Abstract
The serine/threonine kinase LKB1 is a master kinase that regulates a number of critical events such as cell transformation, polarization, development, stress response, and energy metabolism in metazoa. After multiple unsuccessful attempts of generating Dictyostelium lkb1-null cells, an RNAi-based knockdown approach proved effective. Depletion of lkb1 with a knockdown construct displayed severe reduction in prespore cell differentiation and precocious induction of prestalk cells, which were reminiscent of cells lacking GSK3. Similarly to gsk3− cells, lkb1 depleted cells displayed lower GSK3 activity than wild type cells during development and compromised cAMP-mediated inhibition of the DIF-1 mediated ecmB induction. In response to stress insult, the kinase activity of LKB1, but not that of GSK3, increased. Therefore, LKB1 positively functions at the upstream of GSK3 during development and responds to stress insults independently from GSK3.
Keywords: LKB1, GSK3, Development, Stress
Introduction
LKB1 is a multi-tasking kinase that orchestrates metabolism, cell structure, and development (Alessi et al., 2006). Mis-regulation of LKB1 could lead to aberrant cell growth known as Peutz-Jegher’s Syndrome. In multiple cases, LKB1 forms a heterotrimeric complex with the pseudokinase STRAD and the armadillo repeat scaffolding like protein MO25. This complex seems to be critical for coordinating epithelial polarization and energy stress induced 5′AMP-activated protein kinase (AMPK) activation (Baas et al., 2004; Lee et al., 2007). The major downstream effector of LKB1 in response to energy stress is the TSC2/TSC1 complex, which inhibits Rheb GTPase, a mTOR activator. Interestingly, the GAP activity of TSC2 is maintained by the hierarchical phosphorylation of TSC by GSK3 and AMPK (Inoki and Guan, 2006), and is inhibited by GSK3 inhibition (Inoki et al., 2006). Considering that LKB1 is a positive upstream regulator of TSC2, it is puzzling that LKB1 inhibits GSK3 during Xenopus embryogenesis (Ossipova et al., 2003). As such, it will be important to further investigate the mechanisms of LKB1 action in additional signaling contexts and model systems.
It has been suggested that Dictyostelium TOR Complex 1 (TORC1), which is sensitive to Rapamycin, plays a role in growth (Lee et al, 2005), but further studies are necessary to fully understand its function. Compromising TORC2 function displayed aberrant cell movement (Lee et al., 2005) and that of AMPKα exhibited an aberrant response to energy stress (Bokko et al., 2007). However, it is not known if the mediated signaling pathway of LKB1 interacts with that of GSK3 in Dictyostelium cells. GSK3 stimulates prespore cell differentiation and inhibits that of the prestalk cell during development. Dictyostelium GSK3 can either be activated or inhibited by phosphorylation or de-phosphorylation of tyrosine 214 in response to cAMP stimulation (Kim, et al., 2011; Kim et al., 2002; Kim et al., 1999; Plyte et al., 1999).
In this paper, we investigated the role of Dictyostelium LKB1 by using a RNAi mediated knockdown strategy. LKB1 was essential for prespore cell induction, prestalk cell repression, and activation of GSK3. Stress induced phosphorylation of AMPK at the threonine172 in the activation loop was also LKB1 dependent similarly to higher eukaryotes. Therefore, LKB1 is regulating both development and the energy/stress response not only in metazoans, but also in non-metazoan organisms, such as Dictyostelium discoideum.
Materials and Methods
Dictyostelium culture, development, and stress experiments
Dictyostelium cells were cultured as described earlier (Kim, et al., 2002). Cells with selection markers were grown with D3T supplemented with either G418 (20μg/ml to 80μg/ml) or Blasticidin (5μg/ml) as required. Developments on solid substrata were performed on DB agar plates (0.2mM CaCl2, 2mM MgCl2, 24mM NaH2PO4.H2O, 4mM Na2HPO4.7H2O, and 14g Agar/liter). For development in suspension culture, cells were starved for an hour, stimulated with 50nM cAMP pulses at 6 minute intervals for 4 hours, and were further stimulated with 300μM cAMP and/or 100 nM DIF-1 as indicated.
Log phase cells were washed with DB buffer and insulted with either 2mM H2O2 for 15 minutes or 200mM sorbitol for 10 minutes as indicated in each experiment.
Northern blot analysis
Trizol reagent (Invitrogen Inc.) was used for the isolation of total RNA, and each 10μg of total RNA was hybridized with different probes (lkb1, ecmA, ecmB, cotB, pspA, and lkb1). Ig7 was used for loading control. The lkb1 probe was prepared by using the forward primer (5′ATGGAAGTTGAACAACAACCATC3′) and the reverse primer (5′CAATT GGGTGCACTGGTAAACC3′). A Rediprime kit was used to radiolabel the probe (Amersham, Inc.).
