Abstract
Study Objectives:
Evidence supports the use of cognitive behavioral therapies for nightmares in trauma-exposed individuals. This randomized clinical trial replicated a study of exposure, relaxation, and rescripting therapy(ERRT) and extended prior research by including broad measures of mental health difficulties, self-reported physical health problems, and quality of life. Additionally, physiological correlates of treatment-related change assessed from a script-driven imagery paradigm were examined.
Methods:
Forty-seven individuals were randomized to treatment or waitlist control.
Results:
The treatment group demonstrated improvements relative to the control group at the one-week post-treatment assessment. At the 6-month follow-up assessment, significant improvements were found for frequency and severity of nightmares, posttraumatic stress disorder symptoms, depression, sleep quality and quantity, physical health symptoms, anger, dissociation, and tension reduction behaviors. Participants also reported improved quality of life. Treatment-related decreases in heart rate to nightmare imagery were correlated with improvements in sleep quality and quantity; treatment-related decreases in skin conductance to nightmare imagery were correlated with improvements in nightmare severity, posttraumatic stress disorder symptom severity, sleep quality, and fear of sleep; and treatment-related decreases in corrugator activity to nightmare imagery were correlated with improved physical health.
Conclusions:
Findings provide additional support for the use of ERRT in treating nightmares and related difficulties and improving sleep.
Citation:
Davis JL; Rhudy JL; Pruiksma KE; Byrd P; Williams AE; McCabe KM; Bartley EJ. Physiological predictors of response to exposure, relaxation, and rescripting therapy for chronic nightmares in a randomized clinical trial. J Clin Sleep Med 2011;7(6):622-631.
Keywords: Nightmares, posttraumatic stress disorder, sleep quality, treatment, physiological assessment
Nightmares following trauma exposure are consistently associated with sleep disturbance,1 posttraumatic stress disorder (PTSD) severity,2 physiological arousal with or without PTSD,3 and functional impairment over and above PTSD.4,5 Moreover, chronic nightmares (CN) may be a significant maintaining factor of psychological distress, because successful treatment of CN with cognitive behavioral therapy results in the reduction of symptoms of PTSD, depression, and nightmare-related panic.6–8 Therefore, CN may pose a pernicious health problem independent of other psychopathology. Preliminary evidence suggests that psychological treatments which broadly target PTSD may have limited impact on sleep disturbances,9–13 and pharmacological treatments appear to have little effect14 or only a palliative effect15 for some individuals. Thus, cognitive behavioral approaches specifically addressing sleep disturbances are now being evaluated in trauma-exposed samples. The present study is a replication of a randomized controlled trial (RCT) that examined the efficacy of exposure, relaxation, and rescripting therapy(ERRT) to treat CN in trauma-exposed persons7 and expands previous work by assessing the influence of treatment on facets of health that were not included in the first RCT and examining physiological predictors of treatment response.
BRIEF SUMMARY
Current Knowledge/Study Rationale: The purpose of this randomized clinical trial was to replicate a previous study examining the impact of Exposure, Relaxation, and Rescripting Therapy on nightmares and sleep quality. This study expanded previous efforts by including a broader assessment of mental health indices, physical health symptoms, quality of life, and examined physiological correlates of treatment related change.
Study Impact: The present study supports the use of ERRT for trauma-related sleep problems and related psychopathology. Clinicians should assess for nightmares and sleep problems in trauma-exposed persons and consider utilizing interventions that directly address these problems.
The first RCT demonstrated that ERRT reduced nightmare frequency and severity, related psychopathology, and improved sleep in trauma-exposed adults.7 While the mechanisms of change in this treatment are not well understood, it is possible that nightmare-related defensive reactivity and concomitant physiological arousal may promote nightmare-related sleep and psychological problems (e.g., fear of sleep, low waking thresholds).16,17 This may also result in poor daytime functioning, decreased coping resources, and physical health problems. If this supposition is true, then reducing nightmare-related defensive reactivity and related physiological arousal should reduce fear of sleep and improve sleep quality and quantity, thus enhancing daytime functioning. Indeed, previous studies found a generalized impact of changing nightmare content and affect on PTSD symptoms, depression, and general anxiety.7,8,18 Other difficulties related to trauma exposure and sleep deprivation which may also be expected to change following alteration of nightmare content and affect, and subsequent reduction of physiological arousal, have not yet been explored (e.g., anger, irritability, physical health symptoms).
To establish an association between nightmare-related thoughts and physiological reactivity, Rhudy and colleagues3 examined script-driven imagery data collected at the baseline assessment in the current study. A personally relevant nightmare script was presented over headphones to participants who were asked to imagine the nightmare scene as vividly as possible. During imagery, heart rate (HR), skin conductance level (SCL), and facial muscle electromyogram (EMG) were recorded. Results indicated nightmare imagery evoked heightened reactivity as assessed from all physiological outcomes and that autonomic reactions were positively correlated with self-reported global sleep problems, time taken to fall asleep, the degree of panic upon awakening from a nightmare, and self-reported health symptoms. Subsequent analysis revealed that these physiological reactions to nightmare imagery were reduced following ERRT and gains were maintained at 3- and 6-month follow-ups.
Thus, ERRT is able to reduce physiological indices of nightmare-related defensive reactivity.32 What remains unknown, however, is the nature of the relationship between the reduction in these physiological reactions and other measures of treatment outcome. Specifically, it is currently unclear whether changes in physiological defensive reactivity covary with changes in nightmare frequency and severity, mental health, or physical health. Determining which outcome variables covary could provide insight into the mechanisms of nightmare and symptom maintenance. The following hypotheses were made:
As in the first RCT,7 treated participants would show greater improvements across all domains (e.g., nightmare characteristics, sleep quality, and related indices of mental health) at the one-week post assessment compared to the delayed treatment group, and treatment gains for all treated individuals would be maintained across the 6-month time period.
ERRT would reduce the additional mental health symptoms assessed, would reduce physical health problems due to the reduction in nightmare related physiological arousal,19,3 and other areas of participants' lives would demonstrate positive change.
Treatment-related changes in physiological reactivity to nightmare-related imagery would covary with treatment-related changes in psychological functioning.
