizophrenia patients. The data support the continued utilization of the passive gating paradigm for examining P50 gating but strongly suggest that for studies examining gating of the N100 or P200 components, a visual distraction paradigm may enhance the detection of abnormal gating in schizophrenia patients.
Keywords: sensory gating, mid-latency auditory evoked responses, attention, schizophrenia, human
Introduction
Schizophrenia is a severe chronic psychiatric disorder accompanied by a range of positive and negative symptoms and associated with perceptual and cognitive dysfunctions (Strauss ME, 1993; Uhlhaas & Mishara, 2007; Keshavan, Tandon, Boutros, & Nasrallah, 2008). Although attentional deficits have been extensively studied in schizophrenia (Chen & Faraone, 2000; Barr, 2001; Luck & Gold, 2008), the nature of the interaction between attention and the early processing of information in the human brain is less understood in this disorder. Impairments in sensory gating, in addition to deficits in the top-down regulation of attentional resources might be involved in this process (Cullum et al., 1993; Erwin, Turetsky, Moberg, Gur, & Gur, 1998).
Sensory gating refers to the ability of the brain to filter out irrelevant information and is proposed to be a major protective mechanism against flooding of the higher cortical centers with unnecessary information (Venables, 1964). Healthy subjects can efficiently filter out repeating irrelevant auditory and visual information (Adler et al., 1982; Gjini, Sundaresan, & Boutros, 2008). It has been shown that schizophrenia patients fail to suppress the irrelevant repeating auditory information, which is reflected in a decreased or a total lack of amplitude reduction of the auditory evoked responses to repeating stimuli (Adler et al., 1982; Boutros, Zouridakis, & Overall, 1991; Bramon, Rabe-Hesketh, Sham, Murray, & Frangou, 2004; Patterson et al., 2008). Three evoked potential (EP) components are used to examine sensory gating using the PCP: P50, N100, and the P200. P50 is a positive deflection with a latency of about 50 ms from auditory stimuli onset, N100 is a negative deflection peaking at about 100 ms and P200 waveform is a positive deflection peaking about 200 ms post stimulus presentation.
Paired-click paradigms (PCPs) are the standard method used to assess sensory gating. PCPs consist of two identical clicks presented in pairs with a short inter-stimulus interval of 500 ms and an inter-pair interval of 8–10 seconds. The longer inter-pair interval is necessary to assure that the effects of one pair of stimuli do not carry over to the next pair of stimuli (Zouridakis & Boutros, 1992). A significant reduction of the amplitudes of P50, N100, and P200 to the second stimulus (S2) in the pair compared to the first one (S1) is observed in healthy participants reflecting a filtering mechanism of the brain to repeating irrelevant auditory information. An absence or reduction of P50, N100, and/or P200 gating has been demonstrated in patients with schizophrenia (Boutros, Korzyukov, Jansen, Feingold, & Bell, 2004; Boutros et al., 2009). In addition to sensory gating deficits there might be a concurrent deficit in stimulus S1 registration in schizophrenia. In several studies, schizophrenia patients exhibited a P50 amplitude deficit for the S1 stimulus compared to healthy control subjects (Zouridakis, Boutros, & Jansen, 1997; Johanessen et al., 2005; Jin et al., 1997; Blumenfeld & Clementz, 2001; Arnfred, 2006). In other studies, no deficits in stimulus S1 registration were shown (Clementz, Blumenfeld, & Cobb, 1997; Olincy & Martin, 2005). Both deficits (i.e. in sensory gating and stimulus S1 registration) can be present as two independent physiological abnormalities. Our recent examination of a large sample of healthy controls showed that S1 amplitude while significantly contributing to the gating indices, does not completely account for the resulting gating values (Fuerst, Gallinat, & Boutros, 2007).
Effects of factors such as attention and psychological stressors on gating of the P50 have been examined in a small number of studies in healthy subjects, showing either no effect (Jerger, Biggins, & Fein, 1992; White & Yee, 1997), or a relatively small effect (Guterman, Josiassen, & Bashore, 1992) of attention on P50 gating, but a significant influence on the N100 component (White & Yee, 1997). In a recent study in schizophrenia patients, auditory attention was shown to exert a modulatory influence on P50 gating, indicating that the suppression deficit is malleable in schizophrenia without pharmacological agents (Yee et al., 2010). Stress and vigilance have been shown to exert significant influences on both P50 S1 amplitude and gating (Yee & White, 2001; White & Yee, 2006). Rosburg et al. (2009), in a study sample consisting of epilepsy and tumor patients undergoing presurgical evaluation, showed that N100 and P200 gating measures can potentially be affected by attention.
