Abstract
Objective:
Children and adolescents with restless legs syndrome (RLS) are commonly diagnosed with comorbid attention deficit hyperactivity disorder and behavioral disturbances. Uncertainty exists over the significance of other co-occurring psychiatric disorders and their pharmacologic management in children with RLS. The purpose of this study was to determine the prevalence and nature of psychiatric disorders in children with RLS and to describe the use of psychotropic medications in our study cohort.
Methods:
The electronic medical records of children younger than 18 years of age who had been diagnosed with RLS between January 1, 2003, and December 31, 2009, were reviewed. Only those patients whose findings were consistent with the 2003 NIH workshop diagnostic criteria for probable or definite restless legs syndrome were included in this study. The medical records were cross-referenced for encounters with a child psychiatrist or psychologist. Likewise, only psychiatric diagnoses whose medical records explicitly reflected DSM-IV diagnostic criteria for psychiatric disorder(s) were included. Demographic data, serum ferritin, psychotropic medications, and in some cases, the results of pharmacogenomic testing were included in the data analysis in an ad hoc fashion.
Results:
We found 374/922 patients who met diagnostic criteria for childhood onset RLS. The mean age of the subjects was 10.6 years (range 0 to 18) and the male to female ratio was approximately 1:1. Overall, 239/374 (64%) patients with RLS had one or more comorbid psychiatric disorders. Attention deficit hyperactivity disorder was found in 94/374 (25%) patients, mood disturbances were found in 109/374 (29.1%) patients, anxiety disorders in 43/374 (11.5%) patients, and behavioral disturbances in 40/374 (10.9%) patients. Attention deficit hyperactivity disorder and disruptive behavior disorders were more common in males (OR = 1.94 for both), whereas mood disturbances and anxiety disorders were more common in females (OR = 1.6 and 1.26, respectively).
Mean serum ferritin levels derived from all patients without any psychiatric disorder were compared to all patients with one or more psychiatric disorder. No differences were found. The number of new psychotropic medication trials increased significantly with increase in patient age. Stimulants and antidepressant medications were the most commonly prescribed agents. As a part of clinical care, 15 of these patients underwent pharmacogenomic testing. Metabolic abnormalities were predicted by genotyping in 12/15 (80%) patients.
Conclusion:
Comorbid psychiatric conditions occurred in two-thirds of children with RLS, underscoring the need for multidisciplinary management of this condition. An important relationship might exist between psychotropic medication, and possibly pharmacogenomic factors, in children and adolescents with symptoms of restless legs syndrome. These findings are consistent and build on those reported in the adult literature.
Citation:
Pullen SJ; Wall CA; Angstman ER; Munitz GE; Kotagal S. Psychiatric comorbidity in children and adolescents with restless legs syndrome: a retrospective study. J Clin Sleep Med 2011;7(6):587-596.
Keywords: Childhood onset restless legs syndrome, attention deficit hyperactivity disorder, mood disorder, psychotropic medication, serum ferritin
Restless legs syndrome (RLS) is a sensorimotor disorder characterized by uncomfortable sensations (often subjectively described as “creepy,” “crawly,” “tingling,” “pulling,” or “pain”) and an irresistible urge to move, most commonly experienced during the evening or nighttime when the individual is immobile for extended periods of time.1 Approximately 40% of adults diagnosed with RLS have onset of their symptoms before the age of 20, and nearly 10% develop symptoms before the age of 10, thus illustrating the importance of advancing our understanding of childhood onset RLS.2
Using diagnostic criteria developed at a 2003 Consensus Conference sponsored by the National Institutes of Health, Picchietti and colleagues conducted a large survey of over 10,000 subjects, and found the prevalence of definite childhood RLS to be approximately 2%.3 Two recent surveys of over 4300 Turkish children and adolescents and 3300 Turkish adolescents that assessed for symptoms of RLS found the prevalence to be between 2.74% and 3.6%, respectively.4,5 The conclusion from all 3 studies is that childhood RLS is a common disorder, and comorbid mental health conditions are being increasingly recognized.3–5 In a case series of 18 children and adolescents who met diagnostic criteria for pediatric RLS, Picchietti and Stevens described other comorbid disorders such as parasomnias in 7 of 18 subjects, attention deficit hyperactivity disorder (ADHD) in 13 of 18 subjects, oppositional defiant disorder in 4 of 18 subjects, anxiety disorders in 6 of 18 subjects, and depression in 5 of 18 subjects.6 Yilmaz and colleagues also found a high co-occurrence of ADHD symptoms in children with definite RLS.4 However, the scope of psychiatric disorders in children and adolescents with RLS has not been as well studied as in adults. Further complicating matters is the fact that childhood RLS may go unrecognized when there are overwhelming daytime manifestations of comorbid conditions such as ADHD, mood and anxiety disorders.2,7,8 As such, symptoms of RLS may be missed by other providers, such as pediatricians or child psychiatrists, who are at the forefront of treating these comorbid conditions.
BRIEF SUMMARY
Current Knowledge/Study Rationale: Restless legs syndrome is a common sensorimotor disorder that frequently occurs in children and adolescents, and can significantly impact quality of life. Comorbid psychiatric disorders are increasingly being recognized among adults with RLS, but little is known about the prevalence of comorbid psychiatric disorders in children and adolescents with RLS. This was the primary rationale of this study.
Study Impact: Nearly two-thirds of children and adolescents diagnosed with RLS also met diagnostic criteria for one or more psychiatric disorder. This highlights the need for the sleep clinician to assess for psychiatric disturbances in this population, and conversely for the physician treating psychiatric disorders in children and adolescents to assess for RLS.
In adults, several studies have established an association between restless legs syndrome and major depressive disorder and anxiety disorders.9–11 In her paper describing the reciprocal relationship between mood disorders and RLS, Hornyak postulated several mechanisms to help explain the high rate of co-occurrence between mood disorders and RLS that have been observed among adults.11 Both mood disorders and RLS share derangement in dopaminergic metabolism which is demonstrated empirically by the fact that dopaminergic agonists improve RLS symptoms, and can augment antidepressive therapy, whereas dopaminergic antagonists (i.e., antipsychotic medications) may exacerbate RLS symptoms.11–13 Persistent disruption in the quality and quantity of sleep also has profound effects on mood, cognition, and daily functioning.11
An increasing number of clinical reports have also described the triggering or exacerbation of symptoms of RLS by psychotropic medications, which may also help to explain the high co-occurrence rate of RLS and psychiatric disorders in adults.11,14,15 Interestingly, the antidepressant bupropion (Wellbutrin), which acts as both a dopaminergic and noradrenergic agonist, is not known to exacerbate RLS symptoms, thus reinforcing the link between RLS and dopaminergic pathways.11 All studies examining these connections between psychiatric disorders and RLS have been carried out in adults. The role that psychotropic medications might play in exacerbating childhood-onset RLS has not been studied. While ADHD and behavioral disturbances have been previously described in children and adolescents who have RLS, little is known about the occurrence of other psychiatric disorders in childhood RLS. A better understanding of the prevalence of psychiatric disorders and RLS in children and adolescents is a key first step to understanding the putative link between these entities: this was the impetus for our research study.