METHOD
Participants
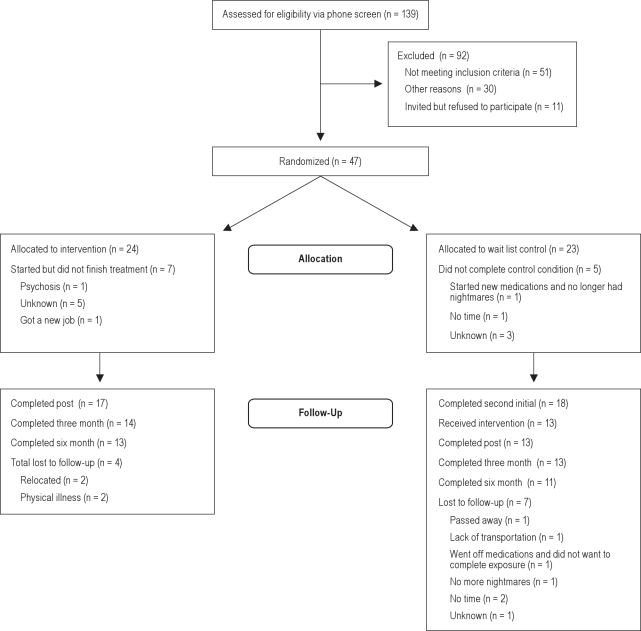
See Figure 1 for the flow chart indicating attrition. Fifty-eight participants met the inclusion criteria and were invited to participate in the treatment study. Eleven individuals refused and did not enter the study. Forty-seven individuals began the study and were randomly assigned to either the treatment condition (n= 24) or the delayed treatment control condition (n = 23). Of these, 40 were randomized to study arms that included psychophysiology assessment (initially the study included a treatment arm that did not include physiological assessment, but this arm was abandoned in order to focus on physiological predictors). Twelve participants (26%) did not complete the Time 2 assessment, and these dropouts did not differ across condition, χ2 (1, N = 47) = 0.34, p = ns. The 18 people in the control group who completed the second initial assessment were invited to participate in the treatment, and 13 did so. A total of 30 individuals (treatment group and delayed treatment group) were treated, and 24 completed the 6-month follow-up.
Figure 1.
Of the 47 participants who entered treatment, the majority were Caucasian (81%; treatment = 91.7%, control = 69.6%), female (75%; treatment = 66.7%, control = 82.6%), married (42.6%; treatment = 29.6%, control = 56.5%) and reported at least some college education (80%; treatment = 70.9%, control = 82.6%). Most were employed (treatment = 54.2%, control = 73.9%). Average age was 47 years (SD = 38.49; Mtreatment = 38.80, Mcontrol = 38.17), and average household income was $60,115 (SD = $70,736; Mtreatment = $52079, Mcontrol = $67,453). Participants reported a mean of 5.5 traumatic events (SD = 2.75 Range = 1-11). The most frequent types of trauma reported were unwanted sexual contact (59.6%), serious accidents (57.4%), and physical assault with a weapon (57.4%). Six individuals (13%) reported combat exposure. Although having a PTSD diagnosis was not a requirement of the study, 53.2% (n= 25) of the participants met criteria, including 54.2% (n= 13) of the treatment group and 52.2% (n= 12) of the control group, χ2 (1, N= 47) = 0.02, ns.On the Clinician-Administered PTSD Scale (CAPS)20, approximately 37% reported moderate, 14% reported severe, and 25% reported extreme PTSD symptoms (M= 56.83, SD= 27.19). Participants reported experiencing nightmares an average of 18 years (SD= 12.41; range 1.5-40) and a mean of 5.92 (SD= 1.74) h sleep/night. Approximately 36% of the sample reported taking over an hour to get to sleep each night. Twenty-six percent reported that their most recurrent and distressing nightmare was exactly like their trauma; 34% reported it was similar to the trauma; 32% reported it was unrelated to their trauma; and 8% did not answer the question.
Measures
Psychological Assessment
Background
The Trauma Assessment for Adults: Self Report Version (TAA)21 assesses lifetime history of potentially traumatic events. The TAA consists of 14 items and includes follow-up questions to assess perceived threat. For the present study, the TAA was modified to include assessment of additional types of traumatic events. Age of first and most recent occurrence is determined for multiple incidents of a given type. Research indicates the TAA has acceptable test-retest reliability and convergent validity.22
Mental Health
The CAPS is a semi-structured clinical interview consisting of 17 items assessing frequency and severity of PTSD symptoms and 5 additional items assess associated symptoms.20 The “F1/I2” rule (frequency coded ≥ 1 and intensity coded ≥ 2 for the past month) was utilized to determine symptom presence and diagnosis. This method has adequate sensitivity and specificity rates and good reliability.20,23 All CAPS interviews were audiotaped, and 25% were randomly selected for evaluation of interrater reliability of PTSD diagnosis. The κ coefficient for the overall PTSD diagnosis was 0.87, with 97.3% interrater agreement.
The Trauma Symptom Inventory (TSI)24 is a 100-item test designed to assess symptoms of PTSD and acute stress disorder in addition to a broader range of symptoms associated with trauma. The TSI includes 3 validity scales (Response level, Atypical Response, Inconsistent Response) and 10 clinical scales. Internal consistencies for the clinical scales range from 0.74 to 0.91, with an overall internal consistency of 0.86. The TSI also demonstrates criterion-related validity.24 For the current study, we only examined those scales that did not overlap in content with the CAPS and we believed had relevance for the present study (e.g., Depression, Anger/Irritability, Dissociation, and Tension Reduction Behavior).
Nightmares and Sleep
The Pittsburgh Sleep Quality Index (PSQI)25 is a self-report measure designed to assess certain qualities and problems associated with sleep. A global sleep quality score is obtained by summing the 7 component scores; higher scores reflect poorer sleep quality. The global score may range from 0-21. The PSQI has good internal homogeneity, test-retest reliability and validity.25 Buysse and colleagues determined a cut-off score of 5 as distinguishing “good” sleepers from “poor” sleepers, with a diagnostic sensitivity of 89.6% and specificity of 86.5%.25
The Trauma Related Nightmare Survey (TRNS)26 was used to assess characteristics of chronic nightmares. Likert-type, categorical, and open-ended questions assess the frequency, severity, and duration of nightmares, as well as cognitions, emotions, and behaviors related to nightmares. While one particular nightmare was targeted in the treatment, this measure assesses nightmares in general. Of interest for the present study, nightmare frequency was assessed as the number of nightmares in the past week and the number of nights with nightmares in the past week. Nightmare severity was rated from 0 “not at all disturbing” to 4 “extremely disturbing.” Test-retest analyses revealed adequate reliabilities and good convergent validity.
Physical Health and Quality of Life
Pennebaker Inventory of Limbic Languidness (PILL).27 The PILL is a 54-item self-report questionnaire that assesses the frequency of common physical symptoms on a 5-point Likert scale ranging from 0 (have never or almost never experienced the symptom)to 4 (experiencing the symptom more than once a week). Pennebaker reports an internal consistency of r= 0.91 and test-retest reliability of r= 0.83.27
Post Treatment Clinical Significance Survey (PTCSS).28 The PTCSS was designed to assess changes in various life areas attributed to treatment. The PTCSS consists of 19 questions assessing to what degree the participant adhered to the treatment components, the helpfulness and difficulty of various treatment components, and change in 9 areas of life (e.g., relations with others, general activity level, anxiety) rated on a 5-point scale ranging from “much worse” to “much better.” The measure was utilized as an index of clinical significance at 6-month follow-up. Psychometric properties of this measure have not been established.