The standard PCP does not control for attentional factors. Subjects are simply instructed to remain awake, and try to decrease their eye movements (at times they are told to fixate their gaze on a spot in front of them). As P50 sensory gating reflects a predominantly preattentional filtering mechanism (Freedman et al., 1987; White & Yee, 1997, 2006) and N100/P200 gating could refer to filter mechanisms involved in triggering and allocation of attention (Lijffijt et al., 2009), we hypothesized that attentional factors (auditory or visual) will influence sensory gating of the N100 and P200 more than the P50 components. We also hypothesized that the influences of attention will be more pronounced in schizophrenia patients thus increasing the differences between schizophrenia patients and healthy controls. Although the effects of auditory attention on sensory gating has been studied in healthy subjects (Jerger et al., 1992; Guterman et al., 1992; White & Yee, 2006) and schizophrenia patients (Yee et al., 2010), the interaction between visual attention and auditory sensory gating has not been studied neither in healthy individuals nor in schizophrenia patients.
In this study we administered a paired-click (S1–S2) sensory gating paradigm in three different attention conditions (passive (standard), auditory attention, and visual attention) to a group of chronic, medicated schizophrenia patients and a group of healthy control subjects in order to assess the effects of attention on the P50, N100, and P200 S1 and S2 responses and their respective gating measures (ratio: S2/S1 × 100).
Materials and methods
Subjects
Twelve schizophrenia patients (11 males/1 female, age mean±sd: 42.5±11.7 years, range: 31–70 years old) and 15 healthy subjects (12 males/3 females, age mean±sd: 38.5±10.9 years, range: 24–69 years old) participated in this study. Schizophrenia subjects were recruited from the outpatient clinics at Wayne State University. They were medicated and clinically stable without change in medications for at least one month. None of the patients were on clozapine and ten of the twelve subjects were on a single atypical antipsychotic medication either risperidone or olanzapine. An agreement among chart and a structured clinical interview (SCID) diagnosis was necessary for inclusion in this study.
Participants in both groups were recruited through flyers that described the study. An initial telephone screening was carried out in order to establish a basic qualification of the participants. Healthy subjects had no history of psychiatric, drug or alcohol abuse, or neurological disorders. They had no first-degree relatives with any Axis-I psychiatric diagnoses including drug use or dependence and were not on any CNS active medications. Exclusion criteria for both groups included: a) history of a seizure disorder or any other neurological problems including head injury leading to loss of consciousness of any length of time; b) currently meeting DSM IV criteria for drug or alcohol dependence; c) positive urine test for any drugs of abuse; d) history of adverse reaction to flickering lights; and e) pregnancy in women.
Written informed consents were obtained from all the subjects. This study was carried out following guidelines for proper human research conduct in accordance with the Declaration of Helsinki. The protocol and study procedures were approved by the Institutional Review Board at Wayne State University.
Conditions
Subjects were seated in a comfortable highback armchair. A paired-click test (S1–S2) was administered in three different attention (passive, auditory attention, visual attention) conditions (STIM presentation software, Neuroscan).
Each of the three conditions was run in 2 blocks in each of two recording days (duration of each run was about 4.5 min), collecting an average of 60 pair of evoked responses per day. Overall 120 paired-click evoked response trials (120 responses for each stimulus, S1 and S2) were collected in the two days for each condition. Blocks were administered in random order.
The passive condition (i.e., standard PCP) consisted of the presentation of two identical stimuli, S1=S2 (4 ms duration clicks, rise/fall: 1ms, intensity: 85 dB, frequency: 1000 Hz). The inter-stimulus and inter-pair intervals were set to vary randomly between 500±25 ms and 8±1 s, respectively. Subjects were instructed to relax, stay awake and try to avoid too much eye movement during the recording session.
The auditory attention condition required attention to frequency/duration deviation present in rare (20%), intermittent different pairs (same S1 as in passive task, S1<S2, S2 parameters: 8 ms duration clicks, rise/fall of 2 ms, intensity: 85 dB, frequency: 2500 Hz). The inter-stimulus and inter-pair intervals were the same as in the passive condition. Subjects were instructed to press a button in response to deviant pairs (basically by paying attention to S2 deviant stimuli). By asking participants to paying attention to S2 stimuli we can assess the effect of the attention on inhibitory mechanisms that are activated during the post S1 stimulus period.
The visual attention condition required attention to visual stimuli during presentation of the auditory click pairs. Visual stimuli were part of a continuous performance task (visual CPT) where consecutive numbers presented on the screen every 2 seconds. The subjects were asked to perform the task in a continuous fashion (compare the presented number with the preceding one), and respond with button press to same events (20%). The CPT is considered a simple visual task which diverts the attention away from the paired-click task. The parameters of auditory stimuli presentation matched with that of the passive condition.
