Abstract
Diarrhea is one of the common symptoms that significantly affects quality of life in patients with inflammatory bowel disease (IBD). The clinical manifestation of diarrhea is mainly dependant on the type of IBD and the location, extent and severity of intestinal inflammation. Understanding the pathophysiologic mechanisms of diarrhea in patients with IBD will be beneficial to developing effective treatments for IBD-associated diarrhea. In recent years, modern molecular techniques have been used intensively to dissect the role of the intestinal microbiota, epithelial barrier and the host immune system in the mechanisms of IBD-induced diarrhea. These studies have significantly advanced our knowledge of the mechanisms of IBD-induced diarrhea. In this article, we focus on the new and critical molecular insights into the contributions of the intestinal microbiota, epithelial tight junctions, proinflammatory cytokines and microRNA as potential mechanisms underlying to IBD-induced diarrhea.
Keywords: diarrhea, epithelial barrier, inflammatory bowel diseases, intestinal microbiota, permeability, proinflammatory cytokines, tight junction
Inflammatory bowel diseases (IBDs) are common, chronic inflammatory disorders of the gut with unknown etiology. The incidence of IBDs have greatly increased in developed, as well as developing countries, in recent years [1]. IBD patients have many symptoms, such as abdominal pain, vomiting, diarrhea, rectal bleeding and weight loss, that can result in significant disability, loss of work, poor quality of life and sometimes even surgery and death.
Diarrhea is one of the common symptoms in patients with IBD [2,3]. Approximately 50% of acute flare-ups of Crohn’s disease (CD) and almost 100% of ulcerative colitis (UC) patients experience diarrhea [4]. The clinical manifestations of diarrhea are diverse in individual patients. It is dependent on the type of IBD and the location, extent and severity of inflammation [5,6]. To effectively treat diarrhea in patients with IBD, it is important to define the pathophysiologic mechanisms in individual patients.
The underlying mechanisms of diarrhea associated with IBD are complex [7,8]. The contributions of inflammation and epithelial tight junction permeability to diarrhea in IBD have recently been studied [9–11]. In this article we focus on recent progress and new molecular insights relevant to the mechanisms of diarrhea in IBD.
General pathophysiology of diarrhea in IBD
To maintain the normal physiological function of the GI tract, the intestinal epithelium has finely tuned mechanisms to maintain ionic balance, fluid absorption and secretion [4,7,8]. The various ionic transport proteins/complexes in the apical, basal or basolateral membranes of intestinal epithelial cells are important for maintaining intestinal homeostasis. Na+, K+, H+ and Cl− ion transport proteins are regulated by endogenous mediators (cAMP, cGMP, PGE2, free radicals, histamine, cytokines and Ca2+) and dietary-related factors (bile acids, short-chain fatty acids through different molecular mechanisms [4,7,8]. In certain pathophysiologic conditions, the balance of ionic and fluid exchange is disrupted and fails to maintain the homeostasis required to compensate for the pro-absorptive/antisecretary mechanisms [4,7,8]. This dysfunctional homeostasis results in excessive secretion of Na+ and Cl− ions followed by the release of a large amount of water into the colonic lumen; this phenomenon is manifested as diarrhea which is a common symptom in patients with IBD [4,7].
The overall fluid absorption in the small and large intestines was described in a recent review [7]. The fluid load to the small intestine is about 8 l per day. The small intestine absorbs about 6 l of fluid per day with ileocecal flow being approximately 2 l per day. The large intestine absorbs about 1.8 l of fluid per day. The maximal absorptive capacity of the colon is approximately 4.5–5.0 l. The fluid in the stool is normally less than 0.2 l per day. The net water absorption is directly proportional to net Na+ and Cl− absorption and is driven by osmotic gradients [4,7,8]. Active Na+ and Cl− secretion/absorption is the driving force for the fluid absorption. Thus, it is critical to understand the role of active Na+ absorption and active Cl− secretion in cellular and molecular mechanisms associated with the diarrhea in IBD. It has been reported that there are at least five different Na+ absorptive processes [7]:
Nutrient-stimulated Na+ absorption is the primary mechanism for either glucose- or amino acid-stimulated post-prandial fluid absorption in the small intestine;
Na+–H+ exchange is responsible for the absorption of large derived from biliary and pancreatic secretion amounts of HCO3 in the duodenum and proximal jejunum. This Na+–H+ exchange is inhibited by increased cAMP levels;
Coupled Na+–Cl− absorption in the distal small intestine and proximal colon is the primary pathway for fluid absorption in the interdigestive period, inhibited by increased cAMP levels and intracellular Ca2+;
Short-chain fatty acid-stimulated Na+ absorption is observed in the colon. Short-chain fatty acids are synthesized by colonic bacteria from nonabsorbed carbohydrates;
Aldosterone-sensitive Na+ absorption via the Na+ channel in the distal colon represents a scavenger mechanism for fluid and Na+ retention.
The homeostasis status of ionic balance, fluid absorption and secretion was studied in patients with UC using perfusion and dialysis technology [12–14]. The molecular mechanisms of disturbed electrolyte transport in intestinal inflammation were reviewed by others [4]. The net Na+, Cl− and water absorption was reduced in UC patients without any evidence of net fluid, Na+ or Cl− secretion [12–14]. Further studies using an in vitro flux chamber to measure the net ionic absorption and short-circuit current in colonic mucosa from UC patients also confirmed the above observations [15,16]. The function of the Na+ channel which is responsible for aldosterone-induced Na+ absorption in the epithelial apical membrane in the distal colon of UC patients was impaired [17]. Other studies indicated that both protein and mRNA levels of down-regulated in adenoma (DRA), which is the apical membrane transporter responsible for colonic Cl−–HCO3− exchange, were reduced in the colonic mucosa of patients with UC [18]. These studies suggest that the impairment of apical membrane transport proteins, Na+ channel for Na+ absorption and DRA for Cl− absorption, results in reduced active Na+ and Cl− absorption and diarrhea in UC [18]. These dysfunctions in ion transport were also observed in the colonic mucosa of patients with active CD [13,15].
The importance of the host immune system, intestinal microorganisms (microbiota) and the intestinal barrier in the mechanisms of diarrhea have been investigated in a large number of studies [7,9–11,19,20]. The interactions between microbiota, intestinal barrier and host immune system are complicated during normal physiological status or under inflammatory stimulation [7,9–11,19,20]. These studies indicate that there are many molecular mechanisms responsible for the generation of diarrhea with a wide range of clinical presentation. Thus, understanding the molecular mechanisms of diarrhea is important in identifying novel molecular targets for the successful treatment of patients with IBD.
The intestinal epithelial barrier plays an important role in host defense by serving as a critical fence between invading pathogens and the host immune system. The integrity of the intestinal barrier depends on both healthy epithelial cells and on an intact paracellular pathway, which appears to be the main route for permeation of macromolecules such as endotoxins [21]. This pathway is a complex array of structures that includes tight junctions between gut epithelial cells. Tight junctions function as gates that regulate intestinal permeability [22–25]. These dynamic tight junctions are highly regulated and are able to change their size under various physiological and pathological conditions [26]. During intestinal inflammation, the intestinal barrier is disrupted. This inflammation-induced intestinal barrier dysfunction results in ions and water passively diffusing from the circulation to the intestinal lumen and causes leak flux diarrhea [8,10,11]. Under normal physiological conditions, only a very limited amount of bacterial antigens and macromolecules cross the epithelial fence [10]. However, during inflammation, the intestinal barrier is disturbed and the passage of antigens is increased [10]. On the luminal side, invading pathogens can disrupt the epithelial barrier and increase intestinal permeability through releasing a variety of agents including pore-forming toxin, cytoskeleton-modifying proteins and bacterial lipopolysaccharide [9]. Thus, some pathogens can cross the epithelial barrier into the basal side to interact with the host immune system. On the basal side, the pathogen-activated immune cells also disrupt the intestinal barrier to increase intestinal permeability and facilitate the pathogen invasion through secreting proinflammatory cytokines such as IFN-γ, TNF-α and IL-1β [9]. The contribution of the microbiota, intestinal barrier and host immune system to the mechanisms of diarrhea are discussed in the following section.
