Abstract
Study Objective:
We sought to determine the effect of severe obstructive sleep apnea (OSA) on long-term outcomes after myocardial infarction. We hypothesized that severe OSA was associated with lower event-free survival rate after ST-segment elevation myocardial infarction (STEMI).
Methods:
A total of 120 patients underwent an overnight sleep study during index admission for STEMI. Severe OSA was defined as apnea hypopnea index (AHI) ≥ 30, and non-severe OSA defined as AHI < 30.
Results:
Among the 105 patients who completed the study, 44 (42%) had severe OSA and 61 (58%) non-severe OSA. The median creatine kinase level and mean left ventricular systolic function were similar between the 2 groups. None of the 105 study patients had received treatments for OSA. Between 1- and 18-month follow-up, the severe OSA group incurred 1 death, 2 reinfarctions, 1 stroke, 6 unplanned target vessel revascularizations, and 1 heart failure hospitalization. In contrast, there were only 2 unplanned target vessel revascularizations in the non-severe OSA group. The incidence of major adverse events was significantly higher in the severe OSA group (15.9% versus 3.3%, adjusted hazard ratios: 5.36, 95% CI: 1.01 to 28.53, p = 0.049). Kaplan-Meier event-free survival curves showed the event-free survival rates in the severe OSA group was significantly worse than that in the non-severe OSA group (p = 0.021, log-rank test).
Conclusion:
42% of the patients admitted with STEMI have undiagnosed severe OSA. Severe OSA carries a negative prognostic impact for this group of patients. It is associated with a lower event-free survival rate at 18-month follow-up.
Citation:
Lee CH; Khoo SM; Chan MY; Wong HB; Low AF; Phua QH; Richards AM; Tan HC; Yeo TC. Severe obstructive sleep apnea and outcomes following myocardial infarction. J Clin Sleep Med 2011;7(6):616–621.
Keywords: Obstructive sleep apnea, myocardial infarction, percutaneous coronary intervention, outcomes
Obstructive sleep apnea (OSA) is increasingly being recognized as an important risk factor in cardiovascular and cerebrovascular diseases.1 The underlying pathophysiological mechanism involves a complex interplay of intermittent hypoxemia, sympathetic activation, endothelial dysfunction, and release of pro-inflammatory cytokines. There are ample data supporting the close association between OSA and hypertension, heart failure, as well as stroke.2–4 However, the relationship between OSA and myocardial infarction has not been well studied.
Previously, we reported that 65.7% of patients presenting with ST-segment elevation myocardial infarction (STEMI) had undiagnosed OSA.5 The role of OSA as an adverse prognostic marker after STEMI, although plausible, remains to be proven. We failed to detect any effect of OSA on microvascular perfusion following primary percutaneous coronary intervention.5 Yet it has been shown that OSA is associated with impaired recovery of left ventricular function after myocardial infarction.6 Findings from published reports on the effect of OSA on clinical outcomes after acute coronary syndrome are conflicting.7,8 Existing reports were limited by short follow-up duration of 6 to 8 months and inclusion of a heterogeneous population including patients with stable angina, unstable angina, and myocardial infarction. Therefore, in this study, we sought to determine the relationship between OSA and long-term event-free survival among patients presenting with STEMI.
BRIEF SUMMARY.
Current Knowledge/Study Rationale:
Obstructive sleep apnea (OSA) is prevalent in patients with acute myocardial infarction. However, the prognostic implication of untreated OSA after acute myocardial infarction is unclear.
Study Impact:
We found that 42% of the patients who presented with ST-segment elevation myocardial infarction (STEMI) had severe OSA. After adjusting for age and body mass index, severe OSA remains an independent predictor of adverse events at 18 months follow-up.
METHODS
Study Design and Patient Population
This was a prospective longitudinal cohort study of 105 patients who presented with STEMI and underwent an overnight sleep study during their index admission. The original study investigating the prevalence of OSA among patients presented with STEMI, as well as the effect of OSA on microvascular perfusion following primary percutaneous coronary intervention, has been published.5 As reported previously, patients aged 21 to 80 years who were admitted to our institution with a first STEMI and had undergone a primary percutaneous coronary intervention were eligible. Exclusion criteria included known OSA, intubation mechanical ventilation, electrical instability with ventricular arrhythmia, cardiogenic shock, previous coronary artery bypass surgery, previous percutaneous coronary intervention to the target vessel, and inability to give informed consent.
