Abstract
Epilepsy and seizures are very common in the early years of life and are often associated with significant morbidity and mortality. Identification of biomarkers for the early detection of epileptogenicity, epileptogenesis, comorbidities, disease progression and treatment implementation will be very important in implementing more effective therapies. This article summarizes the current needs in the search for new early life epilepsy-related biomarkers and discusses the candidate biomarkers that are under investigation, as well as the challenges associated with the identification and validation of these biomarkers.
Keywords: cognitive, development, electrophysiology, epileptogenesis, imaging, pathology
The identification of biomarkers for early life seizures and epilepsies is an essential goal in epilepsy research. Seizures are most common early in life, especially during the first year of life when their incidence peaks [1]. New-onset epilepsies, including many of the intractable epilepsies that persist through adulthood, are often first diagnosed at a young age, spanning from the perinatal period till the beginning of adulthood. Therefore, it is expected that the identification of biomarkers applicable for early life epilepsies may have wider benefits not only in pediatric epilepsies, but possibly also in epilepsies that would persist through adulthood.
However, utilization of the biomarkers identified in adults, whether validated in human patients or animal models, will not typically be the solution. The immature brain is not a micrograph of the adult brain; it operates under different protocols, evolving continuously until adulthood with asynchronous maturation patterns across species and developmental processes (Figure 1 & Table 1). A vivid reflection of this is the evolving behavioral manifestations of early life seizures, which start off as unique types of seizures (i.e., infantile spasms, ‘see-saw’ seizures) that subsequently give way to the classical focal-onset or generalized seizures seen in older patients. The consequences of seizures are also age-specific and further modified by a number of biological, genetic, epigenetic or experiential factors. Finally, drugs that are relatively safe in older subjects carry a risk of associated toxicity if given in certain vulnerable young ages [2], making it impossible to predict the tolerability of a treatment on the basis only of adult toxicology and safety studies.
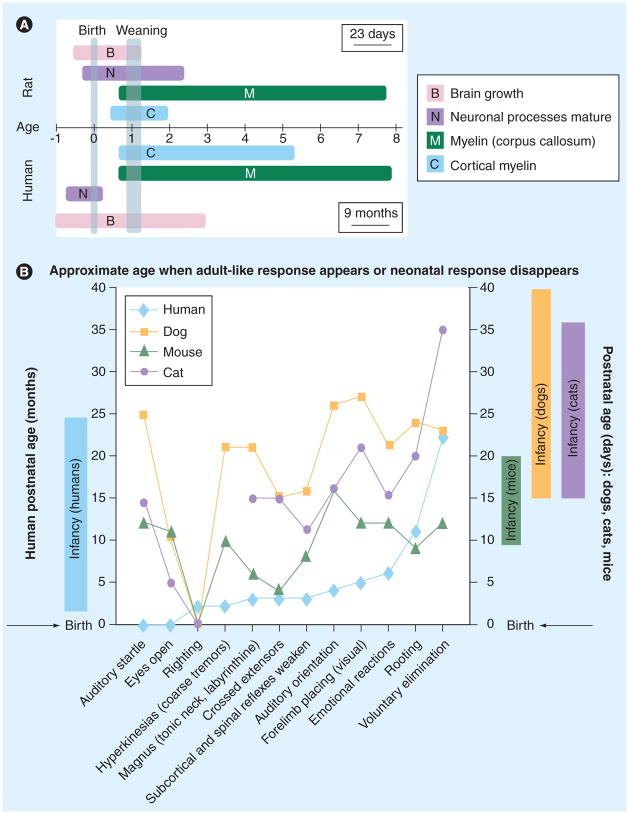
Figure 1. Asynchronous brain development across species and developmental processes.
(A) The temporal evolution of different developmental processes in the human and rat brain. The age units represent periods equivalent to the gestation: 23 days in rats, 9 months in humans [48,49,51].
(B) Developmental milestones in humans, dogs, mice and cats. Developmental milestones are expressed as the approximate age when adult-like response appears or the neonatal response disappears (data are from [50]). The currently accepted developmental periods equivalent to the infantile stage are presented in the boxes adjacent to the Y-axis for humans, mice, dogs and cats. Please note that many reflexes and behaviors that are maturing during the human infantile stage (righting reflex, tonic neck reflex, crossed extensors) have already matured before the equivalent infantile stage in rats.
Table 1.
Milestones in rat development
| Milestones | Age |
|---|---|
| Duration of gestation | 23 days |
| Peak of brain growth (equivalent to human full-term newborn) | PN8 |
| Considered equivalent to human full-term neonates | PN8–13 |
| Eye opening | PN13–15 |
| Weaning from dam | PN21 |
| Puberty onset | PN32–36 |
| Adulthood | >PN60 |
| Life expectancy | 2 years |
PN: Postnatal day.
Clearly, different strategies need to be adopted to satisfy the critical need of developing biomarkers for early life epilepsies. Yet, at present, the challenges are more obvious than the answers. Here, we will briefly highlight the needs, discuss the current status and challenges hindering progress, and attempt to provide strategies to overcome these.
What are the needs?
Biomarkers of epileptogenicity
There are several conditions that lead to the development of epilepsy, such as neonatal hypoxic ischemic encephalopathy, intracranial infections, tuberous sclerosis or brain malformations. However, not all subjects will develop epilepsy, sometimes even after they have experienced one seizure. Early identification of the epileptogenic potential will be paramount in selecting the patient population most likely to benefit from early initiation of a therapy. The desired features of these biomarkers include:
Specificity in differentiating the epileptic state from reactive changes resulting from an initial precipitating event or the first seizure, and from developmental processes that have not yet reached maturity;
Sensitivity in diagnosing epilepsy at the preclinical or early symptomatic stages, when clinical diagnosis has not yet been established;
Ability to detect the reversal of epileptogenicity, to prevent unnecessary continuation of treatments.
Biomarkers of epileptogenesis
The identification of highly predictive indicators of the progressive changes that are required for the development of the epileptic state will help identify:
New age-, sex-, and stage-specific targets for antiepileptogenic treatments;
Epilepsy progression, with sufficient specificity to dissect out potential confounding influences of ongoing developmental processes.
Biomarkers of comorbidities
Cognitive and/or behavioral comorbidities are a common accompaniment of early-onset epilepsies, whether these might be secondary to the primary cause or risk factor associated with the epilepsy or due to the ongoing seizures or their treatments [3,4]. Compared with age-matched peers, young patients with epilepsy have a three-times higher risk of early mortality and an increased risk of autism spectrum disorders [4–6]. Certain epileptic syndromes have a particularly higher association with these comorbidities. For instance, infantile spasms are associated with higher prevalence of mental retardation and autism, as well as increased mortality [6–11]. Biomarkers of risk for development or progression of these comorbidities will be necessary to initiate disease-modifying treatments, as these become available, and improve not only the quality of life, but also the cognitive gains during these formative years. Specifically, desired biomarkers would provide:
Early selection of the subpopulations at higher risk for specific epilepsy-related comorbidities;
Sensitive measures of progression or remission/cure from these comorbidities to guide the implementation of treatments;
Differentiation as to the contribution of underlying etiology, epilepsy or other coincident possible contributors, such as treatments, upon the presence or progression of the comorbidity.
Biomarkers of treatment implementation, tolerability or toxicity
Many antiepilepsy drugs have been shown to cause apoptosis in the brain if given in naive neonatal but not in older pups. Such studies have not yet been reported in pups receiving these treatments to stop seizures. The different biology and vulnerability of the developing brain raises the need to develop methods for early detection and monitoring of the effects of drugs on the developing brain. To better select, guide the implementation and improve the safety of a new treatment, such biomarkers would:
Provide target identification for treatment selection, distinguishing it from age-specific relevant processes;
Define the timing and therapeutic window of treatment administration, based on age- and sex-adapted criteria;
Distinguish the treatment-responsive from the resistant patient populations early;
Provide early risk identification and monitoring of treatment-related toxicities, based on age- and sex-adapted criteria, with sufficient specificity for the administered treatment;
Have the ability to localize the epileptogenic focus accurately and facilitate more effective ablative treatments, if medical treatments are not curative.
