Abstract
Study Objectives:
This study documents both the incidence and effects of central apnea on diagnosis and treatment of OSA at different altitudes in the Mountain West and substantiates the clinical impression that individuals living at altitude with moderate to severe OSA are significantly more difficult to treat with PAP.
Methods:
Split-night polysomnography was compared between sites for patients with a diagnostic AHI > 15 living at 1421 meters (Site 1; N = 150), at 1808 m (Site 2; N = 150) and at 2165 m (Site 3; N = 142). The quality of PAP titration obtained was rated, based on AASM clinical guidelines, from 1 = optimal to 4 = unacceptable. Patients developing central apneas during PAP therapy (CAI > 5.0) were titrated with an alternative O2 > CPAP/Bilevel PAP protocol.
Results:
The mean number of central apneas in the diagnostic portion of studies was significantly higher (p < 0.01) at Sites 2 (19.26) and 3 (12.36) than at Site 1 (3.11). Mean numbers of central apneas/h developing on treatment with PAP varied from 4.8/h at Site 1, to 9.79/h at Site 2, to 19.25/h at Site 3 (p < 0.001). At Site 1, 10.6% had a central apnea index (CAI) > 5.0, while 22% met this criterion at Site 2 and 38.7% at Site 3 (Site 3 vs Site 1, p = 0.01; Site 2 vs Site 1, p = 0.02). Rated titration quality varied significantly between sites. At Site 1, mean titration quality was 1.437 (SD 0.821); for Site 2, 1.569 (SD 0.96), and for Site 3, 1.772 (SD 1.025). Titration quality at Site 3 was significantly worse than at Site 1 (t = 3.22, p < 0.01) and at Site 2 (t = 2.55, p < 0.02). Repeat titration requirement differed significantly (p = 0.025). Analysis of covariance comparing titration across 3 altitude levels, controlling for age, was significant for the effect of altitude (p = 0.017). Utilizing the alternative O2 > C-PAP/Bi-PAP protocol in patients with CAI > 5.0 developing on PAP treatment, an overall optimal or good titration (AASM criteria) was attained in 75/79 (95%) of titrated patients.
Conclusions:
This study demonstrates that central apnea becomes significantly more common at increasing altitude in both diagnostic and treatment portions of split-night polysomnography in patients with significant OSA. An apparent exponential increase in the percentage of OSA patients with a CAI > 5.0 occurs with increasing altitude. Altitude associated central apnea has a significant negative effect on the quality of OSA treatment obtained during PAP titration for patients living at the altitudes addressed in this study.
Citation:
Pagel JF; Kwiatkowski C; Parnes B. The effects of altitude associated central apnea on the diagnosis and treatment of obstructive sleep apnea: comparative data from three different altitude locations in the Mountain West. J Clin Sleep Med 2011;7(6):610-615.
Keywords: Obstructive sleep apnea, central apnea, altitude, CPAP, oxygen
Altitude has a marked effect on respiration during sleep. In the Mountain West, several studies done at altitude indicate that an increase in central apneas (episodes ≥ 10 sec without airflow and no evidence of respiratory effort) occurs even among healthy individuals. 1–4 Overall apnea-hypopnea index (AHI) is higher for individuals with obstructive sleep apnea (OSA), and obstructive events convert to predominately central events at altitude.5 This degree of respiratory disturbance is corrected in part by moving affected individuals from altitude to sea level.6 Clinically, our sleep laboratories at altitude have noted increased difficulty in treating OSA patients with positive airway pressure (PAP). This study, conducted at 3 sleep laboratories at different altitudes in pateints living at these altitudes, documents the effects of changing altitude on the diagnosis and treatment of OSA. It is the authors' hypothesis, as well, that this variation in treatment difficulty occurs secondary to altitude-associated central apnea, affecting both diagnostic polysomnography and PAP therapy of OSA.
BRIEF SUMMARY
Current Knowledge/Study Rationale: This study indicates that altitude associated central apneas increase exponentially with increasing altitude affecting the results attained from diagnostic polysomnography and leading to increasing difficulty with PAP titrations for OSA. This study documents the potential of a clinically utilized alternative O2 > CPAP/BiPAP protocol for treating OSA patients at altitude who develop central apneas during PAP titration.