Diarrhea & the intestinal microbiota
There are a vast range of microorganisms that colonize the mammalian GI tract. These microorganisms are known as intestinal microbiota, that are required for intestinal homeostasis and function and appear to play a role in the pathogenesis of IBD [19,20,27,28]. Host innate and adaptive immune systems respond to intestinal microbiota through different pathways. One of the pathways is that of Toll-like receptors (TLRs), which can recognize conserved microbial molecules. Another pathway is that of Tregs that can maintain tolerance and immune homeostasis to prevent chronic inflammation [20,27,28]. However, the specific contribution of the intestinal mircobiota to the pathogenesis of IBD and the role of the microbiota in mechanisms for IBD-associated diarrhea has not been systematically investigated. The colonization of the intestinal microbiota plays an important role in the host physiological processes, such as nutrient uptake and exchange, the formation and stabilization of the intestinal barrier and the development of the intestinal immune system [19,20,27,28].
The microbial community in the gut is complex. Owing to methodological limitations, the diversity of intestinal microbiota is still incompletely characterized and the role of the microbiota in IBD mechanisms remains poorly defined [19,20]. Modern molecular techniques are rapidly advancing our research progress and knowledge of the intestinal microbiota [19,20,29]. Previous microbiology studies of the intestinal microbiota were mainly dependent on culture techniques. Such cultivation has only been able to characterize a very small fraction of the microbial diversity in the gut. The recent molecular ecological studies based on 16S ribosomal RNA sequences have revealed the characterization and quantification of the gut microbiota [19,20,27–29]. The composition of the mucosa and luminal microbiota has been investigated in humans and mice using large-scale analyses of 16S rDNA genes and metagenomics, which are culture-independent methods [20,30]. The number of species constituting the human gastrointestinal microbiota may be as high as 15,000 species as detected by 16S deep sequencing analyses [20,31]. Recent studies have shown that the intestinal microbiota vary not only between individuals [20,30], but also with anatomical location [20,30], inflammatory status of IBD [32], diet [19,20,33] and alcohol consumption [34]. The impact of gut microbiota on the pathogenesis of mouse IBD models has been extensively reviewed [20]. The gut microbiota of patients with IBD has been studied by several groups [35–38]. Their studies show that there is a compositional shift in the IBD-associated microbiota [32,37], including depletion of specific types of commensal bacteria (Lachnospiraceae and Bacteroidetes) and enrichment in Proteobacteria, using phylogenetic comparisons of the mucosa and luminal microbiota of patients with IBDs and non-IBDs controls [32]. However, the specific species associated with intestinal inflammation or a specific pathogenic pattern of microbiota has not been identified in samples from patients with IBDs [35–38]. In murine intestinal inflammation models, including induction by bacterial infection (Citrobacter rodentium, Salmonella enterica) [39–41], dextran sulfate sodium-induced colitis [40,42] or a genetic deficiency IL-10−/− mouse model [40], the intestinal micro-biota was changed. The major alteration of the microbiota in the inflammatory model was the reduction in both the total number of resident bacteria and the diversity of microbiota [39–41]. Garrett et al. reported that Enterobacteriaceae act in concert with the gut microbiota to induce spontaneous and maternally transmitted colitis [43]. The alteration of the murine gut microbiota during infection with the parasitic helminth Heligmosomoides polygyrus was reported by Walk et al. [44]. A pyrosequencing study in twins showed that the profiles of gut microbiota varied with IBD phenotypes [45]. One recent study demonstrated that the probiotic VLS#3 alters the composition of gut microbiota and these changes correlate with VSL#3-induced disease protection in a 2,4,6-trini-trobenzenesulfonic acid-induced colitis mouse model [46]. Rose et al. reported that starch-entrapped microspheres show a beneficial fermentation profile and decrease in potentially harmful bacterial during in vitro fermentation in fecal microbiota obtained from patients with IBDs [47]. Recently, another study showed that new prebiotics from rice bran ameliorate inflammation in murine colitis models through the modulation of the intestinal mircobiota and the mucosal immune system [48].
In humans, one recent study reported that Escherichia coli phylogenetic group B2 was associated with IBD patients with distal disease activity [49]. Under different environmental or inflammatory conditions, the expressions of some specific bacterial species increased or decreased and the overall compositions of the intestinal microbiota also changed [20,50,51]. These observed changes in intestinal microbiota are termed dysbiosis [20,50,51]. It remains unclear whether the dysbiosis is a causative factor or a side effect in the mechanisms of human IBDs [20]. Thus, the effects of different environmental factors or inflammatory conditions on the intestinal microbiota need to be further investigated.
The dysbiosis in infectious colitis is characterized by a decrease of Faecalibacterium prausnitzii [52]. The metabolites from F. prausnitzii can block NF-κB activation and reduce IL-8 secretion from Caco-2 cells and increase anti-inflammatory IL-10 and reduce proinflammatory IL-12 release from peripheral blood monocytes [53]. In the healthy GI tract, E. coli is the most numerically dominant Gram negative species [19]. One important study reported that the numbers of E. coli in the mucosa of CD patients were increased [54]. These E. coli isolated from the mucosa showed an increased ability to adhere to intestinal epithelial cells and to disrupt the intestinal barrier by producing an α hemolysin [54]. This is a new pathotype of E. coli termed ‘adhesive and invasive E. coli’, which stimulates the release of the proinflammatory cytokine IL-8 [55]. The microfold (M) cells make up approximately 5% of the epithelial cells that overlie Peyer’s patches in the intestine and lyphoid follicles in the colon [56,57]. Recent studies indicate that the M cell is a portal of entry for pathogens, such as the adhesive and invasive E. coli that were found in CD lesions where M cells were located [19,56,57]. M cells have the unique ability to sample antigens from the gut microbiota [19]. They play an important role in mucosal immunity by presenting antigens from a wide range of microorganisms, including bacteria, viruses and parasites, from the gut lumen to dendritic cells and lymphocytes [19,58].
Several studies have indicated that intestinal bacteria such as Campylobacter jejuni RM1221 are able to disrupt the epithelial barrier and increase paracellular permeability to allow noninvasive organisms to translocate through the epithelial layer [19,59]. When the mucosal barrier has been weakened, the TLR5 located on the basolateral aspect of epithelial cells may become accessible to bacterial flagellin [60]. The repositioning of TLRs increases the interaction between epithelial cells and gut microbiota, leading to activation of NF-κB and induction of inflammation [60]. It has been reported that soluble fiber from edible plantains prevents adherence to the epithelium by E. coli isolated from IBD patients [19,61,62].
The host–microbe interaction in the GI tract has been reviewed and summarized by other groups [20,27,40,51,63]. These studies indicated that there are many pathways by which intestinal bacteria can attenuate or aggravate the development of intestinal inflammation [63,64]. These pathways include:
Influencing the inflammatory process and modulating oxidative stress via TLR signaling and NF-κB activation;
Influencing intestinal permeability through regulation of tight junction proteins expression and translocation;
Influencing the composition of the mucus layer. The gut microbiota not only interferes with the expression of MUC genes, but also affects the expression and/or activity of cell glycosyl-transferases, which can induce changes in the carbohydrate repertoire of mucins [64];
Influencing resistance to harmful stimuli and epithelial repair through TLR4 and NF-κB signaling;
Influencing the production and release of immune effective molecules, such as IgA.
More mechanisms for the host–microbe interaction in the GI tract will be revealed in the future as our methods to investigate these interactions become more sophisticated.
In conclusion, recent advances in the molecular technology and microbiology provide us with a much more robust knowledge of the role of the mucosal and fecal microbiota in IBD. The role of the gut microbiota in the mechanisms of IBD and IBD associated diarrhea have been investigated by several groups [35–38]. However, the specific contribution of the intestinal mircobiota to the pathogenesis of IBD and the role of the microbiota in mechanisms for IBD associated diarrhea have not been systematically investigated. One recent study showed that the invasion of bacteria resulted in an increase in the interaction between bacterial components, such as flagellin, with basolateral receptors (e.g., TLR5), leading to activation of NF-κB and inflammation as a consequence [60].
Diarrhea & intestinal barrier function
The intestinal barrier consists of epithelial cells and the tight junctions between the cells [7,9,10,65]. Reduction or disruption of tight junctions results in an increase in intestinal permeability. The role of permeability or tight junctions in mechanisms of IBD have been studied [7,9,10,65]. Disruption and dysfunction of the intestinal barrier in IBD contributes to diarrhea by the leak flux mechanism and also increases the uptake of luminal antigens, which interact with immune cells to induce intestinal inflammation.