Recruited patients were scheduled to undergo an overnight sleep study using a portable diagnostic device between day 2 and 5 after primary percutaneous coronary intervention. Discharge medications were prescribed according to international guidelines. All patients underwent a transthoracic echocardiogram before discharge from the hospital. The study was approved by the local institutional review board (Domain Specific Review Boards. Reference: DSRB-C/06/389), and all patients provided written informed consent.
Overnight Sleep Study
Sleep studies were performed using a portable diagnostic device (Somte; Compumedics, Australia). The parameters measured included nasal airflow (nasal cannula), thoracoabdominal movements (inductive respiratory bands), arterial oxygen saturation (pulse oximetry), snoring episodes (derived from the integrated pressure transducer), limb movement, electrocardiogram (ECG), and body position (continuous actigraphy). This system has been validated against 12-channel “in-hospital” polysomnography for quantifying sleep disordered breathing.9
Outputs from the portable diagnostic device were analyzed by 2 investigators with no knowledge of the clinical characteristics of the patients. An apneic episode was defined as cessation of airflow > 10 sec and hypopnea as a reduction > 50% in airflow lasting > 10 seconds. An event was also considered to be a hypopnea when there was a reduction in airflow that did not reach the 50% criteria, but was associated with an arterial oxygen desaturation > 3%. Apneas were classified as obstructive if there was paradoxical thoracoabdominal movement and as central if there was no thoracoabdominal movement. The apnea-hypopnea index (AHI) was calculated as the number of apneic plus hypopneic episodes per hour of recording time in bed, with the start of recording being the point at which respiration settled to a rhythmic, stable pattern. The end of the recording time was either the waking time recorded by the subject or the point at which the thoracoabdominal tracings became disturbed, consistent with wakefulness. For this study, patients were divided into 2 groups: severe OSA (AHI ≥ 30) and non-severe OSA (AHI < 30) groups.
Long-Term Follow-Up
The 30-day clinical outcomes of the patients have been published in the original report. In this study, the long-term follow-up data of the patient cohort were collected. Clinical outcomes at 6, 12, and 18 months were collected by dedicated research nurses via telephone calls and/or clinic chart reviews, and all the information was entered prospectively. Relevant adverse events included death, reinfarction, stroke, unplanned target vessel revascularization, and heart failure requiring hospitalization.
Statistical Analyses
Continuous variables are described as mean with standard deviation or median with range, whereas dichotomous variables are described as numbers and percentages. Differences in clinical and procedural characteristics between patients in severe OSA and non-severe OSA groups were analyzed by unpaired Student t tests (continuous data) or χ2 tests (dichotomous data).The time to event was calculated from the date of index admission to the date when an adverse event first occurred. Patients in whom there has been no evidence of an adverse event were censored at the date of last follow-up, i.e. 18 months. Event-free survival curves for severe and non-severe OSA groups were constructed using the Kaplan-Meier methods and compared using the log rank test. Cox proportional hazards multivariable analysis adjusting for age and body mass index (BMI) was also performed. All statistical analyses were carried out using SPSS software version 14.0. A p-value < 0.05 was considered significant.
RESULTS
Clinical Characteristics and Sleep Study Results
Between January 2007 and April 2008, overnight sleep studies were commenced in 120 patients and were successfully completed in 105 STEMI patients. The remaining 15 patients could not tolerate the sleep study and therefore discontinued it prematurely. Among the 105 patients who formed the study cohort, the average age was 53 ± 10 years, and the majority (n = 103, 98%) were men.
AHI was ≥ 30 (severe OSA) in 44 (42%) patients; the remaining 61 (58%) patients had AHI < 30 (non-severe OSA). Mean AHIs for severe OSA and non-severe OSA groups were 48.6 ± 13.4 and 14.4 ± 8.2, respectively (p < 0.001). None of the 105 study patients had complained daytime sleepiness or received continuous positive airway pressure (CPAP) or any other treatments for OSA. The baseline demographic and clinical characteristics of the study population are shown in Table 1. The 2 groups were well-matched in the baseline characteristics. The median peak creatine kinase levels and mean left ventricular ejection fraction (by echocardiography on day 2 after admission), which reflects the severity of myocardial injury after STEMI, were similar between the 2 groups. None of the patients in this study had atrial fibrillation, probably due to exclusion of patients with severe heart failure or cardiogenic shock.