Study Impact: The higher frequency of central apnea at altitudes above 6000 ft. indicates that such altitudes may be a relative contraindication for the routine use of limited channel OSA screeners and automatic technologies that are unable to clearly differentiate between central and obstructive apneas. The difficulty of treating OSA due to central apneas occurring on treatment with PAP at altitude is likely to lead to a higher level of untreated OSA among these patients.
Proposed Mechanisms of Altitude-Induced Central Apnea
Apnea is a feature of ‘unstable breathing.'7 Respiratory instability can be due to the disturbance and attempted correction of this self-sustaining oscillatory system in situations in which the magnitude of the correction is greater than the disturbance (this ratio is the loop gain). If the corrective response is opposite and negative (180 degrees out of phase) with the disturbance, the corrective response will augment the disturbance.8 The higher the loop gain, the more likely the instability will occur (i.e., the more likely that central apneas will occur). Factors leading to delays in information processing, increased controller gain, or decreased system damping can potentially result in central apneas. These conditions are present during sleep at altitude.9 Among the primary factors leading to instability in the control of breathing are hypoxemia and respiratory alkalosis, universally seen in subjects exposed to high altitude. This instability in the ventilatory system induced by altitude can lead to the development of periodic breathing and central apneas during sleep. Chronic hypocapnia has been proposed as the common pathway leading to breathing instability during sleep.10 It remains unclear as to whether the pathophysiology leading to altitude-associated central apnea is the same as the pathophysiology contributing to central apneas in patients with congestive heart failure and occurring during PAP titration in OSA patients.
A series of studies indicate that central apnea responds to treatment with supplemental oxygen. For mountaineers at very high altitudes, a primary beneficial effect obtained from the use of supplemental oxygen is an observed decline in apneic events during sleep.11 In the sleep laboratory, supplemental oxygen has been shown to markedly reduced the incidence of central apneas.12,13 Low-flow oxygen has been shown to decrease the rate of sleep disordered breathing during sleep, having particular effects on reported central and mixed apneas, and increasing the percentage of events described as obstructive.14,15
This study was designed in the attempt to document the effects on central apnea on diagnosis and treatment of OSA at different altitudes in the Mountain West and to substantiate the clinical impression that individuals living at altitude with moderate to severe OSA are significantly more difficult to treat with PAP. It is postulated that the increasing difficulty with PAP titration with increasing altitude occurs secondary to an increased frequency of central apnea. While clinically relevant for patients with apnea living at high altitude, this study may have more general importance if, as previously proposed, the diagnosis and treatment of apnea at high altitude can serve as a model for both the development and treatment of central apnea.10
METHODS
Patients
Patients included in this retrospective study were individuals between the ages of 40 and 79 completing a split-night study with a diagnostic AHI > 15 living at the following altitudes: Site 1: 4662 feet (1421 meters), Site 2: 5930 feet (1808 meters), and Site 3: 7100 feet (2165 meters). The 3 locations utilize the same interpreting medical director and diagnostic/treatment protocols. The quality of PAP titration obtained was rated, based on AASM clinical guidelines, as 1 = optimal (AHI < 5.0 with supine REM sleep [Stage R]), 2 = good (AHI < 10.0 with supine Stage R), 3 = adequate (AHI < 15.0 or without supine Stage R), or 4 = unacceptable (AHI > 15.0).16
As per clinical protocols consistent between laboratories, individuals considered not to be titrated to an optimal or good level of titration returned to the lab for a second night of study for retitration. This study was conducted between 2007 and 2009, before study sites had acquired respiratory assist device (RAD) systems. Because of the frequency of central apneas developing on treatment at altitude and the routine failure of routine protocols (CPAP > Bilevel PAP > Bilevel PAP + O2), the medical director of these sites had developed an alternative treatment protocol for patients with a CAI > 5.0 occurring during PAP titration. This protocol is described clinically as an O2 > CPAP/Bilevel PAP titration: (see Appendix, available online only at www.aasmnet.org/jcsm). The same approaches and protocols for evaluation and treatment were used by all 3 sleep labs utilizing the same board certified medical director, as well as partially cross-trained and registered technical staff. Both pressure transducers and oral nasal thermal flow were used to measure respiratory effort and delineate apneas during diagnostic polysomnography. During PAP titration, the airflow signal was derived from the PAP device. There were no changes in clinical approaches to evaluation and treatment of these patients. This study was approved by the medical ethics committee at the University of Colorado School of Medicine.
