Abstract
Background
Genetic resistance is the most effective and sustainable approach to the control of plant pathogens that are a major constraint to agriculture worldwide. In soybean, three dominant R genes, i.e., Rsv1, Rsv3 and Rsv4, have been identified and deployed against Soybean mosaic virus (SMV) with strain-specificities. Molecular identification of virulent determinants of SMV on these resistance genes will provide essential information for the proper utilization of these resistance genes to protect soybean against SMV, and advance knowledge of virus-host interactions in general.
Methodology/Principal Findings
To study the gain and loss of SMV virulence on all the three resistance loci, SMV strains G7 and two G2 isolates L and LRB were used as parental viruses. SMV chimeras and mutants were created by partial genome swapping and point mutagenesis and then assessed for virulence on soybean cultivars PI96983 (Rsv1), L-29 (Rsv3), V94-5152 (Rsv4) and Williams 82 (rsv). It was found that P3 played an essential role in virulence determination on all three resistance loci and CI was required for virulence on Rsv1- and Rsv3-genotype soybeans. In addition, essential mutations in HC-Pro were also required for the gain of virulence on Rsv1-genotype soybean. To our best knowledge, this is the first report that CI and P3 are involved in virulence on Rsv1- and Rsv3-mediated resistance, respectively.
Conclusions/Significance
Multiple viral proteins, i.e., HC-Pro, P3 and CI, are involved in virulence on the three resistance loci and simultaneous mutations at essential positions of different viral proteins are required for an avirulent SMV strain to gain virulence on all three resistance loci. The likelihood of such mutations occurring naturally and concurrently on multiple viral proteins is low. Thus, incorporation of all three resistance genes in a soybean cultivar through gene pyramiding may provide durable resistance to SMV.
Introduction
Plant pathogens, causal agents of numerous devastating crop diseases worldwide, are a major constraint to agriculture and threaten global food security [1]. The use of genetic resistance is considered the most effective and sustainable approach to the control of plant pathogens as it is environmentally-friendly and target-specific, and provides reliable protection without additional labor or material costs during the growing season [2], [3]. The major genetic resistance that has been extensively used in agriculture is mediated by R gene. Such resistance, particularly mediated by natural dominant NBS-LRR R genes is triggered by either direct or indirect interactions between the R gene encoded protein of the host and the avirulence factor produced by the corresponding avirulence (Avr) gene of the invading pathogen [4]–[6]. Two defense responses, i.e., extreme resistance (ER) and hypersensitive response (HR), are often associated with R gene-mediated resistance [7]. In the case of plant viruses, the former is characterized by the arrest of the invading virus at the inoculation site without any visible symptoms or virus accumulation, whereas the latter restricts the virus to the primary infection site by rapid death of infected and neighboring cells [3], [7]. During the coevolutionary arms race of viral pathogens and their host plants, genetic diversity generated by spontaneous mutations (resulting from error-prone replication) and RNA recombination, and the selection force acting on this variability lead to the occurrence of resistance-breaking isolates [3], [8]–[10]. Molecular identification and characterization of virulent determinants from these isolates and their interactions with major resistance genes will advance knowledge of resistance durability, which is essential for developing and utilizing genetic resistance for crop protection [2], [7], [11].
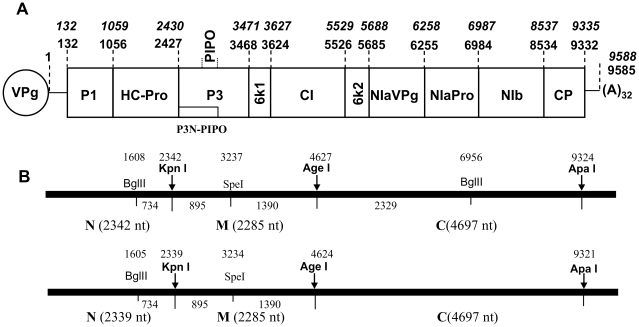
Soybean mosaic virus (SMV), a member of the family Potyviridae, is the most common viral pathogen of soybean [12]. SMV is a seed-borne, aphid-transmitted virus that causes severe yield loss and reduction in seed quality worldwide [13]. Similar to other potyviruses, SMV has a positive-sense, single-stranded RNA molecule as its genome, which is approximately 9,600 nucleotides in length encoding a large polyprotein of ∼350 kDa and a short polyprotein as a result of translational frameshift in the P3 coding region (Fig. 1A). These two polyproteins are processed by three viral proteases (P1 and HC-Pro responsible for autocleavage at their N-terminus and NIA-Pro for all other cleavages) to release 11 mature proteins, from the N-terminus: P1, HC-Pro, P3, P3N-PIPO (resulting from translational slippage or frameshift in P3), 6K1, CI, 6K2 (or 6K), NIa-VPg, NIa-Pro, NIb, and CP [14], [15]. To date, numerous SMV isolates have been reported. In North America, SMV isolates are classified into seven distinct strains, G1 through G7, based on their differential responses on susceptible and resistant soybean cultivars [16]. Screening for resistant soybean germplasm to SMV has identified three independent resistance loci, Rsv1, Rsv3 and Rsv4 [17]–[20]. These three loci are all dominant R genes [5], [18], [21], [22]. Rsv1, found in PI96983 confers extreme resistance to most SMV strains but not to G7, whereas Rsv3-genotype soybean is resistant to higher numbered strain groups including G5 through G7 but susceptible to lower numbered strain groups (G1 through G4) [23]. Rsv4 is the only gene that confers resistance to all the seven strains [12].
Figure 1. Schematic representation of three full-length infectious clones derived from SMV strains G7 and two G2 isolates, L and LRB.
(A). Genomic organization of wild parental viral genomes with their respective viral proteins. Nucleotide position numbers for predicted mature proteins are indicated: italic for G7 and normal for L or LRB. (B). Restriction maps of G7 (top) and L or LRB (bottom). Arrows indicate the division of three cDNA fragments N, M and C and their nucleotide lengths are provided in parentheses. The length of the nucleotides between enzyme sites is given between those sites.
The strain-specific Rsv1- and Rsv3-conferred resistance to SMV is associated with ER and HR, respectively [12], [24]. However, the resistance mechanism of Rsv4 seems different as it is not associated with either ER or HR [12]. As one of the most complex plant-pathogen interactions, the soybean-SMV pathosystem is an excellent model to study R-Avr recognitions. Disturbance of R-SMV interactions can result in escape and spread of the virus to distant tissues. For instance, continued challenge of Rsv1-genotype soybean by SMV isolate N, a G2 isolate, induces a systemic HR (SHR), rather than HR [25]. SHR might be a consequence of delayed occurrence of HR-associated events [26]. Infection of Rsv1-genotype soybean by SMV strain G7, however, triggers a lethal SHR (LSHR), likely due to rapid progression of SHR [27], [28].
In the past several years, many naturally and experimentally evolved SMV resistance-breaking isolates (all the three resistance loci) were documented [3], [8], [11], [27], [29], [30]. As there are only three naturally-occurring resistant sources deployed for soybean breeding programs worldwide, concerns have been raised about the durability of these resistance genes [13]. The great majority of recent studies have focused on the genetic basis of SMV virulence on resistance mediated by each individual resistance gene. Through comparative genomic analyses, virulence proteins responsible for breaking down resistance have been mapped to HC-Pro, P3 and CI depending on the type of resistance [3], [11], [28], [31]–[33].
In this report, SMV strains G7 and G2 (isolates L and LRB) were used as parental viruses to study the gain and loss of SMV virulence on all three resistance loci. SMV chimeras and mutants were created by partial genome swapping and point mutagenesis and then assessed for virulence on soybean cultivars containing different resistance genes. We found that multiple viral proteins participated in virulence on each of the three resistance loci. Based on this study, we suggest that incorporation of all three resistance genes into a soybean cultivar through gene pyramiding may provide durable resistance to SMV.
