Abstract
Study Objectives:
The purpose of this study was to analyze the relationship between food intake and sleep patterns in healthy individuals.
Methods:
Fifty-two healthy volunteers (27 women and 25 men) were recruited to participate in the study. Volunteers underwent sleep evaluation through nocturnal polysomnography and completed a 3-day food diary to evaluate food intake.
Results:
No differences in sleep patterns were observed in either gender, except in the percentage of stage 1 sleep, which was greater in men. Different correlations were observed between sleep and dietary variables according to gender. The correlation between dietary and sleep variables in men indicated a negative relationship between nocturnal fat intake and the sleep latency, including REM sleep. The percentage of nocturnal fat intake correlated with sleep efficiency, sleep latency, REM latency, stage 2 sleep, REM sleep, and wake after sleep onset (WASO) in women. The percentage of nocturnal caloric intake correlated with sleep latency and efficiency in women.
Conclusions:
We conclude that food intake during the nocturnal period is correlated with negative effects on the sleep quality of healthy individuals. Indeed, food intake near the sleeping period (dinner and late night snack) was negatively associated with sleep quality variables. More studies are necessary to elucidate the real effect of food intake on sleep.
Citation:
Crispim CA; Zimberg IZ; dos Reis BG; Diniz RM; Tufik S; de Mello MT. Relationship between food intake and sleep pattern in healthy individuals. J Clin Sleep Med 2011;7(6):659-664.
Keywords: Sleep, food intake, sleep quality, nocturnal caloric intake
Obesity is becoming a worldwide epidemic.1 The etiology of this disease is multifactorial; among the causes are changes in food intake,2 life style, environment,2,3 and genetics,4,5 in addition to physiological6 and psychological7 influences and, more recently, alterations in sleep patterns.8–11
Sleep curtailment has become common due to the demands and opportunities of modern society.12 Recent studies show that alterations in sleep time can influence various aspects associated with the nutritional and metabolic balance of the body, such as the control of body mass,9,11 food intake,10,13 glycemic levels,14,15 and the levels of cholesterol and triacylglycerol.16 Although some studies show that short duration sleep changes the food intake pattern and may cause obesity,9–11 few studies have analyzed whether the opposite casual sequence also occurs; that is, if food intake promotes alterations in the sleep pattern. Indeed, studies that have examined this matter are controversial and use different methodologies. Some studies indicate an impairment of sleep quality when there is an excessive carbohydrate intake.17,18 Driver et al.,19 however, comparing the effect of an evening meal on nocturnal sleep in seven healthy men, did not find any effect. Therefore, this study proposes to analyze the correlation between habitual food intake and sleep patterns in healthy individuals.
BRIEF SUMMARY
Current Knowledge/Study Rationale: Few studies have analyzed if food intake promotes alterations in the sleep pattern. Therefore, the objective of this study was to analyze the correlation between habitual food intake and sleep patterns in healthy individuals.
Study Impact: This study demonstrated that a higher food intake close to the sleeping period is associated with negative aspects of sleep patterns in healthy individuals, especially in women. However, this is an area poorly explored in the literature, and more studies are necessary to elucidate the real influence of food intake upon sleep.
METHODS
Sample
Fifty-two healthy volunteers (27 women and 25 men) between 19 and 45 years old took part in this study. The participants were young adults and were non-obese. They were sedentary and not taking medication. They were all nonsmokers and spent regular times in bed at night (7.5-8.5 h). The subjects did not suffer from sleep disturbances (apnea and hypopnea index [AHI] < 5, and periodic leg movements [PLM] during sleep were < 5, as assessed by polysomnography).20,21 Volunteers were submitted to a clinical examination including blood, urine, and electrocardiogram tests, which were evaluated by a physician who approved their participation in the study. Enrolment was voluntary, and the subjects were informed of the procedures and objectives of the study.
The study was approved by the Committee of Ethics in Research of the Federal University of São Paulo (0019/08). After participants were informed about all the stages of this study and gave their written informed consent.