It is well accepted that mucosal inflammation causes an increase of intestinal epithelial permeability [9]. The inflammation-induced hyperpermeability plays an important role in the pathophysiology of IBD [9,66]. Intestinal barrier dysfunction in CD has been shown in previous studies using in vivo permeability tests [11]. The intestinal barrier dysfunction is positively correlated with the degree of inflammation in IBD patients [67]. Intestinal permeability can predict the clinical relapse of CD [68]. However, the underlying molecular mechanisms for the barrier dysfunction have only recently been elucidated [9,11]. One study reported that occludin, claudin-5 and claudin-8 were downregulated in CD, while the pore forming claudin-2 was upregulated [69]. These changes in expression and distribution of claudins-2, −5 and −8 lead to discontinuous tight junctions and barrier dysfunction in active CD [69]. Epithelial cell apoptosis also increased two to threefold in patients with active CD [69]. Since inflammatory cytokine-induced epithelial cell apoptosis can contribute to intestinal barrier dysfunction [10], it is reasonable to conclude that the epithelial apoptosis contributes to the leakiness and diarrhea in IBDs. NOD2 is the gene product of CARD15, which is expressed in human intestinal epithelial cells and is upregulated synergistically by TNF-α and IFN-γ [70]. CARD15 mutations were found in patients with CD and their first-degree relatives [71,72]. Furthermore, these CARD15 mutations were correlated with barrier dysfunction and increased intestinal permeability in CD patients [71,72]. In clinical studies, diarrhea in IBD patients was significantly improved after treatment with TNF-α antibodies through downregulation of epithelial apoptosis and upregulation of epithelial barrier repair to normalize the elevated intestinal permeability (leakiness) [7]. These studies support the importance of the intestinal barrier in the mechanism of IBD-induced diarrhea. The above observations both in patients with IBD and in animal models of IBD indicate that proinflammatory cytokines can increase intestinal permeability. On the other hand, there is another possibility that the increase of permeability (disruption of intestinal barrier) may be responsible for the initiation of IBD by increasing the translocation of bacteria and bacterial components to the lamina propria and thus increasing the exposure of lamina propria immune cells to bacteria antigens. This possibility is supported by the fact that mucosal permeability was increased in asymptomatic IBD patients and their relatives [7]. Thus, it is still unclear whether the intestinal barrier dysfunction is ‘the chicken or the egg’ for IBD-associated diarrhea.
Taken together, epithelial barrier dysfunction contributes to diarrhea in IBD by a leak flux mechanism and causes an increase of luminal antigen uptake, thus, resulting in mucosal inflammation. Tight junctions are important components of the intestinal epithelial barrier. The detailed molecular pathways for regulation of tight junctions and the cell cytoskeleton in the epithelial barrier during intestinal inflammation have been reviewed in other papers [9,66]. The epithelial tight junctions are regulated by cytokines and other inflammatory factors in the mechanisms of IBD-induced diarrhea. The immune regulation of the epithelial barrier, intestinal permeability and ion transport in IBDs will be discussed in the next section.
Diarrhea & immune dysfunction (T-cell activation, inflammation & cytokines)
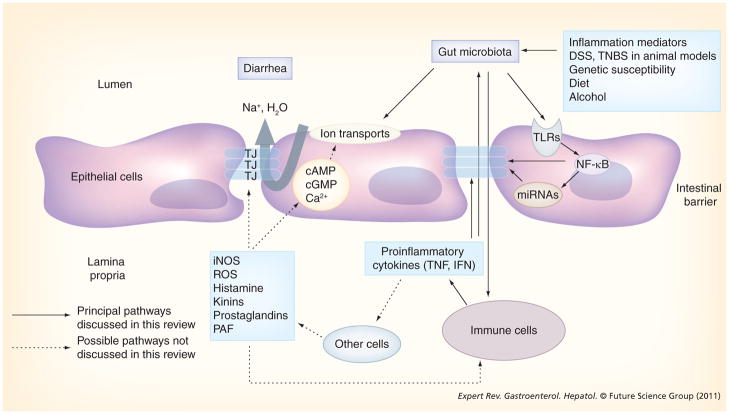
There is significant and persistent inflammation in the colonic mucosa of patients with IBD. The ongoing inflammation might induce diarrhea through triggering the release of proinflammatory cytokines from immune cells. These cytokines not only affect electrolyte transport but also affect the tight junctions in the intestinal epithelium (Figure 1). The cytokine-induced abnormal ion transport and disruption of tight junctions contribute to the inflammation-induced gut leakiness and the mechanisms of IBD-associated diarrhea.
Figure 1. Model for the pathogenesis of irritable bowel disease-induced diarrhea.
DSS: Dextran sulfate sodium; IFN: Interferon; iNOS: Inducible nitric oxide synthase; miRNA: MicroRNA; PAF: Platelet activating factor; ROS: Reactive oxygen species; TJ: Tight junction; TLR: Toll-like receptor; TNBS: 2,4,6-Trinitrobenzenesulfonic acid.
The effects of proinflammatory cytokines on colonic ion transport have been studied by several groups [4,7,8]. Their studies focused on the effects of TNF-α, IFN-γ and IL-1β on the alteration of Na+/H+ exchange, Cl−/HCO3− exchange, Na channels and Na+/K+-ATPase [4,7,8]. These investigations show that these three cytokines downregulate all of the transporters but do not increase Cl− secretion [4,7,8]. These results indicate that proinflammatory cytokines, mainly act on the absorptive processes without inducing secretory processes. These proinflammatory cytokines present in the colonic mucosa of patients with IBD, play an important role in the regulation of intestinal permeability and inflammation. This suggests that the proinflammatory cytokine-induced alteration of ion transport in the colonic mucosa is a principal mechanism responsible for diarrhea in patients with IBDs. Most of the diarrhea occurring in patients with IBDs is characterized by a decrease in the fluid and solute absorption, but not net fluid and solute secretion [7]. The fact that clinical use of monoclonal antibodies to TNF-α (infliximab) can effectively control the diarrhea in patients with IBDs also supports the fact that cytokine-induced changes of ion transport play an important role in the diarrhea mechanism of IBDs [7]. Since only 35–40% of the patients treated with infliximab have a significant remission, it is likely that additional factors may be responsible for the diarrhea in these patients [7]. The tight junctions in the gut epithelium may be one of these additional pathways that proinflammatory cytokines may affect to induce gut leakiness during intestinal inflammation [7].
Mucosal T-cell activation is associated with diarrhea in patients with IBDs [73]. Musch et al. reported that T-cell activation with anti-CD3 monoclonal antibodies induces profound diarrhea in mice, quantified by intestinal weight-to-length ratios [73]. T-cell activation significantly increased permeability to mannitol at 1 h and decreases in electroneutral Na+ absorption, Na+-dependent glucose absorption and cAMP-stimulated anion secretion at 3 h. Furthermore, enteral fluid accumulation was observed in CFTR−/− mice, indicating a minor role of active anion secretion in T-cell activation induced diarrhea. Their studies suggest that diarrhea in IBD is due to TNF-α-mediated abnormal absorption rather than to secretory processes. T-cell activation induces luminal fluid accumulation by increasing mucosal permeability and reducing epithelial Na+/K+-ATPase activity leading to decreased intestinal Na+ and water absorption [73].
Disruption of the intestinal epithelial barrier occurs in IBDs, but neither the mechanisms nor the contribution of the barrier dysfunction to the diarrhea mechanisms have been clearly defined. Clayburgh et al. reported that epithelial barrier dysfunction is required for the development of diarrhea, using a murine model of T-cell-mediated acute diarrhea to investigate the role of the epithelial barrier in diarrheal disease, such as IBD [74]. The T-cell activation-induced diarrhea is characterized by the reversal of net water flux, from absorption to secretion; increased leakage of serum proteins into the intestinal lumen; and altered tight junction structure [74]. T-cell activation was associated with increased phosphorylation of epithelial myosin II regulatory light chain, which is correlated with tight junction regulation and the development of diarrhea [74]. T-cell activation-induced epithelial myosin II regulatory light chain phosphorylation, tight junction disruption, protein leak and diarrhea are prevented in genetic knockout mice of long myosin light chain kinase (MLCK) or treatment of wild-type mice with a highly specific peptide MLCK inhibitor [74]. These studies indicate that barrier dysfunction is critical to the pathogenesis of diarrheal disease in this IBD model and that inhibition of epithelial MLCK may be an effective non-immunosuppressive therapy for treatment of immune-mediated intestinal disease.