Results
Virulence on Rsv1-, Rsv3- and Rsv4-genotype soybeans is determined by multi-viral proteins
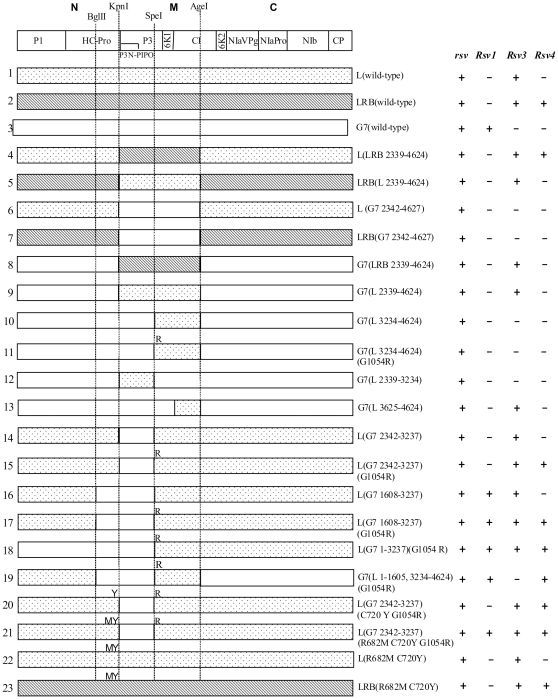
To study genetic determinants of SMV virulence on all three identified resistance loci in soybean, we used SMV strain G7 and two isolates of the G2 strain, L and LRB (Fig. 1). G7 is avirulent on Rsv3- and Rsv4-genotype soybeans and virulent on Rsv1-genotype soybean [12], [22]. In contrast, L infects Rsv3- but not Rsv1- and Rsv4-genotype soybeans, and LRB, a naturally evolved isolate of L differentiated from L by overcoming Rsv4-mediated resistance [8]. The full-length cDNA infectious clones derived from these three SMVs shared pathogenicity similar to their respective parental viruses (Fig. 2). Since P3 is a virulence determinant for Rsv1- and Rsv4-resistance [11], [28] and CI is critical for Rsv3-resistance [3], [32], we first constructed hybrid SMVs by swapping the genomic fragment M (encoding the C-terminal 30 amino acids of HC-Pro, P3, P3-PIPO, 6K1 and the N-terminal two thirds of CI) (Fig. 2). The resulting hybrid SMVs were subjected to pathogenicity assays. Reciprocal exchange of the M fragment of L and LRB did not change their avirulence on Rsv1-genotype and virulence on Rsv3-genotype soybean but resulted in the gain or loss of virulence on Rsv4-genotype soybean, consistent with our published data that P3 of LRB is responsible for breaking down Rsv4-mediated resistance [11]. When the M fragment of L or LRB was replaced with that of G7, the resulting chimeras were avirulent on Rsv1-, Rsv3- and Rsv4-genotype soybeans (Fig. 2). In agreement with previous observations, both the N-terminal P3 (overlapping with P3N-PIPO) and HC-Pro of G7 are required for a G2 isolate to gain virulence on Rsv1-genotype soybean [28], [31] and the CI of G2 is required for G7 to gain virulence on Rsv3-genotype soybean [32]. Chimeric SMVs derived from G7 whose M fragment was substituted with the homologous region of L or LRB lost virulence on Rsv1-genotype soybean, gained virulence on Rsv3-genotype soybean and were avirulent on Rsv4-genotype soybean (Figs. 2 and 3A). The loss of the G7 P3 may account for losing virulence on Rsv1-genotype soybean [28], [32]. The gain of virulence on Rsv3-genotype soybean was not expected as these hybrid SMVs did not contain Rsv3 pathogenetic determinants identified previously, i.e., the C-terminal CI of G7H [3] or both the N- and C-terminal CI of G2 [32]. Since the LRB P3 is responsible for the gain of virulence on Rsv4 soybean [11], it was surprising that G7(LRB 2339-4624), a G7 derivative containing the entire LRB P3, was unable to infect Rsv4-genotype soybean (Figs. 2 and 3A). Thus, it is reasonable to suggest that, in addition to P3, other viral protein(s) or domain(s) of G2 are also required for virulence on Rsv4-conferred resistance. Taken together these results suggest multi-viral proteins constitute virulence determinants for each of the three resistance loci in soybean.
Figure 2. Pathogenicity assays of parental SMV infectious clones L, LRB and G7, chimeric clones and mutants with rsv-, Rsv1-, Rsv3- and Rsv4 -genotype soybeans.
The infectivity of the clones is shown to the right. rsv, Williams 82 (carrying no resistance gene); Rsv1, PI96983; Rsv3, L29; Rsv4, V94-5152; +, positive in ELISA and RT-PCR assays; –, negative in ELISA and RT-PCR assays.
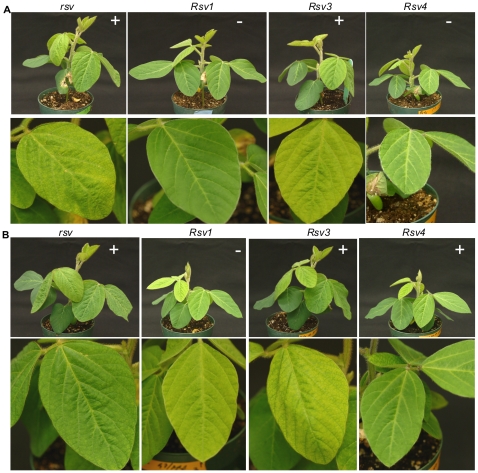
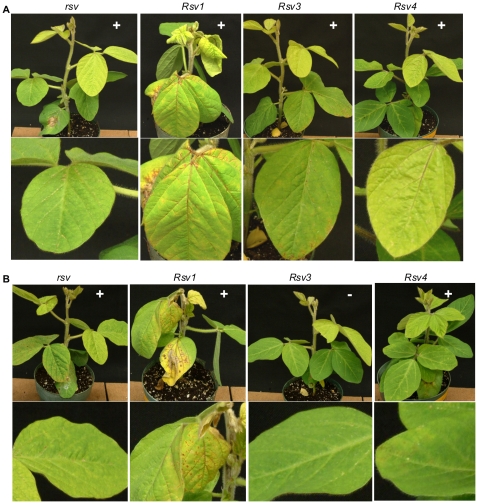
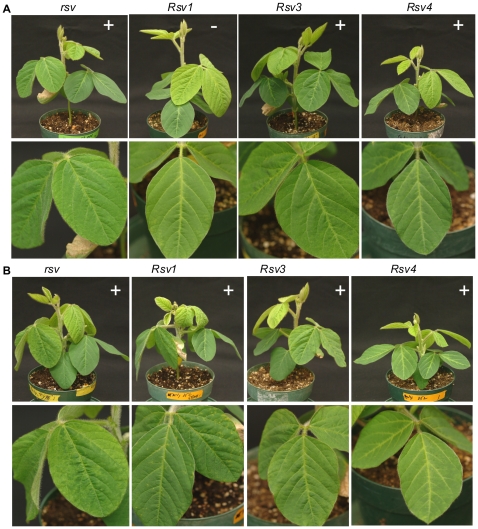
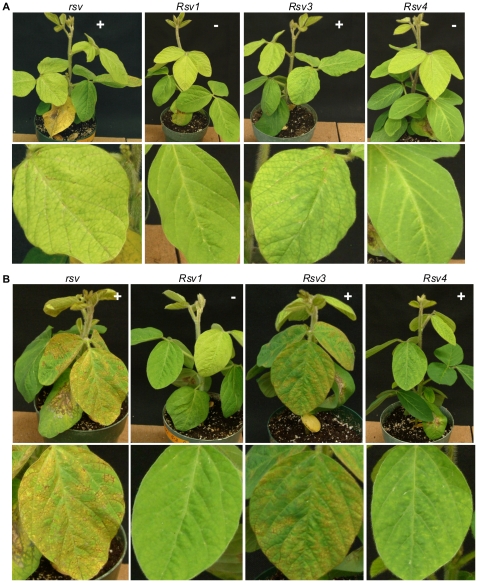
Figure 3. Infectivity and symptoms of soybean inoculated with chimeric SMV clones G7(L 2339-4624) and G7(L 3625-4624).