Food Intake Evaluation
Food intake was determined through a self-administered food diary for 3 non-consecutive days, filled in immediately before the evaluation by polysomnography (PSG). Volunteers were instructed to provide as much detail as possible about the foods and fluids consumed, including brand names and recipes for home-cooked foods. Portion sizes were estimated using common household measurements such as cups, glasses, bowls, teaspoons, and tablespoons in addition to individual food items/units. The diaries were inspected by a researcher together with the subjects to obtain additional explanations and detail, if required. The Nutwin version 1.5 software (University of São Paulo, Brazil, 2002) was used for the quantitative analyses of caloric and macronutrient intakes. At the end of the study, all volunteers received a report containing the analyzed data from their food diary and individual nutritional guidance.
Sleep Evaluation
Volunteers arrived at the sleep laboratory at 21:30 for electrode attachment and went to bed at 23:00. Sleep parameters were recorded by means of 2 PSGs in the laboratory. The PSG was recorded using an EMBLA S7000 digital system (Embla Systems Inc, Medcare Flaga, Reykjavik, Iceland) in 30-sec epochs.
Electroencephalographic recordings were performed according to the international 10-20 system.22 Four channels were used for the electroencephalogram (EEG), 2 channels for the electrooculogram, and 2 channels for the electromyogram (submental and legs). The PSG used a thermistor and a nasal cannula to monitor the airflow, belts to monitor thoracic and abdominal effort, transcutaneous oximetry to record oxygen saturation, and a sensor tracking the position of the trunk during sleep.
Interpretations of the PSG were performed according to standard criteria for sleep classification.23 Analyses included measures of total sleep time (TST), sleep latency, REM sleep latency, sleep efficiency, stages N1, N2, and N3 of NREM sleep and REM sleep, wake after sleep onset (WASO), AHI, oxygen saturation, and PLM. In the results section, data were compared with normative data recommended by Carskadon and Dement.24
Except for periods of exercise and PSG monitoring, the volunteers were asked to go about their normal daytime activities. They were instructed to refrain from any extra exercise, evening tea or coffee, all alcohol, and naps. Adherence to these requests was assessed in the pre-PSG questionnaire.
Anthropometric Evaluation
Body mass and height were measured before the polysomnography examination. For the evaluation of height, a Sanny stadiometer with 0.1 cm precision (American Medical do Brasil,Ltd, Brazil) fixed to the wall was used. A Filizola scale (Star, Filizola, Brazil) with 0.1 kg precision was used to measure body mass. Body mass index (BMI) was calculated by dividing the body weight (in kilograms) by the height (in meters) squared (BMI = weight/height2). All measurements were determined according to the recommended techniques.25
Statistical Analysis
All values were expressed as the mean ± standard deviation (SD) or the mean and 95% confidence intervals. Student's t-tests for independent samples were used for gender comparison between anthropometric, nutritional, and sleep characteristics. Pearson correlation coefficient was used to assess the association of sleep parameters with food intake. Associations between sleep duration and nutritional intake were further analyzed using multiple regression analysis adjusted for age, gender, BMI, and socioeconomic status. The results from the regression analysis are presented as β-values with P-values. The data were analyzed using Statistica 6.0 (StatSoft, Inc., Tulsa, OK, USA). Statistical tests with p ≤ 0.05 were accepted as significant.
RESULTS
Fifty-two participants (25 men and 27 women) completed all measurements. The characteristics of the participants are presented in Table 1. Men had significantly greater body mass, height, BMI, and caloric intake than women (Table 1).
Table 1.