Data Analysis
Statistical analyses were conducted using SPSS version 18.0. Standard descriptive statistics were computed. Tests of significance included independent sample t-tests, one-way analysis of variance and analysis of covariance, controlling for significant demographic variables. Significance of all statistical tests was set at p < 0.05. Anonymity was preserved, with all data evaluated only by the physician and technician involved in the clinical care of these patients. Statistical evaluation was performed after deletion of patient names, without clinical markers, and using numbers to denote data packets.
RESULTS
There was a statistically comparable grouping from each site: Site 1, n = 150; Site 2, n = 150; Site 3, n = 142. Mean age was 58.6 years; mean BMI was 33.2; and 33.6% of the study population were female. Overall titration quality based on AASM criteria was rated as either optimal or good for 79.7% of initial split-night studies: optimal (67.3%), good (12.4%), adequate (14%), and unacceptable (6.3%).16 Comparative demographic analysis between sites indicated a significant age difference for Site 3 (Table 1).
Table 1.
Site comparison
| Altitude, meters (Site) | N | % | Community Population | Lab Beds | Site | Monitor System | Age (SD) | % Female | BMI (SD) |
|---|---|---|---|---|---|---|---|---|---|
| 1421 (1) | 150 | 34 | 145000 | 6 | Hosp | Embla | 58.34 (10.3) | 36.4 | 34.98 (8.0) |
| 1808 (2) | 150 | 34 | 425000 | 4 | IMRT | Alice-5 | 55.26 (9.61) | 33.1 | 33.34 (6.82) |
| 2165 (3) | 142 | 32 | 8500 | 2 | Hosp | Alice-5 | 62.34 (9.50) | 35.2 | 32.74 (6.63) |
| Sig dif. | p = 0.01 |
Data are shown as means.
Rated titration quality varied significantly between sites. At Site 1, mean titration quality (range 1 [optimal] - 4 [unacceptable]) was 1.437 (SD 0.821); for Site 2, 1.569 (SD 0.96), and for Site 3, 1.772 (SD 1.025). Titration quality at Site 3 was significantly worse than at Site 1 (t = 3.22, p < 0.01) and at Site 2 (t = 2.55, p < 0.02). At Site 1, 4 of 150 (2.7%) patients required a repeat titration, while 12 of 150 (8%) at Site 2, and 15 of 142 (10.7%) at Site 3 required repeat titration because of quality ratings of ‘adequate' or ‘unacceptable' during split-night studies. Repeat titration requirement differed significantly among sites (p = 0.025). Analysis of covariance comparing titration across 3 altitude levels, controlling for age, was significant for an effect of altitude (p = 0.017; Table 2). These results demonstrate that increasing altitude has a significant negative effect on the quality of OSA treatment obtained during PAP titration for patients living at altitude.
Table 2.
Comparison of initial split-night study data between sites
| Altitude, meters (Site) | N | AHI (SD) | Low SpO2 (SD) | % CAI > 5.0 in Dx portion | Split-night titration adequacy (SD) | % CAI > 5.0 in Tx portion | Split-night titration rated adequate or unacceptable & 2nd titration requested | # Having second titration |
|---|---|---|---|---|---|---|---|---|
| 1421 (1) | 150 | 45.8 (29.7) | 73.5 (11.3) | 4.7 | 1.44 (0.82) | 4.1 | 9/150 (6%) | 4/150 (3%) |
| 1808 (2) | 150 | 50.1 (35.05) | 75.5 (10.7) | 22.7 | 1.57 (0.96) | 22.6 | 26/150 (17%) | 12/150 (8%) |
| 2165 (3) | 142 | 46.7 (26.8) | 74.2 (9.61) | 33.8 | 1.78 (1.025) | 38.7 | 33/142 (23%) | 15/142 (11%) |
| Sig. dif. | 3 > 1 (p < 0.05) | 3 > 1 (p < 0.01) | 3 > 1 (p = 0.01) | 3 > 1 (p = 0.01) | p = 0.025 | |||
| 2 > 1 (p = 0.02) | 2 > 1 (p = 0.02) |
Analysis of covariance comparing titration adequacy across 3 altitude levels, controlling for age, was significant for the effect of altitude; p = 0.017.
The development of central apneas during treatment with PAP reflected an increase in the number of central apneas in the diagnostic studies with increasing altitude. On the diagnostic portion of these studies, 7/150 patients (4.7%) at Site 1 had CAI > 5.0/h; 34/150 patients (22.7%) at Site 2, and 48/142 at Site 3 (33.8%) had CAI > 5.0/h (Site 3 > Site 1; p < 0.05).