(A). Inoculated with G7(L 2339-4624). Photos were taken 28 days post inoculation. (B). Inoculated with G7(L 3625-4624). Trifoliate leaves are shown underneath. Phtos were taken 21 days post inoculation. Symptoms are evident on rsv- and Rsv3-genotype soybeans. rsv, Williams 82 (carrying no resistance gene); Rsv1, PI96983; Rsv3, L29; Rsv4, V94-5152; +, positive (ELISA and RT-PCR); –, negative (ELISA and RT-PCR).
P3 is involved in virulence on all three resistance loci and CI is essential for breaking down Rsv1- and Rsv3-resistances
The amino acid sequence of HC-Pro, P3, P3N-PIPO, 6K1 and CI was compared among SMV isolates. The very C-terminal HC-Pro amino-acid sequence (downstream of the KpnI site) consisting of 30 amino acids, is identical among all the SMV isolates analyzed including G2 and G7 isolates (Fig. 4). The 6K1 sequence is also highly conserved among SMV isolates with only one substitution (A to V) concerning two similar amino acids for two isolates (Fig. S1). Therefore, these two regions are unlikely to be virulence determinants on the three resistance loci. To further determine the virulence role of P3 (consisting of the embedded P3N-PIPO) (Fig. 5; Fig. S2) and the N-terminal CI (Figs. S3 and S4), two more hybrid SMVs, G7(L 3234-4624) and G7(L 2339-3234) were created (Fig. 2). Both of them lost virulence on Rsv3-genotype soybean (Fig. 2), indicating neither the N-terminal P3 (including P3N-PIPO) of G2 nor the C-terminal P3/N-terminal CI of G2 was sufficient for virulence on Rsv3-genotype soybean. Thus, both P3 and CI were involved in virulence on Rsv3-genotype soybean. Neither of these two viruses restored virulence on Rsv1-genotype soybean (Fig. 2), indicating the N-terminal P3 of G7 as well as the C-terminal P3/N-terminal CI of G7 was essential for G7 to maintain virulence on Rsv1-genotype soybean. Previously, a single mutation (G1054R), downstream of the KpnI site in the C-terminal P3 of isolate NPL, an L derivative (Fig. 5) was sufficient to make isolate L virulent on Rsv4-genotype soybean [11]. This mutation was introduced into the hybrid virus G7(L 3234-4624). The resulting virus G7(L 3234-4624)(G1054R) was unable to infect Rsv4-genotype soybean or other resistant soybeans (Fig. 2). This result again supports that the G2 P3 must function with other viral determinants including CI (see below) for virulence on Rsv4-resistance. To further clarify the virulence role of the N-terminal CI, a chimeric infectious clone G7(L 3625-4624) was constructed. In comparison with wild-type G7, this recombinant virus lost virulence on Rsv1-genotype soybean but gained infectivity on Rsv3-genotype soybean (Figs. 2 and 3B), indicating an essential role of CI for breaking down both Rsv1- and Rsv3-resistances. The fact that this chimeric virus infected Rsv3-genotype soybean, in comparison with recombinant clones G7(L 3234-4624) and G7(L 2339-4624), confirmed the involvement of P3 in breaking down Rsv3-resistance (Figs. 2 and 3). Taken together these data suggest P3 is involved in virulence on all three resistance loci and CI is essential at least for virulence on Rsv-1 and Rsv3-genotype soybeans.
Figure 4. Amino acid sequence alignment of the SMV HC-Pro protein.
Arrow indicates restriction sites BglII and KpnI which were used for plasmid construction. Numbers are the amino acid positions of the deduced polyprotein encoded by the long open reading frame. As shown, the last 30 amino acid sequence (after KpnI) is identical between G7 and G2 strains. The position numbers of two point mutations in this study are indicated.
Figure 5. Amino acid sequence alignment of the SMV P3 protein.
Restriction site SpeI used for clone construction is shown. * indicates two essential amino acids K and R responsible for breaking down Rsv4-mediated resistance.
The N-terminal P3 (including P3N-PIPO) of G2 is not essential for G2 to maintain virulence on Rsv3- and Rsv4-genotype soybeans and that of G7 is insufficient for G2 to gain virulence on Rsv1-genotype soybean
To test if the N-terminal P3 (including P3N-PIPO) is essential for virulence on Rsv3-genotype soybean, the KpnI-SpeI fragment of the L isolate and its mutant containing mutation G1054R that breaks down Rsv4-resistance [11] was replaced with that of G7 to generate hybrid SMVs L(G7 2342-3237) and L(G7 2342-3237)(G1054R) (Fig. 2). As mentioned above, the sequence of the very C-terminal 30 amino acids of HC-Pro downstream of KpnI was identical among G7, L, LRB and other G7 and G2 isolates (Fig. 4). Therefore, the two chimeric SMVs actually obtained about four-fifths of the P3 from the N terminus (including the entire P3N-PIPO) from G7 (Fig. 5, Fig. S2). Both hybrid SMVs retained their infectivity on Rsv3-genotype soybean (Figs. 2 and 6), implying the N-terminal P3 (including P3N-PIPO) of G2 was not essential for virulence on Rsv3-genotype soybean. All Rsv4-genotype soybean plants were susceptible to L(G7 2342-3237)(G1054R) (Figs. 2 and 6), indicating the N-terminal P3 of G2 was not essential for virulence on Rsv4-resistance. Interestingly, both chimeric SMVs acquiring the N-terminal P3 of G7 were avirulent on Rsv1-genotype soybean (Fig. 6) and all Rsv4-genotype soybeans showed resistance to L(G7 2342-3237) (Fig. 6) except for one plant. Sequencing the virus isolated from this plant revealed three amino-acid mutations, i.e., G1054R, S1804G and K2787R. Further mutation analysis showed that the G1504R mutation rather than S1804 within the 6K2 protein and K2787R in CP was responsible for breaking down Rsv4-resistance (data not shown). Thus, the N-terminal P3 (including P3N-PIPO) of G2 is not essential for virulence on Rsv3- and Rsv4-genotype soybeans and this region of G7 is insufficient for virulence on Rsv1-genotype soybean.
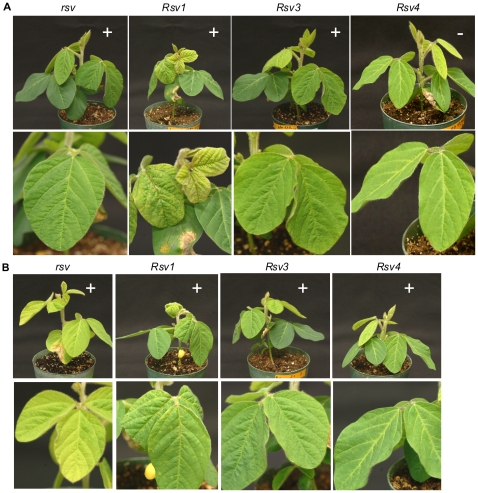
Figure 6. Infectivity and symptoms of soybean inoculated with chimeric SMV clones L(G7 2342-3237) and L(G7 2342-3237)(G1054R).
(A). inoculated with L(G7 2342-3237). (B). Inoculated with L(G7 2342-3237)(G1054R). Trifoliate leaves are shown underneath. Photos were taken 14 days post inoculation. rsv, Williams 82 (carrying no resistance gene); Rsv1, PI96983; Rsv3, L29; Rsv4, V94-5152; +, positive (ELISA and RT-PCR); –, negative (ELISA and RT-PCR).