Table 1.
Demographic and clinical characteristics of the patients
| Characteristics | Overall (n = 105) | AHI ≥ 30 (n = 44) | AHI < 30 (n = 61) | p value |
|---|---|---|---|---|
| Mean age in years (SD) | 52.7 (9.8) | 55.2 (11.0) | 50.9 (8.5) | 0.027 |
| Male sex, n (%) | 103 (98) | 43 (97.7) | 60 (98.4) | 1.000 |
| Smoking, n (%) | 61 (58.1) | 24 (54.6) | 37 (60.7) | 0.531 |
| Hypertension n, (%) | 55 (52.4) | 25 (56.8) | 30 (49.2) | 0.439 |
| Diabetes mellitus, n (%) | 39 (37.1) | 21 (47.7) | 21 (29.5) | 0.057 |
| Hypercholesterolemia, n (%) | 88 (83.8) | 39 (88.6) | 49 (80.3) | 0.254 |
| Family history of coronary artery disease, n (%) | 26 (24.8) | 12 (27.3) | 14 (23.0) | 0.613 |
| Mean Height (SD) | 1.7 (0.1) | 1.7 (0.1) | 1.7 (0.1) | 0.540 |
| Mean weight in kg (SD) | 69.8 (11.2) | 73.6 (12.0) | 67.1 (9.9) | 0.003 |
| Mean body mass index in kg/m2 (SD) | 24.9 (3.3) | 26.1 (3.4) | 24.0 (2.9) | 0.001 |
| Mean systolic blood pressure in mm Hg (SD) | 129.7 (24.3) | 129.3 (26.3) | 130.0 (22.9) | 0.886 |
| Mean diastolic blood pressure in mmHg (SD) | 78.8 (18.1) | 78.8 (3.2) | 78.9 (15.8) | 0.980 |
| Previous myocardial infarction, n (%) | 3 (2.9) | 1 (2.3) | 2 (3.3) | 0.760 |
| Previous PCI to non-culprit artery, n (%) | 5 (4.8) | 2 (4.6) | 3 (4.9) | 0.930 |
| Chronic renal failure, n (%) | 1 (1.0) | 1 (2.3) | 0 (0) | 0.419 |
| Location of infarction, n (%) | ||||
| Anterior | 61 (58.1) | 22 (50.0) | 39 (63.9) | 0.153 |
| Non-anterior | 44 (41.9) | 22 (50.0) | 22 (36.1) |
SD, standard deviation; PCI, percutaneous coronary intervention.
Primary Percutaneous Coronary Intervention
The angiographic and procedural characteristics of the patients are shown in Table 2. Twenty-one (47.7%) and 27 (44.3%) patients in the severe OSA and non-severe OA groups, respectively, had multi-vessel coronary artery disease (p = 0.772). The infarct-related artery was successfully opened in all patients, each of whom received at least 1 stent. Endothelial progenitor cell capturing stents (Genous, Orbus Neich) were implanted in 34 (77.3%) and 40 (65.6%) patients in the severe OSA and non-severe OSA groups, respectively. The corresponding numbers for bare metal stents were 10 (22.7%) and 19 (31.1%) patients, respectively. None of the patients in the severe OSA group and 2 patients in the non-severe OSA group (3.3%) were implanted with a drug-eluting stent. Intravenous glycoprotein IIb/IIIa inhibitors were used in 31 patients (29.5%), and there was no significant difference between the severe OSA (27.3%) and non-severe OSA (31.1%) groups. The median symptom-to-balloon times and door-to-balloon times were similar in severe OSA and non-severe OSA groups. After percutaneous coronary intervention, final TIMI 3 flow was achieved in all except 1 patient (TIMI 2).
Table 2.