Physiological Assessments
Script-Driven Imagery and Nightmare-Related Physiological Reactivity
During the initial psychological assessment, participants were administered a structured interview that assessed for details of their nightmares. The interview asked participants to report on their most recurrent nightmare, or most recent nightmare if a single recurring nightmare was not available. Follow-up questions asked about cognitive, somatic, and sensory details. The physiological assessors used this interview data to construct a personal nightmare script for each participant. Each script was 30 sec long (approximately 100 words), written in second person, and recorded by a female experimenter in a slow-paced voice onto computer.
During physiological assessments, participants were seated alone in a recliner in a sound attenuated chamber while being monitored from an adjoining room by video camera. Before each assessment, participants were instrumented for physiological recording. After being instrumented, the experimenter left the room and participants sat quietly during a 5-min habituation period, followed by a brief (3-min) diaphragmatic breathing exercise presented by computer. Physiological reactivity was then assessed during script-driven imagery. Five imagery scripts (all 30-sec long) were presented which included the participant's personal nightmare script and 4 non-personal emotional scripts (pleasant, action, fear, neutral; script order randomized across subjects and testing periods). Immediately following the computer presentation of a script, participants were instructed to imagine the scene in the script as if it were happening to them. Each imagery period lasted 30 sec. To allow physiological reactivity to return to baseline values in between scripts, there was a 30-sec recovery period; however, HR was monitored during this period, and the recovery period was extended if necessary to allow HR to return to pre-script levels.29 Only physiological reactivity associated with the nightmare script was used for the present analyses.
Physiological Apparatus, Skin Preparation, and Recording Parameters
A PC with dual 17-inch flat panel monitors and A/D board (National Instruments, PCI-6036E) presented scripts and acquired physiological data. Physiological signals were sampled at 250 Hz and collected/filtered using a Model 15LT Bipolar Amplifier (Grass Technologies; West Warwick, RI) with Quad AC (15A54) and Dual DC (15A12) modules. An adaptor (Grass; Model SCA1) for the 15A12 amplifier was used to measure SCL.
Miniature electrodes to measure left corrugator EMG and left lateralis frontalis EMG were placed according to the recommendations of Fridlund and Cacioppo.30 Facial EMG was amplified (×20,000) and bandpass filtered (10-300 Hz) before being stored digitally (while EMG frequencies can range from 1-1000 Hz, most of the signal power is from 10-150 Hz.31,I Electrocardiogram (ECG) was assessed from electrodes placed on each forearm and was converted to HR by calculating the distance between R to R intervals. Facial EMG and ECG electrodes were applied by degreasing the skin, slightly abrading the skin using NuPrep gel (≤ 10 KΩ), and filling the electrodes with EC60 gel (Grass Technologies). SCL was recorded from electrodes filled with isotonic paste (EC33; Grass Technologies) that were placed on the distal volar surface of the non-dominant index and middle fingers. The signals for HR, SCL, and full-wave rectified, non-integrated facial EMG were visually inspected for artifacts in 5-sec bins. Bins that did not contain artifacts were averaged to make a 30-sec pre-script baseline period and a 30-sec imagery period, as noted in the previous section. For additional details about the physiological recording procedures, see the previously published studies by Rhudy and colleagues.3,32
Procedure
The study was approved by the University of Tulsa Institutional Review Board. Participants were recruited via flyers, email, and radio ads and were screened over the phone for inclusion criteria (experiencing a traumatic event and having nightmares at least one time per week for the previous month) and exclusion criteria (apparent psychosis or mental retardation, aged less than 18, active suicidality or recent parasuicidal behaviors, or current drug/alcohol dependence). Eligible individuals were invited to participate in the study and were randomly assigned using a random number generator to either the treatment or the wait-list control group. Each assessment point included psychological and physiological assessments that were conducted by trained upper level graduate students supervised by the papers' first two authors (JLD and JLR).
After the initial assessment (Time 1), the control group was not contacted during the period that the treatment group received the 3-week intervention, with the exception of a phone call to schedule a time for the re-assessment. One week following the completion of treatment, both groups were reassessed (Time 2) and the control group was offered the treatment. Follow-up assessments occurred at 3 months (Time 3) and 6 months (Time 4) post-treatment. Although attempts were made to keep assessors blinded to treatment group assignment, in some instances assessors were un-blinded at the one-week psychological post assessment for several reasons, including participant disclosure of treatment condition, potentially influencing scoring of the structured interview of PTSD symptoms at the one-week post-treatment assessment point. However, similar results were found for PTSD scores in the successfully blinded previous RCT,7 results were consistent with the one-week physiological assessments which were all blinded; and as reported above, interrater reliability PTSD diagnosis was excellent (κ = 0.87; 97.3 % agreement). At each assessment point, participants were provided compensation.
Intervention
Exposure, relaxation, and rescripting therapy (ERRT) is a cognitive behavioral treatment for trauma-related nightmares and is described in detail elsewhere.7,28 ERRT is conducted for 2 h once a week for 3 consecutive weeks and may be used in either an individual or group format. Treatment consists of psychoeducation about trauma, PTSD, and nightmares, relaxation training, modification of sleep habits, written and verbal exposure to the nightmare, rescription of the nightmare based on trauma-related themes (i.e., power, trust, intimacy, esteem, safety),33 and imagery rehearsal of the rescripted dream each night prior to going to sleep.
Data Analytic Strategy
Data were screened for any potential deviations for normality. Most variables were reasonably normal (skewness statistic divided by the standard error of skewness < 1.96). Not surprisingly, the variables that deviated the most from normal were the physiological reactivity variables. To determine whether non-normality (outliers in particular) caused problems for the models, we carefully examined scatterplots between each of the physiological variables and the psychological outcome variables. Using this approach, we did not find that any outliers had particularly strong statistical influence (i.e., outliers in X and Y space) that might be driving the relationships we observed in our analyses. This bolstered our confidence in proceeding with our statistical analyses.
Initially, ANOVA and χ2 tests were used for univariate analyses to assess differences between groups at baseline assessment (Time 1). Treatment response on psychological variables was assessed using multilevel models (SPSS MIXED procedure). Group (immediate treatment vs. delayed treatment) was entered as a nominal between-subjects variable and Time (pre-treatment vs. post-treatment) was entered as a nominal within-subjects variable. Intent-to-treat analyses were conducted on all participants who were randomized. To provide a conservative estimate of initial treatment response for the intent-to-treat analyses, initial baseline scores were carried forward for those who dropped out of the study. Completer analyses were then conducted by selecting only those participants who completed either the post-treatment assessment (treatment group) or second baseline assessment (control group). To assess the magnitude of treatment response for the intent-to-treat and completer analyses, Cohen's d effect sizes were calculated for both groups using baseline and post treatment pooled standard deviations. The overall treatment effect reported is the Cohen's dtreatment minus the Cohen's dcontrol.