What do we have?
As elaborated in the adjoining reports, a number of putative biomarkers have been investigated, mostly in adults, and in certain cases in developing animals or pediatric patients. These include (Box 1):
Box 1. Potential biomarkers that need to be explored in pediatric epilepsy syndromes.
-
Neuroimaging
MRI
MRS
FDG-PET ± MRI
AMT-PET
Tractography
fMRI ± EEG
-
Electrophysiological
-
EEG
Interictal epileptiform activity
Focal slowing
Fast ripples
Paroxysmal fast (EEG)
MEG/MSI
TMS (paired pulse TMS, navigated TMS)
Cardiorespiratory monitoring
-
Cognitive
Immunological
CSF/neuropathology
Genomics
Epigenetics
AMT: α-[11C]-methyl-L-tryptophan; CSF: Cerebrospinal fluid; FDG: Fluorodeoxyglucose; fMRI: functional MRI; MEG: Magnetoencephalography; MRS: Magnetic resonance spectroscopy; MSI: Magnetic source imaging; TMS: Transcranial magnetic stimulation.
Clinical or historical
Electrophysiological
Molecular or metabolic
Anatomical or structural
Clinical or historical
Studies revolving around the prognosis of initial precipitating events (i.e., perinatal hypoxia, intracranial infections, tuberous sclerosis or brain malformations trauma, febrile seizures) and their clinical attributes (i.e., febrile status epilepticus and severity of brain traumatic injury), or the relevance of prior neurological diseases, to the pathogenesis of specific epilepsy syndromes have greatly advanced the field. These risk factors have a low individual predictive value for subsequent epileptogenesis. Nevertheless, they will be useful as landmarks to identify biomarkers of epileptogenicity or epileptogenesis in specific populations and define time windows for early intervention, as exemplified by the Consequences of Prolonged Febrile Seizures in Childhood (FEBSTAT) study, which is discussed in this issue of Biomarkers in Medicine by Gomes and Shinnar [12]. As multiple factors appear to alter outcomes, it is expected that a constellation of such clinical/historical factors (e.g., age at onset, severity, types of events) will be necessary to form the proper context for more accurate interpretation of the validity of the relevant putative biomarker. Cognitive biomarkers, such as neuropsychological tests and behavioral evaluation, are also in use for the early diagnosis and monitoring of progression of cognitive comorbidities, including cognitive decline and autism spectrum disorders.
Electrophysiological
The routine EEG study has been the mainstay of diagnostic evaluation of patients investigated for seizures. In the presence of a first unprovoked seizure, epileptiform or focal EEG abnormalities predict high risk for recurrence of seizures (50–78%) [13–18]. In the absence of a first unprovoked seizure, the utility of EEG abnormalities as potential biomarkers of outcomes following an initial precipitating event has been investigated in several studies. For example, in the acute period following a first episode of febrile status epilepticus, several studies have demonstrated increased incidence of focal slowing in the EEG (34–47%), which was associated with a 33% risk of developing epilepsy [12,19,20]. Longitudinal studies evaluating the evolution and predictive power of electrophysiological abnormalities preceding the onset of clinical seizures and epilepsy in specific clinically recognizable pediatric populations, as currently attempted by the FEBSTAT study, will be invaluable in optimizing their use as surrogate markers or, hopefully, biomarkers in epilepsy and its comorbidities. As elaborated in the article by Worrell and Gotman, in this issue, of particular clinical significance is the use of electrophysiological abnormalities as a means to localize the epileptogenic focus and define the borders of effective resective surgeries in young patients with drug-resistant early-onset epilepsies [21]. Promising localizing biomarkers that are currently under clinical investigation include the interictal fast ripples [22,23], and the interictal paroxysmal fast activities (highly associated with infantile spasms) [24,25]. In addition to EEG studies, interictal magnetoencephalography/magnetic source imaging [26] may be helpful. Furthermore, paired pulse transcranial magnetic stimulation (TMS) is currently under evaluation as a tool to assess cortical excitability for diagnostic or prognostic purposes in patients with generalized epilepsy of genetic origin or with drug-resistant focal epilepsy [27,28]. Navigated TMS has been helpful in preliminary studies as a localizing tool in cortical mapping or for epileptogenic focus delineation in patients with drug-resistant epilepsy, including in pediatric patients [29,30].
Anatomical/structural
Both human and experimental studies have demonstrated that an underlying lesion may influence the development and outcomes of epilepsy. The presence of a lesion, including but not limited to mesial temporal sclerosis, may identify subpopulations of young patients who may not eventually respond to medical treatments [31]. In patients with identifiable initial precipitating events, such as febrile status epilepticus, longitudinal follow-up with MRI, starting at the time of the initial event, showed preliminary promise in the early identification of patients that eventually developed mesial temporal sclerosis and epilepsy [32,33]. However, several challenges need to be accounted for. First, brain growth is not completed yet at the pertinent ages when febrile seizures occur. Hence, not only age- and sex-appropriate volumetric markers need to be used for each MRI, but also longitudinal volumetric studies to monitor the regional brain growth (Figure 1 & Table 1). Second, the acute T2 signal enhancement, seen on the first MRI following the febrile status epilepticus, may eventually normalize, regardless of whether epilepsy may develop [32]. Therefore, the early MRI abnormalities may either be acute reactive but transient changes (i.e., swelling) [34], or may produce functional network disruption or minimal pathology, below the detection threshold of the MRI, which may still be sufficient for epilepsy to develop. It is promising that the preclinical data on the progressive MRI abnormalities in poststatus epilepticus young rats also agree with its potential as a possible early biomarker of epileptogenesis (see review by Nehlig [39]). However, these studies also caution that such imaging abnormalities are not just limited within or specific for the epileptogenic focus, and emphasize the critical importance of correlating them with independent localizing or prognostic biomarkers to improve the predictive value [35]. Indeed, ongoing longitudinal studies are evaluating whether correlation of longitudinal MRI studies with EEG abnormalities or susceptibility genes may provide a more accurate, earlier, and perhaps less expensive approach in selecting out young patients at risk for subsequent epilepsy, following the first febrile status epilepticus [12].
MRI has also been indispensable in the pre-surgical evaluation of drug-resistant epilepsies. To further increase the accuracy of localizing the epileptogenic focus and defining the minimal necessary borders of the region to be resected, a combination of imaging, electrophysiological and/or functional localizing techniques has been used, including combinations of EEG, electrocorticography, fluorodeoxyglucose PET, MRI, magnetoencephalography/magnetic source imaging, functional MRI or paired pulse or navigated TMS, magnetic resonance spectroscopy [22–24,26–30,36,37].
Molecular/metabolic
A number of molecular or metabolism-related targets have been implicated in epileptogenesis, in preclinical studies [38,39]. These include components of different neurotransmitter systems (e.g., GABAergic, glutamatergic), ion channels, inflammatory pathways, transcriptional factors, or signaling pathways that are central to many developmental and immune processes (i.e., mTOR). Their proposed involvement has been documented in specific animal models of temporal lobe epilepsy, of febrile seizures, of generalized seizures, or of hypoxia ± ischemia, as well as in genetic models or models of infantile epileptic encephalopathies. Some of these changes have been better characterized than others, in regards to the timeline they follow, their dependence by age, sex, seizure/epilepsy model, and their importance (rather than contribution) to epileptogenesis in animals. However, more uniform criteria for validation in developmental models of epilepsy, addressing all these factors, will be needed to deduce the significance, cross-dependence with age, sex, genetic, epigenetic or other modifiers and, ultimately, validate these findings in humans, under similar experimental conditions. Although there is supporting evidence in human pathology or imaging studies for a few molecular and metabolism-related targets (i.e., α-[11C]-methyl-L-tryptophan PET [40]), this is mostly derived from patients with intractable epilepsy, at late stages on the disease, as similar studies at earlier stages are not currently technically or ethically feasible. Multicenter studies, linking the phenotype with genomics/proteomics/epigenomics data from specific patient populations, will be necessary to identify relevant biomarkers for susceptibility, clinical or electrophysiological features, and outcomes. Such studies are currently under way: FEBSTAT (principal investigator Shlomo Shinnar, New York, NY, USA); the Epilepsy Phenome/Genome Project (principal investigators Daniel Lowenstein, San Francisco, CA, USA and Ruben Kuzniecky, New York, NY, USA); and the Childhood Absence Epilepsy Trial (principal investigator Tracy Glauser, Cincinnati, OH, USA).