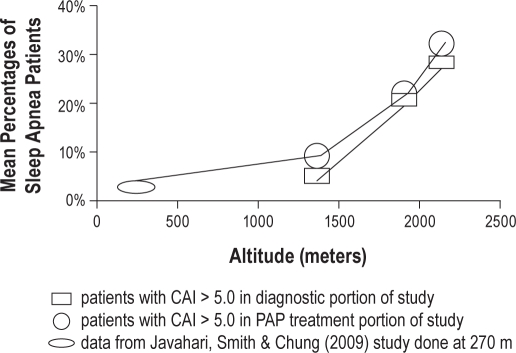
Mean numbers of central apneas/h developing on treatment with PAP varied from 4.8/h at Site 1, to 9.79/h at Site 2, to 19.25/h at Site 3 (significant at p < 0.001). At Site 1 16 of 150 (10.6%) had a CAI > 5.0 on PAP therapy, while at Site 2, 33 of 150 (22%), and at Site 3, 55 of 142 (38.7%) of patients met this criterion (Site 3 > Site 1 χ2 = 28.3, p = 0.01) (Site 2 > Site 1 χ2 = 8.19, p = 0.02; Table 2). When patients with CAI > 5.0 on diagnostic study were eliminated from statistical analysis, no significant differences remained among sites as to the number of patients with CAI > 5.0 on PAP treatment. Graphically, there is an apparent exponential increase with increasing altitude in the percentage of OSA patients with CAI > 5.0 on their diagnostic and treatment portions of split-night studies (Figure 1).
Figure 1.
Comparison of mean percentages of sleep apnea patients with CAI > 5.0 in diagnostic portion of study and CAI > 5.0 in PAP treatment portion of study graphed versus altitude (including treatment data from Javahari, Smith & Chung [2009] study done at 270 m)
Severity of OSA based on AHI and level of lowest SpO2 were not significantly associated with which patients had central apneas. For patients with CAI > 5.0, the following mean indices were obtained: age: 61.7, BMI: 30.8, AHI: 47.9, and low SpO2: 75.6. For patients without CAI > 5.0, the following mean indices were obtained: age: 57.8, BMI: 34.1, AHI: 44.8, and low SpO2: 74.3 (Table 3). CAI > 5.0 was the primary variable affecting PAP titration quality. Titration quality averaged 2.35 for patients with CAI > 5.0 (N = 107), which was significantly worse than the average of 1.34 (N = 335) for non-CAI > 5.0 patients (t = 3.46, p < 0.01; Table 3). When patients with CAI > 5.0 were excluded from analysis, treatment quality at Site 1 (1.36) varied minimally from treatment quality attained at Site 2 (1.35) and Site 3 (1.39; see Table 4).
Table 3.
Comparison of group developing CAI > 5.0 to grouping with CAI < 5.0 during PAP titration
| N | Age (SD) | BMI (SD) | AHI (SD) | Low SpO2 | Titration # 1 Adequacy (SD) | % Re-titration | |
|---|---|---|---|---|---|---|---|
| CAI > 5.0 | 106 | 58.1 (9.34) | 31.6 (6.21) | 56.5 (34.5) | 74.0 (11.93) | 2.35 (1.19) | 12/106 (11%) |
| CAI < 5.0 | 336 | 58.7 (10.26) | 33.8 (7.56) | 44.7 (28.96) | 74.8 (10.20) | 1.3 (0.73) | 20/336 (6%) |
| Sig. dif. | p < 0.01 |
Table 4.
Site comparison excluding CAI > 5.0 during PAP titration grouping (no significant differences)
| Altitude, meters (Site) | Excluding CAI > 5.0 - N | Excluding CAI > 5.0 titration adequacy (SD) | Excluding CAI > 5.0 repeat titrations |
|---|---|---|---|
| 1421 (1) | 133 | 1.36 (0.77) | 1 (0.7%) |
| 1808 (2) | 114 | 1.35 (0.72) | 4 (3.5%) |
| 2165 (3) | 85 | 1.39 (0.66) | 3 (3.5%) |
Titration quality rating: 1-optimal (AHI < 5.0 with supine Stage R), 2-good (AHI < 10.0 with supine Stage R), 3-adequate (AHI < 15.0 or without supine Stage R), or 4-unacceptable (AHI > 15.0)
Utilizing the alternative O2 > CPAP/Bilevel PAP protocol in CAI > 5.0 patients, an optimal or good titration (AASM criteria) was attained during the initial split-night study in 55/107 (51%) patients, and was attained for 20/24 additional patients when they returned for a full-night O2 > CPAP/Bilevel PAP titration. An optimal or good titration was not achieved in 4/79 (5%) of these patients; 28/52 (54%) did not return for their recommended repeat titration to these laboratories (this return rate was consistent for all laboratories). Overall treatment success for CAI > 5.0 patients using this protocol was 75/107 (72%). Excluding non-returning patients, this protocol achieved an optimal/good titration in 75/79 (95%) of these patients.
