SYNOPSIS
Acute graft-versus-host disease (aGVHD) remains a major cause of morbidity and mortality after allogeneic hematopoietic stem cell transplantation (HSCT) in children. Although 30–50% of children respond to corticosteroids as initial therapy, the optimal initial or second-line therapies have not yet been determined. Newer approaches with combination therapy, novel agents, monoclonal antibodies and/or cellular therapies show some promise but require prospective well-designed trials to establish their efficacy, and should include children. This chapter reviews the clinical presentation and treatment of aGVHD, as well as practical management guidelines for children with aGVHD.
Keywords: acute, GVHD, children, management
1. INTRODUCTION
The most significant immunological barrier to successful HSCT is aGVHD, which can result in life-threatening inflammation and tissue destruction. The current model of aGVHD continues to invoke three collaborative phases: (I) preparative regimen induced tissue damage; (II) priming and activation of donor T cells, with CD8 T cells being stimulated by residual host antigen presenting cells (APCs) and CD4 T cells being stimulated by donor APCs presenting host-derived antigens; and (III) target tissue damage induced directly by cytotoxic T cells and indirectly by inflammatory cytokines. In addition to αβ T cells, other cell populations that include natural killer (NK) cells, NK T cells and γδ T cells, conventional and plasmacytoid dendritic cells, and regulatory T cells (Tregs) appear to have important modulatory functions in aGVHD (reviewed in [1]) and further understanding may offer future novel approaches to management. Meanwhile, donor T cells that recognize disparate recipient alloantigens are central mediators of GVHD and remain the focus of current therapies.
The focus of this chapter is to guide the clinician in the various clinical presentations of aGVHD and initial (primary) therapy. Additionally, secondary therapeutic options are reviewed for children who have failed primary therapy and suggestions are offered recognizing that there is no standard approach. Most of what is known about aGVHD therapy has arisen from trials conducted in adults with or without children. Whenever specifics pertain to children, they are highlighted in this paper. The prevention of aGVHD includes the avoidance of known risk factors (predominantly HLA-disparity), immunosuppressive pharmacotherapies and cellular approaches that have been reviewed elsewhere in this book [2–9].
2. DIAGNOSIS AND CLASSIFICATION OF ACUTE GVHD
Historically, GVHD was categorized as 'acute' or ‘chronic’ based on time of presentation; GVHD before day 100 was known as 'acute,' and after day 100 it was known as 'chronic’. This classification was based upon patients transplanted with HLA-identical sibling bone marrow (BM) after receiving myeloablative conditioning. Over the last couple of decades, HCT has become more complex, particularly with the use of different stem cell sources (reviewed elsewhere in this edition), and the development of nonmyeloablative conditioning that is associated with delayed onset aGVHD. These advancements have made the distinction between acute and chronic GVHD based on time of onset no longer accurate. Therefore, it is preferable to recognize aGVHD by the clinicopathological constellation of combinations of inflammatory dermatitis, enteritis, and hepatitis, which reflects T-cell activation with generation of cytotoxic lymphocytes and inflammatory cytokines that cause tissue damage. Chronic GVHD (cGVHD) is now similarly recognized without reference to time after HCT by the presence of diagnostic or distinct cGVHD manifestations which resemble autoimmune diseases and is reviewed in this edition and elsewhere [10]. Thus, for example, secretory diarrhea or erythematous maculopapular dermatitis that follows a relapsing or indolent course is classified as late persistent aGVHD or, alternatively, cGVHD (with overlap syndrome) if classical manifestations of cGVHD are also present. It remains to be determined whether the type or duration of immunosuppressive therapy should differ based on these clinical distinctions.
GVHD Classification
The severity of aGVHD is determined by the degree (or stage) of involvement in each of the main target organs (skin, liver, and upper and lower gastrointestinal (GI) tract) based on accepted criteria that primarily include the extent of rash, magnitude of hyperbilirubinemia, volume of diarrhea, and presence of nausea (Table 1). Various combinations of skin, liver, and GI involvement are then used to assign an overall GVHD severity or grade, as per the modified Glucksberg criteria (Grade I–IV) most commonly or by the International Bone Marrow Transplant Registry Index (Grade A–D) less commonly (Table 2) [11–14].
Table 1.
Acute GVHD Staging
| Stage 0 | Stage 1 | Stage 2 | Stage 3 | Stage 4 | |
|---|---|---|---|---|---|
| Skin | No rash | Rash < 25% BSA | 25–50% | > 50% generalized erythroderma |
Plus bullae and desquamation |
| Gut | Adult: < 500 mL/d |
Adult: 500–1000mL/d |
Adult: 1001–1500 ml/d |
Adult: >1500 ml/d |
Severe abdominal pain +/− ileus, flank blood or melena |
| Child: < 10mL/kg/d |
Child: 10 −19.9 mL/kg/d |
Child: 20 – 30 ml/kg/d |
Child: > 30 ml/kg/d |
||
| UGI | - | Severe nausea/vomiting |
- | - | - |
| Liver | Bilirubin ≤ 2 mg/dL |
2.1–3 mg/dL | 3.1–6 mg/dL | 6.1–15 mg/dL | >15 mg/dL |
Table 2.
Acute GVHD Grading Systems
| Grade* | Skin† | Liver | GI | UGI |
|---|---|---|---|---|
| Consensus | ||||
| I | 1–2 | 0 | 0 | 0 |
| II | 3 | 1 | 1 | 1 |
| III | - | 2–3 | 2–4 | - |
| IV | 4 | 4 | - | - |
| IBMTR§ | ||||
| A | 1 | 0 | 0 | 0 |
| B | 2 | 1–2 | 1–2 | 1 |
| C | 3 | 3 | 3 | - |
| D | 4 | 4 | 4 | - |
Each grade is based on maximum stage for each involved organ
Each column identifies minimum stage for organ grade.
Modified as shown to include UGI GVHD.
Mild aGVHD (grade I or A) is essentially cutaneous GVHD (an erythematous maculopapular rash) involving ≤50% body surface area (BSA) and usually requires no change to systemic GVHD prophylaxis. A rash involving >50% BSA requires additional therapy as discussed below. When cellular injury is severe, skin aGVHD may manifest with bulla formation and skin ulceration. Regardless of surface area involved, this is a severe and often life-threatening form of GVHD (stage 4, overall grade IV).
aGVHD of the GI system may involve the upper GI tract causing anorexia, nausea and vomiting, and/or the lower GI tract causing profuse watery diarrhea with tenesmus, urgency and frequency. If severe (stage 4), lower GI GVHD may cause life-threatening, bloody diarrhea with cramping abdominal pain. Liver involvement is staged according to the degree of hyperbilirubinemia but is often preceded or accompanied by elevations of serum transaminases (especially alanine aminotransferase or ALT) and, slightly later, by elevations of serum alkaline phosphatase. aGVHD of the liver rarely occurs without other organ involvement. aGVHD of the gut and/or liver requires additional systemic therapy regardless of stage.
Diagnosis of GVHD
The clinical signs of aGVHD are not sufficiently pathognomonic to establish the diagnosis, especially when there is isolated organ involvement and a broad differential diagnosis always needs to be considered (Table 3). However, the combination of rash, nausea, and voluminous diarrhea, occurring at the time of, or early after, neutrophil engraftment makes the diagnosis very likely. Tissue biopsy is recommended to confirm a histological diagnosis of aGVHD and, most importantly, to exclude opportunistic infection or drug reaction. Skin biopsies, and/or upper and/or lower GI endoscopy and biopsies should be performed depending on the clinical signs and symptoms. Care should be taken performing duodenal biopsies because there is a greater risk for non-healing ulcerations and intramural hematoma. To avoid bleeding complications, platelet counts should be maintained above 50 × 109/L for at least 3 days after GI biopsies. It is important to note that the interpretation of biopsies performed within 3 weeks of myeloablative therapy may be problematic because it is difficult to separate cellular injury induced by chemoradiotherapy from GVHD. The histological hallmark of GVHD-induced cellular injury is apoptosis, observed in epidermal basal keratinocytes, bile duct or intestinal crypt epithelial cells, and often associated with infiltration by lymphocytes [15,16].
Table 3.
Differential diagnosis of aGVHD
| AGVHD Manifestation | Differential Diagnosis |
|---|---|
| Rash | Drug Reaction |
| Allergic Reaction | |
| Infection | |
| Regimen-related toxicity | |
| Diarrhea | Infection (viral, fungal) |
| Opiate withdrawal | |
| Abdominal Pain | Acute Pancreatitis |
| Acute cholesystitis (biliary sludge, stones, infection) | |
| Narcotic bowel syndrome | |
| Elevated liver enzymes | Sinsusoidal Obstruction Syndrome |
| Medication toxicities (e.g., azoles) | |
| Cholangitis lenta (sepsis) | |
| Biliary sludge syndrome | |
| Viral infections (CMV, EBV, hepatitis B) | |
| Hemolysis |
3. PRIMARY (INITIAL) TREATMENT OF aGVHD
Patients with aGVHD have traditionally continued on GVHD prophylaxis, most commonly a calcineurin inhibitor (CNI), and in some cases the CNI is combined with mycophenolate mofetil (MMF) or sirolimus. Additional therapy depends upon the initial grade of aGVHD, the particular organs involved, and is discussed below.
