Abstract
Study Objectives:
Although periodic limb movements in sleep (PLMS) have been described in multiple pediatric publications, periodic limb movement disorder (PLMD) has not. The aims of this study were to describe the prevalence, sleep-related correlates, and polysomnographic correlates of PLMD in a large pediatric case series, and compare these to pediatric obstructive sleep apnea (OSA).
Methods:
All PLMD cases (defined by International Classification of Sleep Disorders, 2nd edition criteria + respiratory disturbance index [RDI] < 3) and OSA cases (defined by RDI ≥ 3 + PLMS < 5), from a single pediatric sleep practice, over a 2-year time span, were included. Chart, questionnaire, and polysomnographic data were compiled. Of 468 referred children, 66 PLMD cases were identified (14%).
Results:
The PLMD cases, mean age 8.1 years (range 1-17), were clinically characterized by frequent sleep onset and maintenance problems, difficulty awakening, restless sleep, leg pain/discomfort at night, and parasomnias. Compared to 90 OSA children, those with PLMD had a history of significantly more sleep onset and maintenance problems, leg pain/discomfort at night, parasomnias, getting out of bed at night, and family history of restless legs syndrome. Polysomnographically, PLMD cases had more awakenings, stage 1 sleep, stage shifts, and spontaneous arousals.
Conclusions:
These data indicate that pediatric PLMD has important clinical and polysomnographic correlates. In addition, PLMD has many characteristics that are different from pediatric OSA, suggesting that PLMD is a distinct pediatric sleep disorder, of which clinicians should be aware.
Citation:
Gingras JL; Gaultney JF; Picchietti DL. Pediatric periodic limb movement disorder: sleep symptom and polysomnographic correlates compared to obstructive sleep apnea. J Clin Sleep Med 2011;7(6):603-609.
Keywords: Periodic limb movements in sleep, periodic limb movement disorder, sleep disorder, children, parasomnia, obstructive sleep apnea, polysomnography
Although there are multiple publications on pediatric periodic limb movements in sleep (PLMS), little is known about periodic limb movement disorder (PLMD) in children. PLMS are brief leg or arm jerks during sleep, associated with negative physiological consequences, including cardiac acceleration, spikes in blood pressure, and cortical arousals.1–6 PLMS occur in a variety of sleep disorders, including restless legs syndrome (RLS), PLMD, narcolepsy, obstructive sleep apnea (OSA), and REM sleep behavior disorder, or may be induced by certain medications.7,8 Based on the current diagnostic criteria, PLMD is diagnosed when the following are present: (1) PLMS documented by polysomnography; (2) PLMS exceeding norms for age (≥ 5/h for children); (3) clinical sleep disturbance or daytime fatigue; and (4) the absence of another primary sleep disorder or reason for the PLMS, including RLS and OSA.7,9 Prior to revision of the criteria at a workshop at the National Institutes of Health in 2002, PLMD was typically diagnosed by the presence of only criteria 1-2, which do not effectively differentiate PLMS as part of another condition or PLMS as an asymptomatic polysomnographic finding, from PLMD as a distinct disorder. However, there is very little adult or pediatric literature using this current definition, and controversy continues as to the clinical significance of PLMS.10–14
BRIEF SUMMARY
Current Knowledge/Study Rationale: There is very limited medical literature on pediatric periodic limb movement disorder (PLMD) based on the current definition. In this study we identify clinical and polysomnographic features of PLMD in a pediatric sleep center referral population, and compare these to children with obstructive sleep apnea (OSA).
Study Impact: In this large clinical case series, PLMD was found to be common,14% of 468 referred children. Important correlates of pediatric PLMD included disturbed sleep, leg discomfort/pain, parasomnias, and a family history of restless legs, more so than in the children with OSA.
We are aware of only three publications that report pediatric PLMD based on this newer definition. Two were relatively small case series, which emphasized the relationship of pediatric PLMD to family history and subsequent onset of RLS, as well as potential significant associations with parasomnias and attention deficit hyperactivity disorder (ADHD).15,16 In a recent report we looked at daytime behavioral correlates of pediatric PLMD, and found greater daytime mood and behavioral difficulties in children with PLMD when compared to children with OSA.17 In addition, PLMD was found to predict these daytime difficulties better than PLMS index alone. Using a PLMD definition of PLMS > 5/h alone (not using the clinical sleep disturbance criterion), studies have reported pediatric prevalences of 5.6% to 26% in clinical polysomnographic case series,18–23 and a prevalence of 11.9% in the only population-based study.20 Many of the clinical cases were found incidentally in children referred for potential OSA. In a recent pediatric study, correlates of PLMS > 5/h included male gender (61%) and snoring (45%), with PLMS index correlating negatively with total sleep time, sleep efficiency, and percent slow wave sleep on polysomnography.18
The specific aims of this study were to identify the demographic features, prevalence, sleep-related symptoms, and polysomnographic features of PLMD in a large pediatric sleep center referral population, as well as to compare the clinical and polysomnographic correlates of pediatric PLMD to pediatric obstructive sleep apnea (OSA).