The C-terminal moiety of HC-Pro and the N-terminal P3 of G7 together are sufficient for G2 to break down Rsv1-resistance
The C-terminal moiety (the BglII and KpnI fragment) of HC-Pro of L(G7 2342-3237) and L(G7 2342-3237)(G1054R) was further replaced with the corresponding region of G7 to generate chimeric SMVs L(G7 1608-3237) and L(G7 1608-3237)(G1054R) (Figs. 2 and 4). Pathogenicity tests showed that L(G7 1608-3237) retained virulence on Rsv3-genotype soybean, remained avirulent on Rsv4-genotype soybean and gained virulence on Rsv1-genotype soybean. L(G7 1608-3237)(G1054R) retained virulence on Rsv3-genotype soybean and gained virulence on Rsv1- and Rsv4-genotype soybeans (Figs. 2 and 7). To test if the N-terminal virus-encoded polyprotein (upstream of BglII) and the C-terminal polyprotein (downstream of AgeI) affect virulence on Rsv1-, Rsv3- and Rsv4-genotype soybeans, L(G7 1608-3237)(G1054R) was used as a parental clone to produce recombinant viruses L(G7 1-3237)(G1054R) and G7(L 1-1605, 3234-4624)(G1054R). These two chimeric viruses retained infectivity on Rsv1- and Rsv4-genotype soybeans (Fig. 8), indicating either N- (upstream of BglII) or C-termini (downstream of AgeI) of the virus-encoded polyprotein between G2 and G7 does not contain avirulent determinant(s) on Rsv1- and Rsv4-resistances. However, replacement of the C-terminal polyprotein (downstream of AgeI) did affected virulence on Rsv3-genotype soybean (Fig. 8B), suggesting the involvement of this region in breaking down Rsv3-resistance. The symptoms in Rsv1-genotype soybean plants induced by L(G7 1608-3237), L(G7 1608-3237)(G1054R), L(G7 1-3237)(G1054R) and G7(L 1-1605, 3234-4624)(G1054R) were typical of LSHR (Figs. 7, 8 and 9), similar to those induced by G7 (Fig. 9). These results demonstrate that the C-terminal moiety of HC-Pro of G7 and the N-terminal P3 of G7 co-determine virulence on Rsv1-genotype soybean but the corresponding regions of G2 are not required for virulence on Rsv3- and Rsv4-genotype soybeans. As mentioned earlier, L(G7 2342-4627) or LRB(G7 2342-4624) lost virulence on Rsv3- and Rsv4-genotype soybeans and the G2 P3 must function with other viral determinant(s) to maintain virulence on Rsv3- and Rsv4-genotype soybeans. Taken together, these data suggest the G2 CI is critical for virulence on Rsv3-genotype soybean and likely on Rsv4-genotype soybean as well. Indeed, SMVs, i.e., G7(L3234-4624)(G1054R) and G7(L 2339-3234) consisting of CI hybrids (G2/G7) or the entire G7 CI abolished virulence on Rsv3- and Rsv4-genotype soybeans (Fig. 2).
Figure 7. Infectivity and symptoms of soybean inoculated with chimeric SMV clones L(G7 1608-3237) and L(G7 1608-3237)(G1054R).
(A). Inoculated with L(G7 1608-3237). (B). Inoculated with L(G7 1608-3237)(G1054R). Trifoliate leaves are shown underneath. Photos were taken 14 days post inoculation. rsv, Williams 82 (carrying no resistance gene); Rsv1, PI96983; Rsv3, L29; Rsv4, V94-5152; +, positive (ELISA and RT-PCR); –, negative (ELISA and RT-PCR).
Figure 8. Infectivity and symptoms of soybean inoculated with chimeric SMV clones L(G7 1-3237) G1054R and G7(L1-1605)L(3234-4624) G1054R.
(A). Inoculated with L(G7 1-3237) G1054R. (B). Inoculated with G7(L1-1605)L(3234-4624) G1054R. Trifoliate leaves are shown underneath. Photos were taken 3 weeks post inoculation. rsv, Williams 82 (carrying no resistance gene); Rsv1, PI96983; Rsv3, L29; Rsv4, V94-5152; +, positive (ELISA and RT-PCR); –, negative (ELISA and RT-PCR).
Figure 9. Symptoms of Rsv1-genotype soybean inoculated with SMV clones G7, L(G7 1608-3237), L(G7 1608-3237)(G1054R) and L(G7 2342-3237)(R682M, C720Y, G1054R).
Dead leaf tissues resulting from lethal systemic hypersensitive response (LSHR) were evident on Rsv1-genotype soybean inoculated with all four SMVs. Photos were taken 42 days post inoculation.
Alignment of the C-terminal moiety of HC-Pro revealed a difference of five amino acids between L and G7. Point mutagenesis was carried out to determine if any mutations are sufficient for L(G7 2342-3237)(G1054R) to gain virulence on Rsv1-genotype soybean. Single (C720Y) and double mutations (R682M, C720Y) were introduced into L(G7 2342-3237)(G1054R) to produce SMVs L(G7 2342-3237)(C720Y G1054R) and L(G7 2342-3237)(R682M C720Y G1054R). L(G7 2342-3237)(C720Y G1054R) showed no symptoms or infection on Rsv1-genotype soybean but retained virulence on Rsv3- and Rsv4-genotype soybeans (Fig. 10), similar to L (G7 2342-3237)(G1054R). L(G7 2342-3237)(R682M C720Y G1054R), however, was not only virulent on Rsv3- and Rsv4-genotype soybeans as the case for L(G7 2342-3237)(G1054R), but also gained virulence on Rsv1-genotype soybean (Fig. 10). Similar to G7, L(G7 1608-3237) or L(G7 1608-3237)(G1054R), L(G7 2342-3237)(R682M C720Y G1054R) induced typical LSHR 16 or more days post inoculation (dpi) on Rsv1-genotype soybean (Fig. 9). To further test if these two mutations are sufficient for L(wild-type) and LRB9wild-type) to break down Rsv1-resistance, L and LRB mutants, L(R682M C720Y) and LRB (R682M C720Y), were generated. These two mutants failed to infect Rsv1-genotype soybean (Fig. 11). Taken together these data suggest that simultaneous mutations at essential residues of HC-Pro and P3 of G2 are required for the gain of virulence on Rsv1-genotype soybean.
Figure 10. Infectivity and symptoms of soybean inoculated with chimeric SMV clones L(G7 2342-3237)(C720Y) and L(G7 2342-3237)(R682M, C720Y).
(A). Inoculated with L(G7 2342-3237)(C720Y). (B). Inoculated with L(G7 2342-3237)(R682M, C720Y). Trifoliate leaves are shown underneath. Photos were taken 14 days post inoculation. rsv, Williams 82 (carrying no resistance gene); Rsv1, PI96983; Rsv3, L29; Rsv4, V94-5152; +, positive (ELISA and RT-PCR); –, negative (ELISA and RT-PCR).
Figure 11. Infectivity and symptoms of soybean inoculated with chimeric SMV clones L(R682M C720Y) and LRB (R682M C720Y).
(A). Inoculated with L(R682M C720Y). (B). Inoculated with LRB (R682M C720Y). Trifoliate leaves are shown underneath. Photos were taken 3 weeks post inoculation. rsv, Williams 82 (carrying no resistance gene); Rsv1, PI96983; Rsv3, L29; Rsv4, V94-5152; +, positive (ELISA and RT-PCR); –, negative (ELISA and RT-PCR).