Using the same T-cell activation model, Clayburgh et al. demonstrated that although TNF-α-neutralizing antibodies completely restore water absorption after systemic T-cell activation, barrier function is only partially corrected [75]. This suggests that, while barrier dysfunction is critical, other processes must be involved in T-cell-mediated diarrhea. To study the mechanism during these processes in vivo, this group designed a series of experiments to investigate the role of TNF-α and LIGHT [76], a new member of the TNF-α superfamily, in the regulation of barrier function and the mechanisms of T-cell activation-induced diarrhea. Their studies show that both TNF-α and LIGHT caused MLCK-dependent barrier dysfunction [75]. However, while TNF-α caused diarrhea, LIGHT enhanced intestinal water absorption. Moreover, TNF-α, but not LIGHT, inhibited Na+ absorption due to TNF-α-induced internalization of the brush border Na+/H+ exchanger Na+/H+ exchanger (NHE)3 [75]. LIGHT did not cause NHE3 internalization. PKCα activation by TNF-α was responsible for NHE3 internalization and pharmacological or genetic PKCα inhibition prevented NHE3 internalization, Na+ absorption and diarrhea despite continued barrier dysfunction [75]. The studies also indicated that cytokine-induced barrier dysfunction may be an important mechanism in the genesis of diarrhea. However, barrier dysfunction does not appear to be the only cause responsible for IBD-associated diarrhea, other pathways may also be involved as mechanisms promoting IBD diarrhea.
Although these studies provide molecular insight into mechanisms of intestinal water transport by coordinating Na+ absorption and barrier dysfunction in TNF-α-induced diarrhea, there are some contradictory data regarding the critical role of barrier dysfunction and diarrhea. In addition, these studies have some limitations in that all the studies were performed in mice with anti-CD3 monoclonal antibodies and not in patients with IBDs.
Aberrant activation of innate and adaptive immune responses increase mucosal permeability in IBD, although the mechanisms are not completely understood [77]. To examine the role of epithelial NF-κB in IBD-induced enhanced permeability, Tang et al. generated epithelial-specific IκBα mutant (NF-κB super repressor) transgenic (TG) mice [77]. Their studies show that NF-κB activation was inhibited in TG mice following T-cell-mediated immune cell activation using an anti-CD3 monoclonal antibody [77]. Furthermore, T-cell activation-induced diarrhea was inhibited in TG mice. Meanwhile, T-cell activation-induced changes in transepithelial resistance and transmucosal flux of alexa350 (0.35 kDa) and dextran3000 (3 kDa) were blocked in TG mice [77]. They also demonstrate that T-cell activation-induced net water secretion was reversed and T-cell activation-induced luminal flux of different molecular probes (bovine serum albumin, alexa350 and dextran3000) was reduced, using in vivo perfusion loop studies in TG mice [77]. Their studies also confirmed that tight junction proteins (occludin, claudin-1 and zonula occludens-1) are internalized through an NF-κB-dependent pathway [77]. Taken together, these studies suggest that IBD-associated diarrhea results from NF-κB-mediated tight junction protein internalization and increased paracellular permeability [77]. Since epithelial NF-κB activation induced by mucosal T cells occurs in patients with IBDs and actively plays a role in opening paracellular spaces to promote transmucosal fluid effux into the intestinal lumen, NF-κB may be a molecular target for effective therapy of IBD [77]. Reduction of epithelial NF-κB activation in IBDs might thus repair defects in the epithelial barrier and reduce diarrhea.
Although epithelial NF-κB plays an important role in regulation of intestinal barrier function and inflammation in IBD, whether NF-κB directly affects the NHE and/or other epithelial transport function is unclear. Recently, Amin et al. reported that TNF-α represses the expression of NHE2 through NF-κB activation in an intestinal epithelial cell model [78]. TNF-α levels are increased in IBD patients. To study the effect of TNF-α on the expression and activity of NHE2, which is involved in transepithelial Na+ absorption in intestinal epithelial cells and plays an important role in diarrhea mechanism of IBD, they used TNF-α-treated Caco-2 cells. The NHE2 regulations were measured by reverse transcription-PCR, reporter gene assays and Western blot analysis. Their studies showed that the human NHE2 isoform is a direct target of the transcription factor NF-κB. TNF-α-mediated activation of NF-κB decreases the expression and activity of NHE2 in the intestinal epithelial cell line. Their findings indicated that NF-κB plays an important role in the modulation of Na+ absorption during intestinal inflammatory conditions such as IBD where a high level of TNF-α is detected [78].
Diarrhea & microRNA
MicroRNAs (miRNAs) are highly conserved regulatory molecules expressed in eukaryotic cells. They are short noncoding RNAs that regulate gene expression by binding to target mRNAs, which leads to reduced protein synthesis and sometimes decreased steady-state mRNA levels [79]. Although hundreds of miRNAs have been identified, much less is known about their biological function [80–82]. There is evidence that miRNAs affect pathways fundamental to metabolic control in higher organisms such as adipocyte and skeletal muscle differentiation. Furthermore, some miRNAs are implicated in lipid, amino acid and glucose homeostasis [83]. Thus, miRNA abnormalities may contribute to common metabolic diseases and there may be novel therapeutic opportunities based on miRNA targeting [81]. Recently, the profiles of miRNA in IBDs were identified in the mucosa of patients with IBD [84–86]. The role of miRNA in the development of intestinal inflammation was investigated. These studies suggest that miR-NAs are critical regulators for immune response in IBDs and may be novel molecular targets for pharmacological modulation of mucosa inflammation [84–86]. However, the role of miRNA in the mechanisms of IBD-induced diarrhea has not been defined.
In a recent study we showed the possibility that miRNAs are involved in the disruption of tight junction proteins such as tight junction protein zonula occludens-1 (ZO-1) [87]. Thus, inflammation-induced gut leakiness could be owign to the effects of proinflammatory cytokines on the expression of miRNAs that target tight junction genes in intestinal epithelial cells.
We studied the role of miR-212 in alcohol-induced ZO-1 disruption in intestinal epithelial cells because ZO-1 is predicted, in an established miRNA database, to be one of miR-212’s target genes [88,89]. We were the first to show that miR-212 is highly expressed in intestinal tissues using a TaqMan® miRNA realtime PCR assay [87]. We also showed that miR-212 overexpression is accompanied by reductions in ZO-1 protein expression, disruption of ZO-1 and increased permeability of monolayers of Caco-2 cells. miR-212 overexpression correlated with alcohol-induced disruption of monolayer integrity. Most importantly, we found that miR-212 levels in colon biopsy samples in IBD patients were higher than in healthy controls. To see if miR-212 regulates ZO-1 levels, we conducted both overexpression studies using miR-212 precursors and inhibition studies using miR-212-specific antisense oligonucleotide inhibitors (anti-miR-212). The data showed that miR-212 overexpression significantly inhibited ZO-1 protein expression and knocking down of miR-212 expression in Caco-2 cells using anti-miR-212 inhibited alcohol-induced hyperpermeability by 50% (p < 0.05) [87].
The potential importance of miRNAs in intestinal inflammation has recently been demonstrated [84–86]. Wu et al. measured miRNA expression in the intestinal mucosa of patients with active UC, inactive UC, CD, irritable bowel syndrome, infectious colitis and microscopic colitis, as well as in healthy subjects by microarray and reverse transcription-PCR [86]. Their results showed that 11 miRNAs were differentially expressed in active UC (eight increased and three decreased significantly). miR-192 expression was decreased in colonic epithelial cells in active UC [86]. Macrophage inflammatory peptide-2 was identified as a target of miR-192. In colonic epithelial cells, TNF-α, a proinflammatory cytokine that is present in high levels in the inflamed intestine, induced miR-192 reduction and macrophage inflammatory peptide-2 overexpression [86]. miRNAs not only appear to be involved in gut inflammation, but they may also be involved in the regulation of intestinal functions such as fluid secretion. For example, Kapeller et al. reported an association between a functional variant of miR-510, the target site of the serotonin receptor-type 3E gene (HTR3E) and diarrhea predominant irritable bowel syndrome [90]. Their studies indicated that miRNA-510 regulated HTR3E expression and when a cis-regulatory variant was present in the HTR3E gene, the regulation of miR-510 ceased to be effective. This variant of the miRNA target site is associated with female diarrhea predominant irritable bowel syndrome. It remains to be seen whether miRNAs that target genes that are key in regulation of intestinal inflammation also play a role in IBD-associated diarrhea mechanisms.