Cloning of lkb1 cDNA
lkb1 (Dictyostelium gene identification number: DDB_G0279629) cDNA was reverse transcribed from AX3 RNA at 45°C with a OneStep RT-PCR it (QIAGEN), run through a Micropure EZ filter (Millipore) for enzyme removal, amplified by HotMaster-Taq DNA Polymerase (Eppendorf) using the forward primer (5′ATGGAAGTTGA ACAACAACCATC3′) and the reverse primer (5′AAAACTTAATTAATGATACATT TTGAC3′), and cloned into the PCR2.1 vector. The cDNA clone, pTOPO-lkb1, was confirmed to have no mutation by sequencing.
Generation of T7-LKB1
The full-length lkb1 was excised from pTopo-lkb1 and subcloned in-frame into the pCFC5 vector (Chen and Katz, 1998) under the control of Actin-15 promoter downstream to the T7 tag using the EcoRI restriction site. To clone T7-lkb1 under the SP60/CotC promoter, the SL-25 vector was linearized with SpeI and KpnI, and the T7-lkb1 insert was obtained by BglII and XhoI digestion from pCFC5. Both the vector and insert were treated with the Klenow enzyme and were purified before ligation.
Antibodies, western, and immunoprecipitation
Anti-Rabbit T7 (MASMTGGQQMG) affinity purified antibody (1μg/μl) was used to detect T7-tag (QED Biosciences Inc.). Unless otherwise indicated, 20 million cells were lysed at appropriate time points with 1ml of TTG buffer (TTG: 20mM TrisCl (pH7.7), 1% Triton X-100, 5% Glycerol, 0.15M NaCl, 1mM EDTA, 1mM Sodium vanadate, 40μM ammonium molybdate) and with a protease inhibitor cocktail (Roche Inc). Antibodies against phospho-AMPK (#2535), Pan-RAS (Ab-3), GSK3 (4G-1E), and phospho-GSK3 (5G-2F) were purchased from Cell Signaling Technology, CalBiochem, and Upstate Biotechnology.
LKB kinase assay
Cells expressing T7-LKB1 were lysed at appropriate time points and were incubated with 50μl of Protein A Sepharose and 2 μg of T7 antisera for 90 minutes at 4°C. After immunoprecipitation with the T7 anti serum, the immunocomplexes were washed twice with TTG buffer and two more times with freshly made kinase buffer (20mM TrisCl (pH 7.7) and 10mM MgCl2). Washed immunocomplexes were incubated at 30°C for 20 minutes with 5μM ATP, 10μCi of γ32P-ATP, 10μg of Myelin Basic Protein (MBP), 20mM TrisCl (pH 7.7), and 10mM MgCl2. The mixture was resolved on a SDS-PAGE gel and autoradiagraphed.
GSK3 kinase assay
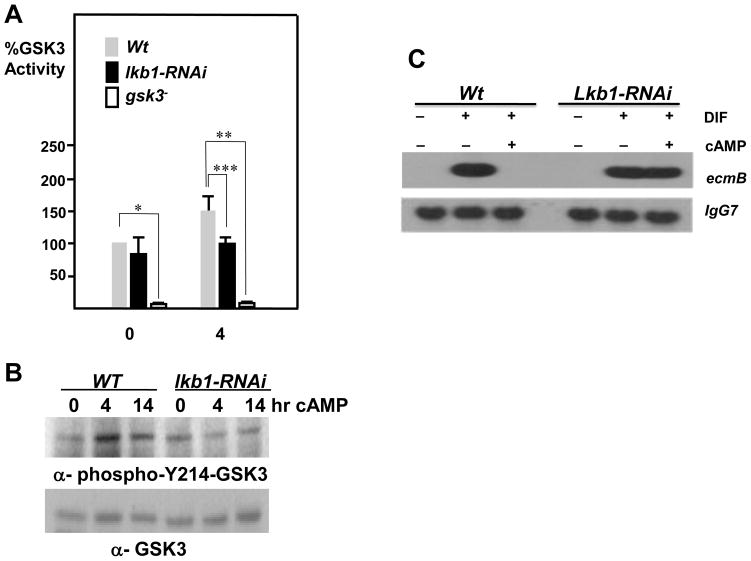
Whole-cell lysates were prepared from cells either pulsed with 50nM cAMP or with additional 4 hours and 15 hours of 300μM cAMP treatment. GSK3-specific kinase activities were determined by a peptide kinase assay (Plyte et al., 1999). GSK3 levels were normalized by western blot analysis with the anti-GSK3 antibody 4G-1E. Mean values of three independent experiments with standard deviations are shown (Fig 5A).
Fig. 5.