Mild aGVHD (skin only)
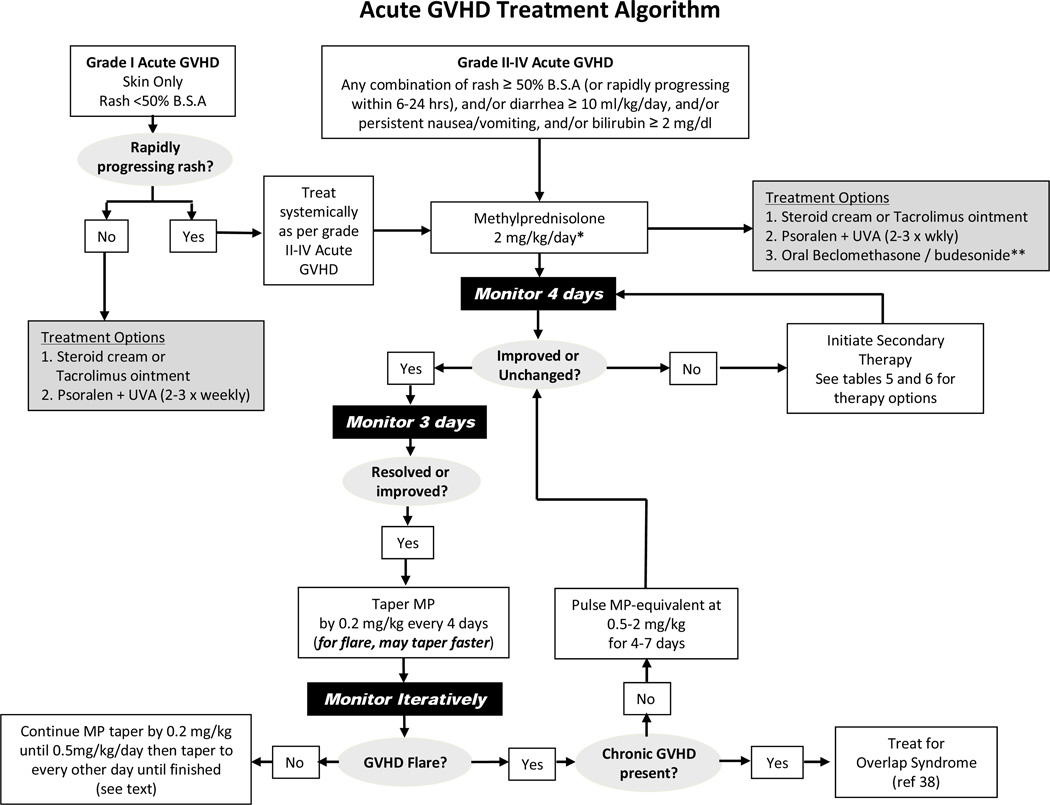
Grade I aGVHD (see Table 2) does usually not require systemic steroid therapy and close observation constitutes acceptable management. A symptomatic rash may be treated with topical therapy using creams or ointments (e.g. 0.1% triamcinolone or 0.1% tacrolimus to the body; 1% hydrocortisone cream to the face) applied 3–4 times daily. Ultraviolet A and psoralen (PUVA) may also be administered up to 3 times per week [17]. If the rash progresses after 3–4 days of topical therapy, or no improvement occurs after 7 days, systemic steroid therapy is needed as outlined below for moderate aGVHD (Figure 1).
Figure 1.
*Some centers choose to begin steroid dosing at < 2 mg/kg/day for grade II aGVHD (see text, Section 4)
**Seattle uses nonabsorbable gut steroids for children with Grade IIa aGVHD defined by anorexia, nausea, emesis or diarrhea < 20 mL/kg/day, no liver involvement, and rash covering < 50% of the body surface area and not progressing rapidly within the first 6–24 hours. Grade IIa aGVHD may be treated with a 10-day induction course of MP 1 mg/kg plus a 50-day course of nonabsorbable glucocorticoids delivered to the GI tract. If GI manifestations are progressing after 3 days of 1 mg/kg and nonabsorbable glucocorticoids then prednisone should be escalated to 2 mg/kg/day. In this latter scenario, the continuation of nonabsorbable glucocorticoids is optional but often invoked as prednisone-sparing therapy. If GI symptoms of anorexia, nausea and diarrhea <10 mL/kg/day without cramping have completely resolved after 10 days, then prednisone is usually successfully tapered rapidly over 1 week to 0.0625 mg/kg/day (or hydrocortisone 7.5 mg/m2 divided into 2–3 daily doses) and continued until 30 days after nonabsorbable glucocorticoids have stopped. This temporary physiological replacement therapy is arguably more relevant in small children compared to adults because adrenal suppression can be clinically relevant due to some amount of systemic absorption of the “nonabsorbable” glucocorticoids. A serum cortisol level < 19 mg/dL after cosyntropin-stimulation, or an early morning baseline cortisol < 3.6 mg/dL are often helpful to confirm a diagnosis of suspected adrenal insufficiency. Children with grade IIa GVHD whose diarrheal volumes are between 10–20 ml/kg/day, especially with abdominal cramping, may require a slower, more standard taper of prednisone.
Obstacles that have likely prevented the widespread adoption of this approach, particularly in children, as a standard practice include the fact that landmark beclomethasone (BDP) trials were conducted with a unique BDP formulation, orBec® (Soligenix, Princeton, NJ), which is an equipotent mix of plain and enteric-coated tablets designed to deliver 1 mg of BDP to the upper and lower GI tracts respectively four times daily in adults. Because the FDA’s Oncology Drugs Advisory Committee voted 7 to 2 that the phase III study results were insufficient for orBec® to garner approval in 2007 (reviewed further in [75]) this therapy remains commercially unavailable. Seattle currently mimics the approach by prescribing one capsule twice daily of EntocortEC® (Prometheus Lab, San Diego, CA) which contains 3 mg of micronized, enterically coated BDE, with 1 mg four times daily of oral BDP (U.S.P. material, Gallipot, St. Paul, MN, www.gallipot.com) emulsion that is compounded typically in corn or olive oil. Only 11 children (5 to 17 years of age) received BPD therapy among 231 patients who were treated during the three orBec® GVHD studies. Therefore, the efficacy of short course prednisone induction and oral BDP therapy for this indication in children has not been validated. Lastly, it is unclear whether children should receive similar dosing of topical GI glucocorticoids as adults. At least two other aGVHD studies of BDP [76] or budesonide (BED) [50] have included children and used adult dosing of BDE and BDP. By analogy in mild to moderate pediatric Crohn’s disease, an international survey found that Europeans favored the use of BDE over conventional glucocorticoid therapy [77]. Randomized controlled studies in pediatric Crohn’s disease have indicated that remission rates were similar in children (mostly teenagers) treated with BDE 3 mg three times daily versus prednisone 40 mg daily but side effects were significantly lower in the BDE treated group (32% vs 71%, p>0.05). Induction dosing of BDE 3 mg four times daily for 1 month followed by a taper resulted in a trend to higher remission rates without an increase in steroid-associated side effects [78].
Moderate to severe aGVHD
Moderate aGVHD is grade II-III aGVHD occurs in 35% – 80% of HCT recipients and is comprised of skin stage 1–3 and/or liver stage 1–3 and/or lower GI stage 1–4, with or without upper GI involvement by the modified Glucksberg criteria [13]. Severe aGVHD is grade IV GVHD at onset, or after progression or no response to moderate GVHD therapy. The conventional first line therapy for grade II-IV aGVHD is systemic glucocorticoids, which are lympholytic and decrease the inflammatory cytokine cascade of aGVHD. The conventional starting dose is 2mg/kg methylprednisolone (or prednisone equivalent) [18–20]. Patients with skin involvement may also receive topical therapy as discussed above.
4. OPTIONAL APPROACHES FOR AGVHD THERAPY
Modification of the methylprednisolone starting dose for patients with grade II aGVHD
An important goal of therapy is to minimize complications associated with high-dose glucocorticoid therapy. Therefore, some centers have attempted to begin treatment with methylprednisolone-equivalent doses < 2 mg/kg for milder GVHD within the spectrum of aGVHD manifestations that warrant systemic therapy as demonstrated by a large retrospective study of 733 patients [21]. This approach requires further validation, particularly for patients with grades III-IV aGVHD who were not well represented in this study. However, the study findings that overall mortality, relapse and non-relapse mortality were similar irrespective of whether patients began therapy with 1 mg/kg or 2 mg/kg methylprednisolone-equivalent doses, certainly seems generalizable for grade II aGVHD. Mean cumulative methylprednisolone-equivalent doses at day 100 remained approximately 50% lower for patients who began therapy at 1 mg/kg versus 2 mg/kg. In the multivariate analysis, the risks of invasive fungal infections (HR, 0.59; 95% CI, 0.3–1.0) and the duration of hospitalization (OR, 0.62; 95% CI, 0.4–0.9) were also reduced in the low dose methylprednisolone group. An important caveat to adopting the approach of intermediate dose glucocorticoid therapy for mild aGVHD is that methylprednisolone-equivalent dosing should be escalated to 2 mg/kg/day if aGVHD manifestations are progressing after 3 days of 1 mg/kg.