Auditory stimuli were administered through ear inserts. Visual stimuli were presented in white on a black background using a 17″ CRT computer monitor.
EEG recording and preprocessing
EEG data were acquired using Brain Vision Recorder software (Brain Products GmbH, Munich, Germany), amplified by a Grass amplifier (model 12C, Grass Instrumentation Co., Quincy, MA, USA) and digitized at 1000 Hz by NI6071e AD card (National Instruments Co., TX, USA). Initially, EEG raw data were recorded and processed from the following sites of the International 10/20 Electrode Placement System: Fz, Cz, Pz, Fp1, Fp2, F3, F4, F7, F8, T3, T4, T5, T6, C3, C4, P3, P4, O1, O2. Two additional channels, VEOG and HEOG, monitored vertical and horizontal eye-movements, respectively. A linked-ears reference was chosen for the data acquisition; the analog bandpass filter was set to 0.01–100 Hz. Electrode impedances were kept below 5 kΩ.
Preprocessing of the EEG data included digital filtering (1–50 Hz), segmentation (−0.1 to 0.4 s from stimuli onset), baseline correction (using prestimulus interval), artifact rejection (amplitudes exceeding ±75μV at Cz and eye movement channels; on average 21% of trials were excluded) and averaging to obtain the auditory evoked potentials of interest (P50, N100, P200). In the auditory attention condition, the epochs obtained for the deviant S2 (and corresponding S1) stimuli were rejected during analysis and not included in the respective averages. Grandaverages were computed for each subject from data recorded in two days, in order to increase the signal to noise ratio, and were further compared between groups and tasks.
Evoked potential analysis
The P50, N100, and P200 amplitudes and basic gating measures (ratios) were scored from data from Cz electrode site.
P50 was scored as the largest positivity in the interval 35–80 ms from S1 and S2 stimuli presentation onset. N100 was scored as the largest negativity in the interval 70–150 ms from S1 and S2 stimuli presentation onset. P200 was scored as the largest positivity in the interval 150–250 ms from S1 and S2 stimuli presentation onset. Peak-to-peak amplitudes were measured between the peak and the preceding trough (for P50 and P200) or positivity (for N100) consistent with our prior work (Boutros et al., 2004). Gating ratios were calculated from the peak-to-peak amplitudes for S1 and S2 (S2/S1×100 in %). Interval of the gating ratios ranged between 0% (maximal gating) and 200% (100 % or more augmentation of S2). A 100% ratio indicated an absence of gating without augmentation of S2.
Statistical analysis
Analysis of Variance (ANOVA) was used to assess differences between measures scores from data collected on two different days. None of the measures differed statistically between the two days of recording. In order to increase the signal-to-noise ratio, grandaverage waveforms were computed for each subject and variable scores from the latter were assessed (Boutros et al., 2009).
Repeated measurement ANOVAs were run to assess differences between the three conditions, with condition as a within-subjects variable (3 attention conditions: auditory attention, passive, visual attention) and group as between-subjects variable (schizophrenia patients and healthy controls). Partial Eta Squared values are reported as indices of the effect size. When sphericity assumption was violated, Greenhouse-Geisser correction was applied. Post hoc analyses in case of significant ANOVA results included pairwise comparisons (paired samples t-tests), which were run separately between each pair of conditions (corrected p-values with Bonferroni method are reported). As previous literature documented decreased EP amplitudes and gating measures in schizophrenia patients for the standard PCP, one-tailed independent samples t-tests were used to test differences in variable means between groups from the data collected in the passive condition (in case of unequal variances, the corresponding version of the t-test was used). Otherwise two-tailed t-tests were used. Statistical tests were run in SPSS software, version 17.0 (SPSS Inc., Chicago, IL).
Results
No statistical difference in age was noted between groups (independent samples two-tailed t-test: t(25)=0.91; p=0.37). The actual sample didn’t allow for a gender based comparison (mainly a sample of male subjects). A higher percentage of schizophrenia patients were smokers compared to healthy controls, although the difference didn’t reach significance (Chi-square = 4.81; p=0.09). In regard to the CPT performance, the percentage of corrects hits was significantly different between groups as expected (healthy: 85%±6, schizophrenia: 57%±14; t(8)=4.16, p=0.003). A t-test against 50% (percentage of correct hits) in schizophrenia patients showed a performance above the chance level (t(8)=1.08, p=0.31). The average response time for correct hits didn’t differ significantly between groups (healthy: 590.72± 48.36; schizophrenia: 599.07±45.42; t(8)=0.28, p=0.78). The CPT behavioral data reported above are from five subjects in each group in which data were successfully collected.