METHODS
This was a retrospective case series of children and adolescents with PLMD referred to a private practice of sleep medicine in a Southeastern urban area over a period of 2 years. The pediatric patients were seen by a single sleep physician (JLG). After institutional review board approval, demographic, chart, questionnaire, and polysomnographic data were compiled. Sleep history was obtained by semi-structured interview during the course of comprehensive clinical assessment, which included consideration of differential diagnosis and other medical conditions. In addition, parents completed a detailed questionnaire developed by author JLG specifically for use in her clinical practice of pediatric sleep medicine and designed to query the behavioral sleep routines, sleep complaints, and daytime mood and behavioral problems in children as perceived by the parent and/or child. Items encompassing sleep-related behavior and routines were analyzed in this study. Daytime mood and behavior items have been reported in a separate study.17 Possible responses to individual items were: 1 = never (0 out of 7 days per week); 2 = sometimes (1-4 out of 7 days); or 3 = frequently (5-7 out of 7 days).
Polysomnography
Polysomnography was performed in an accredited sleep laboratory specifically designed for pediatric studies, attended by a certified sleep technologist trained in pediatric polysomnography, and video recorded. The following parameters were measured: electroencephalogram, eye movements, chin electromyogram, bilateral anterior tibialis electromyogram, electrocardiogram, nasal airflow (Pro-Tech Thermal Airflow Sensors ), chest and abdominal wall motion (Pro-Tech Piezo effort belts), snore recording (Pro-Tech Piezo Snore Sensor), pulse oximetry (Nellcor NPB 295), and body position. The PSGs were reviewed and interpreted by a board-certified Sleep Medicine Specialist also boarded in Pediatrics (JLG).
PLMD and OSA Definitions
PLMD was defined by ICSD-2 criteria: PLMS ≥ 5/h present on PSG; clinical sleep disturbance (difficulty falling or staying asleep, or waking unrefreshed after sufficient sleep); and PLMS were not better explained by another current sleep disorder, medical or neurological disorder, mental disorder, medication use, or substance use disorder.7 PLMS at the termination of respiratory events were not counted. PLMS were defined as a sequence of ≥ 4 limb movements of 0.5–5.0 s in duration, separated by > 5 s and < 90 s, and of an amplitude ≥ 25% of toe dorsiflexion during calibration.7,24 Although the ICSD-2 criteria had not been published at the time of initial assessment for these patients (2001-2002), the criteria may nonetheless be applied retrospectively because they are similar to the ICSD-R criteria used at the time,25 and the interviewer (JLG) was routinely asking for and recording all information relevant to the diagnosis, including “clinical sleep disturbance.”
An obstructive apnea was defined as a decrease ≥ 75% in airflow from the baseline value for ≥ 2 breaths with continuing respiratory effort. A hypopnea was defined as a discernable decrease in airflow from the preceding baseline accompanied by either a decrease in oxygen saturation of ≥ 3% or followed by an arousal. Snore arousals were scored when snoring was associated with EEG arousals. Respiratory disturbance index (RDI) was defined as apneas + hypopneas + snore arousals per hour of sleep.
Sample Selection
From an initial pool of 468 consecutive children referred to a private pediatric sleep medicine practice over a period of 2 years (2001-2002), 347 received a clinical diagnosis of OSA, PLMD, or both. Of the other 121 children, 24 did not complete the diagnostic protocol, 34 did not receive a PSG, and 63 were diagnosed with a disorder outside the scope of the present study, such as narcolepsy (n = 6) or RLS (n = 9). Although narcolepsy and RLS are commonly associated with PLMS, the focus of this study was PLMD. Seventy of the 347 received a dual clinical diagnosis of PLMD and OSA, and because of potential “overlap” were excluded from analysis. Furthermore, to analyze unequivocal cases of PLMD and OSA, more stringent polysomnography-based inclusion criteria were implemented: for the PLMD cases, ICSD-2 defined PLMD and RDI < 3; for the OSA cases, RDI ≥ 3 and PLMS < 5/h. One hundred twenty-one of the remaining 277 children who had received a single clinical diagnosis of OSA or PLMD were excluded from these analyses because they did not meet these stringent study criteria for OSA or PLMD. Eighty-seven of the 121 were OSA cases with PLMS > 5/h but without a clinical diagnosis of PLMD, 30 were PLMD cases with an RDI ≥ 3 but no clinical symptoms for sleep disordered breathing, and 4 had missing data. This resulted in 66 distinct PLMD cases and, for comparison, 90 unequivocal OSA cases.
Although not a primary focus of the study, treatment data were available for 51 of 66 patients with a diagnosis of PLMD (77%). Treatment was open-label, with outcome assessed by clinician impression, through interview of the child and parent at follow-up clinic visits.
Statistical Analysis
Group differences in chart and questionnaire data (counts) were tested using χ2 analyses. In an effort to reduce family-wise error, PSG data were grouped into categories (such as sleep time, wake time, awakenings) and analyzed using multivariate analyses, followed by univariate analyses. Pearson correlations examined associations between variables. An α of p < 0.05 was considered significant. Treatment results were evaluated by Kaplan-Meier survival analysis.