Studies describing RLS and psychiatric comorbidity in adults, as well as the case series reported by Picchietti and Stevens in children were the basis for our first hypothesis: we postulated that there would be a high rate of comorbid mood and anxiety disorders in addition to ADHD and behavioral disturbances among children and adolescents with RLS. Second, since children who have been diagnosed with one or more psychiatric disorder often receive psychotropic medications, we also planned to cross-link patterns of psychotropic medication use to RLS. As already noted studies in adults have demonstrated a link between psychotropic medication use and worsening of RLS symptoms. We hypothesized that our study results would be similar.
Pharmacogenomic testing for hepatic microsomal enzymes such as P450 is presently available at some medical centers including at our Institution. It can help predict how a patient metabolizes certain medications, including many psychotropic medications. This helps determine predicted responses to medications as well as potential susceptibility to side effects.16,17 At our institution, pharmacogenomic testing is often done as part of a comprehensive workup in both child and adult patients with more serious and chronic psychiatric disorders requiring more complicated pharmacologic management. This aids in predicting response to treatment and susceptibility to potential side effects. Therefore, we also wanted to include the results of any clinically relevant pharmacogenomic testing that may have been conducted in patients who coincidentally had both RLS and comorbid psychiatric disorder. This could further our understanding of how psychotropic medications exacerbate RLS symptoms.
The objectives of this study were to: determine what percentage of a cohort of children and adolescents with clearly defined RLS had a comorbid psychiatric disorder and to determine the nature of this disorder and possible relationship to psychotropic medications used.
METHODS
This retrospective study was approved by our institutional review board, in accordance with institutional and federal regulations. We reviewed the electronic medical records of children and adolescents younger than 18 years who had been evaluated for RLS at our Center for Sleep Medicine between January 1, 2003, and December 31, 2009. Patients had been referred to the Center for Sleep by both primary care providers and subspecialists practicing both within and outside of our medical center.
The diagnosis of RLS was based on the 2003 National Institutes of Health Consensus Conference criteria. We included only patients who were evaluated by a sleep medicine specialist, and whose electronic medical records explicitly supported a final sleep diagnosis of definite or probable RLS (Table 1). Patients were excluded from the study if their final diagnosis was possible RLS, there was insufficient history in their medical records to support a diagnosis of definite or probable RLS, an alternate final sleep diagnosis was made, or if an alternate medical or neurological condition better accounted for the patient's final diagnosis. We did not exclude patients with comorbid medical or mental health conditions as long as there was sufficient evidence to support the diagnosis of RLS as being an independent contributing factor to the patient's overall presentation. We combined probable or definite RLS categories into one group, and henceforth use the term “RLS” to denote either probable or definite RLS. We identified 922 patients who were initially evaluated for childhood RLS. We found 374 patients whose medical charts fully supported diagnostic criteria for RLS. Of note, data from 16 of 374 patients were derived from a previous study of senior author18 on the clinical characteristics of childhood-onset restless legs syndrome. The remaining 548 patients did not meet our inclusion/exclusion criteria stated earlier in this paragraph.
Table 1.
2003 NIH Workshop Diagnostic Criteria for RLS in Childhood and Adolescence
Adult Essential Criteria
|
| Definite RLS in children (age 2 to 12 years) |
Definite 1
|
| or |
Definite 2
|
| Definite RLS in adolescence (age 13 to 18 years) |
| The four essential criteria as above. |
| For pediatric and adult RLS: The condition is not better explained by another current sleep disorder, medical, or neurological disorder, mental disorder, medication use, or substance use disorder. |
| Research Criteria (age 0 to 18 years) |
| Probable RLS |
Probable 1
|
| or* |
Probable 2
|
Possible RLS**
|
This last category is intended for young children or cognitively-impaired children, who do not have sufficient language to describe the sensory component of RLS.
Patients who met criteria for possible RLS were excluded from this research study.
The medical records of the 374 patients were reviewed for comorbid psychiatric disorders. We considered only the subjects who had been evaluated by a licensed child psychiatrist or psychologist and whose psychiatric disorder(s) clearly met DSM-IV diagnostic criteria, as described in the electronic medical record. Psychiatric diagnoses were excluded from consideration if there was insufficient history in the medical records to support the stated psychiatric disorder, if there were conflicting diagnoses in medical records, or if another medical condition better explained the patient's symptoms. When describing psychiatric data collected from the medical record, patients were stratified by gender and age (0-9 and 10-18) to account for the relative likelihood that they would receive a particular psychiatric diagnosis. For example, attention deficit hyperactivity disorder is typically diagnosed in younger children, and is more common in boys than girls, whereas depressive disorders tend to be more prevalent in teenage girls.19,20
Levels of serum ferritin were recorded to determine whether iron deficiency exacerbated the risk of comorbid psychiatric disorders in the patients with RLS. Prior to data collection all of the authors met to establish data collection guidelines, and gathering of all relevant data in an ad hoc fashion. Data were primarily collected by 3 of the authors (SJP, ERA, and GEM); however, all authors met regularly to ensure that data collection adhered to the previously established inclusion/exclusion criteria.
In order to determine how well our study cohort generalizes to a larger population we collected demographic data on patient age, gender, and race; the patient's state/country of origin, and the type of medical insurance coverage (private, government, or no insurance) patients or their families carried. Data describing the clinical characteristics (number of patients diagnosed with definite 1, definite 2, probable 1, or probable 2 RLS; number of patients diagnosed with both RLS and a comorbid psychiatric disorder; serum ferritin stratified by age, gender, and ± psychiatric disorder), details of psychiatric comorbidities, and psychotropic medications including data from any patient who underwent pharmacogenomics testing were also collected.