What are the challenges?
Defining normal brain development: the principle of relativity
Brain development is an ongoing set of processes that evolve at different paces for each cell type and network, and are further differentiated by age, sex, species, genetic, biological or epigenetic factors (Figures 1–3 & Table 1). Consequently, in developmental research, the concepts of ‘normal’ and ‘abnormal’ are strictly dependent upon the context in which they are discussed. For example, aberrant reversal of GABAA receptor signaling from hyperpolarizing to depolarizing has been found in the subiculum of resected human temporal lobes of patients with drug-resistant epilepsy, contributing to the generation of interictal epileptic spikes [41,42]. Depolarizing GABA has also been suggested to exist in cortical dysplasias from pediatric patients with drug-resistant epilepsies [43]. The idea that depolarizing GABAA signaling compromises the endogenous ability to inhibit excessive excitation, leading to seizures, and possibly contributes to the refractoriness to GABA-acting antiseizure drugs is tempting and also of interest for possible interventions in pediatric seizures, such as neonatal status epilepticus [44,45]. Yet efforts to validate depolarizing GABAA signaling as a biomarker of epileptogenicity or drug resistance in the early years of life will be faced with a number of challenges. First, depolarizing GABAA receptor signaling early in life is not only normal but necessary to ensure normal age- and sex-specific brain development [46,47]. In fact, a precocious shift of GABAA receptor signaling from depolarizing to hyperpolarizing, in otherwise normal rodents, may impair neuronal differentiation, morphology and synaptic integration [48,49]. Second, efforts to differentiate normal from abnormal depolarizing GABAA receptor signaling, based upon the developmental age, will be hampered by the fact that the normal developmental shift of GABAA signaling occurs asynchronously in different brain regions and cell types in a sex-specific manner, the detail of which is impossible to determine with the current technologies [50,51]. Third, a number of genetic, biological and epigenetic factors may influence the direction of GABAA responses without necessarily rendering the brain epileptic. Therefore, a binary output will not be sufficient to interpret putative in vivo biomarkers that change through development. Quantitative and contextual markers will be necessary to interpret the importance of the specific biomarker within age-, sex- and condition-appropriate parameters.
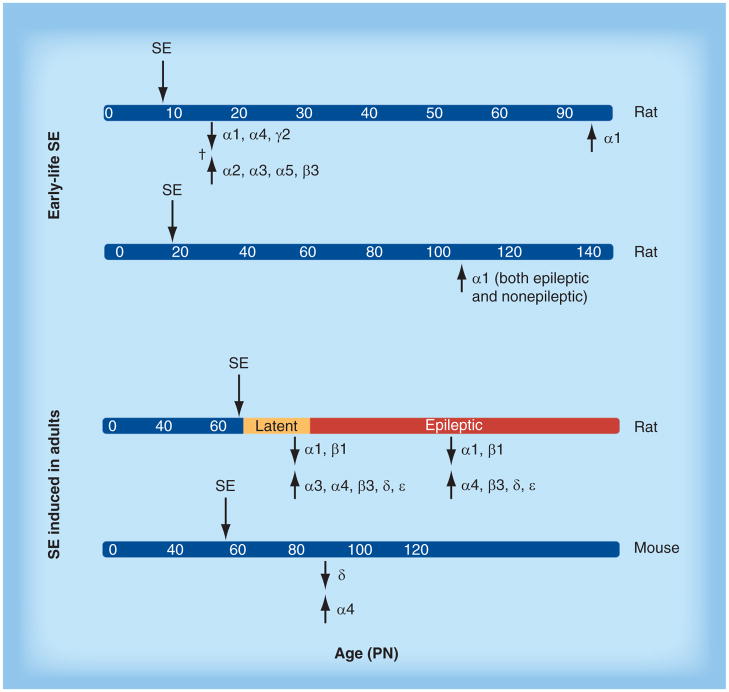
Figure 3. Changes in GABAA receptor expression in the hippocampus after status epilepticus.
SE changes the expression of GABAA receptor subunits in the hippocampus, in a manner that depends upon age at SE induction, subunit type, model of SE induction, and time of observation of changes after the induced SE. All studies are from pilocarpine or lithium–pilocarpine SE, except for the study indicated by †, which utilized kainic acid SE. The data are from representative references on this topic [81–85].
PN: Postnatal day; SE: Status epilepticus.
Reproduced with permission from [47].
Defining equivalence of developmental stages: reality versus utopia?
Preclinical animal research has proven fruitful in understanding normal brain development, and the mechanisms involved in ictogenesis or epileptogenesis, as well as identifying a number of signaling pathways that may help stop or alleviate seizures and their sequelae. However, rodents, and even primates, do not have the same biology and maturation patterns as humans (Figure 1 & Table 1). The currently utilized neonatal and infantile stages in experimental animals have been arbitrarily defined based on relevance to the time of birth and weaning, the body and brain growth rates, the cortical maturation, and the DNA brain content [52,53]. However, these stages only partially recapitulate the developmental processes that occur in human neonates and infants [52–56] Figure 1, Table 1). Currently, in rat pups, a period encompassing several days, from PN8–13, is thought to be developmentally equivalent to the human full-term newborn [52,53,56,57]. Yet, studying the effects of neonatal seizures or other stressors and events more than a week after delivery would miss out on the important influences of the neuroendocrine, neurophysiological and stressor effects occurring during birth. Furthermore, the motor milestones of 2-week-old rats are more advanced compared with human infants – pups are already capable of ambulating and leaving their nest, unlike the 2-month-old human infant [58]. Consequently, studying seizures that depend upon networks that control movements, such as infantile spasms, during developmental stages that are not necessarily equivalent to human stages in terms of motor development may lead to erroneous (false negative or false positive) conclusions as to the relevance of a biomarker for the human condition. Similar issues apply for other structures and networks, as depicted in Table 1. Perhaps routinely testing the validity of a bio-marker at different developmental ages may help clarify its age-relevance and relevance to specific developmental milestones. However, there will be cases when this will not be possible, either due to the age-specific nature of the epilepsy syndrome (i.e., infantile spasms) or other technical reasons.
Differentiating between reactive & pathogenic changes
The immature brain is very vulnerable to change following a variety of stressors, but these changes may not be necessarily epileptogenic. Therefore, distinguishing the reactive changes from those implicated in epileptogenesis and nosogenesis is essential to identify targets for therapeutic intervention or monitoring of outcomes. To achieve this, longitudinal follow-up studies correlating disease evolution with the candidate biomarkers will be needed, as well as interventions that specifically modify the bio-marker and hence prove or disprove its relationship with the tested disease-related outcome.