DISCUSSION
Altitude Associated Central Apneas in OSA Patients: Clinical Implications
This study demonstrates that central apnea was significantly more common at increasing altitude in both diagnostic and treatment portions of split-night polysomnography in patients with significant OSA. The diagnostic findings are supported by a previous study reporting that 46% of patients developing CAI > 5.0 during PAP titration for OSA had CAI > 5.0 on baseline polysomnography.17 Development of central apnea (CAI > 5.0) during PAP therapy has a significant negative association with the quality of OSA treatment obtained during titration for patients with moderate to severe OSA living at high altitude. This decline in treatment quality is associated, as well, with a significant increase in the requirement for a second titration because of inadequate titration during initial split-night studies. These findings, while most apparent at the highest study site (Site 3), were also more prevalent at Site 2 than at Site 1. Site 2 is located at the elevation of the major population centers in the Mountain West. Patients with CAI > 5.0 were significantly more difficult to titrate with PAP than patients with CAI < 5.0 (Table 3). When patients with CAI > 5.0 were excluded from analysis, there was no apparent difference in titration difficulty between sites (Table 4).
Previous studies conducted at low altitude indicate that central apneas develop in 13% to 20% of patients during CPAP titration.17–20 Using the same criteria used in this study, Jahaveri et al. reported that 84 of 1286 patients with OSA developed CAI > 5.0 during PAP titration (overall incidence 6.5%), with 42/84 of these patients (50%) requiring a repeat titration.17 The number of patients developing CAI > 5.0 during PAP titration for OSA in this current study (10.6% at Site 1, 22% at Site 2, and 38.7% at Site 3) were higher than in studies from lower altitude, and increased significantly with increasing altitude. For the site altitudes addressed in this study, there appears to be an exponential increase in the percentage of patients with OSA with central apneas on the diagnostic portion of their study subsequently developing CAI > 5.0 on PAP treatment with increasing altitude. Including data from the study by Javahari et al. (performed in Cincinnati, OH, at an altitude of 270 m) supports the contention that this variation in CAI > 5.0 during the treatment of OSA with PAP with altitude may increase exponentially for elevations higher than those addressed in this study (Figure 1).17 The higher frequency of central apnea at altitudes above 1800 meters (6000 ft.) indicates that such altitudes may be a relative contraindication for the use of limited channel OSA screening devices and automatic technologies that are unable to clearly differentiate between central and obstructive apneas.
As noted in this study, patients with central apnea during PAP titration are more likely than others to require a repeat titration (Table 2). Among patients with CAI > 5.0 for which a repeat titration was recommended, 54% did not return to the lab for that study (a finding consistent for all laboratories studied). The difficulty of treating OSA with PAP in patients developing central apneas during treatment and the associated intolerance of therapy by these patients is likely to lead to a higher level of untreated OSA among these patients.11 Untreated and/or under-treated OSA clearly has negative effects on multiple disease processes, as well as on morbidity and mortality.21–23
This study demonstrates that the described O2 > CPAP/Bilevel PAP titration protocol can be utilized to achieve an optimal/good titration in 95% of OSA patients living at altitude developing central apnea on treatment with PAP. Currently, back-up rate PAP systems are the primary treatment modality marketed for the treatment of central apnea that develop during PAP titration. For patients living at altitude with central sleep apnea syndromes, adaptive servo-ventilation has led to significant improvements in AHI compared to noninvasive PAP.24 Treatment with respiratory assist devices (RADs) such as adaptive servo-ventilation, in patients with central apnea has been shown to lead to a dramatic improvement in achieving AHIs in the optimal treatment range (AHI < 5.0) during PAP titration.25,26 Clinically, utilization of these systems is limited by cost, adaptability to existing titration protocols, and a limitation in the number and quality of applicable research studies. This study indicates that the O2 > CPAP/Bilevel PAP protocol used in this study is effective in treating altitude-associated central apnea in OSA patients as an alternative to RAD systems. This approach has its own difficulties: (1) a level of technical expertise during PAP titration is required beyond that required in the use of RAD systems, and (2) a second night of titration is often required in a patient grouping that demonstrates, at least in this study, a high potential for failure to appear for the second study. It is possible that OSA patients developing central apnea on treatment will be more likely to tolerate RAD systems for treatment However, at present, no comparative studies utilizing alternative treatment modalities for central apnea are available.