Nonabsorbable glucocorticoids for GI tract GVHD
Another strategy that attempts to reduce systemic glucocorticoid exposure has been to incorporate potent topically acting, nonabsorbable, glucocorticoids (beclomethasone dipropionate [BDP] or budesonide [BDE]) into the therapy of GI GVHD (see footnote to Figure 1 for details). McDonald and colleagues have shown that orally administered BDP is effective at controlling milder forms of grade II aGVHD which these studies defined as rash < 50% of the total body surface area (i.e., < stage 2), anorexia, nausea, emesis or diarrhea < 20 mL/kg/day (i.e., < stage 1) and without liver involvement (stage 0) [22–24]. This clinical phenotype has been named “Grade IIa” to delineate it from conventional Grade II disease which also includes more extensive rash and allows mild liver involvement. The latter two of these three BDP trials are the first and only randomized clinical trials to suggest a survival advantage for a new treatment of aGVHD (Table 4). Unfortunately these landmark BDP trials were conducted with a unique BDP formulation, orBec® (DOR BioPharma, Princetown, NJ), which is not currently commercially available. A redesigned Phase III study in adults with the intent of again seeking FDA approval is ongoing. Further trials in children are also warranted to understand the role of nonabsorbable glucocorticoids in the treatment of GI GVHD and to explore appropriate dose regimens for children.
Table 4.
Primary Therapy Trials in Acute GVHD
| Treatment | N | Design | Results and Conclusions | Ref |
|---|---|---|---|---|
| Prednisone* 2mg/kg |
443 | Retrospective single center. Era: 1990–1999; 40% cohort age < 20 yrs. |
Day 28 CR/PR 35%/20%. 1-yr OS 53% (95% CI, 48%–58%). Lower GI ± other organ had worse response. Better OS: age <20, T-replete, Gd I–II at onset, related or matched unrelated donor. |
[20] |
| Prednisone* 1 mg/kg v 2 mg/kg |
733 | Retrospective single center. Compared different prednisone starting doses. Era: 2000–2005. |
For grades II, GVHD control or mortality not compromised at 1 mg/kg v 2 mg/kg. Lower fungal infection rates and duration of hospitalization for 1 mg/kg. For grades III/IV, small numbers precluded definitive conclusions. |
[21] |
| Methylprednisolone 2 mg/kg v 10 mg/kg |
95 | Phase III, multicenter, T- replete MSD BMT. Crossover after 5 days to 10 mg/kg for non-responders at 2 mg/kg. |
Compared to 2 mg/kg, 10 mg/kg did not improve response rates (71% v 68%), TRM, OS, rates of CMV infection, or evolution to grade III-IV aGVHD. TRM 46% among the 55% of non-responders to 5 days of 2 mg/kg who were rescued with MP10 mg/kg versus 16% among responders (P=.007). |
[26] |
| Methylprednisolone 2 mg/kg v 5 mg/kg |
211 | Phase III multicenter. Eligibility: grade I–IV. Day 5 non-responders (N=61) randomized to MP 5 mg/kg or 5 mg/kg + rATG |
Day 5 CR rate 71% and patients tapered MP from D6. 5 yr TRM cumulative incidence 27% v 49% (P=.009), and OS 53% v 35% (P=.007) for responders and nonresponders respectively. No significant difference in response, TRM, OS between non-ATG and ATG groups. |
[33] |
| Prednisone* 1 mg/kg + orBec® v placebo |
129 | Phase III, multicenter. Eligibility: grade IIa (anorexia, vomiting, diarrhea < 1L). Randomized (1:1) to 10 days prednisone + 50 days of oral BDP or placebo. Prednisone rapidly tapered from Day 11. |
Among patients eligible for prednisone taper at study day 10, the risk of GVHD- treatment failure was lower in BDP arm at day 80 (HR 0.38). Day 200 posttransplant mortality lower in BDP arm (HR 0.33, P = .03); mostly explained by 91% reduction in D200 mortality for recipients of unrelated and mismatched grafts (HR 0.09, P = .02). Survival benefit durable to 1 yr after randomization. |
[24] |
| Prednisone* + Budesonide |
22 | Retrospective single center comparison of patients treated for GI GVHD with MP+BDE 3 mg TID to 19 MP-only historical controls. |
CR 77% in BDE group v 42% in controls. Two of 8 CRs in controls developed recurrent GI aGVHD during MP taper vs 0 of 17 in BDE group who continued BDE during MP taper. No severe intestinal infections occurred. |
[50] |
| Prednisone* 2 mg/kg + Anti-CD5 mAb v placebo |
243 | Phase III, single center, double blind trial. |
Higher Day 28 CR rate (40% vs 25% P = .019) but similar Day 42 CR rate (44% vs 38%), and 1 yr survival (49% vs 45%) in anti-CD5 group v placebo; no long term benefit of anti-CD5 immunotoxin. |
[12] |
| Methylprednisolone 60 mg/m2 v 40 mg/m2 + ATG |
96 | Phase III, single center, open label. Eligibility: REL/URD BMT. Intent-to-treat analysis. |
Day 42 CR/PR 76% in both arms. More CMV infections and more pneumonitis in MP/ATG arm. EBV-PTLD uncommon in either arm. Equivalent OS at Day 100, 6 months, and 2 yrs. ATG should be reserved as second-line therapy. |
[27] |
| Methylprednisolone 2mg/kg ± Infliximab |
58 | Phase III, single center, open- label |
CR+PR rates 63% (MP) v 66% (infliximab + MP) were similar. Similar death rates in both arms; mainly due to GVHD and relapse. |
[51] |
| Methylprednisolone 2mg/kg ± Etanercept |
61 | Retrospective single center analysis of Pilot (N=20) plus Phase II (N=41) prospective studies compared to contemporaneous MP only controls (N=99). |
Etanercept resulted in more CRs (69% v 33%; P <.001); similar for REL and URD donors. Elevated plasma TNFR1 levels decreased significantly only in patients with CR |
[29] |
| Methylprednisolone 2mg/kg ± Daclizumab v placebo |
102 | Phase III, multicenter, double- blinded. |
Inferior 1-yr OS in combination arm (29% v 60%; P = .002) attributed partly to increased relapse/GVHD-related mortality. Study closed early: worse 100-day OS in combination arm (77% v 94%; P=.02). Day 42 CR/PR rates (53% v 51%) |
[28] |
| Methylprednisolone 2mg/kg + Human MSCs |
32 | Phase II multicenter. Adults Randomization: 2 × 106 v 8.0 × 106 MSC/kg. GVHD grade at onset: II (21), III (8) and IV (3). |
Day 28 CR/PR 77%/16%. Both MSC doses similarly effective. No infusional toxicities. |
[52] |
| Methylprednisolone 2mg/kg + Human MSCs v placebo (1:1 ratio) |
192 | Phase III multicenter, double- blinded. Third party commercially prepared MSCs (Prochymal®, Osiris) |
No difference in proportion of patients surviving at least 90 days that achieve a CR by day 28 (45% v 46%) |
Osiris, press release |
| Methylprednisolone 2mg/kg + MMF or Etanercept or Pentostatin or Denileukin diftitox |
180 | Randomized phase II BMT CTN trial. MMF prophylaxis recipients (24%) were randomized to a non-MMF arm. At randomization: aGVHD was grade I–II (68%), III-IV (32%). Visceral organ involvement in 53%. |
Day 28 CR rates, 9-month OS %, and C.I. severe infections were: etanercept 26%, 47%, 48%, MMF 60%, 64%, 44%, denileukin 53%, 49%, 62%, pentostatin 38%, 47%, 57%. MMF identified as most promising arm and will be compared to MP alone in phase III. |
[30] |
Abbreviations: ATG; antithymocyte globulin, BDE; budesonide, BDP; beclomethasone dipropionate, CI; confidence interval, CMV; cytomegalovirus, CR; complete response, GI; gastrointestinal, MSD; matched sibling donor, MP; methylprednisolone, OS; overall survival, PR; partial response, REL; related, TID; three times daily, TRM; treatment related mortality, URD; unrelated.
Upfront addition of a second-line agent
Recognizing that the overall response of aGVHD to glucocorticoid therapy is roughly 50% (discussed below), and the durability of those responses is relatively unsatisfactory, several clinical trials have explored ways to improve outcomes; these are summarized in Table 4. These studies have included two randomized trials that showed no benefit to beginning therapy with methylprednisolone doses above 2 mg/kg per day [25,26]. Other studies have shown the potential benefit of adding several second-line agents to methylprednisolone as initial therapy for aGVHD but none has been shown definitively to be more efficacious and safe than methylprednisolone alone. For example, the results of controlled studies that explored the addition of polyclonal or monoclonal anti-T cell antibodies to 2 mg/kg methylprednisolone showed either no benefit [12,27] or resulted in inferior survival [28]. In one historically controlled phase II study, the addition of the anti-TNF fusion protein, etanercept, appeared to induce more complete responses [29]. However, among the 4-arms (etanercept, mycophenolate mofetil (MMF), denileukin diftitox, or pentostatin) of the recently reported prospective BMT Clinical Trials Network, randomized phase II study it was the combination of methylprednisolone plus MMF, rather than methylprednisolone plus etanercept, that showed most promise based on early study endpoints (Table 4) [30]. A follow-up phase III study by the Network to more definitively evaluate MMF in this context is imminent.