GSK3 is aberrantly regulated in lkb1 knockdown cells. (A) Wild type, lkb1 knockdown, and gsk3− cells were pulsed with 50 nM cAMP for 4 hours, and then stimulated with 300 μM cAMP for 4 or 14 hours as indicated. Whole cell lysates were prepared, and GSK3 kinase activities using the GSK3 specific phosphopeptide substrate were measured. Nonspecific kinase activities from LiCl treated samples were subtracted from the total values (Kim et al., 1999). Cells with lkb1-RNAi displayed little GSK3 activation induced by cAMP treatment. Relative values with standard errors are shown. *p<0.001 between wild type and gsk3− samples at time 0, Student’s t-test. **p<0.002 between wild type and gsk3− samples stimulated with cAMP for 4 hours, student’s t-test. ***p<0.0001 between wild type and lkb1-RNAi samples stimulated with cAMP for 4 hours, student’s t-test. (B) Western blot analysis of equivalent protein samples in (A) using anti-phospho-T172-AMPKα, anti-phospho-Y214-GSK3, and anti-total-GSK3. Results revealed lower levels of phospho-T172-AMPKα and phospho-Y214-GSK3 in lkb1 knockdown cells than in wild type cells. (C) Wild type or lkb1 knockdown cells were pulsed for 4 hours and then incubated for four more hours in suspension culture with ligands as indicated. The induction of ecmB messages was analyzed by northern blot analysis.
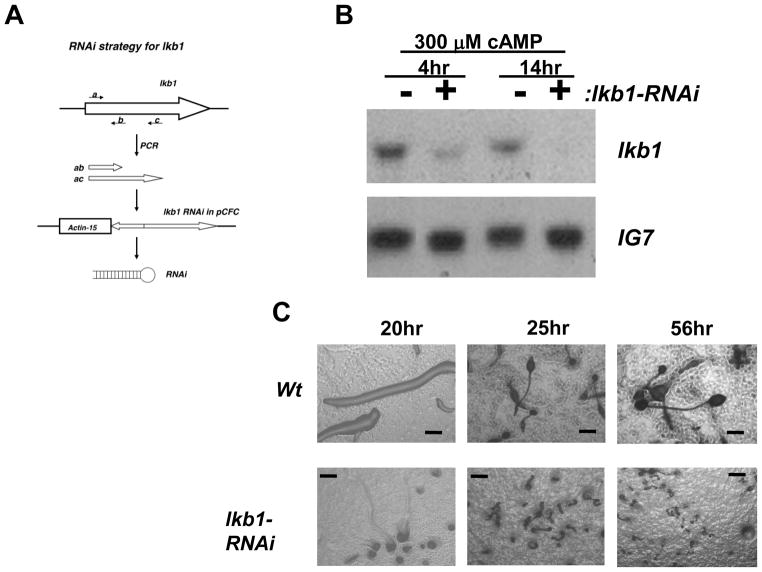
lkb1 RNAi strategy
The two PCR products encoding a partially redundant lkb1 sequence were amplified using the common forward primer (5′CGCCGGAGATCTATGGAAGTTGAACAACAACCATC3′) and either the reverse primer (5′CGCGGAAGCTTCAATTGGGTGC ACTGGTAAACC3′) or (5′CGCCGGAAGCTTCTTTATCGAGATCATTTGGAAATT C3′). The two reverse primers included the HindIII sequence, and the forward primer contained the BglII sequence. The two PCR products were ligated in the tail-to-tail manner through the HindIII site and subcloned into the TOPO vector using the BglII site. The final ligation product was released with EcoRI and cloned into the pCFC5 vector under the Actin-15 promoter, and confirmed by sequencing.
Results
Dictyostelium LKB1
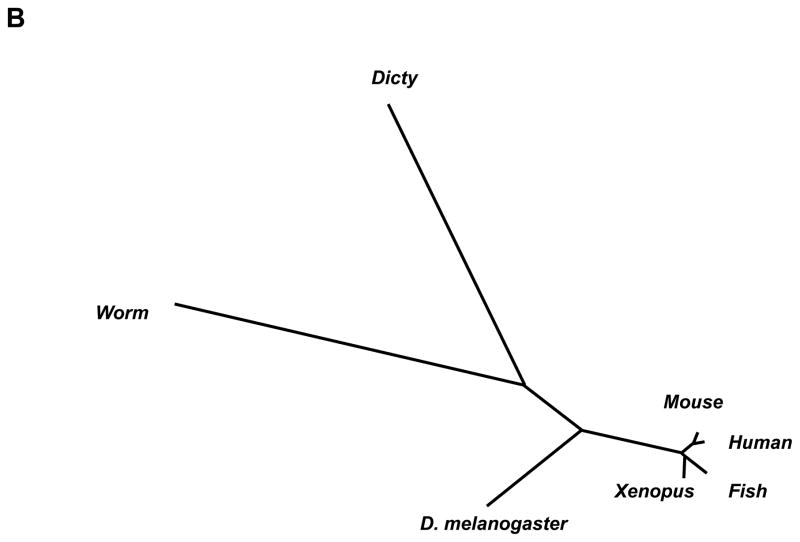
A single Dictyostelium lkb1 was identified and characterized. lkb1 encodes a potential serine/threonine kinase of 66 kD. As shown in Figure 1A, the kinase domain of LKB1 showed 60% of sequence similarity to C. elegans lkb1 ortholog par4. The C-terminal part of the lkb1 contains a number of homo-polymeric tracts and thus shows no significant homology with metazoan lkb1. Seven lkb1 sequences across the phyla were aligned using MAFFT software and the tree diagram was generated by the Neighbor Joining method (Fig. 1B).