Data Analysis
A descriptive statistical approach was used to analyze the demographic and clinical data. A one-way ANOVA and Tukey post hoc analysis was done to compare the number of psychotropic medication trials among all patients by age and gender. A multivariate regression model was used to analyze serum ferritin levels among patients by age, gender, and whether or not the patient carried a psychiatric diagnosis.
RESULTS
Demographic Features of Population Cohort
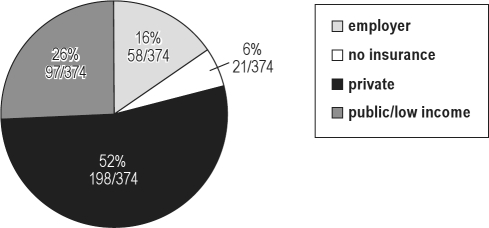
These are described in Table 2. Socioeconomic data are described in Figure 1. Male and female patients shared similar demographic features (Table 2). The overall mean age at the time of the initial sleep evaluation was 10.6 years (range 11 months to 17.5 years; Table 2). The majority of patients were Caucasian (80.5%, 301/374), and identified the state of Minnesota as their home state (61.5%, 230/374), although 27 different states, and 3 additional countries (Canada, Dominican Republic, and Kuwait) were also represented in this population cohort (Table 2). Socioeconomic status was measured by using the patient's listed health insurance. This demonstrated that all socioeconomic strata were well-represented among different age groups, but were likely skewed toward privatized health insurance when compared with the general U.S. population (Figure 1).
Table 2.
Demographic features
| Demographic Data | Female | Male | Total |
|---|---|---|---|
| Number of patients with RLS | 196 | 178 | 374 |
| Number of patients with a psychiatric diagnosis | 121 | 118 | 239 |
| Patient age (years) | |||
| Mean | 11.9 | 9.2 | 10.6 |
| Median | 13.5 | 9 | 11 |
| Range | 1-18 | 0-18 | 0-18 |
| Race/Ethnicity | |||
| Caucasian | 153 | 148 | 301 |
| Hispanic | 10 | 8 | 18 |
| African American | 6 | 6 | 12 |
| Asian | 5 | 1 | 6 |
| Unknown | 21 | 14 | 35 |
| Other | 1 (Native American) | 1 (Middle Eastern) | 2 |
| State/Country of Origin | Number of Patients |
|---|---|
| Minnesota | 230 |
| Iowa | 28 |
| Wisconsin | 21 |
| Illinois | 20 |
| Michigan | 17 |
| Othera | 58 |
Other States/Countries represented included: AR, AZ, CA, Canada, CO, Dominican Republic, FL, GA, ID, IN, KS, Kuwait, KY, MA, MO, NC, ND, NE, NY, OH, OK, OR, SD, TN, and TX.
Figure 1.
Pie chart showing the relative distribution of health insurance coverage listed for all patients in this study cohort
Patients with private health insurance [private] (Blue Cross/Blue Shield, United Health Care, CIGNA, Aetna, Assurant, etc.) represented approximately 53% (198/374) of all patients. Patients with either state or federally-funded health insurance [public/low income] (Medicaid, Minnesota Healthcare Program) represented approximately 26% (98/374) of all patients. Patients with employer-sponsored health insurance [employer] (Mayo Clinic Employee Sponsored Health Plan) represented approximately 15.5% (58/374) of all patients. Approximately 5.6% (21/374) of all patients either did not have health insurance listed or they were listed as receiving charity care [no insurance].
When we cross-referenced these patients for encounters with a licensed child psychiatrist or psychologist, we found that 239/374 (64%; 118 males, 121 females) met DSM-IV diagnostic criteria for one or more comorbid psychiatric conditions during the time for which they were being followed for RLS (Table 2).
Clinical Characteristics of Population Cohort
All patients were subdivided into whether they met diagnostic criteria for the RLS subcategories of definite 1, definite 2, probable 1, or probable 2 (Table 3). Approximately 76.5% (286/374) met criteria for either definite 1 or definite 2 RLS. We did find 49/376 patients who met diagnostic criteria for probable 2 RLS. These patients were either too young or cognitively impaired (diagnosed with either mental retardation or moderate to severe pervasive developmental disorder) to verbally articulate their symptoms, but had all had well-described behavioral manifestations of RLS and had increased periodic limb movements of sleep scored on polysomnography. Additionally, these patients had well-described immediate family history of definitive RLS in their medical records.
Table 3.
Clinical characteristics
| Restless Legs Syndrome Subtype | Definite 1 | Definite 2 | Probable 1 | Probable 2 | |
|---|---|---|---|---|---|
| Number of Patients | 158 | 128 | 39 | 49 | |
| Mean Age | 14.6 | 8.26 | 10.08 | 4.2 | |
| Median Age | 15 | 8 | 9 | 4 | |
| Age Range | 2-18 | 2-13 | 4-18 | 0-15 | |
| Serum Ferritin – Without Psychiatric Diagnoses | Female | Female | Male | Male | |
| Age 0-9 | Age 10-18 | Age 0-9 | Age 10-18 | ||
| Number of Patients | 24 | 43 | 32 | 19 | |
| Mean (μg/L) | 23.33 | 28.51 | 28.47 | 23.20 | |
| Median (μg/L) | 22 | 18 | 26 | 16 | |
| Range (μg/L) | 8-51 | 8-178 | 9-68 | 9-56 | |
| Serum Ferritin – With Psychiatric Diagnoses | |||||
| Number of Patients | 37 | 83 | 60 | 57 | |
| Mean (μg/L) | 28.81 | 25.23 | 25.65 | 32.74 | |
| Median (μg/L) | 25.5 | 20 | 23 | 29 | |
| Range (μg/L) | 9-115 | 4-119 | 5-81 | 4-189 | |
| Referring Specialty | Female | Female | Male | Male | Total |
| Age 0-9 | Age 10-18 | Age 0-9 | Age 10-18 | Patients | |
| Pediatrics | 28 | 43 | 31 | 21 | 123 |
| Pediatric Neurology | 12 | 28 | 20 | 15 | 75 |
| Pediatric Development and Behavior | 4 | 4 | 14 | 7 | 29 |
| Child and Adolescent Psychiatry | 2 | 10 | 3 | 14 | 29 |
| Family Medicine | 4 | 5 | 8 | 3 | 20 |
| Othera | 13 | 43 | 19 | 23 | 98 |
| Total | 63 | 133 | 95 | 83 | 374 |
Other specialties included General Psychiatry, Pain Rehabilitation, Psychology, Ear Nose and Throat, Pediatric Rheumatology, Pediatric Infectious Disease, Pediatric Endocrinology, Pediatric Cardiovascular Medicine, Pediatric Critical Care, Pulmonary Medicine, Internal Medicine, Physical Medicine and Rehabilitation, Allergy, Dermatology, Medical Genetics, Orthopedic Surgery, and Self-Referred.