Nutritional and sleep characteristics of volunteers according to gender*
| Men (n = 25) |
Women (n = 27) |
p value | |||
|---|---|---|---|---|---|
| Mean ± DP | 95% CI | Mean ± DP | 95% CI | ||
| Age (yr) | 27.2 ± 5.9 | 24.8 – 29.6 | 28.8 ± 6.6 | 26.2 – 31.4 | 0.37 |
| Anthropometric Variables | |||||
| Body mass (kg) | 76.6 ± 14.7 | 70.5 – 82.6 | 58.0 ± 8.3 | 54.7 – 61.2 | < 0.01 |
| Height (m) | 1.75 ± 0.1 | 1.72 – 1.78 | 1.61 ± 0.1 | 1.59 – 1.64 | < 0.01 |
| BMI (kg/m2) | 24.9 ± 4.2 | 23.2 – 26.6 | 22.2 ± 2.6 | 21.2 – 23.2 | 0.01 |
| Dietary Variables | |||||
| Caloric intake | |||||
| kcal/day | 2697.6 ± 870.6 | 2338.3 – 3057.0 | 1865.5 ± 502.1 | 1666.9 – 2064.1 | < 0.01 |
| Macronutrients (g) | |||||
| Protein | 108.3 ± 30.1 | 95.9 – 120.8 | 73.2 ± 17.3 | 66.4 – 80.0 | 0.15 |
| Carbohydrate | 359.2 ± 161.8 | 292.4 – 425.9 | 247.4 ± 78.1 | 216.5 – 278.3 | 0.33 |
| Fat | 92.0 ± 27.2 | 80.7 – 103.2 | 64.8 ± 23.8 | 55.4 – 74.2 | 0.44 |
| Macronutrients (%) | |||||
| Protein | 16.6 ± 3.9 | 15.0 – 18.2 | 16.6 ± 5.4 | 14.4 – 18.7 | 0.95 |
| Carbohydrate | 51.9 ± 8.2 | 48.5 – 55.3 | 52.8 ± 5.3 | 50.7 – 54.9 | 0.64 |
| Fat | 31.5 ± 5.9 | 29.0 – 33.9 | 30.7 ± 5.2 | 28.6 – 32.7 | 0.60 |
| Sleep Variables | |||||
| Total sleep time (min) | 369.2 ± 40.2 | 352.6 – 385.9 | 353.7 ± 78.7 | 322.6 – 384.8 | 0.37 |
| Sleep efficiency (%) | 87.3 ± 6.8 | 84.5 – 90.2 | 87.8 ± 7.9 | 84.7 – 90.9 | 0.82 |
| Sleep latency (min) | 16.5 ± 15.5 | 10.2 – 23.0 | 11.3 ± 11.3 | 6.8 – 15.8 | 0.16 |
| REM latency (min) | 95.9 ± 40.7 | 79.1 – 112.8 | 99.8 ± 49.2 | 80.3 – 119.3 | 0.75 |
| N1 (%) | 3.8 ± 2.2 | 2.9 – 2.2 | 2.6 ± 1.8 | 1.9 – 3.3 | 0.03 |
| N2 (%) | 55.0 ± 6.6 | 52.4 – 57.8 | 53.1 ± 7.9 | 50.0 – 56.3 | 0.34 |
| N3 (%) | 23.1 ± 6.5 | 20.5 – 25.9 | 24.6 ± 6.3 | 22.1 – 6.3 | 0.41 |
| REM (%) | 17.9 ± 4.6 | 16.0 – 19.8 | 19.6 ± 5.1 | 17.7 – 21.7 | 0.19 |
| WASO (min) | 37.3 ± 25.7 | 26.7 – 47.9 | 38.0 ± 28.7 | 26.7 – 49.4 | 0.92 |
All values are mean ± SD and 95% CI.
BMI, Body Mass Index; REM, rapid eye movement; WASO, wake after sleep onset.
The data for the observed PSG measures by gender appear in Table 1. Men had a significantly higher percentage of N1 sleep than did women. Although there were no statistically significant differences between genders, the percentage of waking after sleep onset was higher and REM sleep was lower than normative data. Women had reduced total sleep time (≤ 6 h) in comparison with normative data.
The correlation between dietary and sleep variables (Table 2) in men indicated a negative association between nocturnal fat intake, and sleep efficiency, and REM sleep, and a positive association between nocturnal fat intake, and sleep latency, REM sleep latency, N2 sleep, and WASO. In women, there were positive associations between sleep latency and caloric, protein, carbohydrate, and fat nocturnal intake; REM sleep latency and caloric, carbohydrate, and fat nocturnal intake; N2 sleep and caloric, carbohydrate, and fat nocturnal intake; and WASO and caloric, and fat nocturnal intake. In women, negative associations were found between sleep efficiency and caloric, carbohydrate, and fat nocturnal intake, and REM sleep and nocturnal fat intake.