In summary, recent studies provide compelling evidence for the role of miRNAs in the mechanisms underlying IBD-induced diarrhea. Therefore, miRNAs represent new therapeutic targets for the prevention and/or treatment of inflammation-induced diarrhea in patients with IBD.
Diarrhea & other mechanisms
In the above sections we discuss how the intestinal microbiota, epithelial barrier, host immunity and miRNAs contribute to IBD-induced diarrhea through regulating the alteration of ion transport and barrier function during intestinal inflammation. Other mechanisms may also be involved in the diarrhea of patients with IBD [7]. These mechanisms include: ileal dysfunction and bile-acid diarrhea, steatorrhea (fatty acid-induced diarrhea), bacterial overgrowth syndromes, lactose intolerance and short bowel syndrome [7]. These mechanisms were previously briefly reviewed [7]. In order to focus our article on the new and critical molecular insights for IBD-induced diarrhea, other molecules, such as the putative anion transport 1, DRA, cystic fibrosis transmembrane regulator, cAMP, cGMP, Ca2+, iNOS, free radicals, prostaglandins, histamine from mast cells, kinins from neurons, platelet factors and complement cascade components, are not included in this article, even though they may also contribute to the diarrheal mechanisms in IBD [4].
Genetic risk factors of IBD may also be important for the molecular mechanism of diarrhea in IBD patients. Recent studies have indicated some genetic deficiencies associated with the mechanisms of IBD. The genetic susceptibility of IBD has been well reviewed [91]. However, the contribution of genetic risk factors to IBD-induced diarrhea is unknown. To date, no study directly addresses the role of genetic deficiency in the mechanism of diarrhea in IBD. We believe that the genetic deficiency may contribute to the diarrhea mechanism through affects on intestinal microbiota and/or barrier function and/or immune function which are discussed in this article.
Successful treatment of IBD-associated diarrhea is dependant on defining the pathophysiologic mechanism in each individual patient. Diarrhea continues to be a major prevalent symptom in patients with IBD. Physicians should properly evaluate the complaints of diarrhea in IBD patients by assessing both patient symptoms and potential physiologic impacts on fluid and electrolyte status [2,3]. When treating diarrhea, physicians should pay attention to the following conditions: the location, extent and severity of inflammation; malabsorption; altered motility; and iatrogenic causes such as medications, diet and antibiotic-associated colitis (e.g., Clostridium difficile). Therapies for IBD-associated diarrhea include aminosalicylates, corticosteroids, immune modifiers and, most recently, biologic treatment [2,3]. Other medications, including loperamide, diphenoxylate, codeine sulfate and tinctures of opium slow motility and an increase in the absorption of fluids and nutrients [2,3]. The mechanisms of diarrhea in different individual IBD patients are not the same; thus, it is important to select an individual therapy strategy for each patient according to the specific mechanisms that appear to predominate in that patient. Algorithms for selection of antidiarrheal agents have been well reviewed by other studies [2,3].
Conclusion
The molecular mechanisms for IBD-induced diarrhea are summarized in Figure 1. The specific mechanism(s) for individual patients are different. One or more mechanisms may be involved in the pathogenesis of IBDs and IBD-associated diarrhea. Thus, it is important to select therapies for each individual IBD patient according to the specific mechanisms. The roles of the intestinal microbiota, epithelial barrier and host immune system in the mechanisms of IBD-induced diarrhea have been extensively studied. Substantial progress has been achieved recently in understanding the interactions between the microbiota, intestinal barrier and the host immune system during normal physiology or under inflammatory conditions. These studies have significantly improved our knowledge of the mechanisms of IBD-induced diarrhea. Modern molecular techniques provide us with a very useful tool to investigate the role of the mucosal and fecal microbiota in the mechanisms of IBD-induced diarrhea. Intestinal epithelial barrier dysfunctions contribute to the diarrhea in IBD by a leak flux mechanism. The dysfunction of the epithelial barrier results in an increase of intestinal permeability, increase of luminal antigens uptake and initiation of mucosal inflammation. The epithelial tight junctions are regulated by cytokines and other inflammatory factors in the mechanisms of IBD-induced diarrhea. Recent studies show that IBD-associated diarrhea results from NF-κB-mediated tight junction protein internalization and increased paracellular permeability. Epithelial NF-κB activation plays a role in opening paracellular spaces to promote transmucosal fluid effux into the intestinal lumen in patients with IBD. Thus, NF-κB may be a molecular target for effective therapy of IBD. Recent studies also provide new evidence that miRNAs may play a critical role in the regulation of intestinal inflammation and intestinal permeability in the mechanisms underlying IBD-induced diarrhea.
Expert commentary & five-year view
More new molecular techniques, such as mutitag pyrosequencing combined with bioinformatics and network-based modeling, will be used to identify the specific microbiota responsible for IBD-induced diarrhea. The specific pathogenic microorganisms in the human gut as a cause of IBD may be identified soon. Treatment based on the modulation of gut microbiota using specific diets or antibiotics or probiotic bacteria may be developed into clinical trials.
The mechanisms of inflammation induced epithelial barrier dysfunction will be further investigated. The effect of therapeutic agents [92], such as TGF-β, zinc and quercetin, on the intestinal barrier function will be studied. Therapeutic agents will be developed to modulate or repair the epithelial barrier defects in patients with IBD.
Mucosal inflammation-induced increase of epithelial permeability is a common phenomenon in many diseases. The increase of intestinal permeability results in endotoxemia and multi-organ dysfunction. Understanding the mechanisms would be helpful in the development of new therapeutic approaches to prevent and treat these diseases through preserving integrity of the epithelial barrier. To effectively prevent or treat the diarrhea or hyperpermeability in IBD, the molecular mechanism for proinflammatory cytokines to disrupt the tight junctions has to be investigated in patients with IBDs as well as in IBD animal models.
Key issues.
Diarrhea is common in patients with inflammatory bowel diseases (IBDs) and significantly affects the quality of a patient’s life. IBD-induced diarrhea is characterized by a decrease in the fluid and solute absorption, but not net fluid and solute secretion. Understanding the molecular mechanisms of diarrhea is important in identifying novel molecular targets for the successful treatment of patients with IBD.
There are many molecular mechanisms responsible for the generation of diarrhea with a wide range of clinical presentations. The interactions between intestinal microbiota, epithelial barrier and host immune system are complex during normal physiology and become more complex under inflammatory conditions but it is important that we identify how these factors may intereact and play important roles in IBD-induced diarrhea.
Recently, the role of the gut microbiota in the mechanisms of IBDs and IBD-associated diarrhea have been extensively investigated. Modern molecular techniques are rapidly advancing our research progress and knowledge of the intestinal microbiota. The invasion of bacteria results in disruption of the intestinal barrier and in an increase in the interaction between bacterial components and immune cells, leading to activation of NF-κB and inflammation as a consequence.
Intestinal barrier dysfunction (leaky gut) contributes to the diarrhea in IBDs by a leak flux mechanism and causes an increase of luminal antigen uptake, thus, resulting in mucosal inflammation. Tight junctions are important components of the intestinal epithelial barrier. The epithelial tight junctions are regulated by cytokines and other inflammatory factors in the mechanisms of IBD-induced diarrhea.
Epithelial inflammation in IBDs might induce diarrhea through triggering the release of proinflammatory cytokines from immune cells in patients with IBD. These proinflammatory (TNF-α and interferons) cytokines induce abnormal ion transport and disruption of tight junctions that contribute to the inflammation-induced gut leakiness and the mechanisms of IBD-associated diarrhea.
Recent studies indicate that microRNA regulate intestinal permeability and may play an important role in the mechanisms underlying IBD-induced diarrhea.