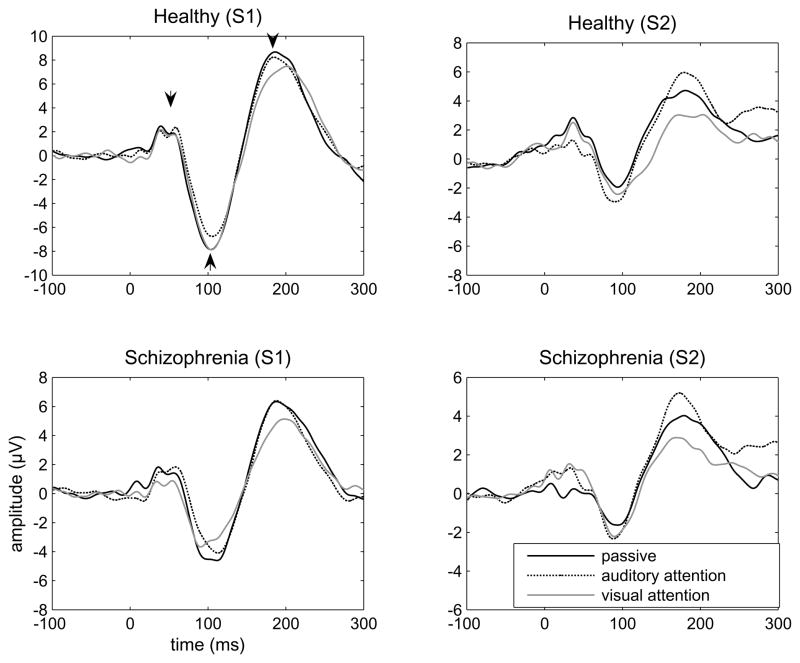
Figure 1 shows grandaverages of auditory evoked responses to S1 and S2 stimuli for both groups under three different attention conditions. Mean amplitudes and standard deviations for P50, N100, P200 responses (to both S1 and S2 stimuli) and gating ratios are presented in Table 1. Below, we begin by reporting the effects of attention from the P50 data then followed by the N100 and P200 data. Then we report the results from the group comparisons in the same order.
Figure 1.
Grandaverages of auditory evoked responses to S1 and S2 stimuli in the paired-click paradigm in schizophrenia patients (N=12) and healthy controls (N=15) under three different attention conditions. In one of the plots, arrowheads point to the scored, consecutive auditory evoked responses: P50, N100 and P200.
Table 1.
Attentional influences in S1, S2 amplitudes for P50, N100, P200 and gating ratios.
Mean amplitudes and standard deviations for P50, N100, P200 responses (to both S1 and S2 stimuli in the paired-click paradigm) and gating ratios in schizophrenia patients (N=12) and healthy controls (N=15) under three different attention conditions.
| Healthy | Schizophrenia | |||||
|---|---|---|---|---|---|---|
| Task Measures | passive | auditory attention | visual attention | passive | auditory attention | visual attention |
| P50 S1 amplitude (μV) | 3.35 (2.36) | 4.27 (2.39) | 3.83 (2.24) | 2.51 (1.40) | 3.64 (2.30) | 2.78 (2.32) |
| P50 S2 amplitude (μV) | 1.82 (1.83) | 2.49 (2.03) | 2.55 (2.86) | 2.25 (2.03) | 1.78 (1.52) | 2.00 (1.26) |
| P50 ratio (S2/S1 × 100) | 62.80 (50.47) | 59.13 (25.1) | 64.96 (50.24) | 88.21 (47.05) | 70.76 (67.61) | 99.55 (65.28) |
| N100 S1 amplitude (μV) | 12.38 (5.93) | 12.18 (6.41) | 12.39 (5.66) | 8.49 * (3.14) | 8.48 (3.15) | 6.71 ** (3.91) |
| N100 S2 amplitude (μV) | 6.28 (4.39) | 6.65 (3.31) | 6.13 (3.99) | 3.48 (1.21) | 4.84 (2.73) | 4.81 (1.70) |
| N100 ratio (S2/S1 × 100) | 43.12 (17.87) | 59.80 (29.87) | 49.39 (16.95) | 44.76 (18.28) | 62.86 (43.76) | 89.06 * (54.25) |
| P200 S1 amplitude (μV) | 19.38 (10.46) | 17.93 (8.89) | 18.43 (11.76) | 13.42 * (4.70) | 12.79 (4.46) | 11.02 (4.31) |
| P200 S2 amplitude (μV) | 8.71 (4.47) | 11.85 (4.90) | 7.94 (4.93) | 6.67 (2.15) | 9.25 (3.52) | 6.56 (1.82) |
| P200 ratio (S2/S1 × 100) | 46.72 (14.56) | 69.99 (19.82) | 48.58 (20.13) | 53.52 (19.25) | 76.55 (31.39) | 69.62 (38.93) |
p<0.05,
p<0.01 (independent samples t-tests between groups)
P50 amplitude and gating measures
Repeated measures ANOVAs revealed a significant effect of attention on S1 amplitudes for P50 (F2,44=3.21, p=0.05, partial eta squared = 0.127), but no condition-by-group interaction (F2,44=0.004, p=0.99, ). The attentional effect was due to a significant increase of the response to S1 stimuli in the auditory attention condition compared to passive condition (t26=2.23; p=0.034). Attention had no significant effect on S2 amplitudes for P50 (F2,44=0.63, p=0.48) in either healthy subjects or schizophrenia patients. Regarding gating measures, effects of attention were not significant for P50 ratios in both groups (F2,44=0.96, p=0.39) or each of them separately.