Fig. 1.

Dictyostelium LKB1. (A) The kinase domain of LKB1 (40–301 amino acids, underlined) resides at the N-terminus, showing 60% of sequence similarity with C. elegans lkb1 ortholog. The C-terminal part of lkb1 contains a number of homo-polymeric tracts and thus shows no significant homology with metazoan lkb1. (B) Dictyostelium lkb1 and six other lkb1 sequences, NP_650302.1 lkb1 isoform B (Drosophila melanogaster), CE27410 (Caenorhabditis elegans), AAH77243.1 (Xenopus laevis), AAH07981.1 (Homo sapiens), NP_001017839.1 (Danio rerio), and BAA76749.1 (Mus musculus), were aligned using MAFFT software, and the tree diagram generated by the Neighbor Joining method is shown.
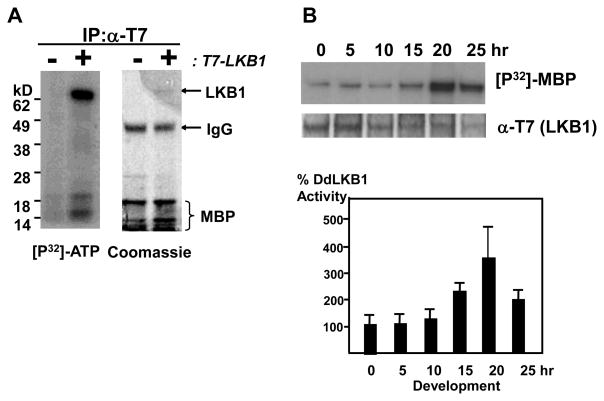
The full-length lkb1 tagged with T7 epitope (T7-LKB1) was constructed under the Actin 15 promoter and introduced into wild type cells. The T7-LKB1 immunopurified from vegetative cells exhibited autophosphorylation and a kinase activity toward Myelin Basic Protein (MBP) as an exogenous substrate (Fig. 2A). The level of Actin-15 promoter controlled LKB1 declined significantly after the aggregation stage, so to circumvent this problem, LKB1 was expressed under the CotC promoter, and the CotC promoter was used for measuring LKB1 kinase activities after 15 hours of development. LKB1 kinase activity was increased up to four fold around 20 hours of development (Fig. 2B).
Fig. 2.
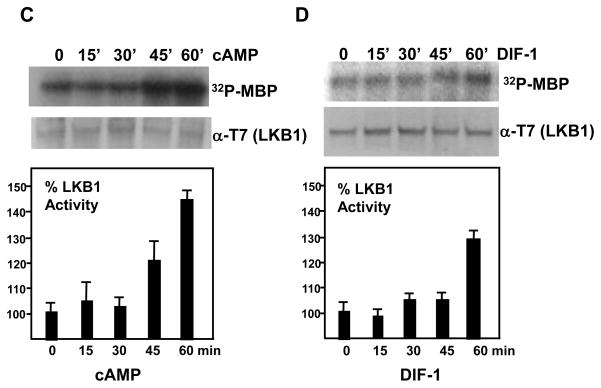
Dictyostelium LKB1 kinase activity increased during development. (A) Whole cell lysates from log phase wild type cells with or without Actin15::T7-LKB1 were prepared, and T7-LKB1 was immunoprecipitated with the anti-T7 antibody and subjected to the kinase assay. (B) LKB1 kinase activities were measured throughout development. T7-LKB1 was expressed under the Actin-15 (0, 5, and 10 hr) or CotC promoters (15, 20, and 25 hr) in wild type cells. LKB1 kinase activities are quantified after normalization of T7-LKB1 levels by western blot. (C) Wild type cells with Actin15::T7-LKB1 were pulsed for 4 hours and stimulated with 300 μM cAMP as indicated, and LKB1 kinase activities were measured. After an hour of cAMP stimulation, LKB1 activity was increased to ~40%. Relative kinase activities with standard errors are shown. (D) Wild type cells with Actin15::T7-LKB1 were pulsed for 4 hours and stimulated with 200nM DIF-1 as indicated. The relative LKB1 kinase activities with standard errors are shown. The LKB1 activity was increased ~30 % after an hour of DIF-1 stimulation.
