Abstract
The distinction between physiological (apoptotic) and pathological (necrotic) cell deaths reflects mechanistic differences in cellular disintegration and is of functional significance with respect to the outcomes that are triggered by the cell corpses. Mechanistically, apoptotic cells die via an active and ordered pathway; necrotic deaths, conversely, are chaotic and passive. Macrophages and other phagocytic cells recognize and engulf these dead cells. This clearance is believed to reveal an innate immunity, associated with inflammation in cases of pathological but not physiological cell deaths. Using objective and quantitative measures to assess these processes, we find that macrophages bind and engulf native apoptotic and necrotic cells to similar extents and with similar kinetics. However, recognition of these two classes of dying cells occurs via distinct and noncompeting mechanisms. Phosphatidylserine, which is externalized on both apoptotic and necrotic cells, is not a specific ligand for the recognition of either one. The distinct modes of recognition for these different corpses are linked to opposing responses from engulfing macrophages. Necrotic cells, when recognized, enhance proinflammatory responses of activated macrophages, although they are not sufficient to trigger macrophage activation. In marked contrast, apoptotic cells profoundly inhibit phlogistic macrophage responses; this represents a cell-associated, dominant-acting anti-inflammatory signaling activity acquired posttranslationally during the process of physiological cell death.
INTRODUCTION
Cell death is vital to the morphological shaping of tissues in development and to the careful sculpting of functionally appropriate cellular repertoires (Surh and Sprent, 1994; Cecconi et al., 1998; Yeh et al., 1998; Yoshida et al., 1998). Selective cell deaths continue to play a role in the homeostasis of mature tissues. For example, the deletion of immune cells in the attenuation of an immune response (Webb et al., 1990; Kawabe and Ochi, 1991) and the elimination of cells that have become functionally inappropriate, including virally infected and transformed cells (Kägi et al., 1995), depend on the selective induction of cell death. The cell death process generally assures both that cells triggered to die will cease to function and that they will be cleared in an orderly manner. Cells that die in these physiological contexts typically are removed rapidly by phagocytic cells, including macrophages (Duvall et al., 1985; Savill et al., 1989). Of primary significance, these cell deaths ensue without inflammatory consequence (Kerr et al., 1972).
Apoptosis is characterized by an orderly sequence of internal events, of which chromatin condensation is one, that precede the loss of cellular integrity (Russell, 1983; Wyllie et al., 1984; Harvey et al., 2000). Early studies also recognized that physiological cell deaths occur in a cell autonomous manner and that bystander cells are unaffected (Ucker et al., 1989; Dhein et al., 1995). Consistent with these observations, engulfing macrophages remove dying cells in the absence of infiltrating immune effectors. The efficient noninflammatory clearance of inappropriate T cells during development in the thymus illustrates this phenomenon dramatically (Surh and Sprent, 1994). In contrast, necrotic cell death, marked by rapid, disorganized swelling and rupture and associated with pathological tissue injury (Henson and Johnson, 1987), elicits inflammatory responses as well as clearance by phagocytic cells (Henson and Johnson, 1987; Stern et al., 1996).
These observations have led to the hypothesis that properties unique to the dying cell must determine the mode and outcome of phagocytic clearance. A variety of molecules have been implicated as putative recognition elements, including phospholipid ligands on the surface of the apoptotic corpse. Alterations in lipid composition occur early in the apoptotic process (Fadok et al., 1992b; Schlegel et al., 1993). Phosphatidylserine (PS), an anionic phospholipid normally cloistered in the inner leaflet of the plasma membrane, is externalized during the cell death process (Fadok et al., 1992b). Phospholipid flipping during cell death is linked to the activation of effector caspases (death-specific proteases) and occurs upstream of nuclear changes (Bratton et al., 1997; Harvey et al., 2000). The belief that this externalized phospholipid serves as a ligand for macrophage recognition follows from studies demonstrating that similar changes target aged erythrocytes for clearance (Schroit et al., 1985; McEvoy et al., 1986). The interaction of dying nucleated cells with macrophages is inhibited partially by phospho-l-serine and PS vesicles, which appear to bind to sites on the macrophage (Fadok et al., 1992b, 1998b; Verhoven et al., 1995; Terpstra et al., 1998).
Complementary studies have focused attention on scavenger receptors as the putative receptors of engulfing macrophages. Class B scavenger receptors, especially CD36 (Savill et al., 1992), constitute an attractive group of candidate recognition molecules that exhibit specificities consonant with the known apoptotic cell surface changes (Fadok et al., 1992b, 1998b; Chang, M.-K. et al., 1999). CD36 has been proposed to function in concert with distinct macrophage integrins (Savill et al., 1990; Albert et al., 1998) as an anchor for a lectin-like thrombospondin bridge to the target (Savill et al., 1992). Other studies have attributed recognition specificity and engulfing activity to class A scavenger receptors and CD68 (Platt et al., 1996; Ramprasad et al., 1996; Terpstra et al., 1997). Of greatest interest, a novel cell surface molecule has been identified recently that is implicated, both by antibody blocking and gene transfer experiments, in PS-dependent clearance of apoptotic cells (Fadok et al., 2000). In addition to these, a number of other molecules have been identified that may be involved in selective cases of target cell interaction with the phagocytic cell (Hart et al., 1997; Schwartz et al., 1999). CD14, a lipopolysaccharide (LPS)-binding molecule normally associated with inflammation, is of particular intrigue (Devitt et al., 1998). Although an essential role for scavenger receptors in the clearance of dead cells is established through genetic studies in Drosophila melanogaster (Franc et al., 1999), similar definitive assignments for mammalian scavenger receptors and other molecules have not been made, in large part because specific inhibitors afford only incomplete interference. As well, loss-of-function mutations in the class A scavenger receptor (Suzuki et al., 1997; Terpstra et al., 1997; Platt et al., 1998) or CD36 (Hughes et al., 1997; Febbraio et al., 1999) confer only marginal reductions in phagocytic recognition in vivo. Of course, it may be that recognition activity cannot be ascribed to any one class of molecules exclusively. Genetic studies of developmental cell death in the worm Caenorhabditis elegans similarly reveal the absence of singularly essential molecules for engulfment, implying that multiple and redundant mechanisms operate for the clearance of dead cells (Ellis et al., 1991).
Technical limitations and differences in experimental approaches also have obscured a resolution of the issues of specificity in the phagocytic process. Few studies have discriminated binding from engulfment, and the low frequency of phagocytic activity in many cases has necessitated subjective evaluation of dead cell interactions with phagocytic cells. Variability also pertains to the different populations of targets and engulfing cells that have been used. Most importantly, the fates of apoptotic and necrotic cells have not been compared rigorously (Hirt et al., 2000); typically apoptotic cells have been contrasted with nonnative targets opsonized with immunoglobulin or complement molecules. Certainly, opsonized cells are phagocytic targets and inflammatory elicitors distinct from true apoptotic cells (Ravetch, 1994), but they are reflective of pathogenic invaders rather than endogenous cells that have suffered a pathological fate.
We have developed a quantitative and objective approach to explore the recognition by macrophages of native cells that have undergone physiological or pathological deaths. Here, we show that recognition of apoptotic and necrotic targets occurs by distinct and noncompeting mechanisms and that PS is not a specific ligand for apoptotic cell recognition. These distinct recognition processes are linked to opposing phlogistic responses. Necrotic cells enhance, but are not sufficient to initiate, macrophage activation. Most significantly, the process of physiological cell death imparts on apoptotic cells an anti-inflammatory activity that functions in a dominant manner to abrogate proinflammatory responses of engulfing macrophages.
EXPERIMENTAL STRATEGY AND METHODS
Strategy for the Quantitation of Macrophage Recognition of Dying Cells
We have developed an objective microwell assay to assess the extent of target cell recognition (as well as the consequent production of proinflammatory cytokines) by phagocytic cells. In essence, recognition of fluorescently labeled target cells can be quantified as the extent of (nonadherent) target cell fluorescence that becomes associated with (adherent) phagocytic cells after their interaction. We also have chosen to work with clonal lines of macrophages and target cells in which we induced physiological death or triggered pathological killing, in order to minimize issues of heterogeneity.