N100 amplitude and gating measures
No significant effect on S1 was noted in the group as a whole (F2,44=1.08, p=0.33). Attention had a significant effect on S2 amplitudes for N100 (F2,44=7.15, p=0.002, partial eta squared = 0.24) when data from both groups were used. No condition-by-group interaction was detected (F2,44=1.38, p=0.26). Pairwise comparisons, run separately for each pair of conditions in the two groups, showed a significant effect of attention on N100 S2 amplitudes which appeared as a significant increase of the response in the auditory attention condition compared to the passive condition in healthy subjects (corrected p=0.032) and as a significant increase of the response in the visual attention condition compared to passive condition in schizophrenia patients (corrected p=0.007). Effects of attention were thus significant for N100 ratios (F2,44=4.62, p=0.015, partial eta squared = 0.17) with a significant condition-by-group interaction (F2,44=4.23, p=0.021, partial eta squared = 0.16). Pairwise comparisons showed an increase for N100 gating ratios (weaker gating) in visual attention condition compared to passive processing condition (corrected p=0.075) in schizophrenia patients.
P200 amplitude and gating measures
In schizophrenia patients a significant decrease of the S1 responses was shown in the visual attention condition compared to the passive condition (corrected p=0.028). Attention also had a significant effect on S2 amplitudes for P200 (F2,44=16.83, p=0.000, partial eta squared = 0.43); but with no condition*group interaction (F2,44=0.93, p=0.40). Pairwise comparisons, run separately for each pair of conditions in the two groups, showed a significant increase of the S2 response in the auditory attention condition compared to passive (corrected p=0.009) and visual attention (corrected p=0.027) conditions in healthy participants only. Visual attention had no significant effects on S2 as compared to the standard passive condition. Regarding P200 gating measures, effects of attention were significant (F2,44=5.89, p=0.005, partial eta squared = 0.21) for the entire sample; with no condition-by-group interaction: (F2,44=0.74, p=0.48). Pairwise comparisons showed that auditory attention to S2 caused a significant increase of P200 gating ratios (weaker gating) compared to passive condition (corrected p=0.008) in healthy participants.
Group differences
P50 measures
In the passive condition and two attention-conditions, schizophrenia patients exhibited no significant differences from controls.
N100 measures
In the passive condition schizophrenia patients exhibited a significant decrease of S1 amplitude compared to controls (t25=2.37, p=0.026, one-tailed t-test), but no difference in N100 gating ratio (t25=0.83, p=0.41, one-tailed t-test). In addition, visual attention to the concurrent CPT task caused a significant decrease of the amplitudes for N100 S1 responses in schizophrenia patients compared to controls (t25=2.95, p=0.006, two-tailed t-test). Auditory sensory gating for N100 in the visual attention condition was significantly impaired in schizophrenia patients compared to healthy controls (two-tailed independent samples t-test: t(25)=2.71; p=0.012).
P200 measures
In the passive condition schizophrenia patients showed a significantly lower P200 response to S1 stimuli as compared to controls (t25=2.16, p=0.04, one-tailed t-test), but no difference in P200 gating ratio (t25=1.47, p=0.15, one-tailed t-test).
Discussion
In this study, by using a standard and two modified paired-click paradigms, we assessed the effects of auditory and visual attention on S1 amplitude (S1 passively processed, or simultaneously with visual attention), S2 amplitude (S2 passively processed or attended, or simultaneously with visual attention), and gating ratios (S2/S1) for the P50, N100, and P200 components. Despite the relatively small sample sizes, three important results emerged.