5. MONITORING OF RESPONSE AND TAPERING GLUCOCORTICOIDS
All patients with aGVHD should be monitored closely. If aGVHD progresses within 3–4 days or no improvement is observed after 5–7 days of methylprednisolone treatment then the GVHD is considered to be steroid refractory (SR-GVHD) and second line therapy is required as discussed further below. Progression is defined as worsening GVHD in ≥1 organ with or without amelioration in any organ.
If on the other hand, GVHD symptoms have resolved after 5–7 days of methylprednisolone therapy it is reasonable to attempt a taper of prednisone by 10% (of the starting dose) at 4 day intervals (depending on the tempo of the response) beginning 6–14 days after starting methylprednisolone. The goal of glucocorticoid therapy is to treat the acute inflammatory manifestations of GVHD and then to gradually taper the doses of prednisone (or methylprednisolone) as soon as possible, over the course of 5–6 weeks. The literature demonstrates that prolonged use of high-dose glucocorticoids for the treatment of aGVHD is associated with an increased risk for infection, relapse and death [26,31–37].
The literature has provided little direction in terms of how best to taper glucocorticoids in aGVHD. In fact, a European survey indicated that only 36% of 87 centers surveyed used a standard taper schedule [31]. Minnesota and Seattle use standardized glucocorticoid tapers and although certain details do vary the principles are similar. If prednisone can be successfully tapered as above then once the total daily dose reaches 0.2–0.3 mg/kg (or ≤20 mg) a transition is made to alternating day prednisone for the remaining 2–3 weeks of the taper in order to facilitate recovery of the adrenal axis.
After aGVHD manifestations have resolved and the prednisone taper proceeds, a recurrence (“flare”) of GVHD activity may emerge. It is important to delineate whether a patient continues to have aGVHD alone, or whether classic cGVHD signs begin to emerge. In the latter scenario of cGVHD with overlap syndrome it is often preferable to incorporate a less aggressive and more protracted course of immunosuppressive therapy into the plan (reviewed in [38]). If cGVHD is not present then it is reasonable to manage the first flare of aGVHD by boosting the dose of prednisone to 20 mg/m2 (0.67 mg/kg) for 7 days (Minnesota), or 2 mg/kg for 2–4 days (Seattle) and then, once the GVHD is under control, the taper resumes promptly towards the methylprednisolone-equivalent dose before the flare plus 0.2–0.4 mg/kg (Figure 1). Once steroids have been tapered off for at least a month, consideration can be made to start tapering other immunosuppressive agents, depending on how far out the patient is from HCT.
6. TREATMENT OF STEROID-RESISTANT ACUTE GVHD AND OUTCOMES
Approximately half of patients with aGVHD will respond to treatment with steroids as initial therapy, with approximately one third of patients having a durable response [18–20,39]. Factors most commonly associated with a favorable response include HLA matching for the GVHD vector, use of related donor grafts, and early onset of GVHD. Failure of primary therapy (steroid resistant [SR-aGVHD]) has been defined operationally as the progression of aGVHD symptoms beyond 3–4 days after starting methylprednisolone. Persistence of grade II-IV GVHD after one week of initial therapy also should be considered failure of response. The prognosis of aGVHD can be related to its overall severity (grade) and response to glucocorticoids [18,40]. It is of no surprise that grade III and IV SR-aGVHD, especially with visceral involvement, generally requires urgent initiation of effective second-line therapy.
Unfortunately, there is no generally accepted therapy for SR-aGVHD but agents that either have been considered, or continue to be explored are listed in Table 5. Published data for each therapy is summarized in Table 6 and includes key study design points, the era of therapy and whether or not the series included children. Polyclonal antithymocyte globulins (ATG), and more recently novel humanized or chimeric monoclonal antibodies, are generally used to treat life-threatening visceral manifestations where urgent control of GVHD is necessary. Unfortunately, longer term survival has been unusual when visceral manifestations are severe [41–46]. However, early administration of ATG within 14 days of primary therapy was reported in one study to be associated with improved survival [47]. It has remained difficult to improve the survival after SR-aGVHD because progressive organ dysfunction is often irreversible, and because second-line therapies constitute a “second hit” to an immune system that has already been impaired by cumulative exposure to high-dose prednisone. While more definitive evidenced based practices are needed, the current approach to second-line therapy in Minnesota for SR-aGVHD is equine ATG. In contrast, Seattle has opted for a customized approach based on the severity of involvement in the most involved organ(s). For the skin, PUVA is considered first for mild to moderate SR-aGVHD, while MMF or sirolimus are considered next for a rash that is moderately severe and/or PUVA resistant/intolerant. Extracorporeal photopheresis (ECP) is reserved for moderate to severe skin manifestations. For the intestinal tract, topical beclomethasone and budesonide are considered first for mild to moderate SR-aGVHD recognizing that topical gut steroids are often incorporated into primary therapy for children in Seattle as detailed in Figure 1 (footnote). The next consideration for moderate to severe intestinal manifestations is infliximab, with or without weekly ECP. Of note, infliximab 5mg/kg is used cautiously and generally for no more than 4 doses spaced weekly or every-other-week depending on the initial response. Since adopting routinely the approach of Ruutu et al [9] using ursodeoxycholic acid prophylaxis in all allogeneic HCT recipients, liver GVHD has occurred infrequently in Seattle. However, MMF and ECP are considered in resistant cases of liver GVHD that is mild to moderate, or moderate to severe respectively.
Table 5.
Therapy options for steroid-refractory aGVHD
| Therapy | Comments |
|---|---|
| SYSTEMIC | |
| Polyclonal | |
| Antithymocyte globulin ATGAMa, Thymoglobulinb | Delayed use appears to be very ineffective. Skin responds best. |
| Monoclonal | |
| Anti-CD2 (OKT3c, visilizumabd) | Currently used infrequently. |
| Anti-IL2 (daclizumabd, basilixumabe, inolimomabc) | Also depletes regulatory T cells. |
| Anti-TNFα (infliximabe) | Consider early for refractory lower GI tract. |
| Anti-CD52 (alemtuzumabd) | Depletes T & B cells (lower risk EBV PTLD) |
| Anti-CD2 (alefaceptf) | Selectively depletes memory T cells; needs further study. |
| Fusion Proteins | |
| Anti-IL2 (denileukin diftitox) | Also depletes regulatory T cells. |
| Anti-TNFα (etanercept) | |
| Macrolides and Antimetabolites | |
| Tacrolimus | Inhibits regulatory T cells |
| Sirolimus | Does not inhibit regulatory T cells. |
| Mycophenolate mofetil | Enteric coated formulation may minimize toxicity but liquid formulation not available |
| Extracorporeal Photopheresis | Particularly effective in skin, infrequently associated with opportunistic infections. |
| Mesenchymal Stem Cells | Large multicenter U.S. trial awaits analysis. |
| TOPICAL | |
| Glucocorticoids | |
| Budesonside | Useful as steroid-sparing agent in GI tract. |
| Beclomethasone | Useful as steroid-sparing agent in GI tract but not commercially available. |
| PUVA | Useful for skin only involvement. |
equine
rabbit
murine
humanized (and not commercially available)
chimeric murine-human
human IgG1-fusion protein
Table 6.