Target cells were labeled covalently, especially with the amine-reactive probe (5,6)-carboxyfluorescein diacetate succinimidyl ester (CFDA) or the lipophilic carbocyanine dye chloromethylated lipophilic carbocyanine dye DiIC18(3) (CM-DiI). CFDA labeled cells uniformly, whereas CM-DiI labeling was less homogenous and more “grainy.” These labels were retained in living cells and throughout the death process. Moreover, staining was maintained quantitatively after dead cell ingestion (our unpublished results). The uptake of propidium iodide (PI) by apoptotic cells was sufficiently stable to permit PI-stained corpses to be visualized after their ingestion; in contrast, PI leaked from necrotic cells during the phagocytosis assay, precluding its use in this analysis (our unpublished results). Of technical note, a cotransfected green fluorescent protein marker (Harvey et al., 2000) also was suitable to track the fate of transfectants upon interaction with macrophages. The intensity of tracker dye signals permitted the interactions of target cells with macrophages to be monitored on a microwell scale, facilitating their objective and quantitative assessment with a fluorescence plate reader. At the same time, culture supernatants could be collected and assayed for the release of cytokines. In the experiments reported here, we have monitored the secretion of TNF-α and IL-6 as a measure of proinflammatory outcome.
Cell Culture and Triggering of Cell Death
Freshly cloned cells were grown at 37°C in a humidified, 5% (vol/vol) CO2 atmosphere in RPMI 1640 medium (Mediatech, Herndon, VA) supplemented with l-glutamine (2 mM), 2-mercaptoethanol (50 μM), and heat-inactivated fetal bovine serum (10% vol/vol; Hyclone Laboratories, Logan, UT). J774A.1 and RAW 264.7 are monocyte-derived macrophage cell lines derived from H-2d mice. Murine S49 thymoma (H-2d) and DO11.10 T-cell hybridoma (H-2d × H-2k) cells were used as targets. Physiological cell death (apoptosis) was induced by treatment of the T cells with the synthetic glucocorticoid dexamethasone (1 μM, 8–22 h) or the macromolecular synthesis inhibitor actinomycin D (200 ng/ml, 8–18 h; Ucker et al., 1989). Cells were killed pathologically (necrotic death) by incubation at 55°C for 10–15 min (until Trypan Blue uptake indicated compromise of membrane integrity).
Fluorescent Labeling of Cells
Target cells were labeled with CFDA (Molecular Probes, Eugene, OR; Exλ = 490 nm; Emλ = 525 nm). Cells (1 × 106 cells/ml in PBS) were incubated with CFDA (5 μM) for 10 min at 37°C and then washed twice in complete medium. Cells were labeled before the induction of physiological cell death or heat killing. Target cells also were labeled with CM-DiI (Molecular Probes; Exλ = 530 nm; Emλ = 645 nm) under similar conditions (2 μM CM-DiI, 30 min at 37°C). For visualization of chromatin, cells were incubated with Hoechst 33342 (Sigma Chemical, St. Louis, MO; 1 μg/ml, 10 min, 37°C), and stained chromatin was visualized (Exλ = 355 nm, Emλ = 465 nm) with a Nikon Diaphot 200 microscope with epi-fluorescence (Nikon, Garden City, NY). The accessibility of phosphatidylserine was revealed by the binding of FITC-conjugated annexin V (PharMingen, San Diego, CA; Exλ = 488 nm, Emλ = 525 nm). Cells were harvested and washed twice with cold PBS. Cells were resuspended in 100 μl of binding buffer (10 mM HEPES, pH 7.4, 140 mM NaCl, 2.5 mM CaCl2) and incubated with 5 μl of FITC-conjugated annexin V for 15 min in the dark at 25°C. After incubation, 400 μl of binding buffer was added per sample, and cells were analyzed cytofluorometrically (FACSCaliber instrument and CellQuest software; Becton Dickinson, San Jose, CA). PI, which was used to assess plasma membrane integrity, was added to cells at 1 μg/ml immediately before cytofluorometric analysis (Exλ = 488 nm, Emλ = 610 nm). Cytofluorometric data were processed with WinMDI software (Joe Trotter, Scripps Research Institute, La Jolla, CA).
Phagocyte Interaction Assays
Two hours before the initiation of phagocytosis assays, macrophages were plated in 96-well flat-bottom tissue culture plates (Costar, Corning, NY) at a density of 2 × 104 cells/0.32-cm2 well to allow semiconfluent monolayer formation. Graded numbers of target cells were added to the macrophage monolayers, and cells were allowed to interact at 4°C (binding) or at 37°C (engulfment as well as binding). In typical interaction assays, the duration of incubation was 60 min. Wells then were washed twice with ice-cold PBS, and plate-bound fluorescence was analyzed on a Cytofluor 2350 Fluorescence Plate Reader (Millipore, Marlborough, MA). For quantitation, a standard curve was prepared with graded number of labeled target cells. The fluorescent labeling of target cells, which varied little (<10%) between experiments, yielded specific fluorescence intensities of ∼1.25 × 103 fluorescence units per 2 × 104 cells (above a background of <50 fluorescence units). That is, in a well with 2 × 104 macrophages, target cell binding, even at level of 0.1 target/macrophage, could be quantified reliably. All data points are the means (± SEM) of triplicate determinations, and each of the experiments presented is representative of multiple (typically ≥10) repetitions. Binding and phagocytosis also were visualized microscopically. For detailed microscopic examination, targets were allowed to interact with macrophages that had been plated at lower density (1 × 105 cells/1.8 cm2 chamber) in microplates with coverslip bottoms (Fisher Scientific, Hanover Park, IL). Digital images were acquired with a SenSys CCD Camera (Photometrics, Tucson, AZ). Images were analyzed using Image-Pro Plus software (Media Cybernetics, Silver Spring, MD).
Quantitation of Cytokine Release
Cytokine production by macrophages was assessed after incubation with target cells for 4 h at 37°C. Where indicated, LPS (Escherichia coli O111:B4; Sigma Chemical) was added to macrophages 2 h before the addition of targets. Culture supernatants were withdrawn from wells and assessed quantitatively for secreted TNF-α and IL-6. Cytokines were assayed by ELISA (Endogen, Woburn, MA), using matched-pair, cytokine-specific capture and biotinylated reporter antibodies. The reporter reaction was developed with HRP-conjugated streptavidin and quantified spectrophotometrically at 450 nm (corrected for turbidity at 550 nm; Microplate Autoreader model EL311; Bio-Tek Instruments, Winooski, VT).
Other Reagents
Small unilamellar vesicles were prepared by sonication from egg phosphatidylcholine (PC; Sigma Chemical) alone or mixed (at a molar ratio of 7:3) with brain PS (Avanti Polar Lipids, Alabaster, AL) as described (Pradhan et al., 1997). Arg-Gly-Asp-Ser (RGDS) and Arg-Gly-Glu-Ser (RGES) tetrapeptides were purchased from Sigma Chemical.
RESULTS
Macrophage Recognition of Dying Cells Is Specific and Efficient
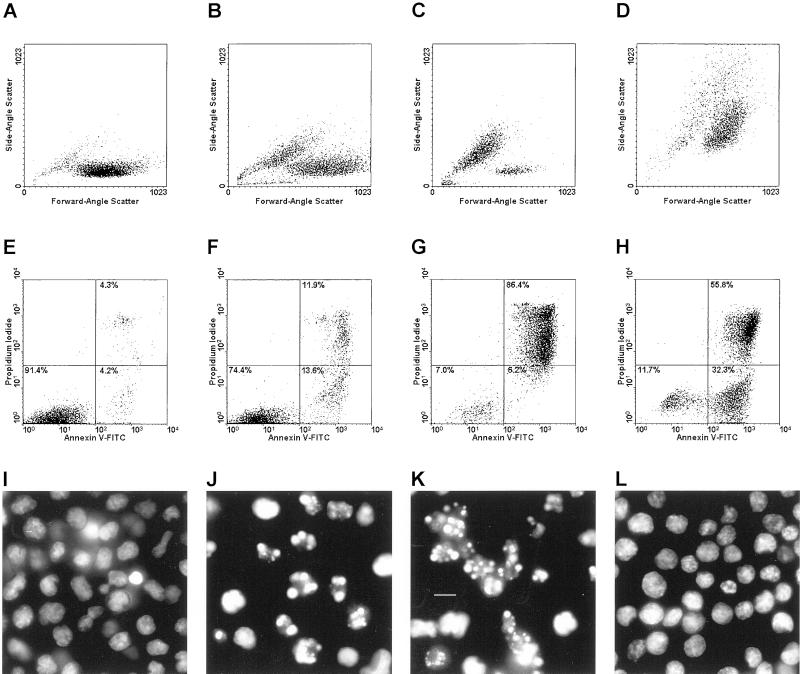
We explored the ability of macrophages to recognize dying cells by challenging them in a dose- and time-dependent manner with distinct populations of targets. Physiological cell death was induced in T-cell targets by treatment with dexamethasone or actinomycin D (Ucker et al., 1989). These cell death responses are associated with the typical hallmarks of apoptosis, including cell shrinkage and chromatin condensation (exemplified in Figure 1, B, C, J, and K, for the T-cell hybridoma DO11.10), as well as caspase activation and genome digestion. Cells were killed with heat to generate pathological cell death targets. Incubation of DO11.10 target cells at 55°C for 10–15 min resulted in an immediate loss of viability, as assessed by the uptake of PI through a compromised plasma membrane (Figure 1H). Heat-killed cells exhibited no apoptotic hallmarks; rather, they were swollen (Figure 1D), and their chromatin was uncondensed (Figure 1L) and not digested (our unpublished results). We examined target cells that had been induced to die physiologically and had suffered an equivalent loss of plasma membrane integrity as well as cells at an earlier stage of the physiological cell death process, when fewer cells had yet lost substantial membrane integrity (cf. Figure 1F and 1G). For brevity, we hereafter use the terms apoptosis and necrosis to reflect the consequences of physiological and pathological cell deaths, respectively.