Our first finding is related to the effects of auditory or visual attention on the S1 amplitude of the P50, N100 and P200 responses. P50 S1 amplitudes increased significantly and similarly in both groups by auditory attention to S2. This was in agreement with the results of recent studies examining the effects of auditory attention on P50 amplitudes in healthy individuals (Yee & White, 2001; White & Yee, 2006) and extended these findings to schizophrenia patients. Additionally, the reduction of N100 and P200 S1 amplitudes in the visual attention condition compared to the passive condition was observed only in schizophrenia patients. As visual attention had no effects on the amplitudes of either S1 or S2 auditory evoked responses in healthy control subjects it underscores pathological responses in schizophrenia patients in both the initial registration of the stimuli (as reflected by the responses to S1) and the ability to habituate to the repeating stimulus (as reflected by S2 responses).
The second finding is that directing attention to the auditory stimulus tended to decrease, and not increase, the P50 gating differences between the two groups. This is of particular interest as focusing subject’s attention on S2 stimuli increased the amplitudes of S1 responses in both groups but increased S2 amplitudes only in healthy subjects (thus no effect on gating). In schizophrenia patients this effect caused improved gating and decreased the differences between the two groups. The latter suggests that directing attention to the second stimulus doesn’t cause an enhancement of P50 amplitude for the S2 stimulus in schizophrenia patients as was shown in a recent study (Yee et al., 2010). These data support the continued utility of the standard passive paradigm to study P50 gating in schizophrenia patients. It is an intriguing thought that the direction of attention of the patients to the auditory input ameliorated the P50 gating deficit with no effects on healthy subjects. It is possible that this observation, if replicated, may have therapeutic implications.
The third finding is related to the sensitivity of gating of the N100 and P200 to visual attentional influences. While in the standard passive paradigm, we did not detect a significant difference between the groups in N100 or P200 gating, when attention was diverted away by a visual distraction task, both N100 and P200 gating became significantly worse in schizophrenia patients. This finding suggests that the visual attention-auditory PCP may be more suitable for examining N100 and P200 gating deviations in schizophrenia patients.
In our sample, significant differences in S1 stimulus registration between healthy subjects and schizophrenia patients were present only for N100 and P200 responses, but not for P50. Our findings do not support a pure S1 registration deficit as the mechanism involved in a decreased P50 suppression of schizophrenia patients (Blumenfeld & Clementz, 2001); instead they are in agreement with the finding that S1 amplitude does not completely account for the resulting gating values (Fuerst et al., 2007). The above data also demonstrate the complex interaction between S1 and S2 changes resulting in the gating ratios. It is worth noting that the simple increase of S1 amplitudes does not automatically result in improved gating as seen in the effects of auditory attention on the P50 amplitudes in healthy controls. In order for the effects on S1 to result in improved gating, the effects on S2 must be less than the effects on S1 (as seen in the schizophrenia patients). This assertion equally applies to the effects of visual attention on gating of the N100 and P200. The inclusion of the visual CPT resulted in a decreases of the amplitudes of the N100 and P200 responses to S1 stimuli in schizophrenia patients with some increase in the amplitudes of the S2 N100 responses, resulting in a significant worsening of the gating ratio without an increase of P200 S2 responses resulting in a lesser worsening of the P200 gating ratio. The allocation of attentional resources away to the visual distraction task might be the main reason behind the latter findings causing schizophrenia patients to be more vulnerable in processing and filtering out efficiently repeating auditory information during a gating phase represented by N100 and P200 responses. The data further suggest that directing attention to the auditory stimuli results in complex interactions that is deserving of further study.
As a limitation of this study, larger sample sizes will be necessary in future studies to further probe other, likely to become significant observations, if the study is powered at a higher level. As in this pilot project the CPT was used simply to divert the attention away from the paired-click task, in a follow-up study based on current findings, a greater attention will be paid in collecting behavioral data for the whole sample while utilizing similar tasks with increasing loads.
Conclusion
The results of this study extend the findings in the existing literature by evaluating the effects of visual attention in addition to auditory attention on early information processing and sensory gating in schizophrenia patients. We propose that the utilization of visual CPT during sensory gating studies may further enhance the differences between patients and healthy controls and may be an avenue to probe visual-auditory pathological interactions in schizophrenia patients.
Acknowledgments
This work was supported by the following grants: RO1 MH63476-01A and RO1 DA019055 from NIMH and NIDA, and by the Joe Young funds to the Department of Psychiatry and Behavioral Neurosciences, Wayne State University.