To determine if LKB1 activity is regulated through cAMP or DIF-1, the well-characterized morphogens of Dictyostelium development, cells were pulsed with cAMP for 4 hours to become differentiation competent, and then were subsequently stimulated with cAMP or DIF-1. In response to both cAMP and DIF-1, LKB1 kinase activities were measured after normalization by western blot analysis. The LKB1 kinase activity remained relatively constant for 30 to 45 minutes post-stimulation. During a prolonged lag period of 30 minutes, LKB1 kinase activity increased up to 30~40% (Fig. 2C and D), which contrasts from that of other cAMP responsive kinases such as ZAK1 and GSK3. The activity of these kinases increased over 200% in 10 minutes of cAMP stimulation right after 4 hours of cAMP pulsing (Kim et al., 1999).
LKB1 regulates prespore gene expression
To determine the role of LKB1, lkb1 knockout constructs were generated. However, even after repeated attempts, it was not possible to create cells lacking lkb1. As an alternative, an RNAi based knockdown construct for lkb1 was generated (Fig. 3A). Cells with the lkb1 knockdown construct displayed near complete ablation of the lkb1 message, whereas the level of Ig7 control transcript was not affected (Fig. 3B). When developed on starvation agar plates, lkb1 knockdown cells exhibited severe developmental defects. Even after 56 hours, no wild-type like fruiting bodies were observed and only minute and aberrantly shaped structures were observed (Fig. 3C).
Fig. 3.
LKB1 knockdown significantly interfered development. (A) LKB1 knockdown strategy to inhibit LKB1 function. The shorter PCR fragment (ab) contains only the common region of 817 base pairs, whereas the longer one (ac) contains extra 546 base pairs. The two PCR products were ligated in a tail-to-tail orientation and subcloned under the Actin-15 promoter to produce the stem loop structure. (B) Total RNA samples prepared from wild type cells or cells expressing the lkb1 knockdown construct (lkb1-RNAi) were pulsed for 4 hours with 50 nM cAMP and further stimulated with 300 μM cAMP for 4 or 14 hours. Levels of lkb1 and Ig7 were determined by northern blot. (C) Wild type cells or cells expressing the lkb1 knockdown construct were plated on DB agar plates. lkb1 knockdown cells displayed delayed aggregation and severely hampered development. Slugs were aberrantly small, and no wild-type like fruiting bodies were observed even at 56 hours. Scale bars are 100 μm.
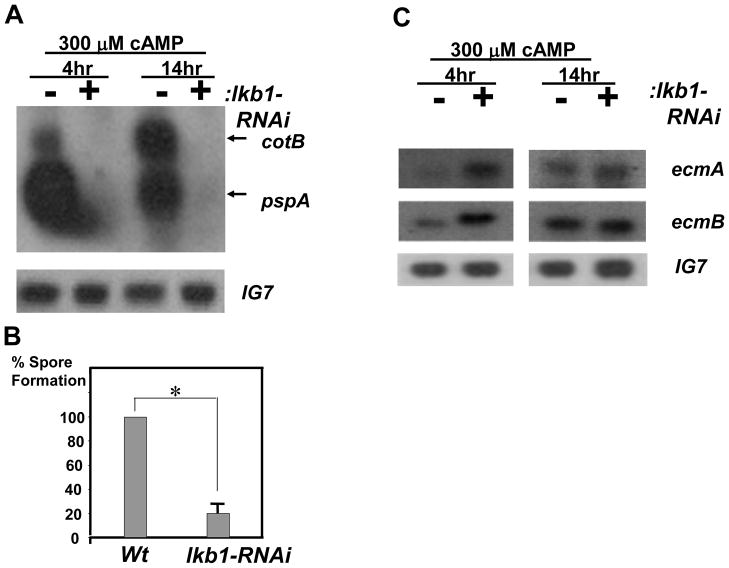
lkb1 knockdown cells expressed little prespore differentiation markers in suspension culture (Fig. 4A) and displayed ~ 20% of spores compared to wild type cells when developed on solid substrata (Fig. 4B). The lkb1 knockdown effect on the prespore/spore pathway was, therefore, less stringent for cells on a solid surface where cell-cell interaction could have played a role in assisting cells to generate low levels of spores. In contrast, lkb1 knockdown cells displayed higher levels of prestalk markers ecmA and ecmB at 4 hours of cAMP stimulation in suspension than wild type cells (Fig. 4C).
Fig. 4.
lkb1 knockdown severely altered prespore and prestalk differentiation. (A) Total RNA samples were prepared from wild type cells or from lkb1-RNAi cellsas previously described in Fig. 3B. Levels of prespore markers pspA and cotB in wild type cells and cells expressing the lkb1-RNAi construct were determined by northern blot. The prespore markers pspA and cotB were not detected in cells expressing the lkb1-RNAi construct, whereas Ig7 transcripts were not affected. (B) The relative efficiency in spore formation of lkb1-RNAi cells compared to wild type cells was determined by counting spores formed on nitrocellulose filters after 25 hours. The mean efficiency of spore formation from lkb1-RNAi cells was 18% with a standard error of 9. The *P value was less than 0.001, Student’s t-test. (C) Levels of prestalk markers ecmA and ecmB in wild type cells or cells expressing the lkb1-RNAi construct were developed in suspension culture as described in (A), and their levels were compared by northern blot analysis.