Serum ferritin levels < 50 μg/L have been associated with RLS or periodic limb movements in sleep in children.20,21 Approximately 80% of subjects with RLS show underlying periodic limb movements in sleep. It is not known whether a difference in serum ferritin level exists between those with a psychiatric disorder and those without one. Therefore, we stratified serum ferritin values by age group, gender, and whether patients had a psychiatric diagnosis to assess for any differences between groups. We identified 355 patients who had their serum ferritin levels checked as part of their evaluation for RLS (Table 3).
A multivariate regression model predicting ferritin levels using groupwise comparisons of age, psychiatric diagnosis, and gender as predictors was not statistically significant (F-value = 0.48, overall p-value = 0.700). This also took into account any statistical outliers that were noted. Age group, defined in the model as either 0-9 or 10-18 (p = 0.447), gender (p = 0.336), or psychiatric diagnosis status (p = 0.467) were not statistically significant in predicting serum ferritin levels. We did not have a large enough sample size to assess for differences in serum ferritin levels between specific psychiatric diagnoses (e.g., major depression vs. generalized anxiety disorder vs. ADHD).
Referral sources from different pediatric disciplines were well represented in our population (Table 3). Primary care pediatricians accounted for 32.9% (123/374) of patient referrals to sleep medicine for evaluation of RLS, and were the single largest referral base to our sleep center. Pediatric disciplines most likely to encounter patients with comorbid psychiatric disorders included General Pediatrics, Pediatric Neurology, Developmental Pediatrics, Child and Adolescent Psychiatry, and Family Medicine and comprised 73.8% (276/374) of the population (Table 3). This was consistent with our finding that of the 239 RLS patients with psychiatric comorbidity, a psychiatric disorder had been diagnosed prior to RLS in 75% (27/36) of females aged 0-9 years, 90.5% (76/84) of females aged 10-18 years, 81% (48/61) of males aged 0-9 years, and 89.6% (52/58) of males aged 10-18 years.
Psychiatric Comorbidities
Overall, we found that 25% (94/374) of RLS patients met the criteria for ADHD; 29.1% (109/374) had either a transient mood disturbance (i.e. Adjustment Disorder) or a recurrent mood disturbance (i.e. Major Depressive Disorder or Bipolar Disorder); 11.5% (43/374) had an anxiety disorder; and approximately 11% (40/374) met the criteria for a behavioral disturbance (Table 4). Mood disturbances and anxiety disorders were more prevalent in females (OR = 1.6 and 1.26, respectively), whereas ADHD and behavioral disorders were more prevalent in males (OR = 1.94). Additional comorbidities are described in Table 4. Among the 239 patients with a psychiatric disorder, 109 patients had been diagnosed with one psychiatric condition, 88 patients with 2 psychiatric conditions, and 42 patients had been diagnosed with ≥ 3 psychiatric conditions.
Table 4.
Psychiatric comorbidity in RLS patients
| Psychiatric Diagnosis | Female | Female | Male | Male |
|---|---|---|---|---|
| Age 0-9 | Age 10-18 | Age 0-9 | Age 10-18 | |
| Attention Deficit Hyperactivity Disorder | 19 | 13 | 31 | 31 |
| Mood Disturbance | 7 | 59 | 15 | 28 |
| Major depressive disorder | 0 | 17 | 1 | 3 |
| Depression NOS | 2 | 23 | 6 | 9 |
| Dysthymic disorder | 0 | 1 | 0 | 0 |
| Bipolar disorder type I | 0 | 3 | 0 | 1 |
| Bipolar disorder NOS | 2 | 0 | 1 | 2 |
| Mood disorder NOS | 0 | 3 | 0 | 4 |
| Adjustment disorder | 3 | 12 | 7 | 9 |
| Anxiety Disorders | 4 | 20 | 10 | 9 |
| Generalized anxiety disorder | 0 | 2 | 0 | 1 |
| Social anxiety disorder | 0 | 2 | 0 | 0 |
| Posttraumatic stress disorder | 0 | 1 | 0 | 1 |
| Panic disorder | 0 | 0 | 1 | 1 |
| Obsessive compulsive disorder | 0 | 0 | 0 | 1 |
| Anxiety disorder NOS | 4 | 14 | 9 | 5 |
| Trichotillomania* | 0 | 1 | 0 | 0 |
| Behavioral Disturbance | 8 | 6 | 18 | 8 |
| Oppositional defiant disorder | 3 | 3 | 2 | 3 |
| Disruptive behavior disorder NOS | 5 | 2 | 16 | 3 |
| Intermittent explosive disorder | 0 | 1 | 0 | 0 |
| Conduct disorder | 0 | 0 | 0 | 2 |
| Pervasive Developmental Disordera | 2 | 0 | 9 | 7 |
| Substance Use Disordersb | 0 | 5 | 0 | 10 |
| Medical Conditions With Psychologic Factorsc | 0 | 20 | 0 | 4 |
| Learning Disordersd | 5 | 2 | 11 | 7 |
| Cognitive Deficite | 3 | 4 | 2 | 6 |
| Otherf | 10 | 4 | 13 | 5 |
| No Psychiatric Diagnosis | 27 | 49 | 35 | 24 |
Pervasive Developmental Disorders included: Asperger's Disorder, Autistic Disorder, and Pervasive Developmental Disorder Not Otherwise Specified.
Substance Use Disorders included: Alcohol, Cannabis, Nicotine, Prescription Drug, Street Stimulant, and Street Opioid misuse, abuse, or dependence disorders.
Medical Conditions with Psychological Factors included: Pain Disorder with medical and psychological components, Non-epileptogenic spells, Conversion Disorder, and Somatoform Disorder Not Otherwise Specified.
Learning Disorders included: Learning Disorder of Reading and Written Expression, Learning Disorder of Mathematics, Learning Disorder of Reading and Mathematics, Non-verbal Learning Disorder, and Learning Disorder Not Otherwise Specified.