Secondary Therapy for Acute GVHD
| Treatment | N | Era | CR/PR % |
OS % |
Comments | Ref |
|---|---|---|---|---|---|---|
| ATGAM | 58 | 1996– 1999 |
30 | <10 | CR/PR: 79% (S), 40% (GI), 66% (L). Deaths: median 40 days after ATG (mostly infection or PD) |
[41] |
| ATGAM | 54 | 1989– 1998 |
NA | 11 | All patients had severe visceral GVHD | Martin (unpubl) |
| ATGAM | 29 | 1981– 1998 |
NA | 12 | 86% (mostly 3-system) Gd III/IV CR/PR: 72% (S), 38% (GI/L). cGVHD in 7/11 survivors. |
[53] |
| ATGAM | 79P | 1990– 1998 |
54 | 32 | Cohort: 43% < age 18 y, 43% Gr III/IV. CR/PR 61% (S ± L/GI) v 27% (GI/L). OS better if ATG within 14d of starting primary therapy (46% v 19%, P=.05) |
[47] |
| ATGAM | 69P | 1980– 1999 |
30 | 5 | Cohort: 59% < age 35 y, 61% Gr III/IV; 74% ≥ 2 organs. ATG initiated at median 25d after starting primary therapy. CR/PR 59% (S), 15% (L), 32% (GI). |
[54] |
| Thymoglobulin | 36 | 1996– 1999 |
59 | 6 | 89% (mostly 3-system) Gd III/IV CR/PR: 96% (S), 46% (GI), 36% (L). Major problem: Infections and 25% EBV PTLD rate |
[42] |
| Daclizumab | 43 | NA | 51 | 40 | Prospective phase I/II. Day 43 CR 47%. Day 120 OS 53% at OBD. Well tolerated. |
[46] |
| Daclizumab | 57P | 1993– 2000 |
54 | 25 | CR/PR 76% if age ≤ 18 yrs. Best responses: skin only and children. No infusion-related toxicity. Infectious complications in 95%. Careful patient selection and aggressive prophylaxis against viral and fungal infections recommended. |
[55] |
| Daclizumab + Infliximab |
22P | 2002– 2007 |
86 | 68 | Single center, consecutively treated children. Median response time 15d from start (2nd course given to 3/7 with recurrent GVHD). 13 viral reactivations, 4 probable fungal infections but only 2 infection related deaths (median f/u 31 mos). 7/22 developed cGVHD. |
[56] |
| Daclizumab | 13P | 2002– 2006 |
92 | 46 | At Day 30, CR 100% (S), CR/PR 50%/30% (GI), CR/PR 11%/55% (L). 50% developed cGHVD. Most effective in skin or low to moderate GI. |
[57] |
| Denileukin Diftitox |
32 | NA | 71 | 30 | Phase I, single center. OS 58% among 7/12 with CR. Reversible hepatic transaminitis in 22% at MTD. |
[45] |
| Infliximab | 21 | 1999– 2001 |
67 | 38 | Single center, retrospective evaluating 21 of 134 with SR-aGVHD who received single agent infliximab. CR 62%. Well tolerated. Particularly active in GI. Infection rates: Fungal (48%), viral (67%) and bacterial (81%). |
[44] |
| Infliximab | 32P | 2000– 2004 |
59 | 41 | Single center, retrospective. Cohort: 22% age < 14 y, Infliximab 2nd/3rd line: 44%/56%. Median of 3 doses. CR/PR 19%/40%. Best responses: GI, age < 35y, longer interval between HCT and infliximab start. Infection rate 72%. 13/19 responders survived a median 449 d (155–842d). 13/13 NR died early. |
[58] |
| Infliximab | 24P | 1998– 2001 |
82 | 46%* | Retrospective single center (all children). Infliximab 10 mg/kg x median 8 (range 1–162) doses. Evaluable (N=22); CR/PR 55%/27%. Good responses: S, GI. *OS 67% at 6 mo, 46% at 12 mo, 21% at 2 y. Recurrent GVHD common after stopping infliximab. Infection rates: 77% bacterial, 32% viral, 14% molds (invasive probable or proven). |
[59] |
| Etanercept | 13 | 1995– 2005 |
46 | 67* | Single center, retrospective (N=21, 8 had aGVHD). Etanercept 25 mg SQ twice/wk x 4 followed by 25 mg/wk x 4. Organ involvement at start of etanercept: S (14), GI (13), L (5), lung (5), oral (4). GI responses: 64%. In aGVHD: CR/PR = 4/2. Well tolerated. Infection rates: 48% CMV reactivation, 14% bacterial, 19% fungal. *median follow-up 429 d (range 71–1007 d) for all 21 patients. |
[60] |
| PUVA | 103 | 1994– 2000 |
24* | 51 | Median onset of PUVA was day 46 after HCT. PUVA 2nd/3rd line: 83%/17%. Median 16 treatments. Generally well tolerated; 8% discontinued for toxicity. *CR 24% (intent to treat); 37% among those who tolerated PUVA for 6 wks. Mean prednisone dose decreased from 1.6 mg/kg to 0.7 mg/kg by week 6. 57% required no additional therapy for skin GVHD. |
[17] |
| ECP | 21 | 1996 - NA |
60* | 57** | Median age 38 y. Donors: SIB or URD. CRs (* at 3 mo) by grade: 100% (II), 67% (III), 12% (IV) and by organ: 60% (S), 67% (L), 0% (GI). Commonest AEs were cytopenias. **Median follow-up 25 mo after HCT. OS-4 y 91% among CRs vs 11% non-CR. |
[61] |
| ECP | 59 | 1996 - NA |
79.5* | 47** | Phase II, single center. Weekly ECP. *CR/PR at a median of 4 cycles (1.3 mo). CR: 82% (S), 61% (L), 61% (GI). **OS at 4 y: 59% among CRs vs 11% non-CRs. Intensified ECP was highly effective in aGVHD. |
[62] |
| ECP | 77P | 1992– 2000 |
Included aGVHD (n = 33) at four pediatric hospitals. CR: 76% (S), 60% (L), 75% (GI). OS- 5y 69% among responders vs 12% for non- responders (P = .001). |
[63] | ||
| ECP | 31P | 1999– 2005 |
UNREL BMT. CR 73%, whereas the GR group had a CR rate of 56% by day 100. 2-y OS/PFS 57%/67% in the GR group v 85%/87% in the ECP group. |
[64] | ||
| ECP | 23 | 1996– 2006 |
52 | 48 | SR-aGVHD [gd II (10), III (7), IV (6)] Median duration of ECP 7 mo (1–33). CR 52% (no responses in Gr. IV). Twelve patients (52%) had complete responses. Average aGVHD grade decreased from 2.8 to 1.4 by day 90 (P=.08). Average MP dose decreased from 2.17 to 0.2 mg/kg/d (P=.004). CR: 66% (S), 27% (L), 40% (GI). Better responses if treated < 35 d from onset aGVHD. |
[65] |
| MMF | 36 | NA | 72 | 37* | Single center. MMF added to CsA and prednisolone. Overall grade improvement of aGVHD was found in 11 of 17 (65%) patients treated with MMF. Most common AEs: mild to modest cytopenias. *5-yr based on 48 pts (12 cGVHD) |
[66,67] |
| MMF | 19 | NA | 42 | 16* | Single center. 6CR and 2 PR. *2-yr |
[68] |
| Sirolimus | 21 | 1996– 1999 |
28* | 34* | Single center pilot trial (all ages). Sirolimus was started 19–78 (median 37) d after HCT for Gd III (10) or IV (11) SR-aGVHD. GVHD. High loading dose and/or high maintenance dosing of sirolimus. *Expected toxicities (cytopenias, hyperlipidemia and HUS) were frequent, associated with high serum concentrations, and likely limited efficacy. Among the18 who received ≥6 doses: 12 responded (5CR, 7PR). |
[69] |
| Pentostatin | 23 | NA | 76 | 26* | Phase I, single center. Universal lymphopenia and late infections was the DLT. Best responses in skin. * Median survival 85 d (5–1,258 d). Suggested dose for phase II was 1.5 mg/m2/d x 3 d. |
[70] |
| MSCs | 1 | NA | - | - | Landmark case of haploidentical hMSCs rescuing an adult with resistant grade IV GI and liver aGVHD. Clinical response was striking. The patient was alive and well after 1 year. |
[71] |
| MSCs | 8 | 2001– 2005 |
75 | 63 | Grades III-IV aGVHD and one with extensive cGVHD. Median MSC dose 1 ×106kg from a variety of donors. CR rate 75%. Five patients alive 2 mo to 3 yrs after HCT. |
[72] |
| MSCs | 55 | 2001– 2007 |
71 | 36 | Multicenter phase II in severe aGVHD using EGBMT ex-vivo MSC expansion procedure. Up to 5 doses MSCs; median dose 1.4×106/kg. CR+PR = 55% + 16%. No infusion toxicities. 2 yr-OS among CRs was 53% vs 16% (p=.018). |
[73] |
| MSCs | 12P | 2005– 2007 |
100* | 50* | Multicenter, Pediatric compassionate use protocol of 3rd party MSCs (Osiris) for Gd III (n=7), IV (n=5). MSCs given 2x/wk for 4 wks at 8×106/kg/dose in first 2 patients; 2×106/kg in next 10 patients. MSC began at median of 119 days post-HCT. No infusion toxicities. Follow-up 102d (range 36–756d). CR+PR = 50%+50%. Six of 12 patients died at median of 68d (range 36–185d) from start of therapy: MOF (3), infection (3). |
[74] |
| Human MSCs v placebo (2:1 ratio) |
260 | 2006– 2008 |
- | - | Phase III multicenter, double-blinded. Third party commercially prepared MSCs (Prochymal®, Osiris). *No improvement in durable CR rates (35% v 30%, primary endpoint) but hMSCs did improve durable liver CRs (29% v 5%, p=.046, N=61) and GI responses (88% v 64%, p=-.018, N=71) |
Osiris press release |
Abbreviations: ATGAM; equine antithymocyte globulin, BDE; budesonide, BDP; beclomethasone dipropionate, CI; confidence interval, CMV; cytomegalovirus, CR; complete response, GI; gastrointestinal, MSD; matched sibling donor, MP; methylprednisolone, OS; overall survival, PR; partial response, REL; related, TID; three times daily, TRM; treatment related mortality, URD; unrelated
included children.