Central Apnea at Altitude: Pathophysiology
The high prevalence of central apnea at altitude potentially offers a model for pathophysiology, diagnosis, and treatment of central apnea developing during PAP therapy for OSA, as well as for central Cheyne Stokes respiratory patterns associated most commonly with congestive heart failure (CHF). It has been previously proposed that CHF and sleeping at high altitude share a common physiologic pathway leading to breathing instability during sleep.10 Possible alternative mechanisms that could lead to the development of central apnea include technical problems with over-titration of PAP, mask leaks, and a decline in sleep quality with the initiation of PAP contributing to an increase in state stability associated central apneas occurring at sleep-wake transition.27,28 However, none of these alternative mechanisms should be altered by altitude, indicating that the most likely pathophysiology contributing to the development of central apnea at altitude is altitude associated dysregulation of CO2 homeostasis, leading to a fall in PaCO2 below the apnea threshold and the development of central apneas.11,29–31 In this study, altitude-associated central apnea in patients with significant OSA responded well to treatment with the alternative O2 > CPAP/Bilevel PAP protocol. This finding brings into focus questions of central apnea pathophysiology at altitude versus the pathophysiology of central apnea occurring in association with CHF and PAP therapy. While it is possible that the clinical response to this O2 > CPAP/Bilevel PAP protocol is typical only of altitude-associated central apnea, this protocol may also be efficacious for other groups of patients with central apnea. If RAD systems are useful as currently marketed for central apnea at altitude, perhaps these systems would also be useful in the treatment and prevention of high altitude syndromes. The chronic intermittent hypoxia seen in patients with central sleep apneas may lead to the same long-term pathophysiological consequences present in patients with untreated OSA.32 Little research has addressed possible long-term consequences of untreated high altitude central apnea. This is an area deserving of further research.
Baseline differences in age, sleep pathology and/or comorbidities among the three groups independent of altitude or technical variations could account for the significant differences between sites reported in this study. However, this seems unlikely due to our attempts to attain methodological consistency between sites and the extent of our robust findings. Altitude effects on OSA treatment with PAP could be further investigated in a follow-up study with a crossover design (e.g., patients with abnormal studies at higher altitude could be restudied at the lower altitude sites after acclimatization, and vice versa). Repeating this study at additional site elevations would be helpful, as well, to determine whether the increase in central apnea is truly exponential with increasing altitude. Data regarding the O2 > CPAP/Bilevel PAP protocol (Appendix) for the treatment of altitude-associated central apnea must be considered preliminary and further comparative studies to O2, PAP, and RAD treatment of central apnea are required.
CONCLUSION
At increasing altitudes in the Mountain West, patients with moderate to severe OSA have a greater number of central apneas and become increasingly difficult to treat with PAP therapy due to the development of central apneas during treatment. This high incidence of central apnea among OSA patients at altitude potentially offers a model for the understanding the pathophysiology of CHF-associated central apnea and the use of PAP therapy in this condition. The difficulty of treating OSA due to central apneas occurring on treatment with PAP at high altitude is likely to lead to a higher level of untreated OSA among these patients. An alternative treatment modality (O2 > CPAP/Bilevel PAP) results in an optimal/good PAP titration for most OSA patients developing central apnea at high altitude.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
Appendix. O2 > CPAP/Bilevel PAP Split night protocol for diagnosing and treating OSA at altitude
Try to obtain 120 minutes of sleep including supine sleep.
Initiate CPAP therapy if AHI is > 15/h and titrate patient to optimal levels of CPAP/BIPAP per routine protocol. For patients developing central apneas on CPAP treatment:
1) lower PAP pressure until centrals are eliminated
2) if central apneas are persistent O2 is added inline at 2 LPM with PAP titration continuing
3) if this approach is unsuccessful and central apneas persist, take patient off PAP therapy and put patient on O2 at 2 LPM by nasal cannula for 30 minutes.