Accounting for age at onset & other modifiers
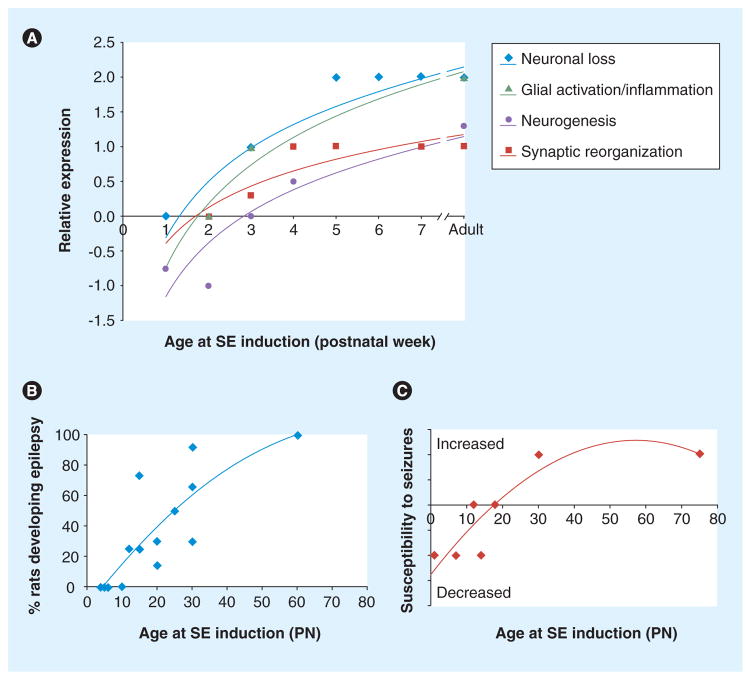
The immature brain is more resilient in developing injury and subsequent epilepsy in response to potentially epileptogenic insults, compared with the adult. In preclinical studies, the risk of neuronal loss, synaptic reorganization, neurogenesis and inflammation, as well as epilepsy following an initial insult (i.e., status epilepticus, brain trauma) increases with the age when this insult is first encountered (Figure 4) [59,60]. In certain cases, epilepsy may ensue in the absence of visible injury following lithium–pilocarpine status epilepticus in PN20 rats [61]. A similar trend has been reported in clinical studies, in which hippocampal sclerosis is less likely to occur in children who were diagnosed with epilepsy at younger ages [62]. These points indicate that biomarkers identified in adult-onset epilepsies may be less frequently applicable in the pediatric epilepsies. Therefore, it will be important to identify new biomarkers for these pediatric patients, based either on the specific pathologies associated with the early-onset epilepsies (i.e., dysplasias), or, in the cases of MRI-negative temporal or extratemporal epilepsies, based on other functional or molecular changes.
Figure 4. Age-specific changes in seizure-induced pathology and epileptogenic changes.
(A) Induction of SE produces neuronal loss, glial activation, inflammation, neurogenesis and synaptic reorganization in a manner that depends upon age at induction of SE. The scale is arbitrary, with positive values if an increase in the stated outcomes is observed and negative values if reduction in the outcomes is seen (i.e., neurogenesis). (B & C) The likelihood of developing epilepsy (B) or increasing susceptibility to induced seizures (assessed by flurothyl or kindling) (C) after SE induced by chemoconvulsants (kainic acid, lithium–pilocarpine) is dependent on the age at induction of the SE.
The data are reviewed in [50,87,88].
PN: Postnatal day; SE: Status epilepticus.
Epigenetic and societal factors, seizures and their treatments, as well as simple nonepileptogenic stressors or experiences may alter the natural course of epilepsy and epileptogenesis [63,64]. Genetic factors may further modify seizure and epilepsy outcomes, sometimes in unpredictable manners [65]. Sex is an important modifier, even during the very early neonatal and infantile stages. It affects not only the function of subcortical networks controlling seizures, such as the basal ganglia, but also the consequences of seizures at the molecular and pathology level [50,66,67].
However, the search for molecular or functional biomarkers for pediatric epilepsies again will need to control for:
The dynamic changes of these parameters during normal development (examples in Figure 2A–D);
The age-, sex-, and time-specific effects of seizures in developing animals (Figure 3);
The impact of additional modifiers (i.e., genetic, epigenetic, biological, comorbid conditions);
Most importantly, differentiate between pathogenic, reactive or fully compensated changes using longitudinal follow-ups.
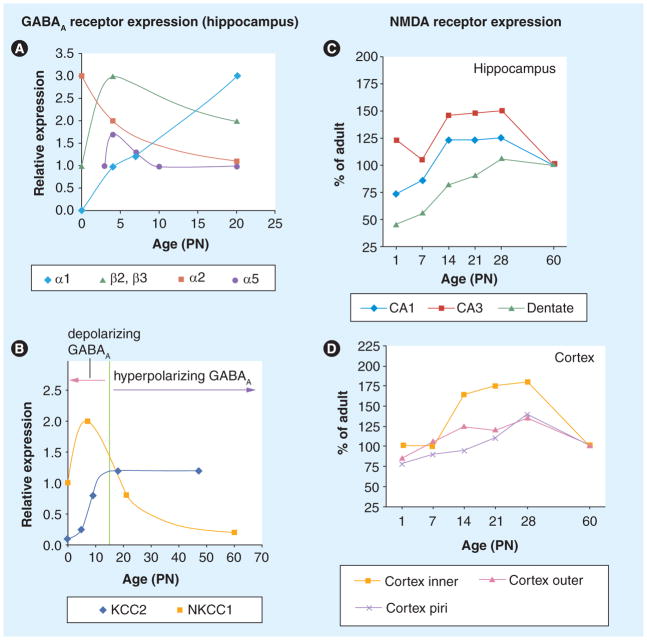
Figure 2. Age-specific changes in the expression and function of neurotransmitter systems.
(A–D) Age-specific changes in the expression of GABAA receptor subunits (A), chloride cotransporters KCC2, NKCC1 and GABAA receptor responses ((B) hippocampus), and NMDA receptors in the hippocampus (C) and cortex (D) of rats. The expression of these receptors and cotransporters changes with age and brain region. In certain cases, these developmental changes alter the function of the relevant receptors, as shown in (B). The developmental increase in KCC2 and decrease in NKCC1 results in the shift of GABAA receptor responses from depolarizing to hyperpolarizing (reviewed in [47,79]).
NMDA: N-methyl-D-aspartate; PN: Postnatal day.
(A & B) Reproduced with permission from [47].
(C & D) Reproduced with permission from [86].
The latter will be a particular challenge, as there is a vast complexity of the molecular pathways controlling neuronal excitability and brain development, which is necessary to compensate for random disruptions in the expression or activity of one signaling pathway. Therefore, it may be worth focusing on age- and sex-appropriate pathway-specific functional biomarkers, and implement them using longitudinal follow-up studies correlating disease evolution with the candidate biomarkers. For example, instead of developing a biomarker for the expression of a specific GABA receptor subunits, it may be worth pursuing a test assessing the impact of selective GABAergic agonists upon the excitability of the suspected epileptogenic network (i.e., using paired pulse TMS). Proof-of-principle studies, not always feasible in clinical trials, will be needed using interventions that specifically modify the biomarker and hence prove or disprove its relationship with a specific disease-related outcome.
Approaches that exemplify these principles are the longitudinal design of the comparative evaluation of combined EEG and MRI as biomarkers of specific epilepsy outcomes in the FEBSTAT study [12], the demonstration in pre-clinical studies that selective neuroprotection of the entorhinal and piriform cortex, but not of the hippocampus, was capable of delaying epileptogenesis [35], as well as the preclinical evidence for neuroprotective effects of phenobarbital [68,69]. Yet, validation of biomarkers with these long-term longitudinal studies will be particularly time-consuming, as well as expensive.
There is a scarcity of animal models of acquired early life onset epilepsy syndromes
At present, there are many genetic animal models of early life epilepsy, but very few models of acquired early life epilepsies and documented onset of spontaneous seizures before adulthood exist. Partly, this may be due to the fact that it is easier to document the epilepsy phenotype than confirming the age at onset of epilepsy, starting the long-term video-EEG recordings in adulthood, when skull growth has been completed and the presumed latent phase has lapsed. However, identification of the age of onset of spontaneous seizures will be essential for the discovery of early preclinical biomarkers of epileptogenicity or epileptogenesis. In immature animals, this will be more laborious, requiring placement of EEG electrodes at different time points and for briefer periods of monitoring per rat, to avoid the constrictive effects of the electrodes upon skull growth. Yet, this may still not be possible in smaller species, as in newborn mice. It should be pointed out that there are few longitudinal studies; the majority of the available data sets have been obtained at specific, discrete, developmental windows.