Cognitive Deficits included: Borderline Intellectual Functioning, Mild Mental Retardation, Moderate Mental Retardation, Severe Mental Retardation, and Cognitive Disorder Not Otherwise Specified.
Other included: Developmental Delay, Expressive Language Disorder, Reactive Attachment Disorder, Eating Disorder Not Otherwise Specified, Schizoaffective Disorder, and Gender Identity Disorder.
Trichotillomania is classified as an Impulse Control Disorder in the DSM-IV diagnostic schema. This disorder commonly occurs in conjunction with other anxiety disorders such as Obsessive Compulsive Disorder, and was thus included under this heading.
Psychotropic Medications
The number of different types of psychotropic medications used by each of the 374 patients was assessed. Patients did not necessarily take these medications all at the same time. In addition, not all patients who were prescribed medications classified as “psychotropic medications” had been diagnosed with a psychiatric disorder, but rather had been diagnosed with other medical conditions where treatment with a psychotropic medication was indicated (e.g., headaches or seizure disorders). Approximately 54% (34/63) of female patients 0-9 years, 69% (92/133) of female patients 10-18 years, 55% (52/95) of male patients 0-9 years, and 71% (59/83) of male patients 10-18 years had been prescribed at least one psychotropic medication during their lifetime (Table 5). The mean number of new psychotropic medication trials started in female patients age in the 0-9 year age group was 1.127 (range 0-8 trials) and 1.932 (range 0-12 trials) in the 10-18 year age group 1.932 (range 0-12 trials). The mean number of new psychotropic medication trials started in male patients was 1.235 (range 0-7 trials) in the 0-9 year age group and 2.085 (range 0-9 trials) in the 10-18 year age group. There was no statistical difference between females and males within the same age group. However, the mean number of new psychotropic medication trials did significantly increase with age (p = 0.0016; Table 5).
Table 5.
Psychopharmacologic data
| Medication Trials | Female | Female | Male | Male |
|---|---|---|---|---|
| Age 0-9 | Age 10-18 | Age 0-9 | Age 10-18 | |
| Mean number of different psychotropic medication trials per patient over lifetimea | 1.13 | 1.93 | 1.23 | 2.09 |
| Range | 0-8 | 0-12 | 0-7 | 0-9 |
| Percentage of patients who were prescribed one or more psychotropic medications before they were diagnosed with RLS | 54% (34/63) | 69% (92/133) | 57% (54/95) | 71% (59/83) |
A statistical difference was found between age groups (0-9 vs 10-18), but not between gender (male vs female) (p ≤ 0.01).
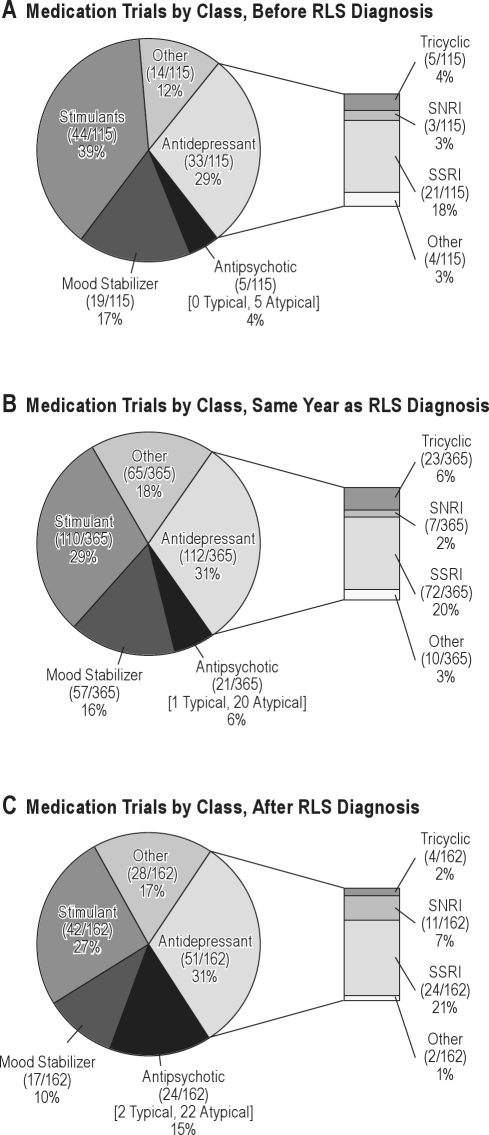
We found that antidepressants (196) and stimulant medications (196) represented over half (61%; 392/642) of the total psychotropic medication classes prescribed to the patients during their lifetime (Figure 2). The absolute number of medications prescribed was highest within the same year that the diagnosis of RLS was made, although the relative percentage of different psychotropic medications classes prescribed to patients remained consistent across time (Figure 2). Given the cross-sectional nature of the study, it was not possible to assess a causal link between prescribing psychotropic medications and the development of RLS.
Figure 2.
Pie charts describe the breakdown of absolute number and relative percentage of psychotropic medications prescribed to patients
Medication groups were organized into different psychotropic medication classes (antidepressants, antipsychotics, mood stabilizers, stimulants, and other). Groups were stratified by year the medications were prescribed relative to when the diagnosis of restless legs syndrome (RLS) was made: (A) at least one year before RLS diagnosis made; (B) same year as RLS diagnosis made; (C) at least one year after RLS diagnosis made.
Pharmacogenomic testing is available at our medical center and is routinely carried out within our department of psychiatry and psychology to aid in predicting psychotropic medication response, and susceptibility to medication side effect. At our Institution pharmacogenomic testing is often carried out in children and adolescents with serious and chronic psychiatric disorders such as schizophrenia, bipolar disorder, or recurrent severe major depression where pharmacologic management can be complicated by poor response to treatment, polypharmacy, and prohibitive medication side effects.