RESULTS
Prevalence and Demographics
Sixty-six of 468 consecutive children referred over a 2-year period were diagnosed with PLMD, a prevalence of 14% in this practice population. The average age was 8.05 years (range 1-17), with 58% males. Comparing children with PLMD to those with OSA, there were not significant group differences for age or gender (Table 1). However, comparing Caucasian to African American children, Caucasian children were more likely to have PLMD than were African American children (49% vs. 26%), while African American children were more likely than Caucasian children to have OSA (74% vs. 51%).
Table 1.
Demographic data: PLMD compared to OSA group
| Parameter | PLMD | OSA | PLMD vs OSA |
|---|---|---|---|
| Age (yrs; mean ± SD) | 8.05 ± 3.93 (range 1-17) | 7.47 ± 4.07 (range 1-15) | n.s. |
| Gender (% male) | 58 | 61 | n.s. |
| Ethnicity* | χ2(3) = 8.88, p = 0.03 | ||
| Caucasian (n = 74) | 36/74 (49%) | 38/74 (51%) | |
| African American (n = 31) | 8/31 (26%) | 23/31 (74%) | |
| Asian (n = 2) | 2 | 0 | |
| Latino (n = 2) | 0 | 2 |
PLMD, periodic limb movement disorder; OSA, obstructive sleep apnea; n.s., not significant;
ethnicity data not available for 47 children.
Chart and Questionnaire Data
As expected, many children with PLMD were reported by the parent and/or child to have sleep onset and maintenance problems (Table 2, chart data). In addition, restless sleep and kicking while asleep were commonly described. Many were also reported to be difficult to awakening in the morning (68%), complain of leg pain or discomfort at night (62%), have parasomnias (61%), and have a family history of RLS (49%). Remarkably, the sleep onset and maintenance problems, leg pain/discomfort at night, kicking in sleep, parasomnias, and family history of RLS were significantly more common in PLMD than OSA, while restless sleep, difficulty awakening, and excessive daytime sleepiness were not.
Table 2.
Sleep-related chart and questionnaire data
| Chart data | PLMD (n = 66) % yes | OSA (n = 90) % yes | PLMD vs OSA |
|---|---|---|---|
| Difficulty falling asleep | 67 | 22 | χ2(1) = 31.09, p < 0.01 |
| Difficulty staying asleep | 50 | 29 | χ2(1) = 7.22, p < 0.01 |
| Difficulty awakening | 68 | 67 | n.s. |
| Restless sleep | 89 | 87 | n.s. |
| Kicking in sleep | 53 | 21 | χ2(1) = 17.14, p < 0.01 |
| Leg pain/discomfort at night | 62 | 18 | χ2(1) = 32.89, p < 0.01 |
| Apnea observed | 15 | 59 | χ2(1) = 30.26, p < 0.01 |
| Excessive daytime sleepiness | 30 | 24 | n.s. |
| Parasomnias | 61 | 36 | χ2(1) = 9.62, p < 0.01 |
| Family history of RLS | 49 | 18 | χ2(1) = 8.40, p < 0.01 |
| Questionnaire data | PLMD (n = 66) % frequent | OSA (n = 90) % frequent | PLMD vs OSA |
| Regular bedtime | 73 | 59 | χ2(1) = 3.19, p = 0.07 |
| Goes to bed awake | 74 | 72 | n.s. |
| Has bedtime ritual | 61 | 48 | n.s. |
| Has caffeine before bed | 3 | 3 | n.s. |
| Uses sleep medication | 8 | 2 | n.s. |
| Refuses to go to bed | 21 | 10 | χ2(1) = 3.81, p = 0.05 |
| Gets out of bed | 35 | 20 | χ2(1) = 4.33, p = 0.04 |
| Refuses to sleep alone | 23 | 18 | n.s. |
| Sleeps with family member | 26 | 19 | n.s. |
| Wakes, goes to parents” bed | 36 | 20 | χ2(1) = 5.18, p = 0.02 |
| Wakes up tired | 39 | 33 | n.s. |
| Wakes irritable, in bad mood | 38 | 31 | n.s. |
| Difficult to awaken | 38 | 34 | n.s. |
| Morning headache | 3 | 8 | n.s. |
| Appears drowsy during day | 9 | 12 | n.s. |
| Falls asleep inappropriately | 24 | 17 | n.s. |
| Moves during sleep | 67 | 54 | n.s. |
| Sleeps in unusual positions | 21 | 24 | n.s. |
| Jerks arms and legs | 21 | 17 | n.s. |
| Crawling, aching | 14 | 8 | n.s. |
| Leg pain | 18 | 9 | χ2(1) = 2.94, p = 0.09 |
| Wets bed | 2 | 10 | χ2(1) = 3.81, p = 0.05 |
| Grinds teeth | 15 | 10 | n.s. |
| Talks in sleep | 23 | 12 | χ2(1) = 3.02, p = 0.08 |
| Walks in sleep | 9 | 3 | n.s. |
| Wakes from nightmares | 12 | 1 | χ2(1) = 8.49, p < 0.01 |
| Frightening dreams | 11 | 2 | χ2(1) = 4.92, p = 0.03 |
| Screams/difficult to fully awaken | 12 | 3 | χ2(1) = 4.88, p = 0.03 |
PLMD, periodic limb movement disorder; OSA, obstructive sleep apnea; RLS, restless legs syndrome; χ2, chi-square; n.s., not significant.