Data from immediate treatment and delayed treatment groups were analyzed for pre-treatment differences. The only difference found was nightmare severity (see Results section), so this variable was controlled for in all analyses, including the follow-up analyses where the groups were combined (N = 47). These data were analyzed using multilevel models (SPSS MIXED procedure) that entered Time (pre-treatment, post-treatment, 3 months, and 6 months) as a nominal within-subjects variable. End state functioning was assessed utilizing only those individuals who completed the 6-month follow-up assessment (n= 24). Effect sizes were calculated with Cohen's d.
Multilevel models examined whether changes in physiological reactivity to nightmare imagery were associated with treatment response on the psychological variables. These analyses included all 40 participants who completed at least one physiological assessment, although one person was excluded because of equipment problems. Because these analyses were largely exploratory, we limited the number of analyses to only those 7 variables that demonstrated significant change in both intent-to-treat and follow-up analyses (see Results section) and health symptoms (PILL) because previously published results found that health symptoms may be related to physiological reactivity to nightmare imagery.3 Therefore, 8 multilevel models were conducted; each included the 4 physiological reactions to nightmare imagery as time-varying covariates. These models controlled for medications that could potentially affect physiological responding (e.g., those that act on cholinergic or adrenergic receptors). No medication variable was a significant predictor in any model, except in the prediction of depression symptoms (not surprisingly, medications that agonize α- or β-adrenergic receptors were more likely to be taken by those more depressed). Thus, to simplify the presentation of the results, medication covariates are not reported in the table of results. Significance of individual physiological predictors in the multilevel models was tested using fixed effects tests (i.e., F tests). The significance of the overall multilevel model was tested using a χ2 difference test that compared the 7 predictor models with the no predictor “null” model. Variance explained by each multilevel model was calculated from η2.34 An autoregressive covariance structure (AR1) was used to model the error structure for repeated measurements in all multilevel models. Follow-up tests for all analyses were conducted using Bonferroni adjusted mean comparisons to control for family-wise type I error rate. Significance level was set at p < 0.05 (2-tailed).
RESULTS
Baseline Analyses
The treatment group (M = 3.29, SD= 0.62) reported greater severity of nightmares at baseline than the control group (M = 2.91, SD= 0.65), F1,47 = 4.13, p < 0.05, so nightmare severity was entered as a covariate in subsequent analyses. The completer group (M= 14.89, SD= 2.49) reported more education than the dropout group (M= 13.17, SD= 2.69), F1,47 = 4.08, p < 0.05. No other differences were found between groups.
Intent-to-Treat and Completer Analyses
Intent-to-treat analyses (see Table 1) revealed a significant interaction of Group × Time for the following variables: nights per week with nightmares, nightmare severity, panic symptoms upon awakening, global sleep quality, hours slept, fear of sleep, TSI depression, and past month CAPS score. When these interactions were decomposed for the simple effect of time, results indicated that the treatment group improved on all of these variables from baseline to post-treatment, while the control group did not change. The remaining variables were unaffected by treatment, although dissociation and anger/irritability decreased over time independent of treatment.
Table 1.
Intent-to-treat data by condition and time
| Variable | Baseline |
One-Week Posttreatment |
d | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatment (n= 24) |
Control (n= 23) |
Treatment (n= 24) |
Control (n= 23) |
Fixed Effects (F-test) |
||||||||
| M | SE | M | SE | M | SE | M | SE | Time | Group | Interaction | ||
| Nights/Week with NM (0-7) | 3.24 | 0.39 | 2.76 | 0.40 | 1.82 | 0.39 | 2.56 | 0.40 | 7.34** | 0.08 | 4.10* | 0.68 |
| NM per Week (0-∞) | 3.19 | 0.57 | 3.50 | 0.58 | 2.85 | 0.57 | 3.63 | 0.59 | 0.10 | 0.50 | 0.49 | 0.21 |
| NM Severity (0-4) | 3.17 | 0.15 | 3.07 | 0.16 | 2.23 | 0.16 | 3.04 | 0.16 | 9.28*** | 4.87* | 8.05** | 0.87 |
| Panic Sxs Upon Waking (0-14) | 6.36 | 0.59 | 4.66 | 0.58 | 4.67 | 0.60 | 5.29 | 0.59 | 1.39 | 0.57 | 6.57** | 0.78 |
| PSQI Sleep Quality (0-21) | 13.55 | 1.02 | 11.81 | 1.06 | 9.03 | 1.01 | 11.49 | 1.07 | 18.07*** | 0.07 | 13.68*** | 0.92 |
| Hours of Sleep (0-24) | 5.68 | 0.34 | 6.18 | 0.34 | 6.61 | 0.34 | 5.94 | 0.34 | 3.45 | 0.04 | 9.79*** | 0.68 |
| Fearful of Sleep (0-4) | 1.85 | 0.21 | 1.20 | 0.22 | 0.85 | 0.21 | 1.20 | 0.22 | 15.33*** | 0.27 | 15.33*** | 0.96 |
| Health Symptoms (0-216) | 74.31 | 6.95 | 75.84 | 7.08 | 67.38 | 6.97 | 76.49 | 7.08 | 1.92 | 0.29 | 2.79 | 0.22 |
| TSI – Depression (0-24) | 12.00 | 1.28 | 10.52 | 1.31 | 9.17 | 1.28 | 10.21 | 1.31 | 7.85** | 0.02 | 5.10* | 0.37 |
| TSI – Anger/Irritability (0-27) | 13.56 | 1.38 | 12.41 | 1.41 | 11.02 | 1.38 | 10.98 | 1.41 | 9.27*** | 0.10 | 0.72 | 0.16 |
| TSI – Dissociation (0-27) | 10.30 | 1.25 | 9.34 | 1.28 | 8.51 | 1.25 | 8.29 | 1.28 | 9.54*** | 0.11 | 0.66 | 0.11 |
| TSI – Tension Reduction (0-24) | 5.65 | 0.81 | 5.24 | 0.83 | 4.19 | 0.81 | 5.11 | 0.83 | 2.94 | 0.05 | 2.05 | 0.36 |
| CAPS Past Month (0-136) | 59.11 | 5.97 | 55.36 | 6.10 | 43.57 | 5.97 | 51.80 | 6.10 | 12.92*** | 0.07 | 5.08* | 0.39 |
p < 0.05.
p < 0.01.
p < 0.001.
NM, nightmare; Sxs, Symptoms; CAPS, Clinician Administered PTSD Scale; TSI, Trauma Symptom Inventory; PSQI, Pittsburgh Sleep Quality Index.
According to Cohen (1988), d = 0.20 is a small effect, d = 0.50 is a medium effect, d = 0.80 is a large effect.