Within our study cohort we identified a subset of 15 patients with RLS and comorbid psychiatric disorder(s) who underwent cytochrome P-450 pharmacogenomic testing for gene polymorphisms encoding for clinically relevant hepatic enzymes, CYP2D6, CYP2C19, and CYP2C9, which are responsible for metabolism of many psychotropic drugs. Allelic variation of genes coding for these 3 enzymes results in abnormal metabolism of these psychotropic drugs. At our institution, variation in metabolic activity is defined as follows: Extensive metabolizers are homozygous for normal genotype and normal drug metabolism. Intermediate metabolizers are heterozygous for one normal allele, and one enzyme-inactivating allele.23 This leads to decreased drug metabolism. There is increased susceptibility to toxic accumulation of parent drug, and side effects at normal drug dosages. We identified 11/15 patients who were intermediate metabolizers of psychotropic medications they were prescribed. Poor metabolizers are homozygous for 2 inactivating alleles. This leads to limited or no drug metabolism. The parent drug often accumulates to toxic levels with serious risk for adverse side effects. We identified one patient who was a poor metabolizer of the psychotropic drugs they were prescribed. Ultra-rapid metabolizers have a duplication of a functional gene resulting in rapid drug metabolism. This results in a decrease in parent drug concentration at normal drug doses and increased risk for poor drug response at normal drug dosages.
A total of 10 patients with reduced metabolic capacity (9 “intermediate metabolizers” and one “poor metabolizer”) were prescribed psychotropic medication metabolized by the corresponding enzyme concurrent with the time they were diagnosed with RLS.
DISCUSSION
The design of this study was a retrospective chart review of pediatric patients who were evaluated RLS at our sleep center from the beginning of 2003 until the end 2009. We selected only those patients with clearly described definite or probable RLS in their medical records, and then cross referenced these records for encounters with a child psychiatrist or psychologist to assess for comorbid psychiatric disorders.
This study was conducted at a Midwest tertiary care facility that receives requests for patient evaluations locally, regionally, nationally, and internationally. The majority of patient evaluations were either local or regional; however this study also included a fair number of national RLS patients and three international RLS patients. Geographically, this study cohort is representative of the Midwest region of the United States. The socioeconomic makeup of this study cohort, as indicated by the breakdown of patient-listed health insurance, was skewed towards patients with privatized health insurance when compared with the general U.S. population. Approximately 26% of patients from our cohort were listed as having either state or federally funded health insurance, and approximately 52% with private health insurance. This reflects a wealthier cohort population when compared with the general U.S. population. The majority of our subjects were Caucasian; thus our cohort is not entirely representative of other regions of the United States. Males and females were equally represented.
Patients with RLS were subcategorized as having definite 1, definite 2, probable 1, or probable 2 RLS based on the available clinical data in the medical record. While most patients met criteria for definite 1 or definite 2 RLS we did find 49 patients that met diagnostic criteria for probable 2 RLS. This contributes to some of the first “probable 2” RLS cases reported in the literature. These patients were some of the youngest in our study cohort (the youngest being 11 months old), and were described as having behavioral manifestations of RLS including increased periodic limb movements noted in their overnight polysomnogram studies. However, they lacked the ability to verbally articulate their symptoms. They also had clearly described family histories of RLS. Some older patients were also diagnosed with probable 2 RLS. These patients were cognitively impaired in some way (either having mental retardation or significant features of autism), and were also limited in their ability to describe their symptoms, but otherwise their clinical features were consistent with RLS.
We used strict inclusion/exclusion criteria that likely led to rejection of a number of patients with legitimate RLS and/or psychiatric disorders because their symptoms had not been adequately described in the medical record. As with any retrospective design, we were able to demonstrate only associations rather than cause and effect, specifically with regard to the relationship between childhood RLS and psychotropic medications. Only prospective evaluation can help determine whether RLS was secondary to psychotropic medication and whether there is a shared biologic link between psychiatric disorders and RLS.
The most significant finding of this study was that nearly two-thirds of children who were diagnosed with RLS also had one or more psychiatric diagnoses, which supported our first hypothesis about the co-occurrence of these disorders. Not surprisingly, ADHD was commonly diagnosed in children with RLS, consistent with what has been previously documented.3–5 Our findings independently support those previously reported results. Additionally, we also identified a high prevalence of other psychiatric disorders. Disturbances in mood were diagnosed even more frequently than ADHD, although this group also included patients with transient mood disturbances such as adjustment disorders. Mood disorders were more prevalent in females and ADHD was encountered more often in males. Psychotropic medications had been started in a majority of subjects prior to formal diagnosis of RLS. This brings up the need for child psychiatrists and psychologists to be more aware of RLS as a possible comorbidity in their patients.
Several studies in adults have shown an important association between RLS and major depression/anxiety disorders.9–11 Lee et al.9 and Hornyak11 have suggested hypotheses to account for this association: first, sleep architecture is adversely affected by both RLS and mood/anxiety disorders, which can exacerbate psychiatric symptoms.9,11 The interplay between decreased sleep quality and quantity on one hand and neurocognitive and psychiatric symptoms on the other hand is well documented.24,25 RLS and some psychiatric disorders may share some similarities in neuropathophysiology, as dopamine is a neurotransmitter central to restless legs syndrome, and plays a key role in psychiatric disorders as well.7,9,26
Somatic disturbances, such as diabetes, cardiovascular disease, and cancer were observed in adult patients with RLS and comorbid depression and anxiety in the study of Winkelmann et al. Even after controlling for this possible confounding variable a strong association was shown between RLS and mood/anxiety disturbances.10 In our study developmental disorders and psychosomatic disorders, such as pain disorders and various medical illnesses with psychological factors were observed among children diagnosed with RLS. We did not control for these diagnoses to focus specifically on depression and anxiety as we were interested in describing any co-occurring psychiatric disturbance. In our study approximately 35% (131/374) of children and adolescents with RLS were diagnosed with more than one psychiatric disorder. This is not surprising because psychiatric disorders commonly co-occur with one another. For example, ADHD and behavioral disturbances commonly co-occur; mood and anxiety disorders also commonly co-occur. We were unable to determine the impact that RLS and comorbid psychiatric conditions had on one another, given the limitations of this study. This would be an important area for ongoing study.
Picchietti and Stevens also described comorbid psychiatric disturbances, such as ADHD, mood disorders, and behavioral disturbances, in a small cohort of children with RLS.6 Our study is consistent with their findings, and demonstrates an important association between childhood RLS and comorbid psychiatric disorders. To the best of our knowledge, this is the largest study to date looking at this association in children and adolescents.