LKB1 is necessary for GSK3 activation
Similar developmental aberrancies exhibited by lkb1 knockdown and gsk3− cells invited an assay to compare GSK3 kinase activities between wild type and lkb1 knockdown cells. A GSK3 kinase assay using a primed peptide substrate specific for GSK3 demonstrated that lkb1 knockdown cells were very inefficient in activating GSK3 compared to wild type cells during cAMP-driven development in suspension culture (Fig. 5A). Consistent with the compromised GSK3 activation, lkb1 knockdown cells displayed lower levels of tyrosine phosphorylation of GSK3 at Y214 (Fig. 5B).
To further corroborate that LKB1 functions upstream of GSK3, we examined the cAMP-mediated inhibition of ecmB induction by DIF-1, which was compromised in cells lacking GSK3 or its activator ZAK1. As shown in Fig. 5C, prestalk cell marker ecmB was induced by DIF-1 but was almost completely inhibited by cAMP addition in wild type cells. In contrast, lkb1 knockdown cells persisted to express the ecmB message in response to DIF-1 and cAMP treatments. With reduced GSK3 activity and the compromised inhibition of DIF-1-mediated ecmB induction by cAMP in lkb1 knockdown cells, it is likely that LKB1 functions upstream of GSK3. It is currently not clear whether LKB1 and ZAK1 function in a single pathway or in parallel ones.
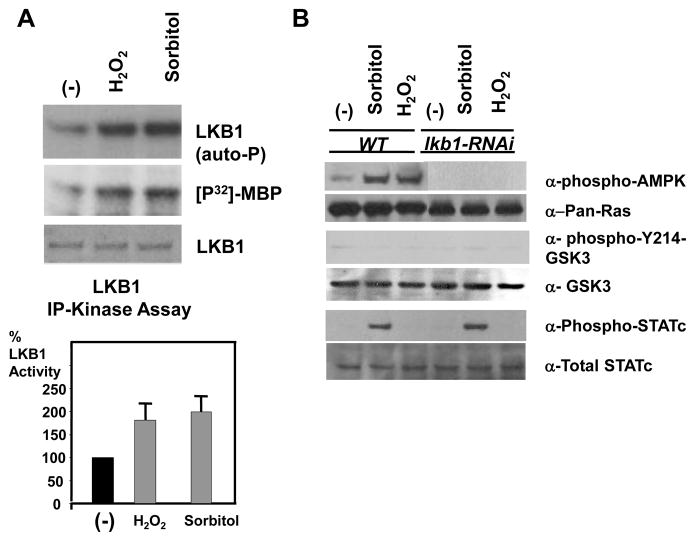
LKB1 is activated by osmotic and oxidative stress insults
LKB1 is essential for the activation of AMPK in response to various stresses in mammals (Woods, et al., 2003; Shaw et al., 2004). To determine if LKB1 responds to stress signals, cells were insulted with Sorbitol or hydrogen peroxide. Both types of stresses induced two-fold LKB1 activation (Fig. 6A), which is in contrast to the previous studies in mammalian cells showing only ~30% of a modest increase (Woods et al., 2003). The well-studied LKB1 target AMPK displays strong kinase activation in response to various stresses in mammalian cells (Woods, et al., 2003; Shaw et al., 2004). In response to osmotic or oxidative stresses, Dictyostelium AMPKα similarly became phosphorylated at Thr 172 of its activation loop in wild type cells, but not in LKB1 knockdown cells (Fig. 6B). In contrast, stress-induced STATc activation (Araki et al., 2003; Na et al., 2007), was not affected in lkb1 knockdown cells (Fig. 6B). Furthermore, LKB1 activation did not lead to GSK3 activation under stress (Fig. 6B), even though LKB1 was necessary for GSK3 activation during development (Fig. 5). Thus, it seems that LKB1 can choose two distinct downstream pathways, one with GSK3 and the other without.
Fig. 6.
Osmotic and oxidative stresses induced LKB1 activation and AMPK phosphorylation. (A) Cells were stressed with 200mM sorbitol or 2mM H2O2, and LKB1 kinase activities were measured. Both insults induced a two fold activation of LKB1 activities with a ~10% of standard error. (B) Levels of specific phosphorylation on AMPK, GSK3, and STATc after stress insults were determined by western blot analysis. Wild type cells stressed with sorbitol or H2O2 displayed increased AMPK and STATc phosphorylation. In contrast, little AMPK phosphorylation was observed from lkb1 knockdown cells in response to sorbitol or H2O2 stress. STATc was phosphorylated normally in lkb1 knockdown cells. Ras, total GSK3, and total STATc western blots were included as normalization controls.