Completer analyses were conducted on those participants who completed therapy and the post assessment (n =17) and those participants who completed the second initial evaluation in the control group (n =18). Results mirrored those reported for the intent-to-treat analyses and, therefore, are not reported here. Data can be obtained from the authors upon request.
Follow-up Analyses
All 47 participants were included in the follow-up analyses. Unlike the intent-to-treat analyses, missing data were not replaced but instead were handled by the multilevel model procedures within SPSS MIXED. Time was entered as the within-subjects factor, and nightmare severity was entered as a continuous covariate (see Table 2). Analyses revealed significant improvements for each variable with the exception of panic symptoms upon awakening from a nightmare. Bonferroni-adjusted mean comparisons indicated statistically significant improvements on all variables between baseline vs. one-week post-treatment, baseline vs. 3-month follow-up, and baseline vs. 6-month follow-up (all p-values < 0.05). The only variables to show significant improvements from one-week post-treatment to 3 months were past month CAPS score and nightmares per week. No variables showed improvement from 3-month to 6-month follow-up; however, it is important to point out that all treatment gains made were maintained throughout the duration of the study (i.e., no variable worsened at any assessment point).
Table 2.
Follow-up analyses (N = 47)
| Variable | Baseline |
1-Week Post |
3-Month F/U |
6-Month F/U |
Time F | d | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| M | SE | M | SE | M | SE | M | SE | |||
| Nights/Week with NM | 3.00 | 0.27 | 1.26 | 0.32 | 1.30 | 0.34 | 0.92 | 0.36 | 13.43*** | 1.16 |
| NM per Week | 3.33 | 0.38 | 2.81 | 0.45 | 1.45 | 0.49 | 1.36 | 0.52 | 4.81** | 0.76 |
| NM Severity | 3.09 | 0.14 | 1.79 | 0.18 | 1.67 | 0.20 | 1.21 | 0.20 | 26.56*** | 2.00 |
| Panic Sxs Upon Waking | 5.47 | 0.43 | 4.39 | 0.51 | 4.05 | 0.60 | 4.22 | 0.64 | 2.34 | 0.45 |
| PSQI Sleep Quality | 12.45 | 0.76 | 7.60 | 0.84 | 7.65 | 0.87 | 7.45 | 0.90 | 25.17*** | 0.96 |
| Hours of Sleep | 5.94 | 0.23 | 6.93 | 0.26 | 6.90 | 0.27 | 6.78 | 0.29 | 8.11*** | 0.57 |
| Fearful of Sleep | 1.51 | 0.14 | 0.68 | 0.16 | 0.48 | 0.17 | 0.41 | 0.18 | 14.85*** | 1.26 |
| Health Symptoms | 74.84 | 4.85 | 64.78 | 5.23 | 63.06 | 5.35 | 59.53 | 5.47 | 6.29** | 0.48 |
| TSI – Depression | 11.04 | 0.89 | 8.70 | 1.00 | 7.71 | 1.07 | 7.47 | 1.15 | 4.78** | 0.52 |
| TSI – Anger/Irritability | 12.84 | 0.89 | 8.74 | 1.02 | 8.90 | 1.10 | 7.93 | 1.19 | 8.39*** | 0.83 |
| TSI – Dissociation | 9.76 | 0.80 | 7.25 | 0.88 | 6.98 | 0.92 | 6.36 | 0.96 | 8.57*** | 0.72 |
| TSI – Tension Reduction | 5.44 | 0.52 | 3.00 | 0.62 | 3.64 | 0.64 | 2.79 | 0.67 | 7.83*** | 0.88 |
| CAPS Past Month | 55.68 | 3.95 | 41.39 | 4.34 | 26.92 | 4.61 | 24.93 | 4.92 | 17.41*** | 1.18 |
p < 0.05.
p < 0.01.
p < 0.001.
Effect sizes calculated for baseline to 6-month differences. According to Cohen (1988), d = 0.20 is a small effect, d = 0.50 is a medium effect, d = 0.80 is a large effect.
NM, nightmare; Sxs, Symptoms; CAPS, Clinician Administered PTSD Scale; TSI, Trauma Symptom Inventory; PSQI, Pittsburgh Sleep Quality Index.
Clinical Significance
Clinical significance was assessed for the 24 participants who completed the 6-month assessment. For nightmare frequency we determined that an absence of nightmares in the past week based on the TRNS constituted a good response to the treatment7; 71% achieved this, reflecting a 58% decrease in number of any nightmares in the past week from baseline to 6-month follow-up. It is important to note that we assessed anynightmares, not just the nightmare targeted in treatment. Approximately 63% of participants had a reduction in CAPS score > 10 points, which is generally considered to be a clinically significant change.35,36 Further, 75% (N =9) of those diagnosed with PTSD at baseline lost their diagnosis. For sleep quality, 9 (37.5%) participants achieved a score ≤ 5 on the PSQI.
We also examined changes in quality of life at the 6-month assessment via the PTCSS (Table 3).28 The majority of participants reported that each area assessed was somewhat or much better following treatment. Further, while participants reported some differences in how helpful they found the treatment techniques, the component with the highest percentage of people stating that it was very helpful was writing out the nightmare (78.3%).
Table 3.
Results from the posttreatment clinical significance survey
| Variable | % |
|---|---|
| Area was somewhat better or much better following treatment | |
| Anxiety | 91.3 |
| Sleep | 90.9 |
| Work | 82.6 |
| Overall PTSD symptoms | 81.9 |
| Relations with others | 78.3 |
| Overall activity level | 78.3 |
| Overall mental health | 78.3 |
| Depression | 60.9 |
| Overall physical health | 56.5 |
| Treatment component was helpful or very helpful | |
| Nightmare education | 100.0 |
| Identification of themes | 91.3 |
| Sleep education | 82.6 |
| Writing out the nightmare | 82.6 |
| Progressive muscle relaxation | 82.6 |
| Changing sleep habits | 78.3 |
| Reading the nightmare aloud | 73.9 |
| Rescripting the nightmare | 73.9 |
| PTSD education | 69.5 |
Treatment Compliance
Treatment compliance was assessed in 2 ways. First, the percentage of between session homework assignments completed was determined. Individuals who completed treatment and at least one follow-up assessment averaged 78.40% (SD= 23.86) compliance with weekly homework. Second, participants were asked at the 6-month assessment how closely they followed the techniques learned; nearly 83% of the sample reported “mostly” or “completely” following the techniques learned in the study.
Associations between Treatment Response and Physiological Reactivity to Nightmare Imagery
Results from these multilevel models are presented in Table 4. The χ2 difference tests indicated all 8 models were an improvement over the intercept only models. Decreases in HR reactivity over time were associated with improvements in sleep quality and hours of sleep. Decreases in SCL reactivity over time were associated with improvements in nightmare severity, PTSD symptoms in the past month (CAPS), sleep quality, and fear of sleep. Facial EMG was generally unrelated to psychological outcomes, except that decreases in corrugator EMG over time were associated with improvements in self-reported health. No physiological variable was significantly associated with nights with nightmares per week or depression. It is noteworthy that the variance explained by those models with significant predictors tended to be large according to η2 values, ranging from 17% variance explained (nightmare severity) to 37% (sleep quality).