The search for biomarkers should preferably span the period including the initial insult and up until the onset of clinically diagnosed epilepsy or comorbidity, as there may not necessarily be a long identifiable ‘latent phase’. In fact, the period required for the establishment of the epileptic state and the specific comorbidity may be either very short or absent, starting during the acute phase of the initial insult. For example, a short latent phase to the onset of infantile spasms exists in the multiple-hit model of infantile spasms, but the model could still be used to develop biomarkers for other types of epilepsy or comorbidities, appearing at later stages [58].
Another gap in our current methodology is the scarcity of animal models that model either unique types of early life epilepsy (i.e., Lennox–Gastaut, electrical status epilepticus in sleep) or epilepsies of unknown etiology, which comprise one third of our patient population. Definitely, more research is needed to develop appropriate animal models to study these age-specific syndromes. Several chronic models of infantile spasms have been recently developed and interesting leads for potential molecular, genetic or electrographic biomarkers have started to be explored [58,70–74].
There are few data with inbred rats of generalized absence epilepsies and the rats with different susceptibility to kindling (slow and fast kindlers). Future studies in the different genetic backgrounds with kindling should take into account that kindling is more easily inducible in immature animals than adults and persists into adulthood [75,76]. The observation that kindling in developing rats leads to spontaneous seizures after 19–20 stimulations [77] compared with more than 348 stimulations in adult rats [78] may need to be further pursued. In fact, little systematic research is being done on the incidence and phenotype of ‘de novo epilepsy’ in otherwise naive experimental animals. How well do the induced models of epilepsy recapitulate the endogenous processes involved in epilepsies of unknown etiology? Utilization of models of de novo epilepsy may help perhaps remove the confounding effects of initial insult-related pathology, as well as identify biomarkers of drug resistance and poor outcome that are selectively present in epilepsies of structural/metabolic rather than unknown etiology. However, it is expected that preclinical research on such low incidence populations with epilepsy of unknown etiology will be only possible through multicenter collaborative studies, rather than through individual labs, unless the specified trait can be propagated through inbreeding. The availability of genetic models of early epilepsy has been an important tool, yet these represent a minority of epilepsy syndromes and the biomarkers identified through them will still need further validation in either different genetic background or acquired early-onset epilepsy models to exclude the possibility that these are specific for the given epilepsy mutation.
Human studies for the identification of biomarkers of epileptogenicity and epileptogenesis will be necessary for the identification and validation of biomarkers, as many pediatric epilepsy syndromes do not have suitable preclinical models. In a few cases these have provided successful or promising biomarkers of epileptogenic focus localization (i.e., α-[11C]-methyl-L-tryptophan PET study, high-frequency oscillations [79,80]). The longitudinal studies, such as the FEBSTAT, will require far longer observation periods and larger sample sizes than preclinical studies would, due to the variability in initial insult severity, treatments, and heterogeneity of population characteristics. Nevertheless they may provide the first insights on whether there are any biomarkers that can be modified by effective treatments. It is worth remembering the old studies in which phenobarbital prevented the development of secondary epileptogenic focus [69], whereas, in the kainic acid status epilepticus model, it decreased the degree of synaptic reorganization [70]. A second step is to identify whether the biomarkers are insult specific. However, the validation and determination of specificity or broader applicability of any identified biomarkers will still require testing in other epilepsy (and nonepileptic) young populations, and is time consuming and expensive. One concern is that human studies have limited potential for target identification of early epileptogenicity/epileptogenesis and are biased towards the identification of biomarkers at late stages of drug-resistant epilepsies, as surgical specimens and localization studies are almost exclusively limited to this drug-resistant epilepsy population. Consequently, it is imperative to find better strategies to optimize the translation of clinical data to preclinical studies and then back to clinical research.
Therefore, to facilitate and accelerate the discovery and validation of biomarkers of epileptogenicity or epileptogenesis relevant for early-onset epilepsies, new animal models of early-onset epilepsy are needed, including acquired models or populations of de novo epilepsy. Careful selection of potential biomarkers and strategies for identification of biomarkers in clinical trials will be essential to limit costs and time spent in failed unnecessary clinical studies. The choice of biomarkers may be influenced by the potential undesirable side effects of certain procedures (e.g., radiation) when PET scans or even MRI may need to be repeated over time.
Conclusion & future perspective
The identification and implementation of biomarkers for early-onset epilepsies has unique intricacies and requirements as they will have to reconcile the evolving biological and functional development of the normal brain with the interacting influences of potentially epileptogenic insults, underlying known and unknown etiologies and an array of epigenetic, genetic and biological factors. Furthermore, the unique features and etiologies of many of the early life epilepsy syndromes demand new syndrome-specific strategies for the design and interpretation of candidate biomarkers. There will not be a ubiquitous solution for this quest. There is a definite need to develop more preclinical models that appropriately model the age-specific features of early-onset epilepsies, including the unique pediatric epilepsy syndromes, and utilize them for target identification and proof-of-principle studies. The utility of candidate biomarkers will have to be evaluated in the context of normal development and function of the targeted brain network and stage of the epilepsy syndrome, as well as the age- and sex-specific effects of the various known etiologies and precipitating events of the studied epilepsy syndromes, preferably using longitudinal studies. To achieve this, a combination of electrophysiological, imaging, functional, genetic or molecular biomarkers will be needed to achieve the best prognostic value.
Executive summary.
-
The current needs for biomarkers of early life epilepsy include age-, sex- and syndrome-appropriate biomarkers of:
Epileptogenicity
Epileptogenesis
Comorbidities
Treatment implementation, tolerability and toxicity
-
Several biomarkers are currently under investigation, including:
Clinical or historical
Electrophysiological
Molecular/metabolic
Anatomical/structural
-
However, developing biomarkers useful for the developing brain presents several challenges, to ensure that proper interpretation and implementation will reconcile:
The evolving biological and functional changes of the developing brain
The age-, sex- and time-specific changes in the selected biomarkers
The distinct features of the variety of early life epileptic syndromes
The modifying effects of other genetic, epigenetic, biological and comorbid factors
Utilization of a combination of the above candidate biomarkers may increase the predictive value of the selected biomarkers.