Discussion
In this study, three SMV isolates of two strains with different responses on Rsv1-, Rsv3- and Rsv4-genotype soybeans were employed to study virulence determinants required for virulence on soybean genotypes carrying different resistance genes. Through comparative genomic analyses, we have provided evidence that P3 is involved in virulence on these resistant cultivars. In recent studies, the potyviral P3 protein (not the embedded PIPO) has been shown as a major determinant for the loss and gain of virulence in a number of potyviral pathosystems. For instance, a mutation (A1047V) in the C-terminus (downstream of PIPO) of the Tobacco etch virus (TEV) P3 protein was found to be associated with the adaptation of TEV to Arabidopsis thaliana [34]. Multiple determinants in the N-terminal P3 (upstream of PIPO) of Pea seed-borne mosaic virus were identified to determine the gain and loss of virulence in Pisum sativum carrying the recessive resistance gene sbm-2 [35]. The virulence determinants of Turnip mosaic virus (TuMV) on Brassica napus cultivars carrying resistance genes TuRB03 or TuRB04 were mapped to both the N- and C-termini of P3 (outside PIPO) [36], [37]. The N-terminal P3 (upstream of PIPO) of TuMV strain TuR1 was also shown to determine the systemic necrosis in Arabidopsis ecotype Ler carrying the dominant gene TuNI through the protein-protein interaction between TuNI and P3 [38]. In the case of SMV, the N-terminal P3 (before PIPO) of G7 was shown to be essential for its virulence on Rsv1-genotype soybean [39]. When the P3 or the N-terminal moiety of P3 of G7 was replaced with the corresponding region from isolates N, L and LRB (three avirulent G2 isolates), the resulting SMVs lost virulence on Rsv1-genotype soybean [39]. We have also reported that the C-terminal P3 (after PIPO) of isolate LRB, a naturally evolved G2 isolate, is responsible for breaking down Rsv4-mediated resistance [11]. A point mutation in the C-terminal P3 (after PIPO) in the isolate L (Q1033K or G1054R) [11] or recombinant SMVs L(G7 2342-3237) (G1054R) and L(G7 1608-3237)(G1054R) in this study is sufficient to alter pathogenicity on Rsv4-genotype soybean. In this report, we have shown that a recombinant G7 containing the entire P3 as well as the N-terminal CI of L, a G2 isolate, gained virulence on Rsv3-genotype soybean (Figs. 2 and 3A). When the N-terminal P3 was reversely swapped back to the G7 type, the resulting recombinant SMV G7(L 3234-4624) lost the ability to infect Rsv3-genotype soybean. Such virulence on Rsv3-genotype soybean could be restored by the chimeric virus G7(L 3625-4624) where the entire P3 was from G7 (Figs. 2 and 3B). These data suggest P3 is not only a virulence determinant on Rsv1- and Rsv4-genotype soybeans [11], [39] but is also required for virulence on Rsv3-genotype soybean. Thus, P3 is a virulence determinant for all three resistance genes in soybean.
In recent studies, the CI protein of G7H and G5H has been shown to be a virulence and avirulence determinant on Rsv3-genotype soybean, respectively [3]. A single amino acid change in the C-terminal of CI was sufficient for G5H to gain virulence on Rsv3-genotype soybean. For G2 and G7 pathotypes, both the N and C termini of CI of the N isolate were required for G7 to break down Rsv3-resistance [32]. In this report, we not only confirmed that CI is involved in virulence on Rsv3-genotype soybean but also provided evidence that CI is essential in virulence on Rsv1-soybean (Figs. 2 and 3B). Previously, CI was suggested to be a virulence determinant for several other potyviruses. For instance, the CI protein of TuMV was shown to be responsible for overcoming TuRB01- and TuRB05-mediated resistance in Brassica napus [40], [41]. In the pepper-Lettuce mosaic virus (LMV) pathosystem, mutations in the C-terminal region of CI granted an avirulent LMV isolate the ability to infect lettuce carrying either recessive resistance genes mol1 or mol2 [41]. Interestingly, a hybrid CI significantly increased the capacity of a Potato virus Y (PVY) isolate to break down pvr2-mediated resistance in pepper [42].
In addition to the N-terminal P3, we show that the C-terminal moiety of HC-Pro of G7 was required for L, a G2 isolate, to gain virulence on Rsv1-genotype soybean and that a point mutation was essential. Our data are consistent with recent findings that concurrent mutations of HC-Pro and P3 are required for G2 (the N isolate) to gain virulence on Rsv1 -genotype soybean [31], [43]. The HC-Pro of Potato virus Y (PVY) has been shown to act as a virulence determinant on potato (Solanum tuberosum) and S. sparsipilum containing PVY resistance genes Nc tbr and Nc spl, respectively [2]. In several other studies, HC-Pro was found to be involved in potyvirus symptom development [44]–[48].
Multi-viral proteins including HC-Pro, P3 and CI are responsible for potyvirus virulence or avirulence on Rsv1-, Rsv3- and Rsv4-genotype soybeans ([3], [11], [28], [31]–[33], this study) and other R-genotype plant species [2], [35]–[38], [40]–[42]. Whether these viral proteins function independently or cooperatively as virulence determinants remains to be elucidated. The function of the potyviral P3 protein is poorly characterized [11]. In addition to its function as a virulence determinant, it has been assumed to play a role in several steps of the potyviral infection cycle such as virus replication, systemic infection, pathogenicity and movement [11], [31], [37], [43], [49]–[52]. Both CI and HC-Pro are multifunctional proteins [15], [43], [53]. CI, having RNA binding, RNA helicase and ATPase activities, has been shown to be essential in virus intra- and intercellular movement and virus replication [54]–[56]. HC-Pro has auto-catalytic proteinase and RNA silencing suppression activities and participates in polyprotein processing, aphid transmission, long-distance movement and viral genome amplification [57]–[59]. Since HC-Pro, P3, 6K1 and CI result from catalytic processing of the large potyviral polyprotein, it is possible that an intermediate precursor protein containing these proteins acts as an elicitor in Rsv1-, Rsv3- and Rsv4-genotype soybeans. As HC-Pro is a cysteine proteinase that efficiently autocleaves the junction between itself and P3, it is more likely that protein complexes formed through protein-protein interactions rather than a single polypeptide play the elicitor role. Indeed, the CI has been shown to bind to other viral proteins including HC-Pro [57], P3 [58] and P3N-PIPO [59]. The essential components of the protein complex may vary depending on the type of resistance. For instance, HC-Pro and P3 are essential for Rsv1-resistance [31], [43] whereas P3 and CI are required for Rsv3- and Rsv4-resistance (this study). As suggested previously [60], overcoming R-mediated resistance may be the outcome of a temporal race of the replication and intercellular movement of the invading virus against the host defense response. Therefore, it is also possible that HC-Pro, P3 and CI may operate separately or as a complex with distinct roles for each of them: one as an elicitor to interact with the R product and the other(s) as a conditioner to regulate virus replication and intercellular movement. This may explain why the absence of the avirulent elicitor is insufficient and a complementary virulence factor is required for the gain of virulence ([31], [43], this study). The functional roles of these viral proteins are beyond current understanding.
In this study, we show that multiple viral proteins are involved in virulence on the three resistance loci and simultaneous mutations at essential positions of different viral proteins are required for an avirulent SMV to gain virulence on all the resistance loci. In nature, spontaneous mutations in RNA viruses occur during virus replication [61]. It is estimated that the mis-incorporation rates catalyzed by the viral RNA-dependent RNA polymerase (RdRp) are in the range of 10−4 to 10−6 per nucleotide each generation [9], [62], [63]. For potyviruses such as SMV, frequent identifications of naturally occurring and lab-experimentally evolved resistance-breaking isolates strongly indicate that the mutation rate introduced by the potyviral RdRp can generate a viral population with adequate genetic variability to break down resistance conferred by a single R gene in a short time period [8], [11], [25], [31], [43], [48], [64]. These resistance-breaking isolates often require just a single point-mutation [11], [48]. However, as shown in this study, overcoming resistance conferred by two resistance genes, i.e., Rsv1 and Rsv4, requires several concurrent mutations at essential residues of HC-Pro and P3 in a G2 isolate. Very likely, for a G7 isolate to gain virulence on Rsv3- and Rsv4-genotype soybeans would involve simultaneous mutations on HC-Pro, P3 and CI. The likelihood for an avirulent isolate to have such concurrent mutations is statistically extremely low. Based on this analysis, incorporation of all three resistance genes into a soybean cultivar through gene pyramiding may provide durable resistance to SMV. As such a resistance-pyramided soybean cultivar exerts high selection pressure that may lead the occurrence of a super strain of SMV [65], developing novel genetic resistance to SMV and related viruses remains a long-term challenge for soybean pathologists and breeders.
Materials and Methods
SMV isolates, soybean cultivars, inoculation and virus detection
Plasmids containing infectious full-length cDNA clones of SMV isolates L (a G2 isolate), LRB (an L-like naturally evolved Rsv4-resistance breaking isolate) and G7 were used as parental viruses [11], [27] to generate G7/G2 chimeric SMVs. Soybean (Glycine max) susceptible cultivar Williams 82 (rsv) and resistant cultivars PI 96983 (Rsv1), L-29 (Rsv3) and V94-5152 (Rsv4) were grown in a growth chamber with 16-hour light at 22 °C and 8-hour dark at 18 °C. All soybean seeds used in this study were harvested from virus-free plants. Biolistic bombardment of plasmid DNA of parental and chimeric SMVs was initially used to establish SMV infections in Williams 82 [8], [11]. The resulting infected leaf tissues were used as inoculums for pathogenicity tests in soybean cultivars of different genotypes by mechanical inoculation. Virus detection was carried out by double-antibody sandwich enzyme-linked immunosorbent assay (DAS ELISA) and reverse-transcription polymerase chain reaction (RT-PCR) as described previously [8], [11].