4) After 30 minutes resume PAP titration continuing inline O2 at 2 LPM
REFERENCES
- 1.Kryger M, Glas R, Jackson D, et al. Impaired oxygenation during sleep in excessive polycythemia of high altitude: Improvement with respiratory stimulation. Sleep. 1978;1:3–17. doi: 10.1093/sleep/1.1.3. [DOI] [PubMed] [Google Scholar]
- 2.Kinsman TA, Hahn AG, Gore CJ, et al. Respiratory events and periodic breathing in cyclists sleeping at 2,650-m simulated altitude. J Appl Physiol. 2002;92:2114–2118. doi: 10.1152/japplphysiol.00737.2001. [DOI] [PubMed] [Google Scholar]
- 3.Zielinski J, Koziej M, Mankowski M, et al. The quality of sleep and periodic breathing in healthy subjects at an altitude of 3200 m. High Alt Med Biol. 2000;1:331–336. doi: 10.1089/15270290050502408. [DOI] [PubMed] [Google Scholar]
- 4.Hoshikawa M, Uchida S, Sugo T, et al. Changes in sleep quality of athletes under normobaric hypoxia equivalent to 2,000-m altitude: a polysomnographic study. J Appl Physiol. 2007;6:2005–2011. doi: 10.1152/japplphysiol.00315.2007. [DOI] [PubMed] [Google Scholar]
- 5.Burgess KR, Cooper J, Rice A, et al. Effect of simulated altitude during sleep on moderate-severity OSA. Respirology. 2006;11:62–69. doi: 10.1111/j.1440-1843.2006.00785.x. [DOI] [PubMed] [Google Scholar]
- 6.Patz D, Spoon M, Corbin R, et al. The effect of altitude descent on obstructive sleep apnea. Chest. 2006;130:1744–1750. doi: 10.1378/chest.130.6.1744. [DOI] [PubMed] [Google Scholar]
- 7.Whitelaw W. Mechanisms of sleep apnea at altitude. Adv Exp Med Biol. 2006;588:57–63. doi: 10.1007/978-0-387-34817-9_6. [DOI] [PubMed] [Google Scholar]
- 8.Kupper T, Schoffl V, Netzer N. Cheyne Stokes breathing at high altitude: a helpful response or a troublemaker? Sleep Breath. 2008;12:123–127. doi: 10.1007/s11325-007-0155-5. [DOI] [PubMed] [Google Scholar]
- 9.Wickramasinge H, Anholm JD. Sleep and breathing at high altitude. Sleep Breath. 1999;3:89–101. doi: 10.1007/s11325-999-0089-1. [DOI] [PubMed] [Google Scholar]
- 10.Day TA, Wilson RJ. A negative interaction between central and peripheral respiratory chemoreceptors may underlie sleep-induced respiratory instability: a novel hypothesis. Adv Exp Med Biol. 2008;605:447–451. doi: 10.1007/978-0-387-73693-8_78. [DOI] [PubMed] [Google Scholar]
- 11.Gilmartin GS, Daly RW, Thomas RJ. Recognition and management of complex sleep-disordered breathing. Curr Opin Pulm Med. 2005;11:485–493. doi: 10.1097/01.mcp.0000183061.98665.b0. [DOI] [PubMed] [Google Scholar]
- 12.McNichols W, Carter J, Rutherford R, et al. Beneficial effect of oxygen in primary alveolar hypoventilation with central sleep apnea. Am Rev Respir Dis. 1982;125:773–775. doi: 10.1164/arrd.1982.125.6.773. [DOI] [PubMed] [Google Scholar]
- 13.White DP. Central sleep apnea. Med Clin North Am. 1985;69:1205–1219. doi: 10.1016/s0025-7125(16)30983-x. [DOI] [PubMed] [Google Scholar]
- 14.Smith PL, Haponik EF, Bleecker ER. The effects of oxygen in patients with sleep apnea. Am Rev Respir Dis. 1984;130:958–963. doi: 10.1164/arrd.1984.130.6.958. [DOI] [PubMed] [Google Scholar]
- 15.Gold AR, Bleedker ER, Smith PL. A shift from central and mixed sleep apnea to obstructive sleep apnea resulting from low flow oxygen. Am Rev Respir Dis. 1985;132:220–223. doi: 10.1164/arrd.1985.132.2.220. [DOI] [PubMed] [Google Scholar]
- 16.Kushida CA, Chediak A, Berry RB, et al. Clinical guidelines for the manual titration of positive airway pressure in patients with obstructive sleep apnea. J Clin Sleep Med. 2008;4:157–171. [PMC free article] [PubMed] [Google Scholar]