Angiographic and procedural characteristics of the patients
| Characteristics | Overall (n = 105) | AHI ≥ 30 (n = 44) | AHI < 30 (n = 61) | p value |
|---|---|---|---|---|
| Target vessel, n (%) | 0.122 | |||
| Left anterior descending artery | 60 (57.1) | 22 (50.0) | 38 (62.3) | |
| Left circumflex artery | 6 (5.7) | 5 (11.4) | 1 (1.6) | |
| Right coronary artery | 38 (36.2) | 17 (38.6) | 21 (34.4) | |
| Lesion site, n (%) | 0.460 | |||
| Proximal | 59 (56.7) | 24 (55.8) | 35 (57.4) | |
| Mid | 31 (29.8) | 13 (30.2) | 18 (29.5) | |
| Distal | 12 (11.5) | 4 (9.3) | 8 (13.1) | |
| Door-to-balloon time, median (min) | 73 (28–1337) | 68.5 (28–261) | 82 (37–1337) | 0.588 |
| Symptom-to-balloon time, median (min) | 213 (55–1614) | 221.5 (64–1614) | 206 (55–967) | 0.846 |
| Peak creatine kinase, median (U/L) | 2424 (114–10139) | 2117.5 (160–10139) | 2451 (114–6591) | 0.943 |
| Mean left ventricular ejection fraction (%) (SD) | 47.6 (10.4) | 47.4 (11.7) | 47.8 (9.5) | 0.870 |
| Mean time from PCI to sleep study (SD) | 46.5 (21.0) | 46.2 (20.9) | 46.8 (21.2) | 0.900 |
| Mean time from admission to sleep study (SD) | 48.1 (21.2) | 47.6 (20.9) | 48.5 (21.6) | 0.840 |
| Baseline TIMI flow, n (%) | 0.745 | |||
| 0 | 81 (77.1) | 34 (77.3) | 47 (77.1) | |
| 1 | 5 (4.8) | 1 (2.3) | 4 (6.6) | |
| 2 | 15 (14.3) | 7 (15.9) | 13.1 (8) | |
| 3 | 4 (3.8) | 2 (4.6) | 3.3 (2) | |
| Final TIMI flow, n (%) | 0.419 | |||
| 2 | 1 (1.0) | 1 (2.3) | 0 | |
| 3 | 104 (99.1) | 43 (97.7) | 61 (100) | |
| Ostial lesion, n (%) | 6 (5.7) | 4 (9.1) | 2 (3.3) | 0.234 |
| Bifurcation lesion, n (%) | 21 (20.2) | 9 (20.5) | 12 (20.0) | 0.955 |
Microvascular Perfusion
The resolution of ST-elevation between the severe OSA and non-severe OSA groups is shown in Table 3. The corrected TIMI frame count and myocardial blush grade results are shown in Table 4. The patients in the severe OSA group had a higher prevalence of microvascular obstruction, as represented by myocardial blush grade 0/1.
Table 3.
Electrocardiographic parameter of microvascular perfusion in AHI ≥ 30 and AHI < 30 patients
| AHI ≥ 30 (n = 44) | AHI < 30 (n = 61) | p value | |
|---|---|---|---|
| ST-segment elevation pre-primary PCI in mm, median (range) | 2 (0–11.5) | 3 (0.5–9) | 0.427 |
| ST-segment elevation post-primary PCI in mm, median (range) | 1 (0–11.5) | 1 (0–5) | 0.792 |
| ST-segment resolution, n (%) | 0.376 | ||
| Complete (> 70%) | 14 (31.8) | 24 (39.3) | |
| Partial (30–70%) | 18 (40.9) | 17 (27.9) | |
| None (< 30%) | 12 (27.3) | 20 (32.8) |
Table 4.