Our study points out those children with RLS may present in the early stages to child psychiatrists or psychologists rather than to sleep specialists for evaluation and management of their behavioral disturbance. Over 50% of all children (ages 0-9 years) and nearly 70% of all adolescents (ages 10-18 years) with RLS had received at least one trial of a psychotropic medication at some point during the course of their care, highlighting the importance of identifying RLS that has been induced or exacerbated by such medications. We found that on average, children had received slightly more than one trial of a psychotropic medication before the age of 10; by the age of 18, adolescents had received nearly two trials of different psychotropic medications. The prescription of psychotropic medications varied considerably, ranging from none to as many as eight or more different medication trials. This variability was reflective of whether a patient had one or more comorbid psychiatric disorders and the severity of that disorder. Those patients diagnosed with more severe or multiple psychiatric disorders tended to receive more trials of different psychotropic medications. While this seems intuitive, it is an important discussion point given that many of these medications can precipitate latent RLS or exacerbate symptoms of RLS.11,14,15,27
We also found that the majority of psychotropic medications were prescribed around the same time that the diagnosis of RLS was made. The implications of this finding are unclear, but as discussed above, psychotropic medications can cause RLS symptoms iatrogenically or exacerbate preexisting symptoms of comorbid RLS. Our finding that patients were being prescribed an increasing number of new psychotropic medication trials as they get older (Table 5) underscores the chronicity of their psychiatric disorder(s) and possible risk for RLS. While we would be cautious about drawing a causal relationship, this should bring increased awareness to providers about the burden of additional medication side effects. Furthermore, this finding illustrates the importance of prospectively studying the mechanism by which psychotropic medications can potentiate RLS symptoms as other studies have failed to find a direct causal relationship between psychotropic medication and RLS symptoms.9,28
Pharmacogenomic studies prior to the initiation of treatment with a selective serotonin reuptake inhibitor or neuroleptic medication (such as risperidone) might also be helpful to assess the potential risk for developing iatrogenic RLS. In humans, the cytochrome P450 enzymes are a group of proteins that are responsible for nearly 75% of oxidative phase I drug metabolism. Allelic variants of several key enzymes, specifically 2D6, 2C9, and 2C19, have clinical relevance in how efficiently some psychotropic drugs are metabolized.29 Pharmacogenetic studies have been shown to be safe and effective to assess for allelic variation in children as well.30 This may have important ramifications for how rapidly a drug is metabolized, and for an individual's susceptibility to a drug's side effects, including symptoms of RLS. In this study we found 12/15 patients with reduced psychotropic drug metabolism predicted by their pharmacogenomic profile. Ten of those patients were taking psychotropic medications around the time they were diagnosed with RLS. This provides a possible mechanism whereby individuals with reduced capacity to metabolize psychotropic drugs are more susceptible to drug side effects such as iatrogenic RLS. However, the widespread use of pharmacogenetics in fields such as psychiatry has not been unanimously received, with concerns raised about cost, predictive power, and clinical utility.31 More research is needed in this emerging field to better define a clinical role for the use of genetic testing.
Iron plays a key role in neurotransmission, neuronal function, and dopaminergic signaling.32 Low serum ferritin plays an important role in RLS pathophysiology and may also contribute to the pathophysiology of some psychiatric disorders such as ADHD.22,32 We were interested in determining if there was a difference in serum ferritin levels in RLS patients without psychiatric disorders compared to those with psychiatric disorders. In this study we found low mean serum ferritin levels (< 50 μg/L) among children and adolescents diagnosed with RLS across age, gender, and the presence/absence of a psychiatric disorder. We did not find a statistically significant difference between patients grouped by gender, age, or whether or not they carried a psychiatric diagnosis. Limited sample sizes prevented us from comparing patients within the “psychiatric disorder” group (ADHD vs. major depression vs. anxiety, etc.). We did not see group-wise differences in serum ferritin in those patients with comorbid psychiatric disorders as compared to patients who did not have a comorbid psychiatric diagnosis. However, our study lacked sufficient power to determine if there were differences in serum ferritin in patients with different psychiatric disorders, and this is an area for further research.
In conclusion, we would propose a bidirectional relationship wherein the sleep disturbance exacerbates mood and attention problems, and the use of psychotropic medications potentially exacerbates the sleep disturbance in some susceptible children. Psychiatric comorbidities occur in two-thirds of children with RLS. This necessitates a comprehensive multidisciplinary approach that involves sleep specialists, child psychiatrists, and psychologists. This study underscores the need for the child psychiatrist/psychologist in assessing for a patient and family history of RLS, as well as being mindful of the role of psychotropic medications in precipitating or exacerbating RLS. It is important for the sleep specialist to look for psychiatric comorbidities; conversely, it is equally important for the child psychiatrist or psychologist to inquire about sleep disorders such as restless legs syndrome, due to the complimentary and at times confounding nature of the illnesses and the need for an integrated management approach.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The work for this study work was performed at Mayo Clinic, Rochester, MN, USA. The authors are grateful to Ms. Lori Solmonson for invaluable assistance in manuscript preparation. The authors are also grateful to Ms. Jennifer R. Geske, M.S., for her work statistically analyzing our data set.