7. ANTIMICROBIAL PROPHYLAXIS AND SUPPORTIVE CARE
aGVHD is by itself profoundly immunosuppressive as are the therapies used to treat it, leading to an extraordinarily high risk for opportunistic infections, and sepsis. Breakdown of skin and intestinal epithelia that occurs in more severe aGVHD forms adds to this risk. High dose prednisone increases the risk for cytomegalovirus (CMV) reactivation [34]. Similarly, invasive aspergillosis occurs more frequently in patients who develop CMV disease and in patients receiving higher doses of prednisone [36]. After nonmyeloablative HCT, high dose prednisone therapy for GVHD at the time of diagnosis of mold infection has been associated with an increased risk for mold infection-related death [37]. Therefore, antimicrobial prophylaxis is standard for these patients and always includes an agent to prevent Pneumocytis jirovecii pneumonia. Prophylactic and/or preemptive antiviral therapy based on serial monitoring of the blood for early viral reactivation are the main approaches used to avoid life-threatening disease caused by CMV and varicella-zoster. In the setting of profound T-cell lymphopenia which is often associated with SR-aGVHD or its treatment, monitoring for Epstein Barr Virus, Human Herpes Virus 6 and adenovirus is prudent to enable pre-emptive therapy, at least until there is some measure of T cell recovery. Fluconazole is used to prevent yeast (but not mold) infections within the first 75–100 days after HCT but mold-active azoles (e.g. voriconazole or posoconazole) or echinocandins are generally substituted when patients have SR-aGVHD or are otherwise receiving high dose glucocorticoids. Some centers use penicillin or extended spectrum quinolones to provide antibacterial prophylaxis for patients with aGVHD. Immunizations are not indicated for patients with SR-aGVHD as they are unlikely to respond immunologically. The exception is that the injectable form of the influenza vaccination is recommended but not the inhaled live attenuated form (i.e. no FluMist) if patients are at least 4 months from HCT [48]. Patients and their family household contacts should also be vaccinated with the injectable vaccine annually as there is the theoretical risk for shedding of live attenuated virus after receipt of the inhaled vaccine.
Adjunctive therapy with ursodeoxycholic acid may improve liver function and a randomized placebo-controlled multicenter study demonstrated that prophylaxis with ursodeoxycholic acid reduced hepatic problems, severe aGVHD, and improved survival after allogeneic HCT [9]. In patients with severe GI GVHD, a period of bowel rest and hyperalimentation are usually necessary. The somatostatin analogue, octreotide, may ameliorate large volume diarrhea to some extent [49]. Iatrogenic glucocorticoid induced myopathy and hyperglycemia needs to be kept in mind during the course of aGVHD management as both these complications require appropriate interventions.
SUMMARY
aGVHD remains a major cause of morbidity and mortality after allogeneic HCT in children. Currently, first-line aGVHD therapy relies on glucocorticoid-induced inhibition of inflammation and donor T cell alloreactivity, usually in combination with one or more of the agents chosen for aGVHD prophylaxis. Newer approaches are needed to improve upon the overall ≤50% durable response rates that have been achieved with systemic glucocorticoids. Because promising agents in small single center studies alone have not generally led to new standards for primary therapy of aGVHD it is essential to encourage (inter)national participation in well designed Phase II, and hopefully definitive Phase III studies so that answers to these important therapy questions can be obtained. Moreover, in the absence of any standard options for the therapy of SR-aGVHD it will be important that the roles of novel cell populations in the pathogenesis of aGVHD be further elucidated, so that follow-on clinical studies can test new approaches with strong rationales in SR-aGVHD. Ideally, the goal of new GVHD therapies should be to minimize the reliance upon glucocorticoid based therapy. Early inclusion of children in GVHD studies is essential so that approaches tested for the most part in adults can have a greater basis when generalized to children.
ACKNOWLEDGEMENTS
This work was supported by Grant Nos. CA18029 from the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Morris ES, Hill GR. Advances in the understanding of acute graft-versus-host disease. Br J Haematol. 2007;137:3–19. doi: 10.1111/j.1365-2141.2007.06510.x. [DOI] [PubMed] [Google Scholar]
- 2.Beatty PG, Hansen JA, Longton GM, et al. Marrow transplantation from HLA-matched unrelated donors for treatment of hematologic malignancies. Transplantation. 1991;51:443–447. doi: 10.1097/00007890-199102000-00034. [DOI] [PubMed] [Google Scholar]
- 3.Lee SJ, Klein J, Haagenson M, et al. High-resolution donor-recipient HLA matching contributes to the success of unrelated donor marrow transplantation. Blood. 2007;110:4576–4583. doi: 10.1182/blood-2007-06-097386. [DOI] [PubMed] [Google Scholar]
- 4.Storb R, Deeg HJ, Whitehead J, et al. Methotrexate and cyclosporine compared with cyclosporine alone for prophylaxis of acute graft versus host disease after marrow transplantation for leukemia. N Engl J Med. 1986;314:729–735. doi: 10.1056/NEJM198603203141201. [DOI] [PubMed] [Google Scholar]
- 5.Beatty PG, Clift RA, Mickelson EM, et al. Marrow transplantation from related donors other than HLA-identical siblings. N Engl J Med. 1985;313:765–771. doi: 10.1056/NEJM198509263131301. [DOI] [PubMed] [Google Scholar]
- 6.Nash RA, Antin JH, Karanes C, et al. Phase 3 study comparing methotrexate and tacrolimus with methotrexate and cyclosporine for prophylaxis of acute graft-versus-host disease after marrow transplantation from unrelated donors. Blood. 2000;96:2062–2068. [PubMed] [Google Scholar]
- 7.Cutler C, Li S, Ho VT, et al. Extended follow-up of methotrexate-free immunosuppression using sirolimus and tacrolimus in related and unrelated donor peripheral blood stem cell transplantation. Blood. 2007;109:3108–3114. doi: 10.1182/blood-2006-09-046219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kernan NA. T-cell depletion for the prevention of graft-versus-host disease. In: Thomas ED, Blume KG, Forman SJ, editors. Hematopoietic Cell Transplantation. 2nd Edition. Boston: Blackwell Science; 1999. pp. 186–196. [Google Scholar]
- 9.Ruutu T, Eriksson B, Remes K, et al. Ursodeoxycholic acid for the prevention of hepatic complications in allogeneic stem cell transplantation. Blood. 2002;100:1977–1983. doi: 10.1182/blood-2001-12-0159. [DOI] [PubMed] [Google Scholar]
- 10.Filipovich AH, Weisdorf D, Pavletic S, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and Staging Working Group report. Biol Blood Marrow Transplant. 2005;11:945–956. doi: 10.1016/j.bbmt.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 11.Glucksberg H, Storb R, Fefer A, et al. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donors. Transplantation. 1974;18:295–304. doi: 10.1097/00007890-197410000-00001. [DOI] [PubMed] [Google Scholar]
- 12.Martin PJ, Nelson BJ, Appelbaum FR, et al. Evaluation of a CD5-specific immunotoxin for treatment of acute graft-versus-host disease after allogeneic marrow transplantation. Blood. 1996;88:824–830. [PubMed] [Google Scholar]
- 13.Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus conference on acute GVHD grading. Bone Marrow Transplant. 1995;15:825–828. [PubMed] [Google Scholar]
- 14.Rowlings PA, Przepiorka D, Klein JP, et al. IBMTR Severity Index for grading acute graft-versus-host disease: retrospective comparison with Glucksberg grade. Br J Haematol. 1997;97:855–864. doi: 10.1046/j.1365-2141.1997.1112925.x. [DOI] [PubMed] [Google Scholar]
- 15.Sale GE. Pathology and recent pathogenetic studies in human graft-versus-host disease. Surv Synth Path Res. 1984;3:235–253. doi: 10.1159/000156929. [DOI] [PubMed] [Google Scholar]
- 16.Sale GE, Shulman HM, Gallucci BB, Thomas ED. Young rete ridge keratinocytes are preferred targets in cutaneous graft-versus-host disease. Am J Pathol. 1985;118:278–287. [PMC free article] [PubMed] [Google Scholar]