Table 4.
Multilevel models examining the associations of relevant treatment outcomes and physiological reactions entered as time-varying covariates after controlling for medications
| Predictors | Dependent Variables |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nights/Wk w/NM |
NM Severity |
Sleep Quality (PSQI) |
Hours of Sleep |
Fearful of Sleep |
Health Symptoms (PILL) |
CAPS past Month |
Depression (TSI) |
|||||||||
| Est. | SE | Est. | SE | Est. | SE | Est. | SE | Est. | SE | Est. | SE | Est. | SE | Est. | SE | |
| Intercept | 1.08 | 0.31 | 1.79 | 0.17 | 5.89 | 0.85 | 7.20 | 0.22 | 0.41 | 0.17 | 61.89 | 7.13 | 24.34 | 4.89 | 6.60 | 1.22 |
| Corr EMG | 1.10 | 1.32 | -0.72 | 0.83 | 1.68 | 2.65 | -1.08 | 0.86 | 0.32 | 0.62 | 28.75 | 12.22 | 19.34 | 15.34 | 5.40 | 3.03 |
| LF EMG | 1.03 | 4.13 | 5.03 | 2.55 | 1.65 | 8.72 | 2.07 | 2.72 | 1.04 | 1.98 | 36.77 | 40.57 | 30.77 | 49.34 | 10.86 | 9.92 |
| HR | 0.07 | 0.04 | 0.02 | 0.02 | 0.20 | 0.08 | -0.08 | 0.02 | 0.00 | 0.02 | 0.07 | 0.35 | 0.52 | 0.44 | -0.02 | 0.09 |
| SC | 0.37 | 0.22 | 0.35 | 0.14 | 1.67 | 0.46 | -0.18 | 0.15 | 0.41 | 0.11 | 4.13 | 2.21 | 9.79 | 2.66 | 0.68 | 0.53 |
| Δχ2 = | 19.91 | 20.87 | 51.41 | 35.58 | 33.08 | 24.57 | 38.73 | 21.04 | ||||||||
| η2 = | 0.16 | 0.17 | 0.37 | 0.19 | 0.23 | 0.22 | 0.30 | 0.11 | ||||||||
Positive associations with PSQI sleep quality indicate that reductions in physiological responses are associated with improvements in sleep quality (higher scores on the PSQI indicate poorer sleep quality). All models with a Δχ2 > 15.51 were statistically significant (i.e., were significant improvements over an intercept only model). η2, eta squared effect size (proportion of variance explained in dependent variable). Corr, corrugator muscle. LF, lateralis frontalis muscle. HR, heart rate. SC, skin conductance. EMG, electromyogram. Est., unstandardized estimate of fixed effect (relationship between predictor and criterion). SE, standard error for the parameter estimate. Estimates in bolded text are significant at p < 0.05.
DISCUSSION
The present study provided a needed replication of the first RCT of this brief cognitive behavioral approach and extended this research by expanding the assessment domains and examining the relationship between change in physiological indicators of nightmare-related defensive reactivity and other measures of treatment response. Results support the use of ERRT for trauma-related sleep problems.
Hypothesis one was supported in that the results of the current study mirror those found in the previous RCT.7 These findings provide additional support for the efficacy of ERRT in reducing chronic nightmares and related psychopathology and improving sleep at the one-week post-treatment assessment and maintaining treatment gains through the six-month follow-up assessment. It is important to note that while only one nightmare was targeted in treatment, the assessment queries all nightmares; so the treatment appears to have a generalized effect. Further, while the effect size for improvement in sleep quality was large, the mean score on the PSQI (7.45) was higher than the cut-off of 5 which distinguishes good sleepers from poor sleepers.25 Hypothesis two was not fully supported at the one-week post-treatment follow-up. Although anger and dissociation changed, this was not related to the treatment but to the passage of time. At the six-month follow-up assessment, statistically significant changes were noted for anger, dissociation, tension reduction, and self-reported physical health. Other measures of clinical significance also show improvement. Participants reported improvements in quality of life across nine areas; further, 75% of participants with a diagnosis of PTSD at baseline who completed the follow-up assessment, had lost this diagnosis at six months post-treatment. This suggests that for some individuals, sleep disturbances may be primary difficulties in need of direct treatment rather than secondary symptoms of PTSD.18 Overall, it is clear that ERRT is successful in producing broad, sustained improvements in a wide range of functioning based on clinical interview and self-report of participants.
Support also exists for the efficacy of ERRT in reducing physiological reactivity to nightmare-related defensive reactivity. A previous study indicated that nightmare sufferers exhibited heightened physiological responses to nightmare imagery3 and that these physiological reactions were significantly reduced by ERRT relative to the control group that showed no change in physiological reactivity to nightmare imagery.32
Rhudy et al.32 argued that physiological reactivity to nightmare imagery may index activation of the fear network associated with the nightmare,37 and that engaging this fear network may be essential for processing emotional material to decrease fear and anxiety37 and improve nightmares and sleep disturbance.38 If true, then changes in physiological reactivity to nightmare imagery should covary with other indicators of treatment outcome. The present study partially supports this hypothesis. Multilevel modeling found that autonomic reactions were significantly associated with improvement in several outcome variables. Decreases in HR reactivity over time were associated with improvement in sleep quality and hours slept per night and decreases in SCL over time were associated with improvements in sleep quality, fear of sleep, nightmare severity, and PTSD symptomatology. These findings could reflect the degree to which nightmare-related thoughts result in defensive reactivity and autonomic arousal that interferes with sleep quality and quantity. The fact that SCL, but not HR, was associated with PTSD symptoms, fear of sleep, and nightmare severity may reflect a stronger association between these outcomes and sympathetic activation; given that SCL is primarily a measure of sympathetic activity. By contrast, HR is influenced by sympathetic and parasympathetic branches of the autonomic nervous system, so the relationship between HR and hours of sleep may reflect a greater involvement of the parasympathetic branch in helping maintain sleep once initiated. At this time these relationships are purely speculative, however. Future research should consider examining the influence of nightmare treatment on metrics of heart rate variability that measure the relative influence of sympathetic and parasympathetic controls over cardiac function. A final relationship observed was between reduction in health symptoms and decreases in corrugator activity. Given the well-established relationship between negative affect and poorer physical health,39 decreases in corrugator could reflect a general decrease in negative affect that was maintaining health symptomatology.