Table 2.
| Variable | Nocturnal Intake (dinner + late-night snack) |
||||
|---|---|---|---|---|---|
| Calories (kcal) | Protein (g) | Carbohydrate (g) | Fat (g) | ||
| Total sleep time (min) | β value | −0.01 | −0.03 | 0.03 | −0.72 |
| p value | 0.67 | 0.95 | 0.82 | 0.18 | |
| Sleep efficiency (%) | β value | −0.004 | −0.06 | −0.01 | −0.17 |
| p value | 0.09 | 0.34 | 0.73 | 0.004 | |
| Sleep latency (min) | β value | 0.01 | 0.18 | 0.03 | 0.21 |
| p value | 0.06 | 0.11 | 0.33 | 0.07 | |
| REM latency (min) | β value | 0.03 | 0.69 | 0.06 | 1.25 |
| p value | 0.03 | 0.06 | 0.51 | 0.001 | |
| N1 (%) | β value | −3.00 | 0.02 | −0.00 | 0.01 |
| p value | 0.96 | 0.19 | 0.39 | 0.44 | |
| N2 (%) | β value | 0.003 | 0.04 | 0.01 | 0.13 |
| p value | 0.12 | 0.51 | 0.36 | 0.03 | |
| N3 (%) | β value | 0.00 | 0.00 | 0.00 | −0.02 |
| p value | 0.91 | 0.94 | 0.90 | 0.69 | |
| REM (%) | β value | −0.00 | −0.06 | −0.01 | −0.12 |
| p value | 0.04 | 0.11 | 0.25 | 0.002 | |
| WASO (min) | β value | 0.01 | 0.12 | −0.00 | 0.55 |
| p value | 0.21 | 0.56 | 0.99 | 0.01 | |
Results are presented as adjusted estimated β values with p values.
β values are adjusted for age, gender, economical status and BMI. REM, rapid eye movement; WASO, wake after sleep onset.
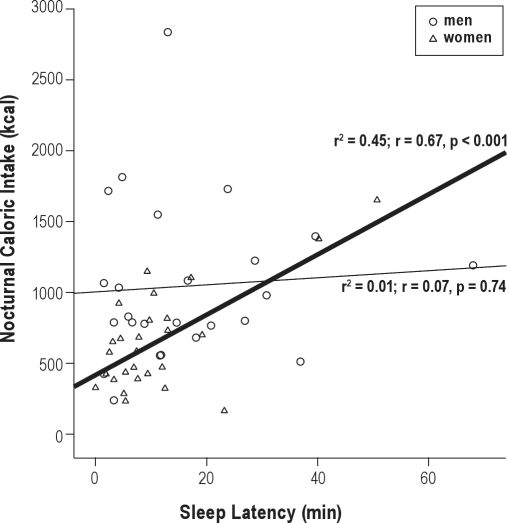
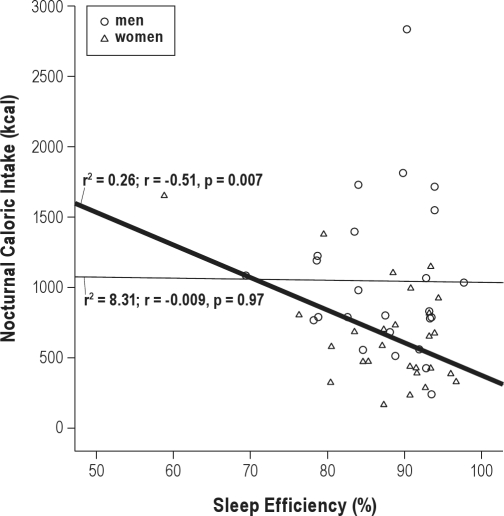
When analyzing the influence of each meal on the sleep variables, we observed that caloric intake in a night (represented as percent of total caloric intake, %) correlated positively with sleep latency in women (r2 = 0.45; r = 0.67, p < 0.001) (n = 15) but not in men (Figure 1). Indeed, caloric intake in a night (%) correlated negatively with sleep efficiency in women (r2 = 0.26; r = −0.51, p = 0.007) (n = 15) (Figure 2) but not in men.
Figure 1.
Relationship between nocturnal caloric intake and sleep latency
Circles represent men and triangles represent women. Linear regression and Pearson's correlation coefficient are shown in each gender best fitting line: men, thinner line; women, thicker line.
Figure 2.
Relationship between nocturnal caloric intake and sleep efficiency
Circles represent men and triangles represent women. Linear regression and Pearson's correlation coefficient are shown in each gender best fitting line: men, thinner line; women, thicker line.