Discussion
Recent studies showed that metazoan LKB1 is involved in a number of cellular events such as the cell cycle, apoptosis, tumor suppression, development, and energy metabolism. In contrast, the function of the LKB1 ortholog outside the metazoan border has largely been unknown. Although yeast has AMPK and AMPK activators Pak1p, Tos3p, and Elm1p, they are likely functional homologues of mammalian CaM Kinase (Hawley et al., 2005; Hong et al., 2003). Dictyostelium LKB1 not only regulates AMPK and exhibits a high degree of sequence homology to mammalian LKB1, but also mediates multicellular development and stress responses. In addition to LKB1, Dictyostelium cells have genes homologous to Mouse protein 25 (MO25, DDB0218587), CaM Kinase (DDB0220010), AMPKα (snfA, DDB0215396), AMPKβ (prkab, DDB0237814), and AMPKγ (prkag, DDB0237813). Components of the mTOR signaling pathway such as TSC2 (DDB0233727), Rheb (DDB0229441), and the TOR kinase complex (reviewed in Wullschleger et al., 2006) were also found in Dictyostelium. The presence of these signaling components in Dictyostelium suggests that LKB1 would be central in orchestrating development and energy metabolism beyond the metazoan border. However, the mechanism of LKB1 mediated signal transduction may show some divergence: no clear orthologs for metazoan pseudokinase STRAD and TSC1 can be found in the Dictyostelium genome.
Similar to LKB1, a single GSK3 exists in Dictyostelium. GSK3 functions as a cell fate regulator for Dictyostelium prespore/prestalk fate pathways. In response to cAMP stimulation, low affinity receptor cAR3 and cAR4 antagonistically regulate GSK3 through tyrosine kinases ZAK1/ZAK2 and through a putative tyrosine phosphatase (Kim, et al., 2002, Kim et al., 2011). Although they are somewhat similar, metazoan Wnt pathways are distinct from Dictyostelium cAR/ZAK/GSK3 pathways. In the canonical Wnt pathway, Frizzled mediated signaling negatively regulates GSK3, whereas in one of the non-canonical Wnt pathways of C. elegans, the opposite is operating. LKB1 is reported to affect the canonical Wnt pathway in Xenopus as a negative regulator of GSK3 (Ossipova et al., 2003). It is, however, not clear if any of Wnt signaling pathways activate LKB1 kinase activity to mediate GSK3 inhibition.
Dictyostelium lkb1 knockdown cells displayed several developmental defects that are reminiscent of gsk3− cells. First, prespore cell differentiation was interfered significantly, and prestalk cells were fully induced precociously. Second, GSK3 kinase activity and phosphorylation of GSK3-Y214 were compromised. Third, the inhibition of DIF-1 induced ecmB expression by cAMP was compromised. These data strongly indicate that LKB1 functions upstream of GSK3. Further study will be necessary to determine how LKB1 functions positively at the upstream of GSK3. A question of interest is whether LKB1 interacts with PP2A/B56 and/or ZAK1/ZAK2.
lkb1 knockdown experiments also demonstrated that while LKB1 is activated during development and stress insults (Fig. 5B and 6B), GSK3 is activated only during development (Fig. 6B). Thus, there exist at least two distinct LKB1-mediated pathways, one mediating development, and the other, stress. It will be important to understand how LKB1 functions at the upstream of both AMPK and GSK3 during development, but excludes GSK3 in response to stress. In addition to the LKB1-mediated AMPK phosphorylation, which may inhibit protein synthesis and/or chemotaxis through TOR complexes, stress insults activate the transcription factor STATc to reorganize the Dictyostelium transcriptome to cope with the stress. Lkb1 knockdown cells displayed that STATc is normally activated by stress, underscoring the specificity of the Lkb1 knockdown phenotype. It would not be unreasonable to speculate that Dictyostelium LKB1 has additional functions besides what is described in this paper. Such possibilities may include cell polarization and morphogenesis during late development, in which LKB1 exhibits its maximal kinase activity.