At this time the relationships observed between measures of physiological reactivity and nightmare-related symptoms primarily help to build a theoretical framework about what might be maintaining nightmares and sleep problems. This will help to generate hypotheses in future studies. Nevertheless, patients may benefit from knowing that physiological arousal stemming from nightmare-related thoughts might promote symptomatology. Thus, the combination of monitoring arousal (e.g., perhaps via a heart rate monitor watch available at most sports stores) and engaging in arousal-reducing interventions (e.g., progressive muscle relaxation, diaphragmatic breathing) could improve symptoms.
Overall, ERRT is found to be effective in alleviating distress specific to nightmares and sleep, underlying physiological indicators of defensive reactivity to nightmare imagery, and broader indices of distress. How this happens is still unclear, however. From an information processing perspective, in order to correct the pathological fear structures, the fear network must be engaged and corrective information needs to be presented and incorporated into the network.37 It is feasible that ERRT activated the fear network through exposure to the nightmare and provided corrective information through conducting exposure in a safe environment, achieving mastery through confronting the nightmare, and changing the storyline with rescription. Changing the fear structure should significantly reduce pre-sleep anticipatory anxiety and physiological arousal, thus enhancing the quality of sleep. An examination of the largest effect sizes (i.e., nightmare severity, past month CAPS score, and fear of sleep) and the association of treatment outcomes with heightened physiological response to nightmare imagery may support this proposition. Given the chronicity of the nightmares reported by this sample (mean of 18 years of experiencing nightmares), the small dose of exposure required is surprising. This is assuming of course that exposure is the key mechanism of change. While this possibility was also supported by the large number of people citing exposure to the nightmare as very helpful, there are several other possible mechanisms of change underlying ERRT including reduced cognitive and physiological arousal via relaxation and modifying sleep habits.7 Another possibility is that rescripting the nightmare may also have changed the cognition, affect, and meaning associated with the nightmare, which may account in part for the generalized impact of the treatment on other indices of distress including depression. More research is needed to determine what may be responsible for our findings.
Limitations
One limitation of this study was the inconsistent blinding for the one-week post assessment and the potential resulting bias of the score of CAPS assessment. However, the fact that the results of this study mirror those of the previous RCT7 and the psychological indices were consistent with the blinded physiological assessments tempers these concerns. The trial included a small sample size comprised primarily of Caucasian women which may limit the generalizability of the results. The 26% dropout rate in this study matches that found in the initial RCT and is fairly typical for trauma-related therapies.40 While the rate in the current study is not exceptional, more research is needed on determining why participants drop out of treatment studies and developing additional strategies to try to increase retention. The use of a wait-list control group does not allow us to speculate about the relative efficacy of ERRT compared to other treatment approaches.41 Future studies will need to conduct direct comparisons to other viable interventions. Additionally, while the study used gold standard interview tools and physiological assessments, future studies should consider including physiological indices of sleep functioning (i.e., polysomnography or actigraphy) and an established measure of clinical significance.
Overall, current results support previous research suggesting that ERRT is an efficacious treatment for trauma-exposed individuals with chronic nightmares and advances the knowledge base in the treatment of nightmares by including an evaluation of the association of physiological and psychological changes over time.
FOOTNOTES
IIt is possible that the sampling frequency of 250 Hz could have led to some EMG aliasing. However, this should not have been a significant problem given that most of the signal power is between 10-15 Hz. Moreover, our confidence in the integrity of the signal is bolstered by our findings that facial EMG reliably increases in response to the nightmare script and these increases are significantly reduced by treatment.32 Therefore, we do not believe aliasing played a significant role in signal degradation in the current study.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
This study was funded with grants from the University of Tulsa Office of Research and Sponsored Programs.
REFERENCES
- 1.Harvey AG, Jones C, Schmidt DA. Sleep and posttraumatic stress disorder: a review. Clin Psychol Rev. 2003;23:377–407. doi: 10.1016/s0272-7358(03)00032-1. [DOI] [PubMed] [Google Scholar]
- 2.Esposito K, Benitez A, Barza L, Mellman T. Evaluation of dream content in combat-related PTSD. J Trauma Stress. 1999;12:681–7. doi: 10.1023/A:1024725319777. [DOI] [PubMed] [Google Scholar]
- 3.Rhudy JL, Davis JL, Williams AE, McCabe KM, Byrd PM. Physiological-emotional reactivity to nightmare-related imagery in trauma-exposed persons with chronic nightmares. Behav Sleep Med. 2008;6:158–77. doi: 10.1080/15402000802162539. [DOI] [PubMed] [Google Scholar]
- 4.Davis JL, Byrd P, Rhudy JL, Wright DC. Characteristics of chronic nightmares in a trauma-exposed treatment-seeking sample. Dreaming. 2007;17:187–98. [Google Scholar]
- 5.Schreuder BJN, Kleijn WC, Rooijmans HGM. Nocturnal re-experiencing more than forty years after war trauma. J Trauma Stress. 2000;13:453–63. doi: 10.1023/A:1007733324351. [DOI] [PubMed] [Google Scholar]
- 6.Forbes D, Phelps AJ, McHugh AF, Debenham P, Hopwood M, Creamer M. Imagery rehearsal in the treatment of posttraumatic nightmares in australian veterans with chronic combat-related PTSD: 12-month follow-up data. J Trauma Stress. 2003;16:509–13. doi: 10.1023/A:1025718830026. [DOI] [PubMed] [Google Scholar]
- 7.Davis JL, Wright DC. Randomized clinical trial for treatment of chronic nightmares in trauma-exposed adults. J Trauma Stress. 2007;20:123–33. doi: 10.1002/jts.20199. [DOI] [PubMed] [Google Scholar]
- 8.Krakow B, Hollifield M, Johnston L, et al. Imagery rehearsal therapy for chronic nightmares in sexual assault survivors with posttraumatic stress disorder: a randomized controlled trial. JAMA. 2001;286:537–45. doi: 10.1001/jama.286.5.537. [DOI] [PubMed] [Google Scholar]