Figure 1.
Morphological discrimination of apoptotic and necrotic cell deaths. DO11.10 cells were left untreated (viable; A, E, and I) or were induced to die. To induce physiological death, cells were treated with actinomycin D (200 ng/ml) for 8 h (early apoptotic; B, F, and J) or for 18 h (late apoptotic; C, G, and K). Pathological death resulted from heating (necrotic, 55°C, 15 min; D, H, and L). Cells were analyzed for attributes of death. Changes in cellular size and granularity were evaluated cytofluorometrically with respect to forward-angle and side-angle light scatter (A–D). Membrane integrity and the accessibility of phosphatidylserine also were assessed cytofluorometrically by staining with propidium iodide (PI; Exλ = 488 nm, Emλ = 610 nm) and FITC-conjugated annexin V (Exλ = 488 nm, Emλ = 525 nm), respectively (E–H). The relative distribution within each population of viable (annexin V− PI−) cells and those with early (annexin V+ PI−) and late (annexin V+ PI+) manifestations of death are indicated. Note that an early population of annexin V+ PI− dying necrotic cells (discussed in the text) is evident in H. Chromatin condensation was visualized microscopically after staining with Hoechst 33342 (Exλ = 355 nm; Emλ = 465; I –L; bar, 5 μm).
Most studies of the interactions between phagocytic cells and their targets have not discriminated between recognition (binding) and engulfment. However, the early study of Duvall et al. (1985) suggested that binding might occur in the absence of engulfment when interactions are allowed to occur at 4°C. Apoptotic, necrotic, and viable CFDA-labeled DO11.10 cells were incubated at 4°C with a monolayer of J774A.1 macrophages without agitation. Graded numbers of targets were added to a constant number of freshly plated macrophages, at input target to macrophage ratios ranging as high as 50:1. At various times of incubation, unbound target cells were removed by washing (with ice-cold buffer), and the extent of target cell binding to the macrophage monolayer was quantified fluorometrically.
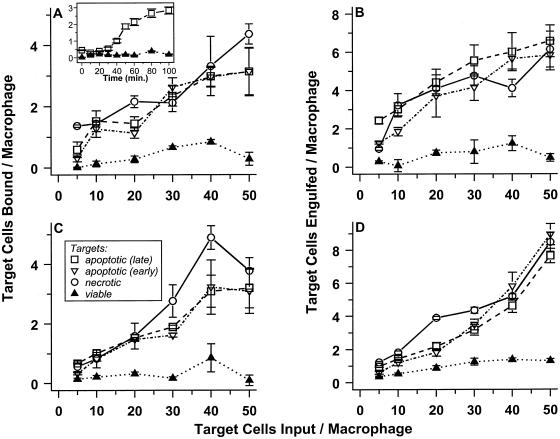
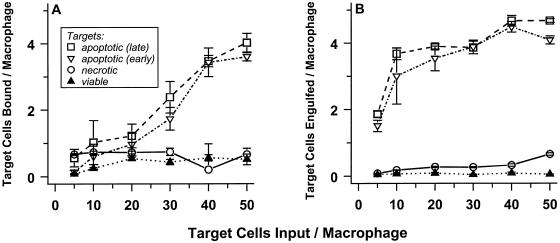
Figure 2A presents the results of one representative experiment, in which target cells were allowed to bind to macrophages for 60 min. Live cells were not bound appreciably to macrophages, whereas apoptotic and necrotic targets were bound to comparable and significant extents. The magnitude of target binding per macrophage displays a roughly linear dependence on target cell dose; in these assays, about 10% of all targets were bound. Most notably, macrophage binding capacity is high: one macrophage can bind more than one target. Cells at early and late stages of the physiological cell death process were bound to similar extents, suggesting that determinants for macrophage recognition appear well before obvious manifestations of cell death.
Figure 2.
J774A.1 macrophages selectively bind and engulf apoptotic and necrotic cells. Target cells were covalently labeled with CFDA and were induced to die physiologically (apoptotic) or pathologically (necrotic). Apoptotic death of DO11.10 cells (A and B) was triggered by treatment with actinomycin D; apoptotic S49 cells (C and D) resulted from treatment with dexamethasone. Populations of early (≤20% Trypan Blue+; ▿) and late (≥65% Trypan Blue+; □) apoptotic targets were prepared. In contrast, necrotic targets (○) were ≥80% Trypan Blue+, whereas untreated (viable; ▴) cells were <8% Trypan Blue+. Graded numbers of labeled cells were mixed with adherent murine J774A.1 macrophages in microwells. After incubation for 60 min at 4°C, unbound cells were removed by washing, and the number of target cells bound was determined by quantifying CFDA fluorescence (Exλ = 490 nm; Emλ = 525 nm; A and C). Similarly, the extent of engulfment (which may include low numbers of target cells that are bound and not engulfed) was determined after incubation for 60 min at 37°C (B and D). In the absence macrophages, binding of target cells to microwells was insignificant.
Typical kinetics by which macrophages bound apoptotic targets cells are shown in the inset of Figure 2A (at a target to macrophage ratio of 40:1). Binding was saturable kinetically, and the same kinetic pattern was followed (with proportionately lower maximum extents of binding) in experiments in which reduced numbers of target cells were added. Scatchard analysis of these data suggest that, on average, each macrophage has five to eight sites for binding apoptotic target cells (but see next). The extents and kinetics of necrotic and apoptotic cell binding to J774A.1 macrophages were equivalent (see Figure 2A and our unpublished results).
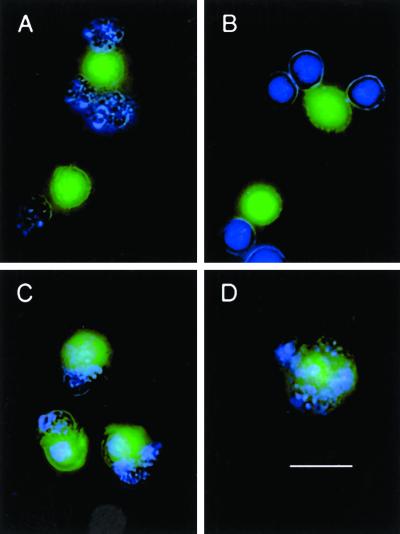
To visualize these interactions microscopically, target cells were stained with Hoechst 33342 to mark cells with apoptotic chromatin condensation (see Figure 1K), and macrophages were labeled with CFDA. The peripheral association of apoptotic targets (blue, with fragmented nuclei) with macrophages (green) confirms that binding without internalization occurs at 4°C (Figure 3A). Under these conditions, macrophages were similarly decorated by bound necrotic (blue, unfragmented) targets (Figure 3B). Surprisingly, approximately 30% of the macrophages were responsible for all target cell binding. Apparently, within the clonal macrophage population, some macrophages are more competent than are others at any time. Fluorometrically derived averages incorporate a mix of macrophages that have bound no targets and others that are “jackpots.”
Figure 3.
Visualization of macrophage binding and engulfment of target cells. J774A.1 macrophages were labeled (green) with 5 μM CFDA and plated in microplates with coverslip bottoms. DO11.10 cells were treated with actinomycin D for 18 h to induce apoptotic targets (A and C); necrotic targets were heat-killed (B and D). After staining (blue) with Hoechst 33342, target cells were added to macrophages at a ratio of 20:1, and cells were incubated for 60 min at 4°C (binding; A and B) and at 37°C (engulfment, as well as binding; C and D). Unbound target cells then were removed by washing; visibly bound targets were not dislodged by this procedure. The image in B illustrates that homotypic target cell association can amplify the apparent extent of binding; on average, this phenomenon distorts the magnitude of specific binding by <10%. Under these conditions, the extent to which target cells aggregated in the absence of macrophages was <3% in all cases. Images are composites of blue Hoechst staining, green CFDA labeling, and phase-contrast illumination of cell outlines. Bar, 10 μm.