Acknowledgments
This work is supported in part by NIH grant SC1CA143958 from the National Cancer Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alessi DR, Sakamoto K, Bayascas JR. LKB1-dependent signaling pathways. Annual Review of Biochemistry. 2006;75:137–163. doi: 10.1146/annurev.biochem.75.103004.142702. [DOI] [PubMed] [Google Scholar]
- Araki T, Tsujioka M, Abe T, Fukuzawa M, Meima M, Schaap P, Morio T, Urushihara H, Katoh M, Maeda M, Tanaka Y, Takeuchi I, Williams JG. A STAT-regulated, stress-induced signalling pathway in Dictyostelium. Journal of Cell Science. 2003;116:2907–2915. doi: 10.1242/jcs.00501. [DOI] [PubMed] [Google Scholar]
- Baas AF, Kuipers J, van der Wel NN, Batlle E, Koerten HK, Peters PJ, Clevers HC. Complete polarization of single intestinal epithelial cells upon activation of LKB1 by STRAD. Cell. 2004;116:457–466. doi: 10.1016/s0092-8674(04)00114-x. [DOI] [PubMed] [Google Scholar]
- Bokko PB, Francione L, Bandala-Sanchez E, Ahmed AU, Annesley SJ, Huang XL, Khurana T, Kimmel AR, Fisher PR. Diverse cytopathologies in mitochondrial disease are caused by AMP-activated protein kinase signaling. Molecular Biology of the Cell. 2007;18:1874–1886. doi: 10.1091/mbc.E06-09-0881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CF, Katz ER. Expression vector containing an N-terminal epitope tag for Dictyostelium discoideum. Biotechniques. 1998;25:22. doi: 10.2144/98251bm03. [DOI] [PubMed] [Google Scholar]
- Hawley SA, Pan DA, Mustard KJ, Ross L, Bain J, Edelman AM, Frenguelli BG, Hardie DG. Calmodulin-dependent protein kinase kinase-beta is an alternative upstream kinase for AMP-activated protein kinase. Cell Metabolism. 2005;2:9–19. doi: 10.1016/j.cmet.2005.05.009. [DOI] [PubMed] [Google Scholar]
- Hong SP, Leiper FC, Woods A, Carling D, Carlson M. Activation of yeast Snf1 and mammalian AMP-activated protein kinase by upstream kinases. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:8839–8843. doi: 10.1073/pnas.1533136100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoki K, Guan KL. Complexity of the TOR signaling network. Trends in Cell Biology. 2006;16:206–212. doi: 10.1016/j.tcb.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Inoki K, Ouyang H, Zhu TQ, Lindvall C, Wang Y, Zhang XJ, Yang Q, Bennett C, Harada Y, Stankunas K, Wang CY, He X, MacDougald OA, You M, Williams BO, Guan KL. TSC2 integrates Wnt and energy signals via a coordinated phosphorylation by AMPK and GSK3 to regulate cell growth. Cell. 2006;126:955–968. doi: 10.1016/j.cell.2006.06.055. [DOI] [PubMed] [Google Scholar]
- Kim L, Brzostowski J, Majithia A, Lee NS, McMains V, Kimmel AR. Combinatorial cell-specific regulation of GSK3 directs cell differentiation and polarity in Dictyostelium. Development. 2011;138(3):421–30. doi: 10.1242/dev.055335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim L, Harwood A, Kimmel AR. Receptor-dependent and tyrosine phosphatase-mediated inhibition of GSK3 regulates cell fate choice. Developmental Cell. 2002;3:523–532. doi: 10.1016/s1534-5807(02)00269-1. [DOI] [PubMed] [Google Scholar]
- Kim L, Liu JC, Kimmel AR. The novel tyrosine kinase ZAK1 activates GSK3 to direct cell fate specification. Cell. 1999;99:399–408. doi: 10.1016/s0092-8674(00)81526-3. [DOI] [PubMed] [Google Scholar]
- Lee S, Comer FI, Sasaki A, McLeod IX, Duong Y, Okumura K, Yates JR, Parent CA, Firtel RA. TOR complex 2 integrates cell movement during chemotaxis and signal relay in Dictyostelium. Molecular Biology of the Cell. 2005;16:4572–4583. doi: 10.1091/mbc.E05-04-0342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Koh H, Kim M, Kim Y, Lee SY, Karess RE, Lee SH, Shong M, Kim JM, Kim J, Chung JK. Energy-dependent regulation of cell structure by AMP-activated protein kinase. Nature. 2007;447:1017–U9. doi: 10.1038/nature05828. [DOI] [PubMed] [Google Scholar]
- Na JB, Tunggal B, Eichinger L. STATc is a key regulator of the transcriptional response to hyperosmotic shock. BMC Genomics. 2007;8 doi: 10.1186/1471-2164-8-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ossipova O, Bardeesy N, DePinho RA, Green JBA. LKB1 (XEEK1) regulates Wnt signalling in vertebrate development. Nature Cell Biology. 2003;5:889–894. doi: 10.1038/ncb1048. [DOI] [PubMed] [Google Scholar]
- Plyte SE, O’Donovan E, Woodgett JR, Harwood AJ. Glycogen synthase kinase-3 (GSK-3) is regulated during Dictyostelium development via the serpentine receptor cAR3. Development. 1999;126:325–333. doi: 10.1242/dev.126.2.325. [DOI] [PubMed] [Google Scholar]
- Shaw RJ, Kosmatka M, Bardeesy N, Hurley RL, Witters LA, DePinho RA, Cantley LC. The tumor suppressor LKB1 kinase directly activates AMP-activated kinase and regulates apoptosis in response to energy stress. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:3329–3335. doi: 10.1073/pnas.0308061100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods A, Johnstone SR, Dickerson K, Leiper FC, Fryer L, Neumann D, Schlattner U, Wallimann T, Carlson M, Carling D. LKB1 is the upstream kinase in the AMP-activated protein kinase cascade. Curr Biol. 2003;13(22):2004–8. doi: 10.1016/j.cub.2003.10.031. [DOI] [PubMed] [Google Scholar]
- Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;127:5–19. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]