Angiographic parameters of microvascular perfusion and quantitative coronary analysis in AHI ≥ 30 and AHI < 30 patients
| AHI ≥ 30 (n = 44) | AHI < 30 (n = 61) | p value | |
|---|---|---|---|
| Final corrected TIMI frame count, median (range) | 21.2 (9.4–68.0) | 20 (9.4–100.0) | 0.362 |
| Myocardial blush grade, n (%) | 0.020 | ||
| 0 | 4 (9.8) | 13 (21.3) | |
| 1 | 16 (39.0) | 8 (13.1) | |
| 2 | 10 (24.4) | 18 (29.5) | |
| 3 | 11 (26.8) | 22 (36.1) | |
| Baseline minimal lumen diameter in mm, median (range) | 0 (0–0.1.92) | 0 (0–0.72) | 0.864 |
| Baseline percent stenosis in %, median (range) | 100 (62–100) | 100 (71–100) | 0.842 |
| Final minimal lumen diameter in mm, mean (SD) | 3.2 (0.57) | 3.0 (0.39) | 0.050 |
| Final percent stenosis in %, median (range) | 1.8 (0–12.5) | 3.7 (0–16.9) | 0.403 |
| Reference vessel diameter in mm, mean (SD) | 3.4 (0.60) | 3.2 (0.40) | 0.038 |
| Lesion length in mm, median (range) | 17.3 (9.00–52.05) | 18.1 (9.36–49.00) | 0.772 |
Discharge Medication and 30-Day Clinical Outcomes
The discharge medications given to the patients were in accordance with international guidelines. The medications included aspirin (96.2%), clopidogrel (100%), β-blockers (90.5%), angiotensin-converting enzyme inhibitors, angiotensin receptor blockers (85.7%), and lipid-lowering agents (96.2%). There was no difference between the severe OSA and non-severe OSA groups in discharge medications (data not shown). Thirty-day clinical follow-up data were available for all patients. There was 1 case of hospitalization for heart failure in the non-severe OSA group. There were no other adverse events.
Effect of OSA on Long-Term Event-Free Survival Rate
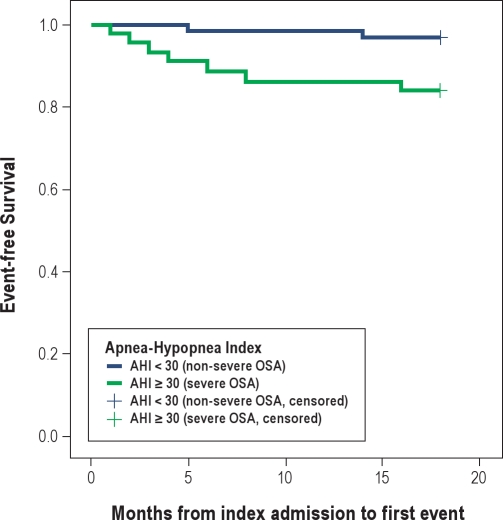
Complete data on 18-month follow-up are available for all 105 patients. Between 1- and 18-month follow-up, the severe OSA group incurred 1 death, 2 reinfarctions, 1 stroke, 6 unplanned target vessel revascularizations (5 percutaneous coronary interventions and 1 coronary artery bypass surgery), and 1 heart failure admission. In contrast, there were only 2 unplanned target vessel revascularizations by percutaneous coronary intervention in the non-severe OSA group. The incidence of major adverse events was significantly higher in the severe OSA group (15.9% versus 3.3%, adjusted HR: 5.36, 95% CI: 1.01 to 28.53, p = 0.049) (Table 5). In Figure 1, the Kaplan-Meier event-free survival curves are presented. The event-free survival rates in the severe OSA group were significantly worse than those in the non-severe OSA group (p = 0.021, log-rank test). There was no incidence of stent thrombosis in this study.
Table 5.
Multivariate logistic regression analysis to discern factors associated with 18-month adverse event rate
| Characteristics | Odds ratio | 90% Confidence Interval | p value |
|---|---|---|---|
| Age | 1.011 | 0.945 - 1.083 | 0.747 |
| Severe OSA | 5.361 | 1.007 - 28.525 | 0.049 |
| Body mass index | 0.959 | 0.771 - 1.193 | 0.710 |
Figure 1.
Kaplan-Meier Curve showing lower event-free survival rate for patients with severe OSA (AHI ≥ 30)
DISCUSSION
In this long-term prospective cohort study of OSA in STEMI patients, we demonstrated that about 40% of the patients admitted with STEMI have undiagnosed severe OSA, defined as AHI ≥ 30. Severe OSA was associated with a negative prognostic impact on both early and late outcomes for this group of patients. After adjusting for age and BMI, severe OSA was associated with a lower event-free survival rate at 18-month follow-up and more unplanned target vessel revascularization compared to patients without severe OSA.