In the questionnaire data (Table 2), where responses are reported as percent frequent responses (5-7 out of 7 days), significantly more children with PLMD than OSA were described as getting out of bed, going to parents” bed, waking from nightmares, having frightening dreams, and having sleep terror-type awakenings, i.e., more sleep onset/maintenance problems and parasomnias. Interestingly, complaints of frequent crawling/aching sensations or leg pain were not significantly more common in children with PLMD. However, when responses of “sometimes” and “frequently” were recoded as “yes” answers for these items, children with PLMD were reported to have crawling/aching sensations and leg pain significantly more often (χ2(1) = 5.00, p = 0.02).
Polysomnographic Data
Based on inclusion criteria, the PLMD and OSA groups had markedly different mean PLMS and respiratory disturbance indices (Table 3), with a mean PLMS index of 23.5 in the PLMD group and mean RDI of 19.2 in the OSA group. In an effort to determine whether the relative severity of each disorder was roughly equivalent, a severity variable was calculated, equal to the standardized PLMS index for children with PLMD and the standardized apnea-hypopnea index (AHI) for children with OSA. The groups were equivalent for this estimate of severity (t154 = 0.79, p = 0.43).
Table 3.
Polysomnographic data (means and standard deviations)
| Parameter | PLMD (n = 66) | OSA (n = 90) | PLMD vs OSA |
|---|---|---|---|
| AHI | 0.6 ± 0.6 | 13.0 ± 17.1 | * |
| RDI | 1.13 ± 0.9 | 19.2 ± 18.1 | * |
| PLMS index | 23.5 ± 17.6 | 1.3 ± 1.5 | * |
| Total recording time (min) | 537.9 ± 77.6 | 531.1 ± 157.8 | n.s. |
| Total sleep time (min) | 450.8 ± 77.2 | 440.5 ± 87.6 | n.s. |
| Sleep efficiency (%) | 83.8 ± 8.5 | 84.9 ± 13.0 | n.s. |
| Sleep onset latency (min) | 41.7 ± 31.7 | 38.3 ± 65.8 | n.s. |
| REM latency (min) | 166.2 ± 80.2 | 171.7 ± 80.0 | n.s. |
| Awake time (mult.) | F1,152 = 3.93, p = 0.02, ηp2 = 0.05 | ||
| # Awakenings | 15.3 ± 10.0 | 11.7 ± 8.3 | F1,153 = 5.97, p = 0.02, ηp2 = 0.04 |
| WASO (min) | 45.4 ± 50.2 | 51.2 ± 93.7 | n.s. |
| Time in each stage (mult.) | F5,150 = 85.82, p < 0.01, ηp2 = 0.10 | ||
| Stage 1 (% TST) | 6.7 ± 3.9 | 4.9 ± 3.19 | F5,150 = 9.26, p < 0.01, ηp2 = 0.06 |
| Stage 2 (% TST) | 57.9 ± 11.3 | 57.5 ± 12.4 | n.s. |
| Stage 3 (% TST) | 8.5 ± 6.2 | 9.9 ± 7.3 | n.s. |
| Stage 4 (% TST) | 11.2 ± 10.2 | 12.6 ± 12.4 | n.s. |
| Stage REM (% TST) | 15.7 ± 5.8 | 15.0 ± 6.6 | n.s. |
| Shifts (mult.) | F5,149 = 2.52, p = 0.03, ηp2 = 0.08 | ||
| Stage shifts | 88.5 ± 33.1 | 66.8 ± 29.2 | F1,153 = 18.76, p < 0.01, ηp2 = 0.11 |
| Stage 1 shifts | 27.3 ± 11.5 | 19.3 ± 11.2 | F1,153 = 18.94, p < 0.01, ηp2 = 0.11 |
| Arousals (mult.) | F5,150 = 85.82, p < 0.01, ηp2 = 0.74 | ||
| Spontaneous arousal index | 3.1 ± 4.1 | 2.0 ± 2.4 | F1,154 = 4.80, p = 0.03, ηp2 = 0.03 |
| AH arousal index | 0.4 ± 0.6 | 11.0 ± 16.6 | F1,154 = 26.83, p < 0.01, ηp2 = 0.15 |
| Snore arousal index | 0.6 ± 0.7 | 6.2 ± 5.0 | F1,154 = 83.32, p < 0.01, ηp2 = 0.15 |
| PLMS arousal index | 9.6 ± 4.6 | 0.8 ± 1.2 | F1,154 = 297.29, p < 0.01, ηp2 = 0.66 |
| Total arousals index | 13.7 ± 7.20 | 20 ± 16.6 | F1,154 = 8.29, p < 0.01, ηp2 = 0.05 |
PLMD, periodic limb movement disorder; OSA, obstructive sleep apnea; AHI, apnea-hypopnea index; RDI, respiratory disturbance index; PLMS, periodic leg movements in sleep; TST, total sleep time; mult, multivariate analysis;
differences defined by group inclusion criteria; n.s., not significant.