Footnotes
For reprint orders, please contact: reprints@futuremedicine.com
Financial & competing interests disclosure
S Moshé has received research support from NIH: R01 NS20253 (PI), R01-NS43209 (investigator) and 2UO1-NS45911 (investigator), and the Heffer Family Foundation. He is the Charles Frost Chair in Neurosurgery and Neurology. He is serving on the Editorial Board of Neurobiology of Disease, Epileptic Disorders, Brain and Development, and Physiological Research. He has received consultancy fees from Eisai. A Galanopoulou has received research support from NIH NINDS/NICHD grant NS62947 and a past research grant from Johnson & Johnson (results not discussed in this paper). She is on the Editorial Board of Neurobiology of Disease and Epilepsy Research. She has also received consultancy fees from Novartis. Albert Einstein College of Medicine holds a patent for the multiple-hit model of infantile spasms (patent 2080216183). The authors do not receive any royalties from this patent. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Bibliography
- 1.Hauser WA, Kurland LT. The epidemiology of epilepsy in Rochester, Minnesota, 1935 through 1967. Epilepsia. 1975;16(1):1–66. doi: 10.1111/j.1528-1157.1975.tb04721.x. [DOI] [PubMed] [Google Scholar]
- 2.Bittigau P, Sifringer M, Genz K, et al. Antiepileptic drugs and apoptotic neurodegeneration in the developing brain. Proc Natl Acad Sci USA. 2002;99(23):15089–15094. doi: 10.1073/pnas.222550499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berg AT, Smith SN, Frobish D, et al. Special education needs of children with newly diagnosed epilepsy. Dev Med Child Neurol. 2005;47(11):749–753. doi: 10.1017/S001216220500157X. [DOI] [PubMed] [Google Scholar]
- 4.Tuchman R, Alessandri M, Cuccaro M. Autism spectrum disorders and epilepsy: moving towards a comprehensive approach to treatment. Brain Dev. 2010;32(9):719–730. doi: 10.1016/j.braindev.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 5.Sillanpaa M, Shinnar S. Long-term mortality in childhood-onset epilepsy. N Engl J Med. 2010;363(26):2522–2529. doi: 10.1056/NEJMoa0911610. [DOI] [PubMed] [Google Scholar]
- 6.Pellock JM, Hrachovy R, Shinnar S, et al. Infantile spasms: a US consensus report. Epilepsia. 2010;51(10):2175–2189. doi: 10.1111/j.1528-1167.2010.02657.x. [DOI] [PubMed] [Google Scholar]
- 7.Saemundsen E, Ludvigsson P, Rafnsson V. Autism spectrum disorders in children with a history of infantile spasms: a population-based study. J Child Neurol. 2007;22(9):1102–1107. doi: 10.1177/0883073807306251. [DOI] [PubMed] [Google Scholar]
- 8.Jambaque I, Chiron C, Dumas C, Mumford J, Dulac O. Mental and behavioural outcome of infantile epilepsy treated by vigabatrin in tuberous sclerosis patients. Epilepsy Res. 2000;38(2–3):151–160. doi: 10.1016/s0920-1211(99)00082-0. [DOI] [PubMed] [Google Scholar]
- 9.Riikonen R, Amnell G. Psychiatric disorders in children with earlier infantile spasms. Dev Med Child Neurol. 1981;23(6):747–760. doi: 10.1111/j.1469-8749.1981.tb02063.x. [DOI] [PubMed] [Google Scholar]
- 10.Chu-Shore CJ, Major P, Camposano S, Muzykewicz D, Thiele EA. The natural history of epilepsy in tuberous sclerosis complex. Epilepsia. 2010;51(7):1236–1241. doi: 10.1111/j.1528-1167.2009.02474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Muzykewicz DA, Costello DJ, Halpern EF, Thiele EA. Infantile spasms in tuberous sclerosis complex: prognostic utility of EEG. Epilepsia. 2009;50(2):290–296. doi: 10.1111/j.1528-1167.2008.01788.x. [DOI] [PubMed] [Google Scholar]
- 12.Gomes WA, Shinnar S. Prospects for imaging-related biomarkers of human epileptogenesis: a critical review. Biomarkers Med. 2011;5(5):599–606. doi: 10.2217/bmm.11.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Camfield P, Camfield C. Epilepsy can be diagnosed when the first two seizures occur on the same day. Epilepsia. 2000;41(9):1230–1233. doi: 10.1111/j.1528-1157.2000.tb00331.x. [DOI] [PubMed] [Google Scholar]
- 14.Ramos Lizana J, Cassinello Garcia E, Carrasco Marina LL, Vazquez Lopez M, Martin Gonzalez M, Munoz Hoyos A. Seizure recurrence after a first unprovoked seizure in childhood: a prospective study. Epilepsia. 2000;41(8):1005–1013. doi: 10.1111/j.1528-1157.2000.tb00286.x. [DOI] [PubMed] [Google Scholar]
- 15.Scotoni AE, Manreza ML, Guerreiro MM. Recurrence after a first unprovoked cryptogenic/idiopathic seizure in children: a prospective study from Sao Paulo, Brazil. Epilepsia. 2004;45(2):166–170. doi: 10.1111/j.0013-9580.2004.16503.x. [DOI] [PubMed] [Google Scholar]
- 16.Winckler MI, Rotta NT. Clinical and electroencephalographic follow-up after a first unprovoked seizure. Pediatr Neurol. 2004;30(3):201–206. doi: 10.1016/j.pediatrneurol.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 17.Shinnar S, Berg AT, Moshé SL, et al. The risk of seizure recurrence after a first unprovoked afebrile seizure in childhood: an extended follow-up. Pediatrics. 1996;98(2 Pt 1):216–225. [PubMed] [Google Scholar]
- 18.Martinovic Z, Jovic N. Seizure recurrence after a first generalized tonic–clonic seizure, in children, adolescents and young adults. Seizure. 1997;6(6):461–465. doi: 10.1016/s1059-1311(97)80021-0. [DOI] [PubMed] [Google Scholar]
- 19.Nordli DR, Moshé SL, Shinnar S. The role of EEG in febrile status epilepticus (FSE) Brain Dev. 2010;32(1):37–41. doi: 10.1016/j.braindev.2009.09.015. [DOI] [PubMed] [Google Scholar]
- 20.Lennox MA. Febrile convulsions in childhood; a clinical and electroencephalographic study. Am J Dis Child. 1949;78(6):868–882. doi: 10.1001/archpedi.1949.02030050887003. [DOI] [PubMed] [Google Scholar]
- 21.Worrell G, Gotman J. High-frequency oscillations and other electrophysiological biomarkers of epilepsy: clinical studies. Biomarkers Med. 2011;5(5):557–566. doi: 10.2217/bmm.11.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu JY, Sankar R, Lerner JT, Matsumoto JH, Vinters HV, Mathern GW. Removing interictal fast ripples on electrocorticography linked with seizure freedom in children. Neurology. 2010;75(19):1686–1694. doi: 10.1212/WNL.0b013e3181fc27d0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bragin A, Wilson CL, Staba RJ, Reddick M, Fried I, Engel J., Jr Interictal high-frequency oscillations (80–500 Hz) in the human epileptic brain: entorhinal cortex. Ann Neurol. 2002;52(4):407–415. doi: 10.1002/ana.10291. [DOI] [PubMed] [Google Scholar]
- 24.Wu JY, Koh S, Sankar R, Mathern GW. Paroxysmal fast activity: an interictal scalp EEG marker of epileptogenesis in children. Epilepsy Res. 2008;82(1):99–106. doi: 10.1016/j.eplepsyres.2008.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nariai H, Nagasawa T, Juhász C, Sood S, Chugani HT, Asano E. Statistical mapping of ictal high-frequency oscillations in epileptic spasms. Epilepsia. 2011;52(1):63–74. doi: 10.1111/j.1528-1167.2010.02786.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu JY, Sutherling WW, Koh S, et al. Magnetic source imaging localizes epileptogenic zone in children with tuberous sclerosis complex. Neurology. 2006;66(8):1270–1272. doi: 10.1212/01.wnl.0000208412.59491.9b. [DOI] [PubMed] [Google Scholar]
- 27.Badawy RA, Curatolo JM, Newton M, Berkovic SF, Macdonell RA. Sleep deprivation increases cortical excitability in epilepsy: syndrome-specific effects. Neurology. 2006;67(6):1018–1022. doi: 10.1212/01.wnl.0000237392.64230.f7. [DOI] [PubMed] [Google Scholar]