- 17.Javaheri S, Smith J, Chung E. The prevalence and natural history of complex sleep apnea. J Clin Sleep Med. 2009;5:205–211. [PMC free article] [PubMed] [Google Scholar]
- 18.Lehman S, Anic N, Thompson C, et al. Central sleep apnea on commencement of continuous airway pressure in patients with the primary diagnosis of obstructive sleep apnea-hyperpnoea. J Clin Sleep Med. 2007;3:462–466. [PMC free article] [PubMed] [Google Scholar]
- 19.Morgenhaler T, Kagramanov V, Hanak V, et al. Complex sleep apnea syndrome: Is it a unique clinical syndrome. Sleep. 2006;29:1203–1208. doi: 10.1093/sleep/29.9.1203. [DOI] [PubMed] [Google Scholar]
- 20.Dernaika T, Tawk M, Nazir S, et al. The significance and outcome of continuous positive airway pressure-related central sleep apnea during split night studies. Chest. 2007;132:81–88. doi: 10.1378/chest.06-2562. [DOI] [PubMed] [Google Scholar]
- 21.Artz M, Young T, Finn L, et al. Association of sleep-disordered breathing and the occurrence of stroke. Am J Respir Crit Care Med. 2005;172:1447–1451. doi: 10.1164/rccm.200505-702OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shahar E, Whitney CW, Redline S, et al. Sleep-disordered breathing and cardiovascular disease: cross-sectional results of the Sleep Heart Health Study. Am J Respir Crit Care Med. 2001;163:19–25. doi: 10.1164/ajrccm.163.1.2001008. [DOI] [PubMed] [Google Scholar]
- 23.Pagel JF. Obstructive sleep apnea (OSA) in primary care: evidence based practice. J Am Board Fam Med. 2007:201–207. doi: 10.3122/jabfm.2007.04.060201. [DOI] [PubMed] [Google Scholar]
- 24.Morgenthaler T, Gay P, Brown L. Adaptive servoventilation versus non-invasive positive pressure ventilation for central, mixed and complex sleep apnea syndromes. Sleep. 2007;30:468–475. doi: 10.1093/sleep/30.4.468. [DOI] [PubMed] [Google Scholar]
- 25.Allam SA, Olsen EJ, Gay PC, Morgenthaler TI. Efficacy of adaptive servoventilation in treatment of complex and central sleep apnea syndromes. Chest. 2007;132:1839–1846. doi: 10.1378/chest.07-1715. [DOI] [PubMed] [Google Scholar]
- 26.Artz M, Wensel R, Montalvan S, et al. Effects of dynamic bilevel positive airway pressure support on central sleep apnea in men with heart failure. Chest. 2008;134:61–66. doi: 10.1378/chest.07-1620. [DOI] [PubMed] [Google Scholar]
- 27.Malhorta A, Bertisch S, Wellman A. Complex sleep apnea: It isn't really a disease. J Clin Sleep Med. 2008;4:1–2. [PMC free article] [PubMed] [Google Scholar]
- 28.Jordan AS, McEvoy RD, Edwards JK, et al. The influence of gender and upper airway resistance on the ventilatory response to arousal in obstructive sleep apnoea in humans. J Physiol. 2004;558:993–1004. doi: 10.1113/jphysiol.2004.064238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Skatrud J, Dempsey J, Badr S, Begle R. Effect of airway impedance on CO2 retention and respiratory muscle activity during NREM sleep. J Appl Physiol. 1988;65:1676–1685. doi: 10.1152/jappl.1988.65.4.1676. [DOI] [PubMed] [Google Scholar]
- 30.Dempsey JA. Crossing the apnoeic threshold: causes and consequences. Exp Physiol. 2005;90:13–24. doi: 10.1113/expphysiol.2004.028985. [DOI] [PubMed] [Google Scholar]
- 31.Wellman A, Jordan A, Malhotra A, et al. Ventilatory control and airway anatomy in obstructive sleep apnea. Am J Respir Crit Care Med. 2004;170:1225–1232. doi: 10.1164/rccm.200404-510OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chiang AA. Obstructive sleep apnea and chronic intermittent hypoxia: A review. Chin J Physiol. 2006;49:234–243. [PubMed] [Google Scholar]