Construction of artificial chimeras between isolates L, LRB and G7 and pathogenicity test
Chimeric SMVs were constructed by using restriction sites indicated (Fig. 1) to swap genomic regions among isolates L, LR and G7 (Fig. 2). For cloning convenience, the full-length cDNA was divided into three fragments designated N, M and C (Fig. 1). Standard DNA manipulation protocols were used for restriction digestions and ligations. DH5α cells (Invitrogen, Burlington, Ontario, Canada) were used for transformation. Plasmid DNA was purified using QIAfilter plasmid midi kit (Qiagen, Toronto, Canada). The purified plasmid was sequenced to confirm identity of the swapped fragment. For pathogenicity tests, each construct was biolistically introduced into three 2-week-old Williams 82 (rsv) seedlings. Infected leaves were harvested 15 days post-bombardment and stored in a –80°C freezer for subsequent experiments. The pathogenicity test was repeated three times and each time, four 2-week-old Williams 82 seedlings and 12 Rsv soybean plants (four for each of three resistant cultivars described above) were mechanically inoculated as described previously [8], [11].
Mutagenesis of HC-Pro
The BglII-KpnI fragment of the L infectious cDNA plasmid was PCR amplified using two pairs of primers containing mutations wherever necessary to generate two PCR products. The amplicons were gel-purified with a QIAquick gel extraction kit (Qiagen, Toronto, Canada) and the purified PCR-products were used as templates to produce a single PCR product with a pair of primers containing BglII and KpnI restriction sites. The PCR products containing a single mutation (C720Y) or double mutations (R682M, C720Y) were digested with BglII and KpnI and cloned into L(G7 2342-3237)(G1054R) (Fig. 2). The resulting plasmids L(G7 2342-3237)(C720Y G1054R) and L(G7 2342-3237) (R682M, C720Y G1054R) were sequenced to confirm mutation(s) and then used for pathogenicity tests.
DAS ELISA, RNA isolation, RT-PCR and sequencing
Virus detection was carried out by DAS ELISA and RT-PCR as described [8], [11]. Approximately 100 mg of leaf from each soybean seedling was sampled at 14, 28 and 42 dpi into an eppendorf tube and flash frozen in liquid nitrogen. The extraction buffer and other ELISA buffers were prepared as described for an SMV DAS ELISA kit (Agdia, Elkhart, Indiana, USA) using alkaline phosphatase conjugated antibodies. For SMV detection by RT-PCR, total RNA was extracted [8] and RT-PCR was performed using two sets of primers (one for SMV and the other for EF 1a serving as a control). SMV mutants were verified by sequencing either PCR products or cloned cDNA as described [8], [11].
Isolation and sequencing of Rsv4-resistance breaking isolate
All recombinant SMVs were maintained in Williams 82 as an inoculum source. After three passages, an Rsv4-genotype soybean seedling inoculated with L (G7 2342-3237) showed typical SMV symptoms and was positive in ELISA and RT-PCR assays. The virus was purified, cloned and completely sequenced essentially as described [8], [11]. The complete genome sequence of this virus was deposited into GenBank with accession number JN416770.
Supporting Information
Amino acid sequence alignment of the SMV 6K1 protein.
(TIF)
Amino acid sequence alignment of the SMV P3N-PIPO protein. Translational frameshift/slippage is indicated by an arrow.
(TIF)
Amino acid sequence alignment of the N-terminal moiety of the SMV CI protein. Restriction site AgeI is indicated.
(TIF)
Amino acid sequence alignment of the C-terminal moiety of the SMV CI protein.
(TIF)
Acknowledgments
The authors are very grateful to Jamie McNeil (Agriculture and Agi-Food Canada, AAFC) for expert technical assistance, Ida van Grinsven (AAFC) for DNA sequencing, and Alex Molnar (AAFC) for photography.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported by AAFC Canadian Crop Genomics Initiative, a research grant from Grain Farmers of Ontario, and a discover grant from the Natural Sciences and Engineering Research Council of Canada. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Dodds PN. Genome evolution in plant pathogens. Science. 2010;330:1486–1487. doi: 10.1126/science.1200245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moury B, Caromel B, Johansen E, Simon V, Chauvin L, et al. The helper component proteinase cistron of Potati virus Y induces hypersensitivity and resistance in potato genotypes carrying dominant resistance genes on chromosome IV. Mol Plant–Microbe Interact. 2011;24:787–797. doi: 10.1094/MPMI-10-10-0246. [DOI] [PubMed] [Google Scholar]
- 3.Seo J-K, Lee S-H, Kim K-H. Strain-specific cylindrical inclusion protein of Soybean mosaic virus elicits extreme resistance and a lethal systemic hypersensitive response in two resistant soybean cultivars. Mol Plant-Microbe Interact. 2009;22:1152–1159. doi: 10.1094/MPMI-22-9-1151. [DOI] [PubMed] [Google Scholar]
- 4.Bonas U, Lahaye T. Plant disease resistance triggered by pathogen-derived molecules: Refined models of specific recognition. Curr Opin Microbiol. 2002;5:44–50. doi: 10.1016/s1369-5274(02)00284-9. [DOI] [PubMed] [Google Scholar]
- 5.Kang BC, Yeam I, Jahn MM. Genetics of plant virus resistance. Annu Rev Phytopathol. 2005;43:581–621. doi: 10.1146/annurev.phyto.43.011205.141140. [DOI] [PubMed] [Google Scholar]
- 6.Soosaar JL, Burch-Smith TM, Dinesh-Kumar SP. Mechanisms of plant resistance to viruses. Nat Rev Microbiol. 2005;3:789–798. doi: 10.1038/nrmicro1239. [DOI] [PubMed] [Google Scholar]
- 7.Bendahmane A, Kanyuka K, Baulcombe DC. The Rx gene from potato controls separate virus resistance and cell death responses. Plant Cell. 1999;11:781–792. doi: 10.1105/tpc.11.5.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gagarinova AG, Babu MR, Poysa V, Hill JH, Wang A. Identification and molecular characterization of two naturally occurring Soybean mosaic virus isolates that are closely related but differ in their ability to overcome Rsv4 resistance. Virus Res. 2008;138:50–56. doi: 10.1016/j.virusres.2008.08.010. [DOI] [PubMed] [Google Scholar]
- 9.Tromas N, Elena SF. The rate and spectrum of spontaneous mutations in plant RNA virus. Genetics. 2010;185:983–989. doi: 10.1534/genetics.110.115915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hajimorad MR, Wen RH, Eggenberger AL, Hill JH, Saghai Maroof MA. Experimental adaptation of an RNA virus mimics natural evolution. J Virol. 2011;85:2557–2564. doi: 10.1128/JVI.01935-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chowda-Reddy RV, Sun H, Chen H, Poysa V, Ling H, et al. Mutations in the P3 protein of soybean mosaic virus G2 isolates determine virulence on Rsv4-genotype soybean. Mol Plant-Microbe Interact. 2011;24:37–43. doi: 10.1094/MPMI-07-10-0158. [DOI] [PubMed] [Google Scholar]
- 12.Gunduz I, Buss GR, Ma G, Chen P, Tolin SA. Genetic and phenotypic analysis of Soybean mosaic virus resistance in PI 88788 soybean. Phytopathol. 2004;94:687–692. doi: 10.1094/PHYTO.2004.94.7.687. [DOI] [PubMed] [Google Scholar]