Footnotes
For reprint orders, please contact reprints@expert-reviews.com
Financial & competing interests disclosure
This work was supported by NIH grants AA18729 (Yueming Tang), AA019405 (Ali Keshavarzian) and AA020216 (Ali Keshavarzian), and in part by a mentoring program of the Department of Internal Medicine of Rush University Medical Center (Yueming Tang). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
• of interest
- 1.Gismera CS, Aladren BS. Inflammatory bowel diseases: a disease(s) of modern times? Is incidence still increasing? World J Gastroenterol. 2008;14(36):5491–5498. doi: 10.3748/wjg.14.5491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shah SB, Hanauer SB. Treatment of diarrhea in patients with inflammatory bowel disease: concepts and cautions. Rev Gastroenterol Disord. 2007;7(Suppl 3):S3–S10. [PubMed] [Google Scholar]
- 3.Payne CM, Fass R, Bernstein H, et al. Pathogenesis of diarrhea in the adult: diagnostic challenges and life-threatening conditions. Eur J Gastroenterol Hepatol. 2006;18(10):1047–1051. doi: 10.1097/01.meg.0000231748.60889.be. [DOI] [PubMed] [Google Scholar]
- 4.Seidler U, Lenzen H, Cinar A, Tessema T, Bleich A, Riederer B. Molecular mechanisms of disturbed electrolyte transport in intestinal inflammation. Ann NY Acad Sci. 2006;1072:262–275. doi: 10.1196/annals.1326.024. [DOI] [PubMed] [Google Scholar]
- 5.Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448(7152):427–434. doi: 10.1038/nature06005. [DOI] [PubMed] [Google Scholar]
- 6.Keighley MR, Stockbrugger RW. Inflammatory bowel disease. Aliment Pharmacol Ther. 2003;18(Suppl 3):66–70. doi: 10.1046/j.0953-0673.2003.01727.x. [DOI] [PubMed] [Google Scholar]
- 7•.Binder HJ. Mechanisms of diarrhea in inflammatory bowel diseases. Ann NY Acad Sci. 2009;1165:285–293. doi: 10.1111/j.1749-6632.2009.04039.x. Concise summary of the pathophysiology of diarrhea in both ulcerative colitis and Crohn’s disease. [DOI] [PubMed] [Google Scholar]
- 8.Field M. Intestinal ion transport and the pathophysiology of diarrhea. J Clin Invest. 2003;111(7):931–943. doi: 10.1172/JCI18326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ivanov AI, Parkos CA, Nusrat A. Cytoskeletal regulation of epithelial barrier function during inflammation. Am J Pathol. 2010;177(2):512–524. doi: 10.2353/ajpath.2010.100168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10•.Schulzke JD, Ploeger S, Amasheh M, et al. Epithelial tight junctions in intestinal inflammation. Ann NY Acad Sci. 2009;1165:294–300. doi: 10.1111/j.1749-6632.2009.04062.x. Describes the different mechanisms causing disruption of epithelial tight junctions in intestinal inflammation. [DOI] [PubMed] [Google Scholar]
- 11.Mankertz J, Schulzke JD. Altered permeability in inflammatory bowel disease: pathophysiology and clinical implications. Curr Opin Gastroenterol. 2007;23(4):379–383. doi: 10.1097/MOG.0b013e32816aa392. [DOI] [PubMed] [Google Scholar]
- 12.Harris J, Shields R. Absorption and secretion of water and electrolytes by the intact human colon in diffuse untreated proctocolitis. Gut. 1970;11(1):27–33. doi: 10.1136/gut.11.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rask-Madsen J, Hammersgaard EA, Knudsen E. Rectal electrolyte transport and mucosal permeability in ulcerative colitis and Crohn’s disease. J Lab Clin Med. 1973;81(3):342–353. [PubMed] [Google Scholar]
- 14.Rask-Madsen J, Jensen PB. Electrolyte transport capacity and electrical potentials of the normal and the inflamed human rectum in vivo. Scand J Gastroenterol. 1973;8(2):169–175. [PubMed] [Google Scholar]
- 15.Archampong EQ, Harris J, Clark CG. The absorption and secretion of water and electrolytes across the healthy and the diseased human colonic mucosa measured in vitro. Gut. 1972;13(11):880–886. doi: 10.1136/gut.13.11.880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hawker PC, McKay JS, Turnberg LA. Electrolyte transport across colonic mucosa from patients with inflammatory bowel disease. Gastroenterology. 1980;79(3):508–511. [PubMed] [Google Scholar]
- 17.Amasheh S, Barmeyer C, Koch CS, et al. Cytokine-dependent transcriptional down-regulation of epithelial sodium channel in ulcerative colitis. Gastroenterology. 2004;126(7):1711–1720. doi: 10.1053/j.gastro.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 18.Yang H, Jiang W, Furth EE, et al. Intestinal inflammation reduces expression of DRA, a transporter responsible for congenital chloride diarrhea. Am J Physiol. 1998;275(6 Pt 1):G1445–G1453. doi: 10.1152/ajpgi.1998.275.6.G1445. [DOI] [PubMed] [Google Scholar]
- 19•.Friswell M, Campbell B, Rhodes J. The role of bacteria in the pathogenesis of inflammatory bowel disease. Gut Liver. 2010;4(3):295–306. doi: 10.5009/gnl.2010.4.3.295. Summary of the role of the mucosal and fecal microbiota in the pathogenesis of inflammatory bowel disease. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20•.Nell S, Suerbaum S, Josenhans C. The impact of the microbiota on the pathogenesis of IBD: lessons from mouse infection models. Nat Rev Microbiol. 2010;8(8):564–577. doi: 10.1038/nrmicro2403. Summarizes the impact of the microbiota on the pathogenesis in mouse infection models. [DOI] [PubMed] [Google Scholar]
- 21.Hollander D. The intestinal permeability barrier. A hypothesis as to its regulation and involvement in Crohn’s disease. Scand J Gastroenterol. 1992;27(9):721–726. doi: 10.3109/00365529209011172. [DOI] [PubMed] [Google Scholar]
- 22.Clayburgh DR, Shen L, Turner JR. A porous defense: the leaky epithelial barrier in intestinal disease. Lab Invest. 2004;84(3):282–291. doi: 10.1038/labinvest.3700050. [DOI] [PubMed] [Google Scholar]
- 23.Turner JR. Molecular basis of epithelial barrier regulation: from basic mechanisms to clinical application. Am J Pathol. 2006;169(6):1901–1909. doi: 10.2353/ajpath.2006.060681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ivanov AI, Nusrat A, Parkos CA. Endocytosis of the apical junctional complex: mechanisms and possible roles in regulation of epithelial barriers. Bioessays. 2005;27(4):356–365. doi: 10.1002/bies.20203. [DOI] [PubMed] [Google Scholar]
- 25.Laukoetter MG, Bruewer M, Nusrat A. Regulation of the intestinal epithelial barrier by the apical junctional complex. Curr Opin Gastroenterol. 2006;22(2):85–89. doi: 10.1097/01.mog.0000203864.48255.4f. [DOI] [PubMed] [Google Scholar]
- 26.Madara JL. Warner-Lambert/Parke-Davis Award lecture. Pathobiology of the intestinal epithelial barrier. Am J Pathol. 1990;137(6):1273–1281. [PMC free article] [PubMed] [Google Scholar]
- 27.Backhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI. Host-bacterial mutualism in the human intestine. Science. 2005;307(5717):1915–1920. doi: 10.1126/science.1104816. [DOI] [PubMed] [Google Scholar]
- 28.Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by Toll-like receptors is required for intestinal homeostasis. Cell. 2004;118(2):229–241. doi: 10.1016/j.cell.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 29.Andoh A, Benno Y, Kanauchi O, Fujiyama Y. Recent advances in molecular approaches to gut microbiota in inflammatory bowel disease. Curr Pharm Des. 2009;15(18):2066–2073. doi: 10.2174/138161209788489186. [DOI] [PubMed] [Google Scholar]
- 30.Qin J, Li R, Raes J, et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464(7285):59–65. doi: 10.1038/nature08821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Salzman NH, de Jong H, Paterson Y, Harmsen HJ, Welling GW, Bos NA. Analysis of 16S libraries of mouse gastrointestinal microflora reveals a large new group of mouse intestinal bacteria. Microbiology. 2002;148(Pt 11):3651–3660. doi: 10.1099/00221287-148-11-3651. [DOI] [PubMed] [Google Scholar]