- 28.Kamida T, Fujiki M, Baba H, Ono T, Abe T, Kobayashi H. The relationship between paired pulse magnetic MEP and surgical prognosis in patients with intractable epilepsy. Seizure. 2007;16(2):113–119. doi: 10.1016/j.seizure.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 29.Saisanen L, Kononen M, Julkunen P, et al. Non-invasive preoperative localization of primary motor cortex in epilepsy surgery by navigated transcranial magnetic stimulation. Epilepsy Res. 2010;92(2–3):134–144. doi: 10.1016/j.eplepsyres.2010.08.013. [DOI] [PubMed] [Google Scholar]
- 30.Schmidt S, Holst E, Irlbacher K, Oltmanns F, Merschhemke M, Brandt SA. A case of pathological excitability located with navigated-TMS: presurgical evaluation of focal neocortical epilepsy. Restor Neurol Neurosci. 2010;28(3):379–385. doi: 10.3233/RNN-2010-0540. [DOI] [PubMed] [Google Scholar]
- 31.Spooner CG, Berkovic SF, Mitchell LA, Wrennall JA, Harvey AS. New-onset temporal lobe epilepsy in children: lesion on MRI predicts poor seizure outcome. Neurology. 2006;67(12):2147–2153. doi: 10.1212/01.wnl.0000248189.93630.4f. [DOI] [PubMed] [Google Scholar]
- 32.Provenzale JM, Barboriak DP, Vanlandingham K, Macfall J, Delong D, Lewis DV. Hippocampal MRI signal hyperintensity after febrile status epilepticus is predictive of subsequent mesial temporal sclerosis. AJR Am J Roentgenol. 2008;190(4):976–983. doi: 10.2214/AJR.07.2407. [DOI] [PubMed] [Google Scholar]
- 33.Vanlandingham KE, Heinz ER, Cavazos JE, Lewis DV. Magnetic resonance imaging evidence of hippocampal injury after prolonged focal febrile convulsions. Ann Neurol. 1998;43(4):413–426. doi: 10.1002/ana.410430403. [DOI] [PubMed] [Google Scholar]
- 34.Scott RC, King MD, Gadian DG, Neville BG, Connelly A. Hippocampal abnormalities after prolonged febrile convulsion: a longitudinal MRI study. Brain. 2003;126(Pt 11):2551–2557. doi: 10.1093/brain/awg262. [DOI] [PubMed] [Google Scholar]
- 35.Andre V, Dube C, Francois J, et al. Pathogenesis and pharmacology of epilepsy in the lithium–pilocarpine model. Epilepsia. 2007;48(Suppl 5):41–47. doi: 10.1111/j.1528-1167.2007.01288.x. [DOI] [PubMed] [Google Scholar]
- 36.Chandra PS, Salamon N, Huang J, et al. FDG-PET/MRI coregistration and diffusion-tensor imaging distinguish epileptogenic tubers and cortex in patients with tuberous sclerosis complex: a preliminary report. Epilepsia. 2006;47(9):1543–1549. doi: 10.1111/j.1528-1167.2006.00627.x. [DOI] [PubMed] [Google Scholar]
- 37.Ogren JA, Wilson CL, Bragin A, et al. Three-dimensional surface maps link local atrophy and fast ripples in human epileptic hippocampus. Ann Neurol. 2009;66(6):783–791. doi: 10.1002/ana.21703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vezzani A, Friedman A. Brain inflammation as a biomarker in epilepsy. Biomarkers Med. 2011;5(5):607–614. doi: 10.2217/bmm.11.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nehlig A. Hippocampal MRI and other structural biomarkers: experimental approach to epileptogenesis. Biomarkers Med. 2011;5(5):585–597. doi: 10.2217/bmm.11.65. [DOI] [PubMed] [Google Scholar]
- 40.Chugani DC. α-methyl-L-tryptophan: mechanisms for tracer localization of epileptogenic brain regions. Biomarkers Med. 2011;5(5):567–575. doi: 10.2217/bmm.11.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huberfeld G, Wittner L, Clemenceau S, et al. Perturbed chloride homeostasis and GABAergic signaling in human temporal lobe epilepsy. J Neurosci. 2007;27(37):9866–9873. doi: 10.1523/JNEUROSCI.2761-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cohen I, Navarro V, Clemenceau S, Baulac M, Miles R. On the origin of interictal activity in human temporal lobe epilepsy in vitro. Science. 2002;298(5597):1418–1421. doi: 10.1126/science.1076510. [DOI] [PubMed] [Google Scholar]
- 43.Cepeda C, Andre VM, Wu N, et al. Immature neurons and GABA networks may contribute to epileptogenesis in pediatric cortical dysplasia. Epilepsia. 2007;48(Suppl 5):79–85. doi: 10.1111/j.1528-1167.2007.01293.x. [DOI] [PubMed] [Google Scholar]
- 44.Kahle KT, Barnett SM, Sassower KC, Staley KJ. Decreased seizure activity in a human neonate treated with bumetanide, an inhibitor of the Na+-K+-2Cl− cotransporter NKCC1. J Child Neurol. 2009;24(5):572–576. doi: 10.1177/0883073809333526. [DOI] [PubMed] [Google Scholar]
- 45.Dzhala VI, Brumback AC, Staley KJ. Bumetanide enhances phenobarbital efficacy in a neonatal seizure model. Ann Neurol. 2008;63(2):222–235. doi: 10.1002/ana.21229. [DOI] [PubMed] [Google Scholar]
- 46.Ben-Ari Y. Excitatory actions of GABA during development: the nature of the nurture. Nat Rev Neurosci. 2002;3(9):728–739. doi: 10.1038/nrn920. [DOI] [PubMed] [Google Scholar]
- 47.Galanopoulou AS. Sexually dimorphic expression of KCC2 and GABA function. Epilepsy Res. 2008;80(2–3):99–113. doi: 10.1016/j.eplepsyres.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang DD, Kriegstein AR. GABA regulates excitatory synapse formation in the neocortex via NMDA receptor activation. J Neurosci. 2008;28(21):5547–5558. doi: 10.1523/JNEUROSCI.5599-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cancedda L, Fiumelli H, Chen K, Poo MM. Excitatory GABA action is essential for morphological maturation of cortical neurons in vivo. J Neurosci. 2007;27(19):5224–5235. doi: 10.1523/JNEUROSCI.5169-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Galanopoulou AS. Dissociated gender-specific effects of recurrent seizures on GABA signaling in CA1 pyramidal neurons: role of GABAA receptors. J Neurosci. 2008;28(7):1557–1567. doi: 10.1523/JNEUROSCI.5180-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Coppola A, Moshé SL. Why is the developing brain more susceptible to status epilepticus? Epilepsia. 2009;50(Suppl 12):25–26. doi: 10.1111/j.1528-1167.2009.02371.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Benjamins JA, McKhann GM. Development, regeneration, and aging. In: Siegel GJ, Albers RW, Katzman R, Agranoff BW, editors. Basic Neurochemistry. Little, Brown; MA, USA: 1972. pp. 365–387. [Google Scholar]
- 53.Dobbing J. The later development of the brain and its vulnerability. In: Davis JA, Dobbing J, editors. Scientific Foundations of Paediatrics. Vol. 565. Saunders; PA, USA: 1974. [Google Scholar]
- 54.Purpura DP. Ontogenetic models in studies of cortical seizure activities. In: Purpura DP, Penry JK, Tower D, Woodbury DM, Walter R, editors. Experimental Models of Epilepsy. Raven Press; NY, USA: 1972. pp. 531–556. [Google Scholar]
- 55.Wiggins RC. Myelination: a critical stage in development. Neurotoxicology. 1986;7(2):103–120. [PubMed] [Google Scholar]
- 56.Avishai-Eliner S, Brunson KL, Sandman CA, Baram TZ. Stressed-out, or in (utero)? Trends Neurosci. 2002;25(10):518–524. doi: 10.1016/s0166-2236(02)02241-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Romijn HJ, Hofman MA, Gramsbergen A. At what age is the developing cerebral cortex of the rat comparable to that of the full-term newborn human baby? Early Hum Dev. 1991;26(1):61–67. doi: 10.1016/0378-3782(91)90044-4. [DOI] [PubMed] [Google Scholar]
- 58.Scantlebury MH, Galanopoulou AS, Chudomelova L, Raffo E, Betancourth D, Moshé SL. A model of symptomatic infantile spasms syndrome. Neurobiol Dis. 2010;37(3):604–612. doi: 10.1016/j.nbd.2009.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sperber EF, Moshé SL. The effects of seizures on the hippocampus of the immature brain. In: Engel JJ, Schwartzkroin PA, Moshé SL, Lowenstein DH, editors. Brain Plasticity and Epilepsy. Academic Press; CA, USA: 2001. pp. 119–139. [DOI] [PubMed] [Google Scholar]