Despite contemporary revascularization strategy and pharmacological treatment, long-term clinical outcomes following STEMI remain unsatisfactory. In the Drug Elution and Distal Protection in Acute Myocardial Infarction (DEDICATION) trial, which included 626 STEMI patients, the incidence of major adverse events at 3-year follow-up after bare metal stent and drug-eluting stent implantation was 11.5% and 18.2%, respectively.10 Effective risk stratification after STEMI is a prerequisite for improving patient outcomes. In an early study by Peker et al., OSA was found to be an independent predictor of cardiovascular mortality at 5-year follow-up in patients with stable coronary artery disease.11 However, this study was conducted more than 15 years ago, and medical therapy for coronary artery disease has refined tremendously during this period. Existing data on the prognostic impact of OSA on acute coronary syndrome are limited and conflicting. Mehra et al. found that despite a high prevalence detected, OSA had no significant impact on 6-month clinical outcomes.7 In contrast, Yumino et al. reported that OSA was associated with lower 8-month event-free survival rates following percutaneous coronary intervention for acute coronary syndrome. The high prevalence of adverse events in the OSA group was mainly driven by high target vessel revascularization rate following planned follow-up angiography.8 The aforementioned studies are limited by small sample size, brief follow-up, and inclusion of a heterogeneous population. Furthermore, patients were divided according to presence (AHI ≥ 10) or absence (AHI < 10) of OSA. Yet, current data suggest that the impact of OSA on coronary artery disease is most pronounced in severe form, with AHI ≥ 30.12,13
To the best of our knowledge, the present study is the first report on the association of untreated severe OSA with long-term clinical outcomes in patients admitted for STEMI. We demonstrate that the adverse event rate in patients with severe OSA (AHI ≥ 30) is five times as high as in those without severe OSA (AHI < 30). Our study population received optimal treatment. The median door-to-balloon time was below 90 minutes. Final TIMI 3 flow was achieved after percutaneous coronary intervention in almost all patients. The use of evidence-based medications on discharge was high.
The reason for the higher adverse event rate for patients with severe OSA remains unclear. The peak cardiac enzyme levels and left ventricular systolic function were similar between the 2 groups. In the present analysis using AHI 30 as a cut-off, there was a higher incidence of suboptimal microvascular perfusion, as measured by myocardial blush grade, in patients with severe OSA. However, no significant differences were detected when using ST-segment resolution and corrected TIMI frame count. Recently, Nakashima reported a higher incidence of impaired ST-segment resolution in STEMI patients with OSA.14 Further studies evaluating the effect of OSA on microvascular perfusion are warranted.
This finding has potential healthcare implications. The current risk stratification for patients admitted with STEMI is imperfect. Our findings suggest that by performing an overnight sleep study during the index admission, we may be able to identify high-risk patients who are likely to have worse long-term prognosis. Although not targeted for STEMI patients, CPAP treatment has been shown to have beneficial effects on long-term cardiovascular outcomes.12,15,16 Studies evaluating the effectiveness of CPAP treatment on STEMI patients with severe OSA are warranted.
In the original publication reporting on this cohort of 105 patients, the diagnosis of OSA was made based on AHI ≥ 15.5 In this follow-up study, instead of comparing the long-term outcomes between patients with AHI ≥ 15 and < 15, we decided to compare patients with AHI ≥ 30 versus AHI < 30 (severe OSA versus no/mild/moderate OSA) because there is evidence suggesting that the vasculopathic effects of OSA are most significant in those with AHI ≥ 30. The mechanism behind this “threshold effect” remains to be determined. In a recently published epidemiological study that included more than 4000 patients, Gottlieb et al. reported that the effect of OSA on coronary artery disease was only observed in patients with AHI ≥ 30.13 In fact, a negligible effect was observed among patients with no (AHI < 5), mild (AHI 5–15) and moderate (AHI 15–30) OSA. In another 10-year observational study on otherwise healthy individuals, severe OSA (AHI > 30) was found to be associated with a higher incidence of fatal and non-fatal cardiovascular events.15 Likewise, the deleterious effect of OSA on left ventricular function recovery following myocardial infarction was most marked in those with AHI > 30.6,17 Finally, the risk of stroke among elderly people was found to be higher in those with severe OSA (AHI > 30).4 When our data were reanalyzed using AHI 15 as the cut-off, there was no significant difference in the incidence of adverse cardiovascular events in patients with AHI < 15 (11.1%) versus AHI ≥ 15 (14.5%) (data not shown).