References
- 1.Adler LE, Pachtman E, Franks R, Pecevich M, Waldo MC, Freedman R. Neurophysiological evidence for a deficit in inhibitor mechanisms involved in sensory gating in schizophrenia. Biological Psychiatry. 1982;17:639–654. [PubMed] [Google Scholar]
- 2.Arnfred SM. Exploration of auditory P50 gating in schizophrenia by way of difference waves. Behavioral & Brain Functions. 2006;28(2):6. doi: 10.1186/1744-9081-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barr WB. Schizophrenia and attention deficit disorder. Two complex disorders of attention. Annals of the New York Academy of Sciences. 2001;931:239–250. doi: 10.1111/j.1749-6632.2001.tb05782.x. [DOI] [PubMed] [Google Scholar]
- 4.Blumenfeld LD, Clementz BA. Response to the first stimulus determines reduced auditory evoked response suppression in schizophrenia: single trials analysis using MEG. Clinical Neurophysioogy. 2001;112(9):1650–1659. doi: 10.1016/s1388-2457(01)00604-6. [DOI] [PubMed] [Google Scholar]
- 5.Boutros NN, Zouridakis G, Overall J. Replication and extension of P50 findings in schizophrenia. Clinical Electroencephalography. 1991;22:40–45. doi: 10.1177/155005949102200109. [DOI] [PubMed] [Google Scholar]
- 6.Boutros NN, Korzyukov O, Jansen B, Feingold A, Bell M. Sensory gating deficits during the mid-latency phase of information processing in medicated schizophrenia patients. Psychiatry Research. 2004;126(3):203–215. doi: 10.1016/j.psychres.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 7.Boutros NN, Brockhaus-Dumke A, Gjini K, Vedeniapin A, Elfakhani M, Burroughs S, Keshavan M. Sensory-gating deficit of the N100 mid-latency auditory evoked potential in medicated schizophrenia patients. Schizophrenia Research. 2009;113(2–3):339–346. doi: 10.1016/j.schres.2009.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bramon E, Rabe-Hesketh S, Sham P, Murray RM, Frangou S. Meta- analysis of the P300 and P50 waveforms in schizophrenia. Schizophrenia Research. 2004;70(2–3):315–329. doi: 10.1016/j.schres.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 9.Chen WJ, Faraone SV. Sustained attention deficits as markers of genetic susceptibility to schizophrenia. American Journal of Medical Genetics. 2000;97(1):52–57. doi: 10.1002/(sici)1096-8628(200021)97:1<52::aid-ajmg7>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 10.Clementz BA, Blumenfeld LD, Cobb S. The gamma band response may account for poor P50 suppression in schizophrenia. Neuroreport. 1997;8(18):3889–3893. doi: 10.1097/00001756-199712220-00010. [DOI] [PubMed] [Google Scholar]
- 11.Cullum CM, Harris JG, Waldo MC, Smernoff E, Madison A, Nagamoto HT, Freedman R. Neurophysiological and neuropsychological evidence for attentional dysfunction in schizophrenia. Schizophrenia Research. 1993;10(2):131–141. doi: 10.1016/0920-9964(93)90048-n. [DOI] [PubMed] [Google Scholar]
- 12.Erwin RJ, Turetsky BI, Moberg P, Gur RC, Gur RE. P50 abnormalities in schizophrenia: relationship to clinical and neuropsychological indices of attention. Schizophrenia Research. 1998;33(3):157–167. doi: 10.1016/s0920-9964(98)00075-9. [DOI] [PubMed] [Google Scholar]
- 13.Freedman R, Adler LE, Gerhardt GA, Waldo MC, Baker N, Rose GM, Franks R. Neurobiological studies of sensory gating in schizophrenia. Schizophrenia Bulletin. 1987;13:669–678. doi: 10.1093/schbul/13.4.669. [DOI] [PubMed] [Google Scholar]
- 14.Fuerst DR, Gallinat J, Boutros NN. Range of sensory gating values and test-retest reliability in normal subjects. Psychophysiology. 2007;44:620–626. doi: 10.1111/j.1469-8986.2007.00524.x. [DOI] [PubMed] [Google Scholar]
- 15.Gjini K, Sundaresan K, Boutros NN. Electroencephalografic evidence of sensory gating in the occipital visual cortex. Neuroreport. 2008;19(15):1519–1522. doi: 10.1097/WNR.0b013e3283108bf3. [DOI] [PubMed] [Google Scholar]
- 16.Guterman Y, Josiassen RC, Bashore TR., Jr Attentional influence on the P50 component of the auditory event-related brain potential. International Journal of Psychophysiology. 1992;12(2):197–209. doi: 10.1016/0167-8760(92)90011-y. [DOI] [PubMed] [Google Scholar]