- 32.Frank DN, St Amand AL, Feldman RA, Boedeker EC, Harpaz N, Pace NR. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc Natl Acad Sci USA. 2007;104(34):13780–13785. doi: 10.1073/pnas.0706625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chapman-Kiddell CA, Davies PS, Gillen L, Radford-Smith GL. Role of diet in the development of inflammatory bowel disease. Inflamm Bowel Dis. 2010;16(1):137–151. doi: 10.1002/ibd.20968. [DOI] [PubMed] [Google Scholar]
- 34.Mutlu E, Keshavarzian A, Engen P, Forsyth CB, Sikaroodi M, Gillevet P. Intestinal dysbiosis: a possible mechanism of alcohol-induced endotoxemia and alcoholic steatohepatitis in rats. Alcohol Clin Exp Res. 2009;33(10):1836–1846. doi: 10.1111/j.1530-0277.2009.01022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gophna U, Sommerfeld K, Gophna S, Doolittle WF, Veldhuyzen van Zanten SJ. Differences between tissue-associated intestinal microfloras of patients with Crohn’s disease and ulcerative colitis. J Clin Microbiol. 2006;44(11):4136–4141. doi: 10.1128/JCM.01004-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bibiloni R, Mangold M, Madsen KL, Fedorak RN, Tannock GW. The bacteriology of biopsies differs between newly diagnosed, untreated, Crohn’s disease and ulcerative colitis patients. J Med Microbiol. 2006;55(Pt 8):1141–1149. doi: 10.1099/jmm.0.46498-0. [DOI] [PubMed] [Google Scholar]
- 37.Kassinen A, Krogius-Kurikka L, Makivuokko H, et al. The fecal microbiota of irritable bowel syndrome patients differs significantly from that of healthy subjects. Gastroenterology. 2007;133(1):24–33. doi: 10.1053/j.gastro.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 38.Othman M, Aguero R, Lin HC. Alterations in intestinal microbial flora and human disease. Curr Opin Gastroenterol. 2008;24(1):11–16. doi: 10.1097/MOG.0b013e3282f2b0d7. [DOI] [PubMed] [Google Scholar]
- 39.Hoffmann C, Hill DA, Minkah N, et al. Community-wide response of the gut microbiota to enteropathogenic Citrobacter rodentium infection revealed by deep sequencing. Infect Immun. 2009;77(10):4668–4678. doi: 10.1128/IAI.00493-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lupp C, Robertson ML, Wickham ME, et al. Host-mediated inflammation disrupts the intestinal microbiota and promotes the overgrowth of Enterobacteriaceae. Cell Host Microbe. 2007;2(2):119–129. doi: 10.1016/j.chom.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 41.Stecher B, Robbiani R, Walker AW, et al. Salmonella enterica serovar typhimurium exploits inflammation to compete with the intestinal microbiota. PLoS Biol. 2007;5(10):2177–2189. doi: 10.1371/journal.pbio.0050244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Heimesaat MM, Fischer A, Siegmund B, et al. Shift towards pro-inflammatory intestinal bacteria aggravates acute murine colitis via Toll-like receptors 2 and 4. PLoS One. 2007;2(7):e662. doi: 10.1371/journal.pone.0000662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Garrett WS, Gallini CA, Yatsunenko T, et al. Enterobacteriaceae act in concert with the gut microbiota to induce spontaneous and maternally transmitted colitis. Cell Host Microbe. 2010;8(3):292–300. doi: 10.1016/j.chom.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Walk ST, Blum AM, Ewing SA, Weinstock JV, Young VB. Alteration of the murine gut microbiota during infection with the parasitic helminth Heligmosomoides polygyrus. Inflamm Bowel Dis. 2010;16(11):1841–1849. doi: 10.1002/ibd.21299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45•.Willing BP, Dicksved J, Halfvarson J, et al. A pyrosequencing study in twins shows that gastrointestinal microbial profiles vary with inflammatory bowel disease phenotypes. Gastroenterology. 2010;139(6):1844–1854. doi: 10.1053/j.gastro.2010.08.049. First study using pyrosequencing in twins demonstrated that bacterial populations differ in abundance among individuals with different phenotypes of inflammatory bowel disease. [DOI] [PubMed]
- 46.Uronis JM, Arthur JC, Keku T, et al. Gut microbial diversity is reduced by the probiotic VSL#3 and correlates with decreased TNBS-induced colitis. Inflamm Bowel Dis. 2010;17(1):289–297. doi: 10.1002/ibd.21366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rose DJ, Venema K, Keshavarzian A, Hamaker BR. Starch-entrapped microspheres show a beneficial fermentation profile and decrease in potentially harmful bacteria during in vitro fermentation in faecal microbiota obtained from patients with inflammatory bowel disease. Br J Nutr. 2010;103(10):1514–1524. doi: 10.1017/S0007114509993515. [DOI] [PubMed] [Google Scholar]
- 48.Komiyama Y, Andoh A, Fujiwara D, et al. New prebiotics from rice bran ameliorate inflammation in murine colitis models through the modulation of intestinal homeostasis and the mucosal immune system. Scand J Gastroenterol. 2010;46(1):40–52. doi: 10.3109/00365521.2010.513062. [DOI] [PubMed] [Google Scholar]
- 49.Petersen AM, Nielsen EM, Litrup E, Brynskov J, Mirsepasi H, Krogfelt KA. A phylogenetic group of Escherichia coli associated with active left-sided inflammatory bowel disease. BMC Microbiol. 2009;9:171. doi: 10.1186/1471-2180-9-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Packey CD, Sartor RB. Commensal bacteria, traditional and opportunistic pathogens, dysbiosis and bacterial killing in inflammatory bowel diseases. Curr Opin Infect Dis. 2009;22(3):292–301. doi: 10.1097/QCO.0b013e32832a8a5d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chow J, Mazmanian SK. A pathobiont of the microbiota balances host colonization and intestinal inflammation. Cell Host Microbe. 2010;7(4):265–276. doi: 10.1016/j.chom.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sokol H, Seksik P, Furet JP, et al. Low counts of Faecalibacterium prausnitzii in colitis microbiota. Inflamm Bowel Dis. 2009;15(8):1183–1189. doi: 10.1002/ibd.20903. [DOI] [PubMed] [Google Scholar]
- 53.Sokol H, Pigneur B, Watterlot L, et al. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc Natl Acad Sci USA. 2008;105(43):16731–16736. doi: 10.1073/pnas.0804812105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Darfeuille-Michaud A, Neut C, Barnich N, et al. Presence of adherent Escherichia coli strains in ileal mucosa of patients with Crohn’s disease. Gastroenterology. 1998;115(6):1405–1413. doi: 10.1016/s0016-5085(98)70019-8. [DOI] [PubMed] [Google Scholar]
- 55.Boudeau J, Glasser AL, Masseret E, Joly B, Darfeuille-Michaud A. Invasive ability of an Escherichia coli strain isolated from the ileal mucosa of a patient with Crohn’s disease. Infect Immun. 1999;67(9):4499–4509. doi: 10.1128/iai.67.9.4499-4509.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lodes MJ, Cong Y, Elson CO, et al. Bacterial flagellin is a dominant antigen in Crohn disease. J Clin Invest. 2004;113(9):1296–1306. doi: 10.1172/JCI20295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Miller H, Zhang J, Kuolee R, Patel GB, Chen W. Intestinal M cells: the fallible sentinels? World J Gastroenterol. 2007;13(10):1477–1486. doi: 10.3748/wjg.v13.i10.1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Garside P, Millington O, Smith KM. The anatomy of mucosal immune responses. Ann NY Acad Sci. 2004;1029:9–15. doi: 10.1196/annals.1309.002. [DOI] [PubMed] [Google Scholar]
- 59.Lamb-Rosteski JM, Kalischuk LD, Inglis GD, Buret AG. Epidermal growth factor inhibits Campylobacter jejuni-induced claudin-4 disruption, loss of epithelial barrier function, and Escherichia coli translocation. Infect Immun. 2008;76(8):3390–3398. doi: 10.1128/IAI.01698-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rhee SH, Im E, Riegler M, Kokkotou E, O’Brien M, Pothoulakis C. Pathophysiological role of Toll-like receptor 5 engagement by bacterial flagellin in colonic inflammation. Proc Natl Acad Sci USA. 2005;102(38):13610–13615. doi: 10.1073/pnas.0502174102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Martin HM, Campbell BJ, Hart CA, et al. Enhanced Escherichia coli adherence and invasion in Crohn’s disease and colon cancer. Gastroenterology. 2004;127(1):80–93. doi: 10.1053/j.gastro.2004.03.054. [DOI] [PubMed] [Google Scholar]