Efficient Engulfment of Recognized Targets Is Temperature Dependent
When DO11.10 cells were incubated with J774A.1 macrophages at 37°C, phagocytosis of the targets ensued. Virtually all targets appeared to be internalized (Figure 3, C and D). The data in Figure 2B demonstrate that macrophages interacted stably with greater numbers of target cells when they were able to engulf as well as bind targets. Selectivity also was evident: macrophages bound and engulfed apoptotic and necrotic targets, but not viable cells, to a similar extensive degree. As visualized microscopically, engulfment appeared to involve the fragmentation of all target cells (Figure 3, C and D).
These macrophages exhibited similar binding and engulfing activity when presented with different target cells and with apoptotic targets that had been induced to die by treatment with different stimuli. The data in Figure 2 (C and D), for example, detail the fate of S49 thymoma targets. In this case, apoptotic targets resulted from treatment with dexamethasone. Even fully allogeneic and xenogeneic apoptotic and necrotic targets, but not viable cells, were recognized specifically and efficiently by J774A.1 macrophages (our unpublished results). For consistency, the data presented below are derived from experiments with a single (semiallogeneic) target cell line, DO11.10.
Recognition of Apoptotic and Necrotic Cells Occurs via Distinct Mechanisms
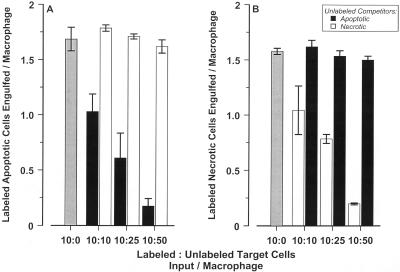
That the extents of uptake of apoptotic and necrotic targets by macrophages were essentially indistinguishable suggested the possibility that a common mechanism exists for the recognition of all native (nonopsonized) corpses. The direct inference of that view is that necrotic corpses should compete with apoptotic ones for macrophage binding. To test this prediction, target cell binding was examined at early times, under conditions where competition for kinetically saturable sites was detectable (see Figure 2A, inset). The ability of the unlabeled (or differentially labeled) targets to interfere with binding of the labeled ones was assessed. (To enhance the magnitude of the signal, the competition was performed at 37°C to allow macrophages to begin to engulf bound targets.) The data in Figure 4A document the ability of apoptotic targets to compete with themselves in a dose-dependent manner. In contrast, the binding of apoptotic targets was unaffected by necrotic cells, although necrotic competitors interfered effectively with labeled necrotic cell binding (Figure 4B). Of importance, microscopic examination revealed that apoptotic and necrotic targets (labeled distinctly with CFDA and CM-DiI) bound to the same macrophage (our unpublished results). The failure of apoptotic and necrotic cells to compete with each other implies that independent mechanisms for the recognition of apoptotic and necrotic targets are involved.
Figure 4.
Apoptotic and necrotic cells do not compete for recognition by J774A.1 macrophages. Recognition of late apoptotic and necrotic CFDA-labeled DO11.10 targets (A and B, respectively) was assessed in the presence or absence of unlabeled target competitors after incubation for 30 min at 37°C, as in Figure 2. Targets were prepared as in Figure 1. Labeled targets (2 × 105 cells/well) were mixed with unstained apoptotic (▪) or necrotic (□) competitors at the indicated ratios and added to a freshly plated monolayer (2 × 104 cells/well) of J774A.1 macrophages.
Binding studies with a different macrophage cell line confirmed this conclusion. RAW 264.7 macrophages bound and engulfed apoptotic targets at a similar rate and to a comparable extent as J774A.1 cells (Figure 5). An estimate of this binding by Scatchard analysis would suggest that the average number and “avidity” of apoptotic sites on RAW 264.7 cells and J774A.1 cells differ by less than threefold. Remarkably, RAW 264.7 cells exhibited no ability to interact with necrotic targets prepared from DO11.10 (Figure 5), S49, or other cell lines (our unpublished results). The dramatic dissociation of apoptotic and necrotic cell recognition by RAW 264.7 macrophages establishes that functionally distinct mechanisms pertain. Moreover, these data suggest that functional as well as morphological attributes of physiological cell death are retained throughout the death process and suggest that arbitrary distinctions drawn between early and late apoptotic cells (e.g., apoptosis versus secondary necrosis) may not be consequential.
Figure 5.
RAW 264.7 macrophages do not recognize necrotic cells. Viable (▴), necrotic (○), and early (▿) and late (□) apoptotic DO11.10 targets were presented to RAW 264.7 macrophages, and binding (A) and engulfment (B) were assessed as in Figure 2.
Phosphatidylserine Is Not a Specific Ligand for the Recognition of Apoptotic Cells
The ability of PS vesicles to compete with apoptotic cells for binding to macrophages has been taken to suggest that exposure of PS on the outer leaflet of the plasma membrane is a sentinel event in the apoptotic cell death process (Fadok et al., 1992b; Verhoven et al., 1995). Our characterization of apoptotic and necrotic targets, however, evidenced that the externalization of PS is not a feature unique to apoptotic cells (see Figure 1). PS exposure, detected by the binding of FITC-conjugated annexin V (Raynal and Pollard, 1994), preceded plasma membrane disintegration, marked by PI uptake, of necrotic as well as apoptotic cells. The annexin V+ PI− cells indicated in Figure 1H represent this intermediate stage of necrotic death. Equivalent apoptotic cells are present at early stages of physiological cell death (Figure 1F) and are less abundant at late stages (Figure 1G).
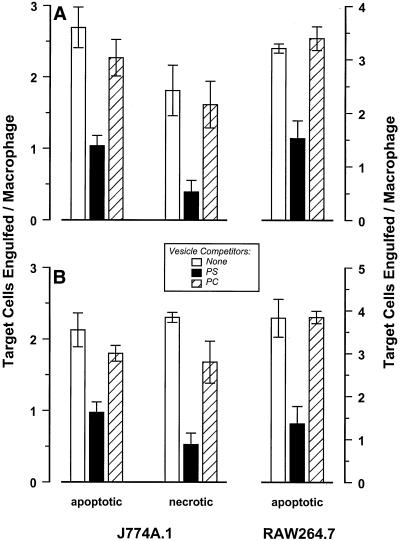
We assessed the role of PS in the binding of apoptotic and necrotic targets. PS vesicles were able to inhibit apoptotic target binding (our unpublished results) and engulfment (Figure 6A) by J774A.1 and RAW 264.7 macrophages. Inhibition was significant although incomplete; vesicles composed of PC, a nonanionic phospholipid, failed to inhibit macrophage interactions with target cells (Figure 6A). PS vesicles, and not PC vesicles, also were effective at blocking necrotic cell interactions with macrophages, consonant with the presence of externalized PS on the necrotic cells. In contrast, PS vesicles were not effective at blocking interactions of macrophages with immunoglobulin-opsonized cells (our unpublished results).
Figure 6.
Phosphatidylserine is not a specific ligand for apoptotic cell recognition. J774A.1 and Raw 264.7 macrophages were plated in microwells and incubated in the absence (A) or in the presence of LPS (0.1 μg/ml, B) for 3 h. DO11.10 targets (as in Figure 1) were added to the macrophage monolayers at a ratio of 10:1. Phosphatidylserine (PS; ▪]) or phosphatidylcholine (PC; square with diagonal line) liposomes were added as indicated to a final phospholipid concentration of 25 μM. Target cell interactions with macrophages in the presence or absence (□) of these liposomes were assessed after incubation at 37°C for 60 min.
Some studies have suggested that activated macrophages rely particularly on a PS-inhibitable mode of target cell recognition, whereas unactivated macrophages use an integrin-dependent process (Fadok et al., 1992a, 1998b; Pradhan et al., 1997). Diagnostically, the integrin-dependent mechanism is inhibitable with a specific integrin-binding tetrapeptide, RGDS (Savill et al., 1990). This prompted us to ask whether the interacting macrophages identified visually as jackpots (Figure 3) are a minor, spontaneously activated (PS-inhibitable and RGDS-uninhibitable) subpopulation within the J774A.1 culture. Contrary to this view, RGDS tetrapeptide also was able to inhibit macrophage binding of apoptotic target cells (Table 1). RGDS peptide was equally effective at inhibiting the binding of necrotic target cells (Table 1).
Table 1.
Evaluation of integrin-dependent target cell binding to macrophages
| Target | Target cells bound per macrophage with
added peptide
|
||
|---|---|---|---|
| None | RGDS | RGES | |
| Apoptotic | 4.33 ± 0.80 | 2.08 ± 0.19 | 4.60 ± 0.63 |
| Necrotic | 4.43 ± 0.13 | 2.74 ± 0.13 | 4.23 ± 0.38 |
The integrin-binding Arg-Gly-Asp-Ser peptide inhibits equally the binding of apoptotic and necrotic targets by J774A.1 macrophages. Binding of DO11.10 targets to J774A.1 macrophages (at a ratio of 40:1), in the presence or absence of Arg-Gly-Asp-Ser (RGDS) or Arg-Gly-Glu-Ser (RGES) tetrapeptides (1 mM), was assessed after 60 min. at 4°C (as in Figure 2).