- 9.Galovski TE, Monson C, Bruce SE, Resick PA. Does cognitive-behavioral therapy for PTSD improve perceived health and sleep impairment? J Trauma Stress. 2009;22:197–204. doi: 10.1002/jts.20418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lydiard RB, Hamner MH. Clinical importance of sleep disturbance as a treatment target in PTSD. Focus. 2009;7:176–83. [Google Scholar]
- 11.Zayfert C, DeViva JC. Residual insomnia following cognitive behavioral therapy for PTSD. J Trauma Stress. 2004;17:69–73. doi: 10.1023/B:JOTS.0000014679.31799.e7. [DOI] [PubMed] [Google Scholar]
- 12.Davis JL, De Arellano M, Falsetti SA, Resnick HS. Treatment of nightmares related to post-traumatic stress disorder in an adolescent rape victim. Clin Case Stud. 2003;2:283–94. [Google Scholar]
- 13.Forbes D, Creamer M, Biddle D. The validity of the PTSD checklist as a measure of symptomatic change in combat-related PTSD. Behav Res Ther. 2001;39:977–86. doi: 10.1016/s0005-7967(00)00084-x. [DOI] [PubMed] [Google Scholar]
- 14.Friedman M. Drug treatment for PTSD: Answers and questions. Ann New York Acad Sci. 1997;821:359–71. doi: 10.1111/j.1749-6632.1997.tb48292.x. [DOI] [PubMed] [Google Scholar]
- 15.Raskind MA, Peskind ER, Kanter ED, et al. Reduction of nightmares and other PTSD symptoms in combat veterans by prazosin: a placebo-controlled study. Am J Psychiatry. 2003;160:371–3. doi: 10.1176/appi.ajp.160.2.371. [DOI] [PubMed] [Google Scholar]
- 16.Davis JL. Treating post-trauma nightmares: a cognitive behavioral approach. New York: Springer Publishing Company; 2009. [Google Scholar]
- 17.Krakow B, Tandberg D, Scriggins L, Barey M. A controlled comparison of self-rated sleep complaints in acute and chronic nightmare sufferers. J Nerv Mental Dis. 1995;183:623–7. doi: 10.1097/00005053-199510000-00002. [DOI] [PubMed] [Google Scholar]
- 18.Spoormaker VI, Montgomery P. Disturbed sleep in post-traumatic stress disorder: secondary symptom or core feature? Sleep Med Rev. 2008;12:169–84. doi: 10.1016/j.smrv.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 19.Boscarino J, Chang J. Electrocardiogram abnormalities among men with stress-related psychiatric disorders: Implications for coronary heart disease and clinical research. Ann Behav Med. 1999;21:227–34. doi: 10.1007/BF02884839. [DOI] [PubMed] [Google Scholar]
- 20.Blake DK, Weathers FW, Nagy LM, et al. A therapist rating scale for assessing current and lifetime PTSD: The CAPS-1. Behav Ther. 1990;13:187–8. [Google Scholar]
- 21.Resnick HS, Best CL, Kilpatrick DG, Freedy JR, Falsetti SA. Charleston, SC: National Crime Victims Research and Treatment Center, Medical University of South Carolina; 1993. Trauma Assessment for Adults - Self-Report Version. Unpublished Scale. [Google Scholar]
- 22.Gray MJ, Elhai JD, Owen JR, Monroe R. Psychometric properties of the Trauma Assessment for Adults. Depress Anxiety. 2009;26:190–5. doi: 10.1002/da.20535. [DOI] [PubMed] [Google Scholar]
- 23.Weathers FW, Ruscio AM, Keane TM. Psychometric properties of nine scoring rules for the Clinician-Administered Posttraumatic Stress Disorder Scale. Psychol Assess. 1999;11:124–33. [Google Scholar]
- 24.Briere J. Trauma symptom inventory. Odessa: Psychological Assessment Resources; 1995. [Google Scholar]
- 25.Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh sleep quality index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 26.Davis JL, Wright D, Borntrager C. Tulsa: The University of Tulsa; 2001. The trauma-related nightmare survey. [Google Scholar]
- 27.Pennebaker JW. The psychology of physical symptoms. New York: Springer-Verlag; 1982. [Google Scholar]
- 28.Davis JL, Wright DC, Byrd P, Rhudy JL. Post treatment clinical significance survey. In: Davis JL, editor. Treating post-trauma nightmares: A cognitive behavioral approach. New York: Springer Publishing Company; 2009. [Google Scholar]
- 29.Keane TM, Kolb LC, Kaloupek DG, et al. Utility of psychophysiology measurement in the diagnosis of posttraumatic stress disorder: Results from a department of Veteran's Affairs cooperative study. J Consult Clin Psychol. 1998;66:914–23. doi: 10.1037//0022-006x.66.6.914. [DOI] [PubMed] [Google Scholar]
- 30.Fridlund AJ, Cacioppo JT. Guidelines for human electromyographic research. Psychophysiology. 1986;23:567–89. doi: 10.1111/j.1469-8986.1986.tb00676.x. [DOI] [PubMed] [Google Scholar]
- 31.Stern RM, Ray WJ, Quigley KS. Muscles: Electromyography. Psychophysiological recording New York: Oxford University Press; 2001. pp. 109–24. [Google Scholar]
- 32.Rhudy JL, Davis JL, Williams AE, et al. Cognitive-behavioral treatment for chronic nightmares in trauma-exposed persons: assessing physiological reactions to nightmare-related fear. J Clin Psychol. 2010;66:365–82. doi: 10.1002/jclp.20656. [DOI] [PubMed] [Google Scholar]
- 33.Resick PA, Schnicke MK. Cognitive processing therapy for sexual assault victims: A treatment manual. Newbury Park: Sage Publications; 1993. [DOI] [PubMed] [Google Scholar]
- 34.Tabachnick BG, Fidell LS. Using multivariate statistics. 5th ed. Boston: Allyn and Bacon; 2007. [Google Scholar]
- 35.Weathers FW, Keane TM, Davidson JR. Clinician-administered PTSD scale: a review of the first ten years of research. Depress Anxiety. 2001;13:132–56. doi: 10.1002/da.1029. [DOI] [PubMed] [Google Scholar]
- 36.Schnurr PP, Friedman MJ, Foy DW, et al. Randomized trial of trauma-focused group therapy for posttraumatic stress disorder: results from a Department of Veterans Affairs Cooperative Study. Arch Gen Psychiatry. 2003;60:481–9. doi: 10.1001/archpsyc.60.5.481. [DOI] [PubMed] [Google Scholar]
- 37.Foa EB, Kozak MJ. Emotional processing of fear: Exposure to corrective information. Psychol Bull. 1986;99:20–35. [PubMed] [Google Scholar]
- 38.Levin R, Nielsen TA. Disturbed dreaming, posttraumatic stress disorder, and affect distress: A review and neurocognitive model. Psychol Bull. 2007;133:482–528. doi: 10.1037/0033-2909.133.3.482. [DOI] [PubMed] [Google Scholar]
- 39.Cohen S, Rodriguez MS. Pathways linking affective disturbance and physical disorders. Health Psychol. 1995;14:374–80. doi: 10.1037//0278-6133.14.5.374. [DOI] [PubMed] [Google Scholar]
- 40.Schottenbauer MA, Glass CR, Arnkoff DB, Tendick V, Gray SH. Nonresponse and dropout rates in outcome studies on PTSD: review and methodological considerations. Psychiatry. 2008;71:134–68. doi: 10.1521/psyc.2008.71.2.134. [DOI] [PubMed] [Google Scholar]
- 41.Cook JM, Harb GC, Gehrman PR, et al. Imagery rehearsal for posttraumatic nightmares: A randomized controlled trial. J Trauma Stress. 2010;23:553–63. doi: 10.1002/jts.20569. [DOI] [PubMed] [Google Scholar]