- 13.Cui X, Chen X, Wang A. Detection, understanding and control of Soybean mosaic virus. In: Sudarić A, editor. Soybean – Molecular Aspects of Breeding. Rijeka, Croatia: Intech; 2011. pp. 335–354. [Google Scholar]
- 14.Chung BY, Miller WA, Atkins JF, Firth AE. An overlapping essential gene in the Potyviridae. Proc Natl Acad Sci USA. 2008;105:5897–5902. doi: 10.1073/pnas.0800468105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Urcuqui-Inchima S, Haenni AL, Bernardi F. Potyvirus proteins: a wealth of functions. Virus Res. 2001;74:157–175. doi: 10.1016/s0168-1702(01)00220-9. [DOI] [PubMed] [Google Scholar]
- 16.Cho EK, Goodman RM. Strains of soybean mosaic virus: Classification based on virulence in resistant soybean cultivars. Phytopathol. 1979;69:467–470. [Google Scholar]
- 17.Gunduz I, Buss GR, Chen P, Tolin SA. Characterization of SMV resistance genes in Tousan 140 and Hourei soybean. Crop Sci. 2002;42:90–95. doi: 10.2135/cropsci2002.9000. [DOI] [PubMed] [Google Scholar]
- 18.Hayes AJ, Ma G, Buss GR, Saghai Maroof MA. Molecular marker mapping of Rsv4, a gene conferring resistance to all known strains of Soybean mosaic virus. Crop Sci. 2000;40:1434–437. [Google Scholar]
- 19.Liao L, Chen P, Buss GR, Yang Q, Tolin SA. Inheritance and allelism of resistance to Soybean mosaic virus in Zao 18 soybean from China. J Hered. 2002;93:447–452. doi: 10.1093/jhered/93.6.447. [DOI] [PubMed] [Google Scholar]
- 20.Zheng C, Chen P, Gergerich R. Characterization of resistance to Soybean mosaic virus in diverse soybean germplasm. Crop. 2005;Sci.45:2503–2509. [Google Scholar]
- 21.Gore MA, Hayes AJ, Jeong SC, Yu YG, Buss GR, et al. Mapping tightly linked genes controlling potyvirus infection at the Rsv1 and Rpv1 region in soybean. Genome. 2002;45:592–599. doi: 10.1139/g02-009. [DOI] [PubMed] [Google Scholar]
- 22.Buss GR, Ma G, Kristipati S, Chen P, Tolin SA. A new allele at the Rsv3 locus for resistance to Soybean mosaic virus. In: Kauffman HE, editor. Proc World Soybean Res Conf, Chicago, 4–7 Aug. 1999. VI. Champaign, IL: Superior Printing; 1999. 490 [Google Scholar]
- 23.Jeong SC, Kristipati S, Hayes AJ, Maughan PJ, Noffsinger SL, et al. Genetic and sequence analysis of markers tightly linked to the Soybean mosaic virus resistance gene, Rsv3. . Crop Sci. 2002;42:265–270. [PubMed] [Google Scholar]
- 24.Saghai Maroof MA, Tucker DM, Skoneczka JA, Bowman BC, Tripathy S, et al. Fine mapping and candidate gene discovery of the Soybean mosaic virus resistance gene, Rsv4. . Plant Genome. 2010;3:14–22. [Google Scholar]
- 25.Hajimorad MR, Hill JH. Rsv1-mediated resistance against Soybean mosaic virus-N is hypersensitive response-independent at inoculation site, but has the potential to initiate a hypersensitive response-like mechanism. Mol Plant-Microbe Interact. 2001;14:587–598. doi: 10.1094/MPMI.2001.14.5.587. [DOI] [PubMed] [Google Scholar]
- 26.Dinesh-Kumar SP, Tham WH, Baker BJ. Structure-function analysis of the Tobacco mosaic virus resistance gene N. Proc Natl Acad Sci USA. 2000;97:14789–14794. doi: 10.1073/pnas.97.26.14789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hajimorad MR, Eggenberger AL, Hill JH. Evolution of Soybean mosaic virus-G7 molecularly cloned genome in Rsv1-genotype soybean results in emergence of a mutant capable of evading Rsv1-mediated recognition. Virology. 2003;314:497–509. doi: 10.1016/s0042-6822(03)00456-2. [DOI] [PubMed] [Google Scholar]
- 28.Hajimorad MR, Eggenberger AL, Hill JH. Loss and gain of elicitor function of Soybean mosaic virus G7 provoking Rsv1-mediated lethal systemic hypersensitive response maps to P3. J Virol. 2005;79:1215–1222. doi: 10.1128/JVI.79.2.1215-1222.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim YH, Kim OS, Lee BC, Moon JK, Lee SC, et al. G7H, a new Soybean mosaic virus strain: its virulence and nucleotide sequence of CI gene. Plant Dis. 2003;87:1372–1375. doi: 10.1094/PDIS.2003.87.11.1372. [DOI] [PubMed] [Google Scholar]
- 30.Choi BK, Koo JM, Ahn HJ, Yum HJ, Choi CW, et al. Emergence of Rsv-resistance breaking Soybean mosaic virus isolates from Korean soybean cultivars. Virus Res. 2005;112:42–51. doi: 10.1016/j.virusres.2005.03.020. [DOI] [PubMed] [Google Scholar]
- 31.Eggenberger AL, Hajimorad MR, Hill JH. Gain of virulence on Rsv1-genotype soybean by an avirulent Soybean mosaic virus requires concurrent mutations in both P3 and HC-Pro. Mol Plant-Microbe Interact. 2008;21:931–936. doi: 10.1094/MPMI-21-7-0931. [DOI] [PubMed] [Google Scholar]
- 32.Zhang C, Hajimorad MR, Eggenberger AL, Tsang S, Whitham SA, et al. Cytoplasmic inclusion cistron of Soybean mosaic virus serves as a virulence determinant on Rsv3-genotype soybean and a symptom determinant. Virology. 2009;391:240–248. doi: 10.1016/j.virol.2009.06.020. [DOI] [PubMed] [Google Scholar]
- 33.Wen RH, Saghai Maroof MA, Hajimorad MR. Amino acid changes in P3, and not the overlapping pipo-encoded protein, determine virulence of Soybean mosaic virus on functionally immune Rsv1-genotype soybean. Mol Plant Pathol. 2011;12:799–807. doi: 10.1111/j.1364-3703.2011.00714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Agudelo-Romero P, Carbonell P, Perez-Amador MA, Elena SF. Virus adaptation by manipulation of host's gene expression. PLoS ONE. 2008;3:e2397. doi: 10.1371/journal.pone.0002397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hjulsager CK, Olsen BS, Jensen DMK, Cordea MI, Krath BN, et al. Multiple determinants in the coding region of Pea seed-borne mosaic virus P3 are involved in virulence against sbm-2 resistance. Virology. 2006;355:52–61. doi: 10.1016/j.virol.2006.07.016. [DOI] [PubMed] [Google Scholar]
- 36.Jenner CE, Tomimura K, Ohshima K, Hughes SL, Walsh JA. Mutations in Turnip mosaic virus P3 and cylindrical inclusion proteins are separately required to overcome two Brassica napus resistance genes. Virology. 2002;300:50–59. doi: 10.1006/viro.2002.1519. [DOI] [PubMed] [Google Scholar]
- 37.Jenner CE, Wang X, Tomimura K, Ohshima K, Ponz F, et al. The dual role of the potyvirus P3 protein of Turnip mosaic virus as a symptom and avirulence determinant in brassicas. Mol Plant-Microbe Interact. 2003;16:777–784. doi: 10.1094/MPMI.2003.16.9.777. [DOI] [PubMed] [Google Scholar]
- 38.Kim BM, Suehiro N, Natsuaki T, Inukai T, Masuta C. The P3 protein of Turnip mosaic virus can alone induce hypersensitive response-like cell death in Arabidopsis thaliana carrying TuNI. Mol Plant-Microbe Interact. 2010;23:144–152. doi: 10.1094/MPMI-23-2-0144. [DOI] [PubMed] [Google Scholar]
- 39.Hajimorad MR, Eggenberger AL, Hill JH. Strain-specific P3 of Soybean mosaic virus elicits Rsv1-mediated extreme resistance, but absence of P3 elicitor function alone is insufficient for virulence on Rsv1-genotype soybean. Virology. 2006;345:156–166. doi: 10.1016/j.virol.2005.09.055. [DOI] [PubMed] [Google Scholar]
- 40.Jenner CE, Sanchez F, Nettleship SB, Foster GD, Ponz F, et al. The cylindrical inclusion gene of Turnip mosaic virus encodes a pathogenic determinant to the Brassica resistance gene TuRB01. Mol Plant–Microbe Interact. 2000;13:1102–1108. doi: 10.1094/MPMI.2000.13.10.1102. [DOI] [PubMed] [Google Scholar]