A previous study showed that after bare metal stent implantation, OSA was associated with higher late lumen loss (1.28 ± 0.84 mm versus 0.69 ± 0.81 mm) and binary restenosis rate (37% versus 15%) compared to absence of OSA.8 The target vessel revascularization rate of the OSA group was four times as high as those without OSA. This is in line with our study, which shows that the target vessel revascularization rate among patients with severe OSA was three times as high as those in the non-severe OSA group. Although two-thirds of our patients received endothelial progenitor cell capturing (Genous) stents, we have recently reported that the late lumen loss of endothelial progenitor cell capturing stent was similar to that of bare metal stent.18
Study Limitations
The sample size of this study is relatively small. We are unable to determine the effect of severe OSA on late lumen loss following endothelial progenitor cell capturing stent implantation due to lack of planned repeat angiography. Data on OSA-related symptoms were not captured. However, the relations between OSA and cardiovascular diseases seem not to be related to the presence of symptoms of sleep apnea.19 Patients who were sick and intubated were excluded due to inability to obtain written informed consent. It is conceivable that the effect of OSA could be even more pronounced if all patients admitted for STEMI comers had been included. The mean body mass index of our Asian study population (approximately 25 kg/m2) was different from that of Western patients. Hence, whether the results can be generalized to the western population remains unclear.
CONCLUSION
In conclusion, 42% of the patients admitted with STEMI have undiagnosed severe OSA. Severe OSA carries a negative prognostic impact for this group of patients. It is associated with a lower event-free survival rate at 18-month follow-up.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors thank the Publications Support Unit of the National University Health System, Singapore, for its help in the preparation of this manuscript. This study was supported by Cardiac Department Fund, National University Heart Centre Singapore.
Authors' Contributions: Study design: Dr. Lee, Dr. Khoo, Dr. Chan, Dr. Wong, Dr. Richards, Dr. Yeo. Research funding application: Dr. Lee, Dr. Tan, Dr. Yeo. Application for ethics approval: Dr. Lee, Dr. Phua, Dr. Yeo. Data collection, data analysis and critical review of the findings: Dr. Lee, Dr. Khoo, Dr. Chan, Dr. Phua, Dr. Low, Dr. Richards, Dr. Tan, Dr. Yeo. Manuscript draft: Dr. Lee, Dr. Khoo, Dr. Chan, Dr. Phua. Manuscript review and intellectual inputs: Dr. Lee, Dr. Richards, Dr. Wong, Dr. Tan, Dr. Yeo.