Comparing PLMD to OSA, total recording time, total sleep time, and REM latency did not differ. However, children with PLMD had significantly more awakenings, stage 1 sleep, stage shifts, stage 1 shifts, and spontaneous arousals (Table 1). Conversely, children with OSA had significantly more total arousals. Both PLMD and OSA groups had equivalent, but remarkably low sleep efficiencies of 83.8% and 84.9%, and long sleep latencies of 41.7 and 38.3 minutes, respectively. Comparable pediatric polysomnographic normative values are 89.3% to 90.8% for sleep efficiency and 23 minutes for sleep latency.26,27
The PLMS index was not significantly correlated with age, total sleep time, sleep efficiency, sleep onset latency, awakenings, waking after sleep onset, percent slow wave sleep, or minimum SaO2 NREM. It was significantly correlated with stage shifts (r(155) = 0.27, p < 0.01), stage 1 shifts (r(155) = 0.27, p < 0.01), percent stage 1 sleep (r(155) = 0.17, p = 0.03), and minimum SaO2 REM (r(153) = 0.19, p < 0.02), but negatively correlated with snore arousals (r(155) = −0.38, p < 0.01).
Treatment
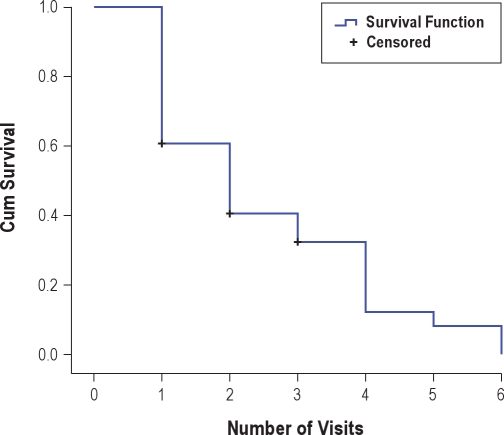
Treatment data were available for 77% (51/66) of the PLMD cases. Of the 15 children not included in the treatment analysis, 14 never began treatment, and one saw another provider for follow-up (data unavailable). Children who began treatment but subsequently were lost to follow-up or their treatment was not successful during the period of the analysis were included in the analysis. In this cohort, 76% (39/51) were successfully treated as defined by resolution of their sleep complaint, determined by clinician assessment at follow-up visits. Sixty percent of these successes occurred by the second visit, and 88% by the fourth visit. The mean number of visits to successful treatment was 2.54 visits (see Figure S1). Although treatment choices were individualized, dopamine agonists (pramipexole or ropinirole) were tried first in 88% of cases, with clonazepam as first choice in 6%. Final treatment status is summarized in Table S1. Fourteen percent of children who were treated reported adverse effects. The most common to the dopamine agonists were a paradoxical alerting reaction (7%) and headache (3%). Less than 1% reported nausea. The most common adverse effect to clonazepam was a paradoxical alerting reaction (13%). Iron status was not routinely assessed, and iron supplementation was not used because at the time there was not evidence for efficacy of iron in PLMD.
DISCUSSION
This is the largest case series to date of pediatric PLMD, as defined by current diagnostic criteria,7,9 that reports sleep-related symptoms and polysomnographic correlates. In addition, it is the first large case series to compare pediatric PLMD to pediatric OSA.
As expected, prominent sleep onset and maintenance problems were reported in PLMD children, as was difficulty awakening in the morning. About one-third got out of bed many nights per week and went to their parents” bed, significantly more than in the OSA group. While restless sleep was commonly described for children with PLMD, this was equally as often reported for children with OSA and, thus, not a differentiating feature. Similarly, for both groups fewer than one-third were described as exhibiting signs of excessive sleepiness, frequent drowsiness, or frequent falling asleep inappropriately. Supporting previous work, which indicates history alone is not accurate for the determination of PLMS, almost 50% of children with PLMD were not noted by families to have kicking or frequent jerking during sleep.15,21,28,29 Also supportive of previous studies related to pediatric PLMS, an unusually high rate of parasomnias were reported, particularly sleep terrors and nightmares.15,16,28,30 Arousals associated with PLMS are the presumed mechanism for the triggering of parasomnias.30 Interestingly, parasomnias in this study were significantly more in PLMD than OSA children, in spite of the OSA children having somewhat higher total arousal rates, suggesting other possible factors.