- 17.Furlong T, Leisenring W, Storb R, et al. Psoralen and ultraviolet A irradiation (PUVA) as therapy for steroid-resistant cutaneous acute graft-versus-host disease. Biol Blood Marrow Transplant. 2002;8:206–212. doi: 10.1053/bbmt.2002.v8.pm12014809. [DOI] [PubMed] [Google Scholar]
- 18.Martin PJ, Schoch G, Fisher L, et al. A retrospective analysis of therapy for acute graft-versus-host disease: Initial treatment. Blood. 1990;76:1464–1472. [PubMed] [Google Scholar]
- 19.Weisdorf DJ, Snover DC, Haake R, et al. Acute upper gastrointestinal graft-versus-host disease: Clinical significance and response to immunosuppressive therapy. Blood. 1990;76:624–629. [PubMed] [Google Scholar]
- 20.MacMillan ML, Weisdorf DJ, Wagner JE, DeFor TE, Burns LJ, Ramsay NK, Davies SM, Blazar BR. Response of 443 patients to steroids as primary therapy for acute graft-versus-host disease: comparison of grading systems. Biol Blood Marrow Transplant. 2002;8:387–394. doi: 10.1053/bbmt.2002.v8.pm12171485. [DOI] [PubMed] [Google Scholar]
- 21.Mielcarek M, Storer BE, Boeckh M, et al. Initial therapy of acute graft-versus-host disease with low-dose prednisone does not compromise patient outcomes. Blood. 2009;113:2888–2894. doi: 10.1182/blood-2008-07-168401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baehr PH, Levine DS, Bouvier ME, et al. Oral beclomethasone dipropionate for treatment of human intestinal graft-versus-host disease. Transplantation. 1995;60:1231–1238. [PubMed] [Google Scholar]
- 23.McDonald GB, Bouvier M, Hockenbery DM, et al. Oral beclomethasone dipropionate for treatment of intestinal graft-versus-host disease: a randomized, controlled trial. Gastroenterology. 1998;115:28–35. doi: 10.1016/s0016-5085(98)70361-0. [DOI] [PubMed] [Google Scholar]
- 24.Hockenbery DM, Cruickshank S, Rodell TC, et al. A randomized, placebo-controlled trial of oral beclomethasone dipropionate as a prednisone-sparing therapy for gastrointestinal graft-versus-host disease. Blood. 2007;109:4557–4563. doi: 10.1182/blood-2006-05-021139. [DOI] [PubMed] [Google Scholar]
- 25.Vogelsang GB, Hess AD, Santos GW. Acute graft-versus-host disease: Clinical characteristics in the cyclosporine era. Medicine. 1988;67:163–174. doi: 10.1097/00005792-198805000-00003. [DOI] [PubMed] [Google Scholar]
- 26.van Lint MT, Uderzo C, Locasciulli A, et al. Early treatment of acute graft-versus-host disease with high- or low-dose 6-methylprednisolone: a multicenter randomized trial from the Italian Group for Bone Marrow Transplantation. Blood. 1998;92:2288–2293. [PubMed] [Google Scholar]
- 27.Cragg L, Blazar BR, DeFor T, et al. A randomized trial comparing prednisone with antithymocyte globulin/prednisone as an initial systemic therapy for moderately severe acute graft-versus-host disease. Biol Blood Marrow Transplant. 2000;6:441–447. doi: 10.1016/s1083-8791(00)70036-x. [DOI] [PubMed] [Google Scholar]
- 28.Lee SJ, Zahrieh D, Agura E, et al. Effect of up-front daclizumab when combined with steroids for the treatment of acute graft-versus-host disease: results of a randomized trial. Blood. 2004;104:1559–1564. doi: 10.1182/blood-2004-03-0854. [DOI] [PubMed] [Google Scholar]
- 29.Levine JE, Paczesny S, Mineishi S, et al. Etanercept plus methylprednisolone as initial therapy for acute graft-versus-host disease. Blood. 2008;111:2470–2475. doi: 10.1182/blood-2007-09-112987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alousi AM, Weisdorf DJ, Logan BR, et al. Etanercept, mycophenolate, denileukin, or pentostatin plus corticosteroids for acute graft-versus-host disease: a randomized phase 2 trial from the Blood and Marrow Transplant Clinical Trials Network. Blood. 2009;114:511–517. doi: 10.1182/blood-2009-03-212290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ruutu T, Niederwieser D, Gratwohl A, Apperley JF. A survey of the prophylaxis and treatment of acute GVHD in Europe: a report of the European Group for Blood and Marrow Transplantation (EBMT). Chronic Leukaemia Working Party of the EBMT. Bone Marrow Transplant. 1997;19:759–764. doi: 10.1038/sj.bmt.1700745. [DOI] [PubMed] [Google Scholar]
- 32.Ruutu T, Hermans J, van Biezen A, et al. How should corticosteroids be used in the treatment of acute GVHD? EBMT Chronic Leukemia Working Party. European Group for Blood and Marrow Transplantation. Bone Marrow Transplant. 1998;22:614–615. doi: 10.1038/sj.bmt.1701377. [DOI] [PubMed] [Google Scholar]
- 33.van Lint MT, Milone G, Leotta S, et al. Treatment of acute graft-versus-host disease with prednisolone: significant survival advantage for day +5 responders and no advantage for nonresponders receiving anti-thymocyte globulin. Blood. 2006;107:4177–4181. doi: 10.1182/blood-2005-12-4851. [DOI] [PubMed] [Google Scholar]
- 34.Nichols WG, Corey L, Gooley T, et al. Rising pp65 antigenemia during preemptive anticytomegalovirus therapy after allogeneic hematopoietic stem cell transplantation: risk factors, correlation with DNA load, and outcomes. Blood. 2001;97:867–874. doi: 10.1182/blood.v97.4.867. [DOI] [PubMed] [Google Scholar]
- 35.Hakki M, Riddell SR, Storek J, et al. Immune reconstitution to cytomegalovirus after allogeneic hematopoietic stem cell transplantation: impact of host factors, drug therapy, and subclinical reactivation. Blood. 2003;102:3060–3067. doi: 10.1182/blood-2002-11-3472. [DOI] [PubMed] [Google Scholar]
- 36.Marr KA, Carter RA, Boeckh M, et al. Invasive aspergillosis in allogeneic stem cell transplant recipients: changes in epidemiology and risk factors. Blood. 2002;100:4358–4366. doi: 10.1182/blood-2002-05-1496. [DOI] [PubMed] [Google Scholar]
- 37.Fukuda T, Boeckh M, Carter RA, et al. Risks and outcomes of invasive fungal infections in recipients of allogeneic hematopoietic stem cell transplants after nonmyeloablative conditioning. Blood. 2003;102:827–833. doi: 10.1182/blood-2003-02-0456. [DOI] [PubMed] [Google Scholar]
- 38.Lee SJ, Vogelsang G, Flowers MED. Chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2003;9:215–233. doi: 10.1053/bbmt.2003.50026. [DOI] [PubMed] [Google Scholar]
- 39.Weisdorf D, Haake R, Blazar B, et al. Treatment of moderate/severe acute graft-versus-host disease after allogeneic bone marrow transplantation: An analysis of clinical risk features and outcome. Blood. 1990;75:1024–1030. [PubMed] [Google Scholar]
- 40.Hings IM, Severson R, Filipovich AH, et al. Treatment of moderate and severe acute GVHD after allogeneic bone marrow transplantation. Transplantation. 1994;58:437–442. doi: 10.1097/00007890-199408270-00008. [DOI] [PubMed] [Google Scholar]
- 41.Khoury H, Kashyap A, Adkins DR, et al. Treatment of steroid-resistant acute graft-versus-host disease with anti-thymocyte globulin. Bone Marrow Transplant. 2001;27:1059–1064. doi: 10.1038/sj.bmt.1703032. [DOI] [PubMed] [Google Scholar]
- 42.McCaul KG, Nevill TJ, Barnett MJ, et al. Treatment of steroid-resistant acute graft-versus-host disease with rabbitt antithymocyte globulin. J Hematother Stem Cell Res. 2000;9:367–374. doi: 10.1089/15258160050079470. [DOI] [PubMed] [Google Scholar]
- 43.Carpenter PA, Lowder J, Johnston L, et al. A phase II multicenter study of visilizumab, humanized anti-CD3 antibody, to treat steroid-refractory acute graft-versus-host disease. Biol Blood Marrow Transplant. 2005;11:465–471. doi: 10.1016/j.bbmt.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 44.Couriel DR, Saliba RM, Giralt S, et al. Acute and chronic graft-versus-host disease after ablative and nonmyeloablative conditioning for allogeneic hematopoietic transplantation. Biol Blood Marrow Transplant. 2004;10:178–185. doi: 10.1016/j.bbmt.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 45.Ho VT, Zahrieh D, Hochberg E, et al. Safety and efficacy of denileukin diftitox in patients with steroid-refractory acute graft-versus-host disease after allogeneic hematopoietic stem cell transplantation. Blood. 2004;104:1224–1226. doi: 10.1182/blood-2004-01-0028. [DOI] [PubMed] [Google Scholar]
- 46.Przepiorka D, Kernan NA, Ippoliti C, et al. Daclizumab, a humanized anti-interleukin-2 receptor alpha chain antibody, for treatment of acute graft-versus-host disease. Blood. 2000;95:83–89. [PubMed] [Google Scholar]