REFERENCES
- 1.Bradley TD, Floras JS. Obstructive sleep apnoea and its cardiovascular consequences. Lancet. 2009;373:82–93. doi: 10.1016/S0140-6736(08)61622-0. [DOI] [PubMed] [Google Scholar]
- 2.Shahar E, Whitney CW, Redline S, et al. Sleep-disordered breathing and cardiovascular disease: cross-sectional results of the Sleep Heart Health Study. Am J Respir Crit Care Med. 2001;163:19–25. doi: 10.1164/ajrccm.163.1.2001008. [DOI] [PubMed] [Google Scholar]
- 3.Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342:1378–84. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- 4.Munoz R, Duran-Cantolla J, Mart́inez-Vila E, et al. Severe sleep apnea and risk of ischemic stroke in the elderly. Stroke. 2006;37:2317–21. doi: 10.1161/01.STR.0000236560.15735.0f. [DOI] [PubMed] [Google Scholar]
- 5.Lee CH, Khoo SM, Tai BC, et al. Obstructive sleep apnea in patients admitted for acute myocardial infarction. Prevalence, predictors, and effect on microvascular perfusion. Chest. 2009;135:1488–95. doi: 10.1378/chest.08-2336. [DOI] [PubMed] [Google Scholar]
- 6.Nakashima H, Katayama T, Takagi C, et al. Obstructive sleep apnoea inhibits the recovery of left ventricular function in patients with acute myocardial infarction. Eur Heart J. 2006;27:2317–22. doi: 10.1093/eurheartj/ehl219. [DOI] [PubMed] [Google Scholar]
- 7.Mehra R, Principe-Rodriguez K, Kirchner HL, Strohl KP. Sleep apnea in acute coronary syndrome: high prevalence but low impact on 6-month outcome. Sleep Med. 2006;7:521–8. doi: 10.1016/j.sleep.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 8.Yumino D, Tsurumi Y, Takagi A, Suzuki K, Kasanuki H. Impact of obstructive sleep apnea on clinical and angiographic outcomes following percutaneous coronary intervention in patients with acute coronary syndrome. Am J Cardiol. 2007;99:26–30. doi: 10.1016/j.amjcard.2006.07.055. [DOI] [PubMed] [Google Scholar]
- 9.Cunnington D, Menagh J Cherry G, Teichtahl H. Comparison of full in-laboratory polysomnography to a portable sleep data acquisition device. Am J Resp Crit Care Med. 2003;167:A405. (Abstract) [Google Scholar]
- 10.Kaltoft A, Kelbaek H, Thuesen L, et al. Long- term outcome after drug-eluting versus bare-metal stent implantation in patients with ST-segment elevation myocardial infarction: 3-year follow-up of the randomized DEDICATION (Drug Elution and Distal Protection in Acute Myocardial Infarction) Trial. J Am Coll Cardiol. 2010;56:641–5. doi: 10.1016/j.jacc.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 11.Peker Y, Hedner J, Kraiczi H, Löth S. Respiratory disturbance index: an independent predictor of mortality in coronary artery disease. Am J Respir Crit Care Med. 2000;162:81–6. doi: 10.1164/ajrccm.162.1.9905035. [DOI] [PubMed] [Google Scholar]
- 12.Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365:1046–53. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]
- 13.Gottlieb DJ, Yenokyan G, Newman AB, et al. Prospective study of obstructive sleep apnea and incident coronary heart disease and heart failure: the Sleep Heart Health Study. Circulation. 2010;122:352–60. doi: 10.1161/CIRCULATIONAHA.109.901801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nakashima H, Muto S, Amenomori K, Shiraishi Y, Nunohiro T, Suzuki S. Impact of obstructive sleep apnea on myocardial tissue perfusion in patients with ST-segment elevation myocardial infarction. Circ J. 2011;75:890–6. doi: 10.1253/circj.cj-10-0768. [DOI] [PubMed] [Google Scholar]
- 15.Milleron O, Pillière R, Foucher A, et al. Benefits of obstructive sleep apnoea treatment in coronary artery disease: a long-term follow-up study. Eur Heart J. 2004;25:728–34. doi: 10.1016/j.ehj.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 16.Cassar A, Morgenthaler TI, Lennon RJ, Rihal CS, Lerman A. Treatment of obstructive sleep apnea is associated with decreased cardiac death after percutaneous coronary intervention. J Am Coll Cardiol. 2007;50:1310–4. doi: 10.1016/j.jacc.2007.06.028. [DOI] [PubMed] [Google Scholar]
- 17.Shamsuzzaman AS, Gersh BJ, Somers VK. Obstructive sleep apnea: implications for cardiac and vascular disease. JAMA. 2003;290:1906–14. doi: 10.1001/jama.290.14.1906. [DOI] [PubMed] [Google Scholar]
- 18.Low AF, Lee CH, Teo SG, et al. Effectiveness and safety of the Genous endothelial progenitor cell-capture stent in acute ST-elevation myocardial infarction. Am J Cardiol. 2011;108:202–5. doi: 10.1016/j.amjcard.2011.03.024. [DOI] [PubMed] [Google Scholar]
- 19.Young T, Skatrud J, Peppard PE. Risk factors for obstructive sleep apnea in adults. JAMA. 2004;291:2013–6. doi: 10.1001/jama.291.16.2013. [DOI] [PubMed] [Google Scholar]