REFERENCES
- 1.Allen RP, Picchietti D, Hening WA, Trenkwalder C, Walters AS, Montplaisir J. Restless legs syndrome: diagnostic criteria, special considerations, and epidemiology. A report from the restless legs syndrome diagnosis and epidemiology workshop at the National Institutes of Health. Sleep Med. 2003;4:101–19. doi: 10.1016/s1389-9457(03)00010-8. [DOI] [PubMed] [Google Scholar]
- 2.Simakajornboon N, Kheirandish-Gozal L, Gozal D. Diagnosis and management of restless legs syndrome in children. Sleep Med Rev. 2009;13:149–56. doi: 10.1016/j.smrv.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Picchietti D, Allen RP, Walters AS, Davidson JE, Myers A, Ferini-Strambi L. Restless legs syndrome: prevalence and impact in children and adolescents--the Peds REST study. Pediatrics. 2007;120:253–66. doi: 10.1542/peds.2006-2767. [DOI] [PubMed] [Google Scholar]
- 4.Yilmaz K, Kilincaslan A, Aydin N, Kor D. Prevalence and correlates of restless legs syndrome in adolescents. Dev Med Child Neurol. 2011;53:40–7. doi: 10.1111/j.1469-8749.2010.03796.x. [DOI] [PubMed] [Google Scholar]
- 5.Turkdogan D, Bekiroglu N, Zaimoglu S. A prevalence study of restless legs syndrome in Turkish children and adolescents. Sleep Med. 2011;12:315–21. doi: 10.1016/j.sleep.2010.08.013. [DOI] [PubMed] [Google Scholar]
- 6.Picchietti DL, Stevens HE. Early manifestations of restless legs syndrome in childhood and adolescence. Sleep Med. 2008;9:770–81. doi: 10.1016/j.sleep.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 7.Muhle H, Neumann A, Lohmann-Hedrich K, et al. Childhood-onset restless legs syndrome: clinical and genetic features of 22 families. Mov Disord. 2008;23:1113–21. doi: 10.1002/mds.22016. quiz 203. [DOI] [PubMed] [Google Scholar]
- 8.Picchietti MA, Picchietti DL. Restless legs syndrome and periodic limb movement disorder in children and adolescents. Semin Pediatr Neurol. 2008;15:91–9. doi: 10.1016/j.spen.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 9.Lee HB, Hening WA, Allen RP, et al. Restless legs syndrome is associated with DSM-IV major depressive disorder and panic disorder in the community. J Neuropsychiatry Clin Neurosci. 2008;20:101–5. doi: 10.1176/jnp.2008.20.1.101. [DOI] [PubMed] [Google Scholar]
- 10.Winkelmann J, Prager M, Lieb R, et al. “Anxietas tibiarum”. Depression and anxiety disorders in patients with restless legs syndrome. J Neurol. 2005;252:67–71. doi: 10.1007/s00415-005-0604-7. [DOI] [PubMed] [Google Scholar]
- 11.Hornyak M. Depressive disorders in restless legs syndrome - epidemiology, pathophysiology, and management. CNS Drugs. 2010;24:89–98. doi: 10.2165/11317500-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 12.Montagna P, Hornyak M, Ulfberg J, et al. Randomized trial of pramipexole for patients with restless legs syndrome (RLS), and RLS-related impairment of mood. Sleep Med. 2011;12:34–40. doi: 10.1016/j.sleep.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 13.Benes H, Mattern W, Peglau I, et al. Ropinirole improves depressive symptoms and restless legs syndrome severity in RLS patients: a multicentre, randomized, placebo-controlled study. J Neurol. 2011;258:1046–54. doi: 10.1007/s00415-010-5879-7. [DOI] [PubMed] [Google Scholar]
- 14.Yang C, White DP, Winkelman JW. Antidepressants and periodic limb movements of sleep. Biol Psychiatry. 2005;58:510–14. doi: 10.1016/j.biopsych.2005.04.022. [DOI] [PubMed] [Google Scholar]
- 15.Rottach KG, Schaner BM, Kirch MH, et al. Restless legs syndrome as a side effect of second generation antidepressants. J Psychiat Res. 2009;43:70–5. doi: 10.1016/j.jpsychires.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 16.Mrazek DA. Current applications of clinical genetic testing for psychiatric practice. Minn Med. 2007;90:42–3. [PubMed] [Google Scholar]
- 17.Mrazek DA. Psychiatric pharmacogenomic testing in clinical practice. Dialogues Clin Neurosci. 2010;12:69–76. doi: 10.31887/DCNS.2010.12.1/dmrazek. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kotagal S, Silber MH. Childhood-onset restless legs syndrome. Ann Neurol. 2004;56:803–7. doi: 10.1002/ana.20292. [DOI] [PubMed] [Google Scholar]
- 19.Practice parameters for the assessment and treatment of children and adolescents with depressive disorders. AACAP. J Am Acad Child Adolesc Psychiatry. 1998;37:63S–83S. doi: 10.1097/00004583-199810001-00005. [DOI] [PubMed] [Google Scholar]
- 20.Green M, Wong M, Atkins D, Taylor J, Feinleib M. Diagnosis of attention deficit/hyperactivity disorder. AHRQ Technical reviews and summaries. Report No. 99-0050 19991-120. [Google Scholar]
- 21.National Heart Lung and Blood Institute Working Group on Restless Legs Syndrome. Restless legs syndrome: detection and management in primary care. National Heart, Lung, and Blood Institute Working Group on Restless Legs Syndrome. Am Fam Physician. 2000;62:108–14. [PubMed] [Google Scholar]
- 22.Picchietti M, Picchietti D. Advances in pediatric restless legs syndrome: iron, genetics, diagnosis, and treatment. Sleep Med. 2010;11:643–51. doi: 10.1016/j.sleep.2009.11.014. [DOI] [PubMed] [Google Scholar]
- 23.Communique. Cytochrome P-450 enzyme genotyping: optimizing patient care through pharmacogenetics. Mayo Reference Services. 2005;30:1–9. [Google Scholar]
- 24.Lofthouse N, Gilchrist R, Splaingard M. Mood-related sleep problems in children and adolescents. Child Adolesc Psychiatr Clin N Am. 2009;18:893–916. doi: 10.1016/j.chc.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 25.O'Brien LM. The neurocognitive effects of sleep disruption in children and adolescents. Child Adolesc Psychiatr Clin N Am. 2009;18:813–23. doi: 10.1016/j.chc.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 26.Connor JR, Wang XS, Allen RP, et al. Altered dopaminergic profile in the putamen and substantia nigra in restless leg syndrome. Brain. 2009;132:2403–12. doi: 10.1093/brain/awp125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cohrs S, Rodenbeck A, Hornyak M, Kunz D. Restless legs syndrome, periodic limb movements, and psychopharmacology. Nervenarzt. 2008;79(1263-4):6–72. doi: 10.1007/s00115-008-2575-2. [DOI] [PubMed] [Google Scholar]
- 28.Brown LK, Dedrick DL, Doggett JW, Guido PS. Antidepressant medication use and restless legs syndrome in patients presenting with insomnia. Sleep Med. 2005;6:443–50. doi: 10.1016/j.sleep.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 29.Ingelman-Sundberg M. Pharmacogenetics of cytochrome P450 and its applications in drug therapy: the past, present and future. Trends Pharmacol Sci. 2004;25:193–200. doi: 10.1016/j.tips.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 30.Wall CA, Oldenkamp C, Swintak C. Safety and efficacy of pharmacogenomics in pediatric psychopharmacology. Prim Psychiatry. 2010;17:53–8. [Google Scholar]
- 31.Arranz MJ, Kapur S. Pharmacogenetics in psychiatry: are we ready for widespread clinical use? Schizophr Bull. 2008;34:1130–44. doi: 10.1093/schbul/sbn114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Calarge C, Farmer C, DiSilvestro R, Arnold E. Serum ferritin and amphetamine response in youth with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2010;20:495–502. doi: 10.1089/cap.2010.0053. [DOI] [PMC free article] [PubMed] [Google Scholar]