- 41.Abdul-Razzak A, Guiraud T, Peypelut M, Water J, Houvenaghel MC, et al. Involvement of the cylindrical inclusion (CI) protein in the overcoming of an eIF4E-mediated resistance against Lettuce mosaic potyvirus. Mol Plant Pathol. 2009;10:109–113. doi: 10.1111/j.1364-3703.2008.00513.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Montarry J, Doumayrou, Simon V, Moury B. Genetic background matters: a plant-virus gene-for-gene interaction is strongly influenced by genetic contexts. Mol Plant Pathol. 2011;12:911–920. doi: 10.1111/j.1364-3703.2011.00724.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hajimorad MR, Eggenberger AL, Hill JH. Adaptation of Soybean mosaic virus avirulent chimeras containing P3 sequences from virulent strains to Rsv1-genotype soybeans is mediated by mutations in HC-Pro. Mol Plant-Microbe Interact. 2008;21:937–946. doi: 10.1094/MPMI-21-7-0937. [DOI] [PubMed] [Google Scholar]
- 44.Sáenz P, Salvador B, Simón-Mateo C, Kasschau KD, Carrington JC, et al. Host-specific involvement of the HC protein in the long-distance movement of potyvirus. J Virol. 2002;76:1922–1931. doi: 10.1128/JVI.76.4.1922-1931.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shiboleth YM, Haronsky E, Leibman D, Arazi T, Wassenegger M, et al. The conserved FRNK box in HC-Pro, a plant vira suppressor of gene silencing, is required for small RNA binding and mediates symptom development. J Virol. 2007;81:13135–13148. doi: 10.1128/JVI.01031-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Torres-Barcelo C, Martin S, Daros JA, Elena SF. From hypo- to hypersuppression: effects of amino acid substitution on the RNA-silencing suppressor activity of the tobacco etch potyvirus HC-Pro. Genetics. 2008;180:1039–1049. doi: 10.1534/genetics.108.091363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yambao MLM, Yagihashi H, Sekiguchi T, Sasaki T, Sato M, et al. Point mutations in helper component protease of Clover yellow vein virus are associated with the attenuation of RNA silencing suppression activity and symptom expression in broad bean. Arch Virol. 2008;153:105–115. doi: 10.1007/s00705-007-1073-3. [DOI] [PubMed] [Google Scholar]
- 48.Seo J-K, Sohn S-H, Kim K-H. A single amino acid change in HC-Pro of soybean mosaic virus alters symptom expression in a soybean cultivar carrying Rsv1 and Rsv3. Arch Virol. 2011;156:135–141. doi: 10.1007/s00705-010-0829-3. [DOI] [PubMed] [Google Scholar]
- 49.Klein PG, Klein RR, Rodríguez-Cerezo E, Hunt AG, Shaw JG. Mutational analysis of the tobacco vein mottling virus genome. Virology. 1994;204:759–769. doi: 10.1006/viro.1994.1591. [DOI] [PubMed] [Google Scholar]
- 50.Chu M, Lopez-Moya JJ, Llave-Correas C, Pirone TP. Two separate regions in the genome of Tobacco etch virus contain determinants of the wilting response of Tabasco pepper. Mol Plant-Microbe Interact. 1997;10:472–480. doi: 10.1094/MPMI.1997.10.4.472. [DOI] [PubMed] [Google Scholar]
- 51.Merits A, Guo D, Järvekülg L, Saarma M. Biochemical and genetic evidence for interactions between potato A potyvirus-encoded proteins P1 and P3 and proteins of the putative replication complex. Virology. 1999;263:15–22. doi: 10.1006/viro.1999.9926. [DOI] [PubMed] [Google Scholar]
- 52.Cui X, Wei T, Chowda-Reddy RV, Sun G, Wang A. The Tobacco etch virus P3 protein forms mobile inclusions via the early secretory pathway and traffics along actin microfilaments. Virology. 2010;397:56–63. doi: 10.1016/j.virol.2009.11.015. [DOI] [PubMed] [Google Scholar]
- 53.Ala-Poikela M, Goytia E, Haikonen T, Rajamäki M-L, Valkonen JPT. Helper component proteinase of the genus potyvirus is an interaction partner of translation initiation factor eIF(iso)4E and eIF4E and contains a 4E binding motif. J Virol. 2011;85:6784–6794. doi: 10.1128/JVI.00485-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Carrington JC, Jensen PE, Schaad MC. Genetic evidence for an essential role for potyvirus CI protein in cell-to-cell movement. Plant J. 1998;14:393–400. doi: 10.1046/j.1365-313x.1998.00120.x. [DOI] [PubMed] [Google Scholar]
- 55.Kekarainen T, Savilahti H, Valkonen JPT. Functional genomics on Potato virus A: virus genome-wide map of sites essential for virus propagation. Genome Res. 2002;12:584–594. doi: 10.1101/gr.220702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wei T, Zhang C, Hong J, Xiong R, Kasschau KD, et al. Formation of complexes at plasmodesmata for potyvirus intercellular movement is mediated by the viral protein P3N-PIPO. PLoS Pathogens. 2010;6:e1000962. doi: 10.1371/journal.ppat.1000962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Blanc S, López-Moya J-J, Wang R, García-Lampasona S, Thornbury DW, et al. A specific interaction between coat protein and helper component correlates with aphid transmission of a potyvirus. Virology. 1997;231:141–147. doi: 10.1006/viro.1997.8521. [DOI] [PubMed] [Google Scholar]
- 58.Kasschau KD, Carrington, JC A counter defensive strategy of plant viruses: suppression of posttranscriptional gene silencing. Cell. 1998;95:461–470. doi: 10.1016/s0092-8674(00)81614-1. [DOI] [PubMed] [Google Scholar]
- 59.Kasschau KD, Carrington JC. Long-distance movement and replication maintenance functions correlate with silencing suppression activity of potyviral HC-Pro. Virology. 2001;285:71–81. doi: 10.1006/viro.2001.0901. [DOI] [PubMed] [Google Scholar]
- 60.Culver JN, Lindbeck AGC, Dawson WO. Virus-host interactions: induction of chlorotic and necrotic responses in plants by tobamoviruses. Annu Rev Phytopathol. 1991;29:193–217. [Google Scholar]
- 61.Gacía-Arenal F, Fraile A, Malpica JM. Variation and evolution of plant virus populations. Int Microbiol. 2003;6:225–232. doi: 10.1007/s10123-003-0142-z. [DOI] [PubMed] [Google Scholar]
- 62.Drake JW, Holland JJ. Mutation rates among RNA viruses. Proc Natl Acad Sci USA. 1999;96:13910–13913. doi: 10.1073/pnas.96.24.13910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Malpica JM, Fraile A, Moreno I, Obies CI, Drake JW, et al. The rate and character of spontaneous mutations in an RNA virus. Genetics. 2002;162:1505–1511. doi: 10.1093/genetics/162.4.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Seo J-K, Ohshima K, Lee H-G, Son M, Choi H-S, et al. Molecular variety and genetic structure of the population of Soybean mosaic virus based on the analysis of complete genome sequences. Virology. 2009;393:91–103. doi: 10.1016/j.virol.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 65.Janzac B, Fabre F, Palloix A, Moury B. Constraints on evolution of virus avirulence factors predict the durability of corresponding plant resistances. Mol Plant Pathol. 2009;10:599–610. doi: 10.1111/j.1364-3703.2009.00554.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Amino acid sequence alignment of the SMV 6K1 protein.
(TIF)
Amino acid sequence alignment of the SMV P3N-PIPO protein. Translational frameshift/slippage is indicated by an arrow.
(TIF)
Amino acid sequence alignment of the N-terminal moiety of the SMV CI protein. Restriction site AgeI is indicated.
(TIF)
Amino acid sequence alignment of the C-terminal moiety of the SMV CI protein.
(TIF)