Necrotic Targets Enhance the Proinflammatory Responses of Engulfing Macrophages, but Are Not Sufficient to Trigger Macrophage Activation
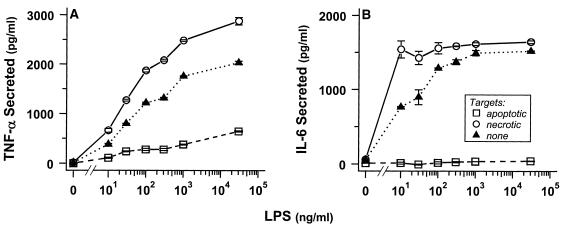
We characterized the release of the proinflammatory cytokines TNF-α and IL-6 after target cell interactions with macrophages as an indicator of inflammatory outcome. Necrotic and apoptotic targets alone did not elicit J774A.1 and RAW 264.7 macrophages to secrete TNF-α and IL-6 (Figure 7 [note the absence of cytokines when LPS is absent] and our unpublished results). LPS (Figure 7) and opsonized targets (our unpublished results), on the other hand, were able to activate macrophages to secrete those proinflammatory cytokines.
Figure 7.
Engulfed apoptotic cells inhibit proinflammatory cytokine release. J774A.1 macrophages were incubated at 37°C without or with LPS at the indicated concentrations for 2 h before the addition of late apoptotic (□) or necrotic (○) DO11.10 targets (prepared as in Figure 1; target to macrophage ratio of 20:1) or without added targets (▴). Culture supernatants were collected 4 h after target addition, and levels of secreted TNF-α and IL-6 were quantified. Under these conditions, target cells themselves produced no detectable levels of cytokines (<10 pg/ml TNF-α and < 15 pg/ml IL-6).
We investigated whether necrotic target cells could augment a proinflammatory LPS signal. Indeed, when macrophages were primed with suboptimal concentrations of LPS, their incubation with necrotic cells resulted in a modest elevation of cytokine secretion relative to macrophages treated with LPS alone (Figures 7 and 8). Secreted cytokines were the products of the macrophages, because necrotic cells did not secrete detectable TNF-α or IL-6, even when treated with LPS (our unpublished results). Of note, cytokine release does not appear to be a consequence of macrophage rupture, because engulfing macrophages remained viable and adherent throughout these assay (see Figure 3).
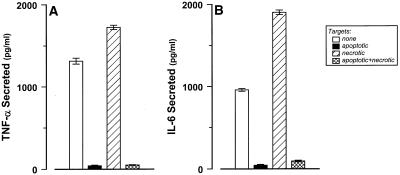
Figure 8.
Inhibition of cytokine release from macrophages exerted by apoptotic cells is a dominant effect. J774A.1 macrophages were incubated with LPS (100 ng/ml) for 2 h DO11.10 targets (as in Figure 1) were added to wells subsequently (at the indicated target to macrophage ratios): late apoptotic (▪; 10:1), necrotic (▨; 10:1), apoptotic and necrotic (⊠; 10 + 10:1). Secreted TNF-α and IL-6 levels were quantified 4 h after target addition or in the absence of any added targets (□).
Engulfment of necrotic cells in vivo is believed to result in an inflammatory response. Our results establish this phenomenon in a simplified cell culture system and also suggest that the interaction between a necrotic corpse and its engulfing macrophage is not sufficient for this response. These data demonstrate that neither do macrophages need to be activated to bind and engulf targets, nor do they become activated simply because they do engulf. Rather, necrotic cells trigger the enhanced secretion of proinflammatory cytokines from independently activated macrophages.
Inhibition of Proinflammatory Macrophage Responses by Engulfed Apoptotic Targets Represents an Apoptotic Gain-of-Function
In contrast to the augmentation of cytokine secretion afforded by necrotic targets, macrophages that were incubated with apoptotic targets did not release TNF-α or IL-6, even when primed with optimal doses of LPS (Figure 7). In fact, apoptotic cells (induced to die with actinomycin D [Figure 7] or dexamethasone [our unpublished results]) strongly inhibited the secretion of proinflammatory cytokines from macrophages. Macrophages pretreated with LPS retained their ability to bind and engulf target cells (Figure 6B).
The absence of a cytokine response associated with apoptotic cell interaction might reflect the absence of a proinflammatory stimulatory signal on apoptotic targets. However, the observation that IL-6 and TNF-α secretion by LPS-activated macrophages is abrogated after engulfment of apoptotic targets strongly suggests that apoptotic cells actively antagonize (LPS-derived) proinflammatory signals. To test this model, a mixture of apoptotic and necrotic targets was presented to LPS-activated macrophages. The inhibitory effect of the apoptotic targets was completely dominant to the stimulatory effect of the necrotic cells (Figure 8). The absence of proinflammatory cytokines is not due to simple absorption by the apoptotic cells, because the levels of already-secreted cytokines after LPS stimulation were not reduced by the addition of apoptotic targets (our unpublished results). Neither is this inhibition mediated through soluble factors released from apoptotic cells. In all experiments, apoptotic cells were washed twice before they were presented to macrophages; furthermore, apoptotic cell supernatants were not inhibitory (our unpublished results). We conclude that during the process of physiological cell death, apoptotic cells acquire a cell-associated, dominant-acting, anti-inflammatory signaling activity that overrides proinflammatory macrophage responses.
The notion that apoptotic cells can inhibit proinflammatory macrophage responses is consistent with previous work, including studies of LPS-activated, monocyte-derived macrophages (Voll et al., 1997; Fadok et al., 1998a; McDonald et al., 1999). Henson and coworkers (Fadok et al., 1998a; McDonald et al., 1999) have argued that apoptotic inhibition, observed after long periods (14–18 h) of incubation, is effected in a paracrine manner, through the induced release by macrophages of antagonistic factors, including platelet-activating factor, prostaglandin E2, and especially TGF-β. A distinct issue is raised by the observation that the inhibition imposed by apoptotic cells is rapid. The secretion of TNF-α and IL-6 by macrophages, which was detectable after less than 2 h of LPS stimulation (see Figure 7), was halted by the addition of apoptotic cells, even when incubation in the presence of LPS was continued (Figures 7 and 8 and our unpublished results). These results suggest that a virtually immediate and direct anti-inflammatory effect of apoptotic cells must be exerted on the engulfing macrophage, proximal to proinflammatory signaling. It remains to be determined on what level this blockade is enforced.
DISCUSSION
Macrophages Discriminate Precisely between Targets that Die Physiologically and those that Die Pathologically
Cell death is critical in normal organismal development and homeostasis, particularly for shaping and maintaining appropriate cellular networks. We have addressed previously the fundamental question of whether a common cell-autonomous effector mechanism pertains in distinct cases of cell death. That work has led to the identification of a thematically conserved, ordered pathway for cellular destruction. The ability of a dying cell to trigger phagocytosis without eliciting an inflammatory response likely is the overriding biological purpose of the physiological cell death process. The crucial questions in this context are how recognition without inflammatory response is assured and where within the apoptotic process signals for phagocytic clearance are expressed.
Our data demonstrate that macrophages discriminate innately between cells that have undergone a physiological death and those that have suffered a pathological death. The distinction drawn between apoptotic and necrotic corpses may have seemed arbitrary, especially in light of the similar binding behaviors and inhibitor (including phospholipid vesicle) profiles that we observed for the two classes of targets. However, competition experiments between different targets and binding studies with the RAW 264.7 macrophage cell line establish that macrophage recognition of the products of physiological and pathological cell deaths occur by distinct mechanisms. Clearly, it will be important to extend these finding to primary macrophage populations. At the same time, the RAW 264.7 macrophage cell line, which lacks an intact recognition mechanism for necrotic cells, is an intriguing subject for genetic reconstitution. Of greater interest would be a complementary macrophage lacking apoptotic cell recognition function.
Altogether, our data confirm that the distinction between apoptotic and necrotic cells is real and that independent, noncompeting modes of binding are involved in the recognition of native cells that have died by distinct modes of death. It is significant that clearance and phlogistic outcomes attributed to the processes of physiological cell death and pathological death in vivo are recapitulated reliably in a simple cell culture setting. These observations imply that the interaction of a macrophage with its target cell alone is sufficient to effect these responses.