Although we excluded cases of pediatric RLS for this study, to better define the correlates of PLMD in childhood, there were significantly more children with PLMD than OSA who complained of leg pain/discomfort at night (62% vs. 18%) during the clinical interview. However, in the questionnaire data, children with PLMD were not more likely to report to their parents crawling/aching sensations or leg pain, when analyzed as a percent of frequent responses (5-7 nights/week). Nonetheless, when “sometimes” and “frequent” responses were combined, complains of crawling/aching sensations and leg pain were significantly more likely in children with PLMD, suggesting relatively infrequent sensations or reporting of sensations to parents. In combination with a remarkably high family history of RLS (49% vs. 18%), these data suggest that pediatric PLMD may be a precursor to RLS in some cases, as other studies have indicated.15,16 Because of the relatively young mean age in this sample (8.1 years, range 1-17), we would expect some of these children to meet RLS criteria over time, simply due to the development of better language and cognitive skills, which are needed to adequately report RLS symptoms.15,31
The polysomnographic results provide objective data that document sleep disruption in pediatric PLMD, which was compared to that in pediatric OSA. Both groups had long sleep latencies, relatively low sleep efficiencies, increased WASO, and frequent arousals. Although we did not have concurrent normal controls, historical normative data clearly support the impairment of sleep in these cases.26,27,32 Some of the polysomnographic findings were more common in children with PLMD as compared to the cases with OSA—awakenings, stage 1 sleep, stage shifts, stage 1 shifts, and spontaneous arousals—while children with OSA had significantly more total arousals. These differences, the careful attention to only scoring PLMS independent of overt or subtle respiratory events, and the differences in clinical symptoms, indicate that PLMD (as currently defined) is not simply a variant of OSA, as has been suggested.22
While the prevalence of 14% in this pediatric sleep clinic population is not directly comparable to other studies, which defined PLMD as PLMS > 5/h only (not using the clinical sleep disruption criterion), it is within the broad range of those other studies, 5.6% to 26%.18–23 Importantly, PLMD was purposely assessed for in this clinical population, rather than found as an incidental finding. Consistent with previous literature we found PLMS/PLMD to be significantly more common in Caucasian children and OSA more common in African American children,33 but interpretation should be cautious since in about 30% ethnicity was not reported.
It is relevant to note that there are no FDA-approved treatments for PLMD, or for most other sleep disorders, in children. The need for rigorous treatment trials in children is evident based on this study and other studies.34,35 While we have included the results of medications tried clinically for this cohort of children with PLMD, the open-label use and non-polysomnographic outcome assessment limit conclusions about treatment based on these data. Two recent review papers more comprehensively discuss potential treatment considerations, including iron assessment and therapy.13,14 In addition, a newer small, double-blind, placebo-controlled study has shown benefit of dopaminergic treatment for PLMS in children.36
The major strengths of this study are the large sample size, the fact that PLMD was purposefully assessed for based on ICSD-2 criteria, and the stringent criteria used to define distinct PLMD and OSA groups. A strength and limitation of this study was the exclusion of “overlap” cases, children with both PLMD and OSA. This was done to accurately characterize PLMD and compare it to OSA. However, in clinical practice overlap does occur, often with uncertainty about the potential significance of PLMD. Other limitations include that we did not have data available on inter-movement interval for PLMS, which new research suggests may be important in distinguishing various causes for PLMS.37 Also, we did not have data on autonomic or “subcortical” arousals related to PLMS, which have been shown to occur in children38 and may mediate the cardiovascular morbidity reported with RLS.39 Lastly, we did not have consistently obtained information on ADHD, depression, anxiety, or iron deficiency, conditions that have been associated with RLS, PLMS, and PLMD.13,14,40
In conclusion, these data support PLMD as a distinct pediatric disorder, with significant sleep-related symptom correlates, polysomnographic correlates, and, when combined with our previous work, significant daytime mood and behavior correlates. Rather than dismissing PLMS as an incidental polysomnographic finding in children, we suggest that clinicians consider these data to help sort out the potential significance of a diagnosis of PLMD in pediatric sleep assessment. Future research on pediatric PLMD should include full iron assessment and other research techniques, such as home infrared videography, actigraphy, and leg accelerometry to assess sleep and leg movement patterns over extended periods of time.
DISCLOSURE STATEMENT
This project was sponsored in part by a researcher-initiated grant by GlaxoSmithKline (GSK), Inc (to Dr. Gingras, PI), and support from United Sleep Medicine Centers, Charlotte, NC. Support from these organizations was used for data collection only. Dr. Picchietti has served as a consultant to Boehringer Ingelheim and UCB Pharma, and has received research support from Boehringer Ingelheim for other projects. Dr. Gaultney has indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors thank Dr. Kunal Merchant, Ph.D., for her assistance in the initial organization of this project. Dr. Merchant was employed by GSK when this project was begun, and is currently employed by Salix Pharmaceuticals, Inc. Portions of these data were presented at the Pediatric Sleep Medicine Conference in Amelia Island, FL in March 2006 and at the Association of Professional Sleep Societies meeting in Salt Lake City, UT in June 2006. Contributions/roles: STUDY DESIGN: Drs. Gingras and Picchietti; COLLECTION OF DATA: Dr. Gingras; ANALYSIS AND INTERPRETATION OF DATA: Drs. Gingras, Gaultney and Picchietti; WRITING OF THE REPORT: Drs. Gingras, Gaultney and Picchietti; REVIEW OF THE MANUSCRIPT: Drs. Gingras, Gaultney and Picchietti.
Figure S1.
Number of clinical visits to attain successful treatment by Kaplan-Meier survival analysis
Table S1.
Treatment outcomes for PLMD patients (N = 51)
| Outcome | Cases |
|---|---|
| Dopamine agonist | 28 |
| Clonazepam | 6 |
| Dopamine agonist + Clonazepam | 5 |
| Lost to follow up or not successful | 12 |
PLMD, periodic limb movement disorder.