- 60.Cernak I, Chang T, Ahmed FA, et al. Pathophysiological response to experimental diffuse brain trauma differs as a function of developmental age. Dev Neurosci. 2010;32(5–6):442–453. doi: 10.1159/000320085. [DOI] [PubMed] [Google Scholar]
- 61.Raol YS, Budreck EC, Brooks-Kayal AR. Epilepsy after early-life seizures can be independent of hippocampal injury. Ann Neurol. 2003;53(4):503–511. doi: 10.1002/ana.10490. [DOI] [PubMed] [Google Scholar]
- 62.Riney CJ, Harding B, Harkness WJ, Scott RC, Cross JH. Hippocampal sclerosis in children with lesional epilepsy is influenced by age at seizure onset. Epilepsia. 2006;47(1):159–166. doi: 10.1111/j.1528-1167.2006.00382.x. [DOI] [PubMed] [Google Scholar]
- 63.Galanopoulou AS, Velisek L, Moshé SL. Seizures and antiepileptic drugs: does exposure alter normal brain development in animal models? In: Janigro D, editor. Mammalian Brain Development. Humana Press; NY, USA: 2009. pp. 105–132. [Google Scholar]
- 64.Young D, Lawlor PA, Leone P, Dragunow M, During MJ. Environmental enrichment inhibits spontaneous apoptosis, prevents seizures and is neuroprotective. Nat Med. 1999;5(4):448–453. doi: 10.1038/7449. [DOI] [PubMed] [Google Scholar]
- 65.Glasscock E, Qian J, Yoo JW, Noebels JL. Masking epilepsy by combining two epilepsy genes. Nat Neurosci. 2007;10(12):1554–1558. doi: 10.1038/nn1999. [DOI] [PubMed] [Google Scholar]
- 66.Lemmens EM, Lubbers T, Schijns OE, Beuls EA, Hoogland G. Gender differences in febrile seizure-induced proliferation and survival in the rat dentate gyrus. Epilepsia. 2005;46(10):1603–1612. doi: 10.1111/j.1528-1167.2005.00252.x. [DOI] [PubMed] [Google Scholar]
- 67.Veliskova J, Moshé SL. Sexual dimorphism and developmental regulation of substantia nigra function. Ann Neurol. 2001;50(5):596–601. doi: 10.1002/ana.1248. [DOI] [PubMed] [Google Scholar]
- 68.Sutula T, Cavazos J, Golarai G. Alteration of long-lasting structural and functional effects of kainic acid in the hippocampus by brief treatment with phenobarbital. J Neurosci. 1992;12(11):4173–4187. doi: 10.1523/JNEUROSCI.12-11-04173.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Morrell F, Baker L. Effects of drugs on secondary epileptogenic lesions. Neurology. 1961;11:651–664. doi: 10.1212/wnl.11.8.651. [DOI] [PubMed] [Google Scholar]
- 70.Frost JD, Jr, Lee CL, Hrachovy RA, Swann JW. High frequency EEG activity associated with ictal events in an animal model of infantile spasms. Epilepsia. 2011;52(1):53–62. doi: 10.1111/j.1528-1167.2010.02887.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lee CL, Frost JD, Jr, Swann JW, Hrachovy RA. A new animal model of infantile spasms with unprovoked persistent seizures. Epilepsia. 2008;49(2):298–307. doi: 10.1111/j.1528-1167.2007.01377.x. [DOI] [PubMed] [Google Scholar]
- 72.Raffo E, Coppola A, Ono T, Briggs SW, Galanopoulou AS. A pulse rapamycin therapy for infantile spasms and associated cognitive decline. Neurobiol Dis. 2011;43(2):322–329. doi: 10.1016/j.nbd.2011.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Marsh E, Fulp C, Gomez E, et al. Targeted loss of Arx results in a developmental epilepsy mouse model and recapitulates the human phenotype in heterozygous females. Brain. 2009;132(Pt 6):1563–1576. doi: 10.1093/brain/awp107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Price MG, Yoo JW, Burgess DL, et al. A triplet repeat expansion genetic mouse model of infantile spasms syndrome, Arx (GCG)10+7, with interneuronopathy, spasms in infancy, persistent seizures, and adult cognitive and behavioral impairment. J Neurosci. 2009;29(27):8752–8763. doi: 10.1523/JNEUROSCI.0915-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Haas KZ, Sperber EF, Benenati B, Stanton PK, Moshé SL. Idiosyncracies of limbic kindling in developing rats. In: Corcoran ME, Moshé SL, editors. Kindling. Vol. 5. Plenum; NY, USA: 1998. pp. 15–26. [Google Scholar]
- 76.Baram TZ, Hirsch E, Schultz L. Short interval electrical amygdala kindling in infant rats: the paradigm and its application to the study of age-specific convulsants. In: Corcoran ME, Moshé SL, editors. Kindling. Vol. 5. Plenum; NY, USA: 1998. pp. 35–44. [Google Scholar]
- 77.Haas KZ, Sperber EF, Moshé SL. Kindling in developing animals: interactions between ipsilateral foci. Brain Res Dev Brain Res. 1992;68(1):140–143. doi: 10.1016/0165-3806(92)90257-w. [DOI] [PubMed] [Google Scholar]
- 78.Pinel JP. Effects of diazepam and diphenylhydantoin on elicited and spontaneous seizures in kindled rats: a double dissociation. Pharmacol Biochem Behav. 1983;18(1):61–63. doi: 10.1016/0091-3057(83)90252-6. [DOI] [PubMed] [Google Scholar]
- 79.Kumar A, Asano E, Chugani HT. α-[11C]-methyl-L-tryptophan PET for tracer localization of epileptogenic brain regions: clinical studies. Biomarkers Med. 2011;5(5):577–584. doi: 10.2217/bmm.11.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Staba RJ, Bragin A. High-frequency oscillations and other electrophysiological biomarkers of epilepsy: underlying mechanisms. Biomarkers Med. 2011;5(5):545–556. doi: 10.2217/bmm.11.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Brooks-Kayal AR, Shumate MD, Jin H, Rikhter TY, Coulter DA. Selective changes in single cell GABAA receptor subunit expression and function in temporal lobe epilepsy. Nat Med. 1998;4(10):1166–1172. doi: 10.1038/2661. [DOI] [PubMed] [Google Scholar]
- 82.Friedman LK, Pellegrini-Giampietro DE, Sperber EF, Bennett MV, Moshé SL, Zukin RS. Kainate-induced status epilepticus alters glutamate and GABAA receptor gene expression in adult rat hippocampus: an in situ hybridization study. J Neurosci. 1994;14(5 Pt 1):2697–2707. doi: 10.1523/JNEUROSCI.14-05-02697.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lauren HB, Lopez-Picon FR, Korpi ER, Holopainen IE. Kainic acid-induced status epilepticus alters GABA receptor subunit mRNA and protein expression in the developing rat hippocampus. J Neurochem. 2005;94(5):1384–1394. doi: 10.1111/j.1471-4159.2005.03274.x. [DOI] [PubMed] [Google Scholar]
- 84.Raol YH, Zhang G, Lund IV, Porter BE, Maronski MA, Brooks-Kayal AR. Increased GABAA-receptor α1-subunit expression in hippocampal dentate gyrus after early-life status epilepticus. Epilepsia. 2006;47(10):1665–1673. doi: 10.1111/j.1528-1167.2006.00640.x. [DOI] [PubMed] [Google Scholar]
- 85.Peng Z, Huang CS, Stell BM, Mody I, Houser CR. Altered expression of the δ subunit of the GABAA receptor in a mouse model of temporal lobe epilepsy. J Neurosci. 2004;24(39):8629–8639. doi: 10.1523/JNEUROSCI.2877-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Insel TR, Miller LP, Gelhard RE. The ontogeny of excitatory amino acid receptors in rat forebrain – I. N-methyl-D-aspartate and quisqualate receptors. Neuroscience. 1990;35(1):31–43. doi: 10.1016/0306-4522(90)90117-m. [DOI] [PubMed] [Google Scholar]
- 87.Haas KZ, Sperber EF, Opanashuk LA, Stanton PK, Moshé SL. Resistance of immature hippocampus to morphologic and physiologic alterations following status epilepticus or kindling. Hippocampus. 2001;11(6):615–625. doi: 10.1002/hipo.1076. [DOI] [PubMed] [Google Scholar]
- 88.Lynch M, Sayin U, Bownds J, Janumpalli S, Sutula T. Long-term consequences of early postnatal seizures on hippocampal learning and plasticity. Eur J Neurosci. 2000;12(7):2252–2264. doi: 10.1046/j.1460-9568.2000.00117.x. [DOI] [PubMed] [Google Scholar]