Identities of the Ligands and Receptors for the Recognition of Apoptotic and Necrotic Cells Remain Elusive
It is surprising that even within clonal populations of macrophages, only a minority of cells is competent to bind and engulf targets at any time. Previous studies with primary macrophages (of peritoneal or monocytic origin) have established that phagocytic activity increases after in vitro culture (“maturation”; Newman et al., 1982), and that treatment with particulate stimuli also enhances subsequent activity (Fadok et al., 1993, 1998b). Binding that is inhibitable with PS vesicles, moreover, is reportedly restricted to activated macrophages (Pradhan et al., 1997; Fadok et al., 1998b). Consistent with this view, expression of the candidate PS-dependent receptor as well as scavenger receptors and CD68, molecules that could be responsible for anionic phospholipid binding, is elevated after activation (Fukasawa et al., 1996; Ramprasad et al., 1996; Murao et al., 1997; Fadok et al., 2000). Integrin-dependent (RGDS-inhibitable) interactions, in comparison, have been attributed to unactivated cells (but see Savill et al., 1990; Pradhan et al., 1997; Fadok et al., 1998b). Although J774A.1 cells have been characterized as resembling “unactivated” macrophages (Pradhan et al., 1997), the assays we use here evince substantially higher levels of binding and engulfment than reported previously (also see McDonald et al., 1999) and reveal significant inhibition of their target cell interactions by PS vesicles. These data suggest that PS-inhibitable interactions may not be restricted to activated macrophages and that modes of binding inhibitable by RGDS and by PS are not mutually exclusive.
More importantly, our data indicate that PS is unlikely to be involved specifically in the recognition of apoptotic target cells. Both necrotic and apoptotic cells display externalized PS, yet relative to apoptotic cells, necrotic cells are not recognized equivalently (and not at all in one case). It seems clear that PS exposure cannot be sufficient for recognition (see also Pradhan et al., 1997). That PS vesicles, presumably binding to the macrophage (Terpstra et al., 1998), inhibit partially the recognition of both classes of corpses suggests that PS-specific binding sites on the macrophage may facilitate interactions with target cells or that bound vesicles may interfere sterically with accessibility of targets for other recognition molecules.
Apoptotic corpses derived from syngeneic and congenic cells induced by disparate suicidal stimuli are recognized and engulfed with equivalent specificity and efficiency in this system. Human targets also are recognized efficiently by these murine macrophages (our unpublished results; also see McDonald et al., 1999), implying that apoptotic cell determinants for macrophage recognition are widely conserved. Among the apoptotic stimuli we have used, inhibitors of macromolecular synthesis illuminate further that the appearance of these recognition determinants is not dependent on de novo synthesis (also see Flora et al., 1996).
Are Physiological Cell Death Determinants for Phagocytic Recognition and for Anti-inflammatory Effect Distinct?
Distinct macrophage mechanisms for the recognition of apoptotic and necrotic cells are associated with opposing phlogistic outcomes. A critical question is whether the anti-inflammatory signals acquired by an apoptotic cell are distinct from the recognition determinants it expresses. We have begun to explore this issue by asking where within the ordered physiological cell death process signals for macrophage recognition are expressed. Our initial experiments map the expression on the apoptotic cell of determinants for both recognition and inhibition of inflammatory response downstream of the action of Bcl-2 (our unpublished results). Target cells treated with a death-inducing concentration of actinomycin D and spared from death by transfected Bcl-2 were not recognized by J774A.1 cells and were unable to inhibit the LPS-mediated induction of TNF-α from those macrophages. These results extend earlier findings that Bcl-2 spared cells from phagocytic recognition (Flora et al., 1996, but see Lagasse and Weissman, 1994).
In a larger context, the issue of an obligatory linkage between apoptotic death and noninflammatory outcome is posed profoundly with the death of macrophages themselves. Macrophages do not die when they engulf (Meagher et al., 1992; Bellingan et al., 1996), but they can be triggered to undergo physiological cell death, for example, by pathogens or immune effectors (Richter-Dahlfors et al., 1997; Oddo et al., 1998). This macrophage death is associated with the processing and release of the potent proinflammatory cytokine IL-1β (Hogquist et al., 1991). It will be of great importance to understand whether the apoptotic death of a macrophage is necessarily proinflammatory. Perhaps it is just this unusual apoptotic death that signals immunological “danger” (Matzinger, 1994).
An Anti-inflammatory Signal Is Acquired during the Physiological Cell Death Process
It is striking that the anti-inflammatory effect of apoptotic targets is dominant to the inflammatory enhancement of necrotic cells. This dispels the commonly held notion that the phagocytosis of apoptotic cells occurs prelytically so as to circumvent the inflammatory response that ensues after the release of noxious intracellular contents from ruptured corpses (Ren et al., 1995; Stern et al., 1996; Fadok et al., 1998b). Although the failure of apoptotic cells to promote the maturation of dendritic cells has not been associated with a dominant inhibitory effect (Sauter et al., 2000), the absence of an inflammatory macrophage response to apoptotic cells clearly reflects an active inhibitory signal, not simply the absence of a proinflammatory one. The acquisition of this anti-inflammatory signal represents a gain-of-function that occurs independently of de novo macromolecular synthesis. Like the induction of death-associated effector activities (SH Chang, KJ Harvey, M Cvetanovic, and D.S. Ucker, unpublished results), the appearance of determinants for recognition and inhibition of inflammation must occur primarily on a posttranslational level. If macrophages need receive this signal affirmatively in order not to produce proinflammatory cytokines, the loss of recognition function, for example, by mutation, likely would manifest a chronically uninhibited inflammatory response, akin to the loss of TGF-β (Shull et al., 1992; Kulkarni et al., 1993). This provides a stringent criterion in the evaluation of macrophage receptors for apoptotic cells.
Finally, the direct abrogation by apoptotic cells of proinflammatory cytokine release raises the issue of whether these inflammatory responses, like target cell interactions, are limited to a fraction of the macrophage population. Inhibition exerted independently of soluble factors could only be manifest if macrophages that secrete cytokines are exclusively the ones that interact with targets. It will be intriguing to determine whether that assessment of cytokine production on the level of the individual cell independently identifies macrophages visualized as jackpots by virtue of their target cell interactions.
ACKNOWLEDGMENTS
We are grateful to Lin Tao (University of Illinois, College of Dentistry) and Richard Ye (University of Illinois, College of Medicine) for providing J774A.1 and RAW 264.7 cells, respectively, and to Hayat Onyuksel (University of Illinois, College of Pharmacy) for preparing liposomes. We thank our colleagues Oscar Colamonici, Jim Cook, Daniel Floryk, Amy Kenter, Dunja Lukovic, Navreet Nanda, Bellur Prabhakar, William Walden, and Richard Ye for their constructive comments. This work was supported by grants to D.S.U. from the National Institutes of Health and a generous fellowship to R.E.C. from the International Foundation for Ethical Research.