REFERENCES
- 1.Ali NJ, Davies RJ, Fleetham JA, Stradling JR. Periodic movements of the legs during sleep associated with rises in systemic blood pressure. Sleep. 1991;14:163–5. [PubMed] [Google Scholar]
- 2.Sforza E, Nicolas A, Lavigne G, Gosselin A, Petit D, Montplaisir J. EEG and cardiac activation during periodic leg movements in sleep: support for a hierarchy of arousal responses. Neurology. 1999;52:786–91. doi: 10.1212/wnl.52.4.786. [DOI] [PubMed] [Google Scholar]
- 3.Pennestri MH, Montplaisir J, Colombo R, Lavigne G, Lanfranchi PA. Nocturnal blood pressure changes in patients with restless legs syndrome. Neurology. 2007;68:1213–8. doi: 10.1212/01.wnl.0000259036.89411.52. [DOI] [PubMed] [Google Scholar]
- 4.Siddiqui F, Strus J, Ming X, Lee IA, Chokroverty S, Walters AS. Rise of blood pressure with periodic limb movements in sleep and wakefulness. Clin Neurophysiol. 2007;118:1923–30. doi: 10.1016/j.clinph.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 5.Ferri R, Zucconi M, Rundo F, Spruyt K, Manconi M, Ferini-Strambi L. Heart rate and spectral EEG changes accompanying periodic and non-periodic leg movements during sleep. Clin Neurophysiol. 2007;118:438–48. doi: 10.1016/j.clinph.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 6.Guggisberg AG, Hess CW, Mathis J. The significance of the sympathetic nervous system in the pathophysiology of periodic leg movements in sleep. Sleep. 2007;30:755–66. doi: 10.1093/sleep/30.6.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.American Academy of Sleep Medicine. International classification of sleep disorders, 2nd ed.: diagnostic and coding manual. Rev. ed. Westchester, IL: American Academy of Sleep Medicine; 2005. [Google Scholar]
- 8.Silber MH. Commentary on controversies in sleep medicine. Montplaisir et al.: Periodic leg movements are not more prevalent in insomnia or hypersomnia but are specifically associated with sleep disorders involving a dopaminergic mechanism. Sleep Med. 2001;2:367–9. doi: 10.1016/s1389-9457(01)00106-x. [DOI] [PubMed] [Google Scholar]
- 9.Allen RP, Picchietti D, Hening WA, Trenkwalder C, Walters AS, Montplaisir J. Restless legs syndrome: diagnostic criteria, special considerations, and epidemiology. A report from the restless legs syndrome diagnosis and epidemiology workshop at the National Institutes of Health. Sleep Med. 2003;4:101–19. doi: 10.1016/s1389-9457(03)00010-8. [DOI] [PubMed] [Google Scholar]
- 10.Picchietti D. Periodic limb movements in sleep: irrelevant epiphenomenon, marker for a potential problem, or a disorder? J Clin Sleep Med. 2006;2:446–7. [PubMed] [Google Scholar]
- 11.Hogl B. Periodic limb movements are associated with disturbed sleep. Pro. J Clin Sleep Med. 2007;3:12–4. [PubMed] [Google Scholar]
- 12.Mahowald MW. Periodic limb movements are NOT associated with disturbed sleep. Con. J Clin Sleep Med. 2007;3:15–7. [PubMed] [Google Scholar]
- 13.Picchietti MA, Picchietti DL. Restless legs syndrome and periodic limb movement disorder in children and adolescents. Semin Pediatr Neurol. 2008;15:91–9. doi: 10.1016/j.spen.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 14.Khatwa U, Kothare SV. Restless legs syndrome and periodic limb movements disorder in the pediatric population. Curr Opin Pulm Med. 2010;16:559–67. doi: 10.1097/MCP.0b013e32833f11ae. [DOI] [PubMed] [Google Scholar]
- 15.Picchietti D, Stevens HE. Early manifestations of restless legs syndrome in childhood and adolescence. Sleep Med. 2008;9:770–81. doi: 10.1016/j.sleep.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 16.Picchietti DL, Rajendran RR, Wilson MP, Picchietti MA. Pediatric restless legs syndrome and periodic limb movement disorder: parent-child pairs. Sleep Med. 2009;10:925–31. doi: 10.1016/j.sleep.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 17.Gaultney JF, Merchant K, Gingras JL. Parents of children with periodic limb movement disorder versus sleep-disordered breathing report greater daytime mood and behavior difficulties in their child: the importance of using ICSD-2nd Edition criteria to define a PLMD study group. Behav Sleep Med. 2009;7:119–35. doi: 10.1080/15402000902976655. [DOI] [PubMed] [Google Scholar]
- 18.Bokkala S, Napalinga K, Pinninti N, et al. Correlates of periodic limb movements of sleep in the pediatric population. Pediatr Neurol. 2008;39:33–9. doi: 10.1016/j.pediatrneurol.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 19.Kirk VG, Bohn S. Periodic limb movements in children: prevalence in a referred population. Sleep. 2004;27:313–5. doi: 10.1093/sleep/27.2.313. [DOI] [PubMed] [Google Scholar]