- 62.Geier MS, Butler RN, Howarth GS. Inflammatory bowel disease: current insights into pathogenesis and new therapeutic options; probiotics, prebiotics and synbiotics. Int J Food Microbiol. 2007;115(1):1–11. doi: 10.1016/j.ijfoodmicro.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 63.van Vliet MJ, Harmsen HJ, de Bont ES, Tissing WJ. The role of intestinal microbiota in the development and severity of chemotherapy-induced mucositis. PLoS Pathog. 2010;6(5):e1000879. doi: 10.1371/journal.ppat.1000879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hooper LV, Gordon JI. Commensal host-bacterial relationships in the gut. Science. 2001;292(5519):1115–1118. doi: 10.1126/science.1058709. [DOI] [PubMed] [Google Scholar]
- 65.Blumberg RS, Li L, Nusrat A, Parkos CA, Rubin DC, Carrington JL. Recent insights into the integration of the intestinal epithelium within the mucosal environment in health and disease. Mucosal Immunol. 2008;1(5):330–334. doi: 10.1038/mi.2008.29. [DOI] [PubMed] [Google Scholar]
- 66.Turner JR. Intestinal mucosal barrier function in health and disease. Nat Rev Immunol. 2009;9(11):799–809. doi: 10.1038/nri2653. [DOI] [PubMed] [Google Scholar]
- 67.Murphy MS, Eastham EJ, Nelson R, Pearson AD, Laker MF. Intestinal permeability in Crohn’s disease. Arch Dis Child. 1989;64(3):321–325. doi: 10.1136/adc.64.3.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wyatt J, Vogelsang H, Hubl W, Waldhoer T, Lochs H. Intestinal permeability and the prediction of relapse in Crohn’s disease. Lancet. 1993;341(8858):1437–1439. doi: 10.1016/0140-6736(93)90882-h. [DOI] [PubMed] [Google Scholar]
- 69.Zeissig S, Burgel N, Gunzel D, et al. Changes in expression and distribution of claudin 2, 5 and 8 lead to discontinuous tight junctions and barrier dysfunction in active Crohn’s disease. Gut. 2007;56(1):61–72. doi: 10.1136/gut.2006.094375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rosenstiel P, Fantini M, Brautigam K, et al. TNF-α and IFN-γ regulate the expression of the NOD2 (CARD15) gene in human intestinal epithelial cells. Gastroenterology. 2003;124(4):1001–1009. doi: 10.1053/gast.2003.50157. [DOI] [PubMed] [Google Scholar]
- 71.Buhner S, Buning C, Genschel J, et al. Genetic basis for increased intestinal permeability in families with Crohn’s disease: role of CARD15 3020insC mutation? Gut. 2006;55(3):342–347. doi: 10.1136/gut.2005.065557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.D’Inca R, Annese V, di Leo V, et al. Increased intestinal permeability and NOD2 variants in familial and sporadic Crohn’s disease. Aliment Pharmacol Ther. 2006;23(10):1455–1461. doi: 10.1111/j.1365-2036.2006.02916.x. [DOI] [PubMed] [Google Scholar]
- 73.Musch MW, Clarke LL, Mamah D, et al. T cell activation causes diarrhea by increasing intestinal permeability and inhibiting epithelial Na+/K+-ATPase. J Clin Invest. 2002;110(11):1739–1747. doi: 10.1172/JCI15695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Clayburgh DR, Barrett TA, Tang Y, et al. Epithelial myosin light chain kinase-dependent barrier dysfunction mediates T cell activation-induced diarrhea in vivo. J Clin Invest. 2005;115(10):2702–2715. doi: 10.1172/JCI24970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Clayburgh DR, Musch MW, Leitges M, Fu YX, Turner JR. Coordinated epithelial NHE3 inhibition and barrier dysfunction are required for TNF-mediated diarrhea in vivo. J Clin Invest. 2006;116(10):2682–2694. doi: 10.1172/JCI29218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mauri DN, Ebner R, Montgomery RI, et al. LIGHT, a new member of the TNF superfamily, and lymphotoxin α are ligands for herpesvirus entry mediator. Immunity. 1998;8(1):21–30. doi: 10.1016/s1074-7613(00)80455-0. [DOI] [PubMed] [Google Scholar]
- 77•.Tang Y, Clayburgh DR, Mittal N, et al. Epithelial NF-κB enhances transmucosal fluid movement by altering tight junction protein composition after T cell activation. Am J Pathol. 2010;176(1):158–167. doi: 10.2353/ajpath.2010.090548. Demonstrated the role of epithelial NF-κB in the mechanisms of T-cell activation-induced diarrhea and T-cell activation-induced disruption of intestinal tight junctions. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Amin MR, Orenuga T, Tyagi S, Dudeja PK, Ramaswamy K, Malakooti J. Tumor necrosis factor-α represses the expression of NHE2 through NF-κB activation in intestinal epithelial cell model, C2BBe1. Inflamm Bowel Dis. 2010;17(3):720–731. doi: 10.1002/ibd.21419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Carthew RW. Gene regulation by microRNAs. Curr Opin Genet Dev. 2006;16(2):203–208. doi: 10.1016/j.gde.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 80.Ambros V. The functions of animal microRNAs. Nature. 2004;431(7006):350–355. doi: 10.1038/nature02871. [DOI] [PubMed] [Google Scholar]
- 81.Krutzfeldt J, Stoffel M. MicroRNAs: a new class of regulatory genes affecting metabolism. Cell Metab. 2006;4(1):9–12. doi: 10.1016/j.cmet.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 82.Krutzfeldt J, Poy MN, Stoffel M. Strategies to determine the biological function of microRNAs. Nat Genet. 2006;38(Suppl):S14–S19. doi: 10.1038/ng1799. [DOI] [PubMed] [Google Scholar]
- 83.Gauthier BR, Wollheim CB. MicroRNAs: ‘ribo-regulators’ of glucose homeostasis. Nat Med. 2006;12(1):36–38. doi: 10.1038/nm0106-36. [DOI] [PubMed] [Google Scholar]
- 84.Fasseu M, Treton X, Guichard C, et al. Identification of restricted subsets of mature microRNA abnormally expressed in inactive colonic mucosa of patients with inflammatory bowel disease. PLoS One. 2010;5(10):e13160. doi: 10.1371/journal.pone.0013160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wu F, Zhang S, Dassopoulos T, et al. Identification of microRNAs associated with ileal and colonic Crohn’s disease. Inflamm Bowel Dis. 2010;16(10):1729–1738. doi: 10.1002/ibd.21267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wu F, Zikusoka M, Trindade A, et al. MicroRNAs are differentially expressed in ulcerative colitis and alter expression of macrophage inflammatory peptide-2 α. Gastroenterology. 2008;135(5):1624–1635.e1624. doi: 10.1053/j.gastro.2008.07.068. [DOI] [PubMed] [Google Scholar]
- 87.Tang Y, Banan A, Forsyth CB, et al. Effect of alcohol on miR-212 expression in intestinal epithelial cells and its potential role in alcoholic liver disease. Alcohol Clin Exp Res. 2008;32(2):355–364. doi: 10.1111/j.1530-0277.2007.00584.x. [DOI] [PubMed] [Google Scholar]
- 88.Blow MJ, Grocock RJ, van Dongen S, et al. RNA editing of human microRNAs. Genome Biol. 2006;7(4):R27. doi: 10.1186/gb-2006-7-4-r27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Griffiths-Jones S, Grocock RJ, van Dongen S, Bateman A, Enright AJ. miRBase: microRNA sequences, targets and gene nomenclature. Nucleic Acids Res. 2006;34(Database issue):D140–D144. doi: 10.1093/nar/gkj112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kapeller J, Houghton LA, Monnikes H, et al. First evidence for an association of a functional variant in the microRNA-510 target site of the serotonin receptor-type 3E gene with diarrhea predominant irritable bowel syndrome. Hum Mol Genet. 2008;17(19):2967–2977. doi: 10.1093/hmg/ddn195. [DOI] [PubMed] [Google Scholar]
- 91.Cho JH, Brant SR. Recent insights into the genetics of inflammatory bowel disease. Gastroenterology. 2010;140(6):1704–1712. doi: 10.1053/j.gastro.2011.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hering NA, Schulzke JD. Therapeutic options to modulate barrier defects in inflammatory bowel disease. Dig Dis. 2009;27(4):450–454. doi: 10.1159/000233283. [DOI] [PubMed] [Google Scholar]