Abbreviations used:
- CFDA
succinimidyl ester of (5,6)-carboxyfluorescein diacetate
- CM-DiI
chloromethylated lipophilic carbocyanine dye DiIC18(3)
- LPS
lipopolysaccharide
- PC
phosphatidylcholine
- PI
propidium iodide
- PS
phosphatidylserine
- RGDS
Arg-Gly-Asp-Ser tetrapeptide
- RGES
Arg-Gly-Glu-Ser tetrapeptide
REFERENCES
- Albert ML, Pearce SFA, Francisco LM, Sauter B, Roy P, Silverstein RL, Bhardwaj N. Immature dendritic cells phagocytose apoptotic cells via aVβ5 and CD36, and cross-present antigens to cytotoxic T lymphocytes. J Exp Med. 1998;188:1359–1368. doi: 10.1084/jem.188.7.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellingan GJ, Caldwell H, Howie SEM, Dransfield I, Haslett C. In vivo fate of the inflammatory macrophage during the resolution of inflammation: inflammatory macrophages do not die locally, but emigrate to the draining lymph nodes. J Immunol. 1996;157:2577–2585. [PubMed] [Google Scholar]
- Bratton DL, Fadok VA, Richter DA, Kailey JM, Guthrie LA, Henson PM. Appearance of phosphatidylserine on apoptotic cells requires calcium-mediated nonspecific flip-flop and is enhanced by loss of the aminophospholipid translocase. J Biol Chem. 1997;272:26159–26165. doi: 10.1074/jbc.272.42.26159. [DOI] [PubMed] [Google Scholar]
- Cecconi F, Alvarez-Bolado G, Meyer BI, Roth KA, Gruss P. Apaf1 (CED-4 homolog) regulates programmed cell death in mammalian development. Cell. 1998;94:727–737. doi: 10.1016/s0092-8674(00)81732-8. [DOI] [PubMed] [Google Scholar]
- Chang M-K, Bergmark C, Laurila A, Hörkkö S, Han K-H, Friedman P, Dennis EA, Witztum JL. Monoclonal antibodies against oxidized low-density lipoprotein bind to apoptotic cells and inhibit their phagocytosis by elicited macrophages: evidence that oxidation-specific epitopes mediate macrophage recognition. Proc Natl Acad Sci USA. 1999;96:6353–6358. doi: 10.1073/pnas.96.11.6353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devitt A, Moffatt OD, Raykundalia C, Capra JD, Simmons DL, Gregory CD. Human CD14 mediates recognition of phagocytosis of apoptotic cells. Nature. 1998;392:505–509. doi: 10.1038/33169. [DOI] [PubMed] [Google Scholar]
- Dhein J, Walczak H, Bäumler C, Debatin K-M, Krammer PH. Autocrine T-cell suicide mediated by APO-1/(Fas/CD95) Nature. 1995;373:438–441. doi: 10.1038/373438a0. [DOI] [PubMed] [Google Scholar]
- Duvall E, Wyllie AH, Morris RG. Macrophage recognition of cells undergoing programmed cell death (apoptosis) Immunology. 1985;56:351–358. [PMC free article] [PubMed] [Google Scholar]
- Ellis RE, Jacobson DM, Horvitz HR. Genes required for the engulfment of cell corpses during programmed cell death in Caenorhabditis elegans. Genetics. 1991;129:79–94. doi: 10.1093/genetics/129.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadok VA, Savill JS, Haslett C, Bratton DL, Doherty DE, Campbell PA, Henson PM. Different populations of macrophages use either the vitronectin receptor or the phosphatidylserine receptor to recognize and remove apoptotic cells. J Immunol. 1992a;149:4029–4035. [PubMed] [Google Scholar]
- Fadok VA, Voelker DR, Campbell PA, Cohen JJ, Bratton DL, Henson PM. Exposure of phosphatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophages. J Immunol. 1992b;148:2207–2216. [PubMed] [Google Scholar]
- Fadok VA, Laszlo DJ, Noble PW, Weinstein L, Riches DWH, Henson PM. Particle digestibility is required for induction of the phosphatidylserine recognition mechanism used by murine macrophages to phagocytose apoptotic cells. J Immunol. 1993;151:4274–4285. [PubMed] [Google Scholar]
- Fadok VA, Bratton DL, Konowal A, Freed PW, Westcott JY, Henson PM. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-β, PGE2, and PAF. J Clin Invest. 1998a;101:890–898. doi: 10.1172/JCI1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadok VA, Warner ML, Bratton DL, Henson PM. CD36 is required for phagocytosis of apoptotic cells by human macrophages that use either a phosphatidylserine receptor or the vitronectin receptor (aVβ3) J Immunol. 1998b;161:6250–6257. [PubMed] [Google Scholar]
- Fadok VA, Bratton DL, Rose DM, Pearson A, Ezekowitz RAB, Henson PM. A receptor for phosphatidylserine-specific clearance of apoptotic cells. Nature. 2000;405:85–90. doi: 10.1038/35011084. [DOI] [PubMed] [Google Scholar]
- Febbraio M, Abumrad NA, Hajjar DP, Sharma K, Cheng W, Pearce SF, Silverstein RL. A null mutation in murine CD36 reveals an important role in fatty acid and lipoprotein metabolism. J Biol Chem. 1999;274:19055–19062. doi: 10.1074/jbc.274.27.19055. [DOI] [PubMed] [Google Scholar]
- Flora PK, Devitt A, Johnson GD, Milner AE, Gregory CD. Bcl-2 delays macrophage engulfment of human B cells induced to undergo apoptosis. Eur J Immunol. 1996;26:2243–2247. doi: 10.1002/eji.1830260941. [DOI] [PubMed] [Google Scholar]
- Franc NC, Heitzler P, Ezekowitz RAB, White K. Requirement for Croquemort in phagocytosis of apoptotic cells in Drosophila. Science. 1999;284:1991–1994. doi: 10.1126/science.284.5422.1991. [DOI] [PubMed] [Google Scholar]
- Fukasawa M, Adachi H, Hirota K, Tsujimoto M, Arai H, Inoue K. SRB1, a class B scavenger receptor, recognizes both negatively charged liposomes and apoptotic cells. Exp Cell Res. 1996;222:246–250. doi: 10.1006/excr.1996.0030. [DOI] [PubMed] [Google Scholar]
- Hart SP, Dougherty GJ, Haslett C, Dransfield I. CD44 regulates phagocytosis of apoptotic neutrophil granulocytes, but not apoptotic lymphocytes, by human macrophages. J Immunol. 1997;159:919–925. [PubMed] [Google Scholar]
- Harvey KJ, Lukovic D, Ucker DS. Caspase-dependent Cdk activity is a requisite effector of apoptotic death events. J Cell Biol. 2000;148:59–72. doi: 10.1083/jcb.148.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson PM, Johnson RB., Jr Tissue injury in inflammation: oxidants, proteinases, and cationic proteins. J Clin Invest. 1987;79:669–674. doi: 10.1172/JCI112869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirt UA, Gantner F, Leist M. Phagocytosis of nonapoptotic cells dying by caspase-independent mechanisms. J Immunol. 2000;164:6520–6529. doi: 10.4049/jimmunol.164.12.6520. [DOI] [PubMed] [Google Scholar]
- Hogquist KA, Nett MA, Unanue ER, Chaplin DD. Interleukin 1 is processed and released during apoptosis. Proc Natl Acad Sci USA. 1991;88:8485–8489. doi: 10.1073/pnas.88.19.8485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes J, Liu Y, Damme JV, Savill J. Human glomerular mesangial cell phagocytosis of apoptotic neutrophils. J Immunol. 1997;158:4389–4397. [PubMed] [Google Scholar]
- Kägi D, Seiler P, Pavlovic J, Ledermann B, Zinkernagel RM, Hengartner H. The roles of perforin- and Fas-dependent cytotoxicity in protection against cytopathic and noncytopathic viruses. Eur J Immunol. 1995;25:3256–3262. doi: 10.1002/eji.1830251209. [DOI] [PubMed] [Google Scholar]
- Kawabe Y, Ochi A. Programmed cell death and extrathymic reduction of Vβ8+ CD4+ T cells in mice tolerant to Staphylococcus aureus enterotoxin B. Nature. 1991;349:245–248. doi: 10.1038/349245a0. [DOI] [PubMed] [Google Scholar]
- Kerr JFR, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;26:239–256. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni AB, Huh C-G, Becker D, Geiser A, Lyght M, Flanders KC, Roberts AB, Sporn MB, Ward JM, Karlsson S. Transforming growth factor β1 null mutation in mice causes excessive inflammatory response and early death. Proc Natl Acad Sci USA. 1993;90:770–774. doi: 10.1073/pnas.90.2.770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagasse E, Weissman IL. bcl-2 inhibits apoptosis of neutrophils but not their engulfment by macrophages. J Exp Med. 1994;179:1047–1052. doi: 10.1084/jem.179.3.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzinger P. Tolerance, danger, and the extended family. Annu Rev Immunol. 1994;12:991–1045. doi: 10.1146/annurev.iy.12.040194.005015. [DOI] [PubMed] [Google Scholar]
- McDonald PP, Fadok VA, Bratton D, Henson PM. Transcriptional and translational regulation of inflammatory mediator production by endogenous TGF-β in macrophages that have ingested apoptotic cells. J Immunol. 1999;163:6164–6172. [PubMed] [Google Scholar]
- McEvoy L, Williamson P, Schlegel RA. Membrane phospholipid asymmetry as a determinant of erythrocyte recognition by macrophages. Proc Natl Acad Sci USA. 1986;83:3311–3315. doi: 10.1073/pnas.83.10.3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meagher LC, Savill JS, Baker A, Fuller RW, Haslett C. Phagocytosis of apoptotic neutrophils does not induce macrophage release of thromboxane B2. J Leukoc Biol. 1992;52:269–272. [PubMed] [Google Scholar]