- 20.Crabtree VM, Ivanenko A, O'Brien LM, Gozal D. Periodic limb movement disorder of sleep in children. J Sleep Res. 2003;12:73–81. doi: 10.1046/j.1365-2869.2003.00332.x. [DOI] [PubMed] [Google Scholar]
- 21.Martin BT, Williamson BD, Edwards N, Teng AY. Parental symptom report and periodic limb movements of sleep in children. J Clin Sleep Med. 2008;4:57–61. [PMC free article] [PubMed] [Google Scholar]
- 22.Martinez S, Guilleminault C. Periodic leg movements in prepubertal children with sleep disturbance. Dev Med Child Neurol. 2004;46:765–70. doi: 10.1017/s0012162204001318. [DOI] [PubMed] [Google Scholar]
- 23.Chervin RD, Archbold KH. Hyperactivity and polysomnographic findings in children evaluated for sleep-disordered breathing. Sleep. 2001;24:313–20. doi: 10.1093/sleep/24.3.313. [DOI] [PubMed] [Google Scholar]
- 24.Recording and scoring leg movements. The Atlas Task Force. Sleep. 1993;16:748–59. [PubMed] [Google Scholar]
- 25.ASDA. International classification of sleep disorders, revised: Diagnostic and coding manual. Rev. ed. Rochester, MN: American Sleep Disorders Association; 1997. [Google Scholar]
- 26.Montgomery-Downs HE, O'Brien LM, Gulliver TE, Gozal D. Polysomnographic characteristics in normal preschool and early school-aged children. Pediatrics. 2006;117:741–53. doi: 10.1542/peds.2005-1067. [DOI] [PubMed] [Google Scholar]
- 27.Uliel S, Tauman R, Greenfeld M, Sivan Y. Normal polysomnographic respiratory values in children and adolescents. Chest. 2004;125:872–8. doi: 10.1378/chest.125.3.872. [DOI] [PubMed] [Google Scholar]
- 28.Picchietti DL, Walters AS. Moderate to severe periodic limb movement disorder in childhood and adolescence. Sleep. 1999;22:297–300. doi: 10.1093/sleep/22.3.297. [DOI] [PubMed] [Google Scholar]
- 29.Hilbert J, Mohsenin V. Can periodic limb movement disorder be diagnosed without polysomnography? A case-control study. Sleep Med. 2003;4:35–41. doi: 10.1016/s1389-9457(02)00238-1. [DOI] [PubMed] [Google Scholar]
- 30.Guilleminault C, Palombini L, Pelayo R, Chervin RD. Sleepwalking and sleep terrors in prepubertal children: what triggers them? Pediatrics. 2003;111:e17–25. doi: 10.1542/peds.111.1.e17. [DOI] [PubMed] [Google Scholar]
- 31.Picchietti DL, Arbuckle R, Abetz L, et al. Pediatric restless legs syndrome: analysis of symptom descriptions and drawings. J Child Neurol. 2011;26:1365–76. doi: 10.1177/0883073811405852. [DOI] [PubMed] [Google Scholar]
- 32.Scholle S, Beyer U, Bernhard M, et al. Normative values of polysomnographic parameters in childhood and adolescence: Quantitative sleep parameters. Sleep Med. 2011;12:542–9. doi: 10.1016/j.sleep.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 33.O'Brien LM, Holbrook CR, Faye Jones V, Gozal D. Ethnic difference in periodic limb movements in children. Sleep Med. 2007;8:240–6. doi: 10.1016/j.sleep.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 34.Owens JA, Rosen CL, Mindell JA. Medication use in the treatment of pediatric insomnia: results of a survey of community-based pediatricians. Pediatrics. 2003;111:e628–35. doi: 10.1542/peds.111.5.e628. [DOI] [PubMed] [Google Scholar]
- 35.Owens JA, Rosen CL, Mindell JA, Kirchner HL. Use of pharmacotherapy for insomnia in child psychiatry practice: A national survey. Sleep Med. 2010;11:692–700. doi: 10.1016/j.sleep.2009.11.015. [DOI] [PubMed] [Google Scholar]
- 36.England SJ, Picchietti DL, Couvadelli BV, et al. L-Dopa improves restless legs syndrome and periodic limb movements in sleep but not attention-deficit-hyperactivity disorder in a double-blind trial in children. Sleep Med. 2011;12:471–7. doi: 10.1016/j.sleep.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ferri R, Zucconi M, Manconi M, et al. Different periodicity and time structure of leg movements during sleep in narcolepsy/cataplexy and restless legs syndrome. Sleep. 2006;29:1587–94. doi: 10.1093/sleep/29.12.1587. [DOI] [PubMed] [Google Scholar]
- 38.Walter LM, Foster AM, Patterson RR, et al. Cardiovascular variability during periodic leg movements in sleep in children. Sleep. 2009;32:1093–9. doi: 10.1093/sleep/32.8.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Walters AS, Rye DB. Review of the relationship of restless legs syndrome and periodic limb movements in sleep to hypertension, heart disease, and stroke. Sleep. 2009;32:589–97. doi: 10.1093/sleep/32.5.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Picchietti MA, Picchietti DL. Advances in pediatric restless legs syndrome: Iron, genetics, diagnosis and treatment. Sleep Med. 2010;11:643–51. doi: 10.1016/j.sleep.2009.11.014. [DOI] [PubMed] [Google Scholar]