- 17.Jerger K, Biggins C, Fein G. P50 suppression is not affected by attentional manipulations. Biological Psychiatry. 1992;31(4):365–377. doi: 10.1016/0006-3223(92)90230-w. [DOI] [PubMed] [Google Scholar]
- 18.Jin Y, Potkin SG, Patterson JV, Sandman CA, Hetrick WP, Bunney WE., Jr Effects of P50 temporal variability on sensory gating in schizophrenia. Psychiatry Research. 1997;70(2):71–81. doi: 10.1016/s0165-1781(97)03091-6. [DOI] [PubMed] [Google Scholar]
- 19.Johannesen JK, Kieffaber PD, O’Donnell BF, Shekhar A, Evans JD, Hetrick WP. Contributions of subtype and spectral frequency analyses to the study of P50 ERP amplitude and suppression in schizophrenia. Schizophrenia Research. 2005;78(2–3):269–284. doi: 10.1016/j.schres.2005.05.022. [DOI] [PubMed] [Google Scholar]
- 20.Keshavan MS, Tandon R, Boutros NN, Nasrallah HA. Schizophrenia, “just the facts”: what we know in 2008 Part 3: neurobiology. Schizophrenia Research. 2008;106(2–3):89–107. doi: 10.1016/j.schres.2008.07.020. [DOI] [PubMed] [Google Scholar]
- 21.Lijffijt M, Lane SD, Meier SL, Boutros NN, Burroughs S, Steinberg JL, Swann AC. P50, N100, and P200 sensory gating: relationships with behavioral inhibition, attention, and working memory. Psychophysiology. 2009;46(5):1059–1068. doi: 10.1111/j.1469-8986.2009.00845.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luck SJ, Gold JM. The construct of attention in schizophrenia. Biological Psychiatry. 2008;64(1):34–39. doi: 10.1016/j.biopsych.2008.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Olincy A, Martin L. Diminished suppression of the P50 auditory evoked potential in bipolar disorder subjects with a history of psychosis. American Journal of Psychiatry. 2005;162(1):43–49. doi: 10.1176/appi.ajp.162.1.43. [DOI] [PubMed] [Google Scholar]
- 24.Patterson JV, Hetrick WP, Boutros NN, Jin Y, Sandman C, Stern H, Bunney WE., Jr P50 sensory gating ratios in schizophrenics and controls: a review and data analysis. Psychiatry Research. 2008;158(2):226–247. doi: 10.1016/j.psychres.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 25.Rosburg T, Trautner P, Elger CE, Kurthen M. Attention effects on sensory gating-intracranial and scalp recordings. Neuroimage. 2009;48(3):554–563. doi: 10.1016/j.neuroimage.2009.06.063. [DOI] [PubMed] [Google Scholar]
- 26.Strauss ME. Relations of symptoms to cognitive deficits in schizophrenia. Schizophrenia Bulletin. 1993;19(2):215–231. doi: 10.1093/schbul/19.2.215. [DOI] [PubMed] [Google Scholar]
- 27.Uhlhaas PJ, Mishara AL. Perceptual anomalies in schizophrenia: integrating phenomenology and cognitive neuroscience. Schizophrenia Bulletin. 2007;33(1):142–156. doi: 10.1093/schbul/sbl047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Venables P. Input dysfunction in schizophrenia. Progress in Experimental Personality and Psychopathology Research. 1964;1:1–47. [PubMed] [Google Scholar]
- 29.White PM, Yee CM. Effects of attentional and stressor manipulations on the P50 gating response. Psychophysiology. 1997;34(6):703–711. doi: 10.1111/j.1469-8986.1997.tb02145.x. [DOI] [PubMed] [Google Scholar]
- 30.White PM, Yee CM. P50 sensitivity to physical and psychological state influences. Psychophysiology. 2006;43(3):320–328. doi: 10.1111/j.1469-8986.2006.00408.x. [DOI] [PubMed] [Google Scholar]
- 31.Yee CM, White PM. Experimental modification of P50 suppression. Psychophysiology. 2001;38(3):531–539. doi: 10.1017/s0048577201981454. [DOI] [PubMed] [Google Scholar]
- 32.Yee CM, Williams TJ, White PM, Nuechterlein KH, Ames D, Subotnik KL. Attentional modulation of the P50 suppression deficit in recent-onset and chronic schizophrenia. Journal of Abnormal Psychology. 2010;119(1):31–39. doi: 10.1037/a0018265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zouridakis G, Boutros NN. Stimulus parameter effects on the P50 evoked response. Biological Psychiatry. 1992;32(9):839–841. doi: 10.1016/0006-3223(92)90088-h. [DOI] [PubMed] [Google Scholar]
- 34.Zouridakis G, Boutros NN, Jansen BH. A fuzzy clustering approach to study the auditory P50 component in schizophrenia. Psychiatry Research. 1997;69(2–3):169–181. doi: 10.1016/s0165-1781(96)02979-4. [DOI] [PubMed] [Google Scholar]