- 47.MacMillan ML, Weisdorf DJ, Davies SM, et al. Early antithymocyte globulin therapy improves survival in patients with steroid-resistant acute graft-versus-host disease. Biol Blood Marrow Transplant. 2002;8:40–46. doi: 10.1053/bbmt.2002.v8.pm11858189. [DOI] [PubMed] [Google Scholar]
- 48.Tomblyn M, Chiller T, Einsele H, et al. Guidelines for preventing infectious complications among hematopoietic cell transplant recipients: a global perspective. Bone Marrow Transplant. 2009;44:453–455. doi: 10.1038/bmt.2009.254. [DOI] [PubMed] [Google Scholar]
- 49.Ippoliti C, Champlin R, Bugazia N, et al. Use of octreotide in the symptomatic management of diarrhea induced by graft-versus-host disease in patients with hematologic malignancies. J Clin Oncol. 1997;15:3350–3354. doi: 10.1200/JCO.1997.15.11.3350. [DOI] [PubMed] [Google Scholar]
- 50.Bertz H, Afting M, Kreisel W, et al. Feasibility and response to budesonide as topical corticosteroid therapy for acute intestinal GVHD. Bone Marrow Transplant. 1999;24:1185–1189. doi: 10.1038/sj.bmt.1702055. [DOI] [PubMed] [Google Scholar]
- 51.Antin JH, Chen AR, Couriel DR, et al. Novel approaches to the therapy of steroid-resistant acute graft-versus-host disease. Biol Blood Marrow Transplant. 2004;10:655–668. doi: 10.1016/j.bbmt.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 52.Kebriaei P, Isola L, Bahceci E, et al. Adult human mesenchymal stem cells added to corticosteroid therapy for the treatment of acute graft-versus-host disease. Biol Blood Marrow Transplant. 2009;15:804–811. doi: 10.1016/j.bbmt.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 53.Remberger M, Aschan J, Barkholt L, et al. Treatment of severe acute graft-versus-host disease with anti-thymocyte globulin (Review) Clin Transplant. 2001;15:147–153. doi: 10.1034/j.1399-0012.2001.150301.x. [DOI] [PubMed] [Google Scholar]
- 54.Arai SM. Poor outcome in steroid-refractory graft-versus-host disease with antithymocyte globulin treatment. Biol Blood Marrow Transplant. 2002;8:155–160. doi: 10.1053/bbmt.2002.v8.pm11939605. [DOI] [PubMed] [Google Scholar]
- 55.Perales MA, Ishill N, Lomazow WA, et al. Long-term follow-up of patients treated with daclizumab for steroid-refractory acute graft-vs-host disease. Bone Marrow Transplant. 2007;40:481–486. doi: 10.1038/sj.bmt.1705762. [DOI] [PubMed] [Google Scholar]
- 56.Rao K, Rao A, Karlsson H, et al. Improved survival and preserved antiviral responses after combination therapy with daclizumab and infliximab in steroid-refractory graft-versus-host disease. J Pediatr Hematol Oncol. 2009;31:456–461. doi: 10.1097/MPH.0b013e31819daf60. [DOI] [PubMed] [Google Scholar]
- 57.Miano M, Cuzzubbo D, Terranova P, et al. Daclizumab as useful treatment in refractory acute GVHD: a paediatric experience. Bone Marrow Transplant. 2009;43:423–427. doi: 10.1038/bmt.2008.331. [DOI] [PubMed] [Google Scholar]
- 58.Patriarca F, Sperotto A, Damiani D, et al. Infliximab treatment for steroid-refractory acute graft-versus-host disease. Haematologica. 2004;89:1352–1359. [PubMed] [Google Scholar]
- 59.Sleight BS, Chan KW, Braun TM, et al. Infliximab for GVHD therapy in children. Bone Marrow Transplant. 2007;40:473–480. doi: 10.1038/sj.bmt.1705761. [DOI] [PubMed] [Google Scholar]
- 60.Busca A, Locatelli F, Marmont F, et al. Recombinant human soluble tumor necrosis factor receptor fusion protein as treatment for steroid refractory graft-versus-host disease following allogeneic hematopoietic stem cell transplantation. Am J Hematol. 2007;82:45–52. doi: 10.1002/ajh.20752. [DOI] [PubMed] [Google Scholar]
- 61.Greinix HT, Volc-Platzer B, Kalhs P, et al. Extracorporeal photochemotherapy in the treatment of severe steroid-refractory acute graft-versus-host disease: a pilot study. Blood. 2000;96:2426–2431. [PubMed] [Google Scholar]
- 62.Greinix HT, Knobler RM, Worel N, et al. The effect of intensified extracorporeal photochemotherapy on long-term survival in patients with severe acute graft-versus-host disease. Haematologica. 2006;91:405–408. [PubMed] [Google Scholar]
- 63.Messina C, Locatelli F, Lanino E, et al. Extracorporeal photochemotherapy for paediatric patients with graft-versus-host disease after haematopoietic stem cell transplantation. Br J Haematol. 2003;122:118–127. doi: 10.1046/j.1365-2141.2003.04401.x. [DOI] [PubMed] [Google Scholar]
- 64.Calore E, Calo A, Tridello G, et al. Extracorporeal photochemotherapy may improve outcome in children with acute GVHD. Bone Marrow Transplant. 2008;42:421–425. doi: 10.1038/bmt.2008.174. [DOI] [PubMed] [Google Scholar]
- 65.Perfetti P, Carlier P, Strada P, et al. Extracorporeal photopheresis for the treatment of steroid refractory acute GVHD. Bone Marrow Transplant. 2008;42:609–617. doi: 10.1038/bmt.2008.221. [DOI] [PubMed] [Google Scholar]
- 66.Basara N, Blau WI, Kiehl MG, et al. Efficacy and safety of mycophenolate mofetil for the treatment of acute and chronic GVHD in bone marrow transplant recipient. Transplant Proc. 1998;30:4087–4089. doi: 10.1016/s0041-1345(98)01351-7. [DOI] [PubMed] [Google Scholar]
- 67.Basara N, Kiehl MG, Blau W, et al. Mycophenolate Mofetil in the treatment of acute and chronic GVHD in hematopoietic stem cell transplant patients: four years of experience. Transplant Proc. 2001;33:2121–2123. doi: 10.1016/s0041-1345(01)01968-6. [DOI] [PubMed] [Google Scholar]
- 68.Nash RA, Furlong T, Storb R, et al. Mycophenolate mofetil (MMF) as salvage treatment for graft-versus-host-disease (GVHD) after allogeneic hematopoietic stem cell transplantation (HSCT): safety analysis. Blood. 1997;90 Suppl. 1:105a. #459. [Abstract] [Google Scholar]
- 69.Benito AI, Furlong T, Martin PJ, et al. Sirolimus (Rapamycin) for the treatment of steroid-refractory acute graft-versus-host disease. Transplantation. 2001;72:1924–1929. doi: 10.1097/00007890-200112270-00010. [DOI] [PubMed] [Google Scholar]
- 70.Bolanos-Meade J, Jacobsohn DA, Margolis J, et al. Pentostatin in steroid-refractory acute graft-versus-host disease. J Clin Oncol. 2005;23:2661–2668. doi: 10.1200/JCO.2005.06.130. [DOI] [PubMed] [Google Scholar]
- 71.Le Blanc K, Rasmusson I, Sundberg B, et al. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet. 2004;363:1439–1441. doi: 10.1016/S0140-6736(04)16104-7. [DOI] [PubMed] [Google Scholar]
- 72.Ringdén O, Uzunel M, Rasmusson I, et al. Mesenchymal stem cells for treatment of therapy-resistant graft-versus-host disease. Transplantation. 2006;81:1390–1397. doi: 10.1097/01.tp.0000214462.63943.14. [DOI] [PubMed] [Google Scholar]
- 73.Le Blanc K, Frassoni F, Ball L, et al. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet. 2008;371:1579–1586. doi: 10.1016/S0140-6736(08)60690-X. [DOI] [PubMed] [Google Scholar]
- 74.Prasad VK, Lucas KG, Kleiner GI, et al. Use of mesenchymal stem cells to treat pediatric patients with severe (grade III-IV) acute graft versus host disease refractory to steroid and other agents on a compassionate use basis. Blood. 2007;118(Part 1) 872A–873A #2971. [Abstract] [Google Scholar]
- 75.McDonald GB. Oral beclomethasone dipropionate: a topically active corticosteroid for the treatment of gastrointestinal graft-versus-host disease following allogeneic hematopoietic cell transplantation (Review) Expert Opinion on Investigational Drugs. 2007;16:1709–1724. doi: 10.1517/13543784.16.10.1709. [DOI] [PubMed] [Google Scholar]
- 76.Iyer RV, Hahn T, Roy HN, et al. Long-term use of oral beclomethasone dipropionate for the treatment of gastrointestinal graft-versus-host disease. Biol Blood Marrow Transplant. 2005;11:587–592. doi: 10.1016/j.bbmt.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 77.Levine A, Weizman Z, Broide E, et al. A comparison of budesonide and prednisone for the treatment of active pediatric Crohn disease. Journal of Pediatric Gastroenterology and Nutrition. 2003;36:248–252. doi: 10.1097/00005176-200302000-00017. [DOI] [PubMed] [Google Scholar]
- 78.Levine A, Kori M, Dinari G, et al. Comparison of two dosing methods for induction of response and remission with oral budesonide in active pediatric Crohn's disease: a randomized placebo-controlled trial. Inflammatory Bowel Diseases. 2009;15:1055–1061. doi: 10.1002/ibd.20881. [DOI] [PubMed] [Google Scholar]