- Murao K, Terpstra V, Green SR, Kondratenko N, Steinberg D, Quehenberger O. Characterization of CLA-1, a human homologue of rodent scavenger receptor BI, as a receptor for high density lipoprotein and apoptotic thymocytes. J Biol Chem. 1997;272:17551–17557. doi: 10.1074/jbc.272.28.17551. [DOI] [PubMed] [Google Scholar]
- Newman SL, Henson JE, Henson PM. Phagocytosis of senescent neutrophils by human monocyte-derived macrophages and rabbit inflammatory macrophages. J Exp Med. 1982;156:430–442. doi: 10.1084/jem.156.2.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oddo M, Renno T, Attinger A, Bakker T, MacDonald HR, Meylan PR. Fas ligand-induced apoptosis of infected human macrophages reduces the viability of intracellular Mycobacterium tuberculosis. J Immunol. 1998;160:5448–5454. [PubMed] [Google Scholar]
- Platt N, Suzuki H, Kurihara Y, Kodama T, Gordon S. Role for the class A macrophage scavenger receptor in the phagocytosis of apoptotic thymocytes in vitro. Proc Natl Acad Sci USA. 1996;93:12456–12460. doi: 10.1073/pnas.93.22.12456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt N, da Silva RP, Gordon S. Recognizing death: the phagocytosis of apoptotic cells. Trends Cell Biol. 1998;8:365–372. doi: 10.1016/s0962-8924(98)01329-4. [DOI] [PubMed] [Google Scholar]
- Pradhan D, Krahling S, Williamson P, Schlegel RA. Multiple systems for recognition of apoptotic lymphocytes by macrophages. Mol Biol Cell. 1997;8:767–778. doi: 10.1091/mbc.8.5.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramprasad MP, Terpstra V, Kondratenko N, Quehenberger O, Steinberg D. Cell surface expression of mouse macrosialin and human CD68 and their role as macrophage receptors for oxidized low density lipoprotein. Proc Natl Acad Sci USA. 1996;93:14833–14838. doi: 10.1073/pnas.93.25.14833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravetch JV. Fc receptors: rubor redux. Cell. 1994;78:553–560. doi: 10.1016/0092-8674(94)90521-5. [DOI] [PubMed] [Google Scholar]
- Raynal P, Pollard HB. Annexins: the problem of assessing the biological role for a gene family of multifunctional calcium- and phospholipid-binding proteins. Biochim Biophys Acta. 1994;1197:63–93. doi: 10.1016/0304-4157(94)90019-1. [DOI] [PubMed] [Google Scholar]
- Ren Y, Silverstein RL, Allen J, Savill J. CD36 gene transfer confers capacity for phagocytosis of cells undergoing apoptosis. J Exp Med. 1995;181:1857–1862. doi: 10.1084/jem.181.5.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter-Dahlfors A, Buchan AMJ, Finlay BB. Murine salmonellosis studied by confocal microscopy: Salmonella typhimurium resides intracellularly inside macrophages and exerts a cytotoxic effect on phagocytes in vivo. J Exp Med. 1997;186:569–580. doi: 10.1084/jem.186.4.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell JH. Internal disintegration model of cytotoxic lymphocyte-induced target damage. Immunol Rev. 1983;72:97–118. doi: 10.1111/j.1600-065x.1983.tb01074.x. [DOI] [PubMed] [Google Scholar]
- Sauter B, Albert ML, Francisco L, Larsson M, Somersan S, Bhardwaj N. Consequences of cell death: exposure to necrotic tumor cells, but not primary tissue cells or apoptotic cells, induces the maturation of immunostimulatory dendritic cells. J Exp Med. 2000;191:423–434. doi: 10.1084/jem.191.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savill JS, Wyllie AH, Henson JE, Walport MJ, Henson PM, Haslett C. Macrophage phagocytosis of aging neutrophils in inflammation: programmed cell death in the neutrophil leads to its recognition by macrophages. J Clin Invest. 1989;83:865–875. doi: 10.1172/JCI113970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savill J, Dransfield I, Hogg N, Haslett C. Vitronectin receptor-mediated phagocytosis of cells undergoing apoptosis. Nature. 1990;343:170–173. doi: 10.1038/343170a0. [DOI] [PubMed] [Google Scholar]
- Savill J, Hogg N, Ren Y, Haslett C. Thrombospondin cooperates with CD36 and the vitronectin receptor in macrophage recognition of neutrophils undergoing apoptosis. J Clin Invest. 1992;90:1513–1522. doi: 10.1172/JCI116019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlegel RA, Stevens M, Lumley-Sapanski K, Williamson P. Altered lipid packing identifies apoptotic thymocytes. Immunol Lett. 1993;36:283–288. doi: 10.1016/0165-2478(93)90101-7. [DOI] [PubMed] [Google Scholar]
- Schroit AJ, Madsen JW, Tanaka Y. In vitro recognition and clearance of red blood cells containing phosphatidylserine in their plasma membranes. J Biol Chem. 1985;260:5131–5138. [PubMed] [Google Scholar]
- Schwartz BR, Karsan A, Bombeli T, Harlan JM. A novel β1 integrin-dependent mechanism of leukocyte adherence to apoptotic cells. J Immunol. 1999;162:4842–4848. [PubMed] [Google Scholar]
- Shull MM, Ormsby I, Kier AB, Pawlowski S, Diebold RJ, Yin M, Allen R, Sidman C, Proetzel G, Calvin D, Annunziata N, Doetschman T. Targeted disruption of the mouse transforming growth factor-β1 gene results in multifocal inflammatory disease. Nature. 1992;359:693–699. doi: 10.1038/359693a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern M, Savill J, Haslett C. Human monocyte-derived macrophage phagocytosis of senescent eosinophils undergoing apoptosis: mediation by aVβ3/CD36/thrombospondin recognition mechanism and lack of phlogistic response. Am J Pathol. 1996;149:911–921. [PMC free article] [PubMed] [Google Scholar]
- Surh CD, Sprent J. T-cell apoptosis detected in situ during positive and negative selection in the thymus. Nature. 1994;372:100–103. doi: 10.1038/372100a0. [DOI] [PubMed] [Google Scholar]
- Suzuki H, Kurihara Y, Takeya M, Kamada N, Kataoka M, Jishage K, Ueda O, Sakaguchi H, Higashi T, Suzuki T, Takashima Y, Kawabe Y, Cynshi O, Wada Y, Honda M, Kurihara H, Aburatani H, Doi T, Matsumoto A, Azuma S, Noda T, Toyoda Y, Itakura H, Yazaki Y, Horiuchi S, Takahashi K, Kruijt JK, van Berkel TJC, Steinbrecher UP, Ishibashi S, Maeda N, Gordon S, Kodama T. A. role for macrophage scavenger receptors in atherosclerosis and susceptibility to infection. Nature. 1997;386:292–296. doi: 10.1038/386292a0. [DOI] [PubMed] [Google Scholar]
- Terpstra V, Kondratenko N, Steinberg D. Macrophages lacking scavenger receptor A show a decrease in binding and uptake of acetylated low-density lipoprotein and of apoptotic thymocytes, but not of oxidatively damaged red blood cells. Proc Natl Acad Sci USA. 1997;94:8127–8131. doi: 10.1073/pnas.94.15.8127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terpstra V, Bird DA, Steinberg D. Evidence that the lipid moiety of oxidized low density lipoprotein plays a role in its interaction with macrophage receptors. Proc Natl Acad Sci USA. 1998;95:1806–1811. doi: 10.1073/pnas.95.4.1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ucker DS, Ashwell JD, Nickas G. Activation-driven T cell death I. Requirements for de novo transcription and translation and association with genome fragmentation. J Immunol. 1989;143:3461–3469. [PubMed] [Google Scholar]
- Verhoven B, Schlegel RA, Williamson P. Mechanisms of phosphatidylserine exposure, a phagocyte recognition signal, on apoptotic T lymphocytes. J Exp Med. 1995;182:1597–1601. doi: 10.1084/jem.182.5.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voll RE, Herrmann M, Roth EA, Stach C, Kalden JR, Girkontaite I. Immunosuppressive effects of apoptotic cells. Nature. 1997;390:350–351. doi: 10.1038/37022. [DOI] [PubMed] [Google Scholar]
- Webb S, Morris C, Sprent J. Extrathymic tolerance of mature T cells: clonal elimination as a consequence of immunity. Cell. 1990;63:1249–1256. doi: 10.1016/0092-8674(90)90420-j. [DOI] [PubMed] [Google Scholar]
- Wyllie AH, Morris RG, Smith AL, Dunlop D. Chromatin cleavage in apoptosis: association with condensed chromatin morphology and dependence on macromolecular synthesis. J Pathol. 1984;142:67–77. doi: 10.1002/path.1711420112. [DOI] [PubMed] [Google Scholar]
- Yeh W-C, de la Pompa JL, McCurrach ME, Shu H-B, Elia AJ, Shahinian A, Ng M, Wakeham A, Khoo W, Mitchell K, El-Deiry WS, Lowe SW, Goeddel DV, Mak TW. FADD: essential for embryo development and signaling from some, but not all, inducers of apoptosis. Science. 1998;279:1954–1958. doi: 10.1126/science.279.5358.1954. [DOI] [PubMed] [Google Scholar]
- Yoshida H, Kong Y-Y, Yoshida R, Elia AJ, Hakem A, Hakem R, Penninger JM, Mak TW. Apaf1 is required for mitochondrial pathways of apoptosis and brain development. Cell. 1998;94:739–750. doi: 10.1016/s0092-8674(00)81733-x. [DOI] [PubMed] [Google Scholar]