DISCUSSION
This study demonstrated that food intake, mainly in the evening period, is correlated with several variables related to sleep patterns (sleep efficiency, sleep latency, N2 sleep, REM sleep latency, REM percentage, and WASO). These results indicate that a higher food intake close to the sleeping period is associated with negative aspects of sleep patterns in healthy individuals, especially in women. Studies have shown pronounced gender differences in the occurrence of sleep disorders.26,27 It has been suggested these differences in sleep, while subtle under baseline conditions, may increase in magnitude under biological or environmental challenges.26,27 However, the role of food intake pattern in these differences is unknown, and further research should examine this relationship.
There is evidence that an adequate sleep pattern acts to protect against a series of nutritional and metabolic disorders such as obesity,28,29 dyslipidemia,16 diabetes, and insulin resistance.30,31 These disorders probably occur because sleep plays an important role in the metabolic control of the body.10,11 Therefore, alterations in the quantity and quality of sleep lead to hormonal disturbances; these might be capable of altering the physiological homeostasis and consequently generate diseases.10,11
According to our results, high caloric food intake preceding the sleeping period was correlated with greater sleep latency in women (nocturnal caloric intake correlated positively with sleep latency). In general, this meal took place between 30 and 60 minutes before the volunteers went to bed. Contrary to previous studies,18,19 our results suggest that intake of high fat and carbohydrate foods preceding the sleeping period are associated with higher sleep latency. Other studies19,20 have demonstrated that individuals who ingested a high-glycemic index, carbohydrate-rich meal 4 hours prior to sleep presented a decrease in sleep latency. In addition to the amount of carbohydrates, the glycemic index may have an important influence on sleep patterns, especially in inducing sleepiness. Different types of fat have also been shown to influence sleep. Grandner et al. found that actigraphic nocturnal sleep duration was negatively associated with total fat, monounsaturated fat, trans fat, saturated fat, and polyunsaturated fat, and that higher intakes of fat were associated with less sleep and subjective napping.32
Another factor that could have influenced our findings is that increased gastric volume leads to greater physical discomfort and thus impairs the consolidation of sleep onset. It has been demonstrated that sleep decreases the activity of the digestive tract,33 which may have an overloaded activity during the night if food intake is excessive.
Data from this study suggest that individuals who consume meals with increased fat content in the nocturnal period seem predisposed to have decreased REM sleep. Indeed, previous studies have shown that individuals who ingested a fatty meal felt sleepier.34,35 However, there are no studies associating food intake and sleep stages; thus a comparison with our results is not possible.
One issue to consider is the possibility that nocturnal food intake leads to deterioration in sleep patterns and can cause metabolic and nutritional problems. It is widely documented in the literature that changes in sleep patterns can alter the sensations of hunger and appetite and consequently lead to increased body mass.10,11 On the other hand, we showed recently that fat intake at night is associated with higher values for body fat percentage, body mass index, and waist circumference.36 This leads us to believe that the mechanism by which nocturnal food intake may trigger an increase in body mass can be modulated by sleep.
Further studies in this area should explore the effect of energy density (the amount of energy in a given weight of food), not just the calorie content, on the sleep patterns of both genders individuals. Studies have indicated that people tend to consume a fairly consistent weight of food over the course of a few days; therefore, the consumption of low-energy-dense foods that contain less energy per gram may decrease overall energy intake.37 However, the real effect of energy density on sleep patterns is unclear. Consuming water- and fiber-rich foods such as fruit and vegetables is one strategy for decreasing the energy density of the diet.38 It would be of great interest to understand whether this strategy would promote an effect on sleep patterns.
This study indicates that food intake, primarily preceding sleep, seems to exert a negative influence on sleep quality. However, our research had some limitations, such as the small number of participants. Therefore, more studies are needed, on a larger sample and wider range of individuals; evidence from older people is also required because age may affect sleep and nutrition patterns.There is also prominent response-set bias evident in the answers to methods used to investigate food intake. Volunteers may alter their diet records due to social desirability (the tendency to respond in such a way as to avoid criticism) and social approval (the tendency to seek praise).39 Indeed, this is an area poorly explored in the literature, and more studies are necessary to elucidate the real influence of food intake upon sleep.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
This study was supported by the AFIP, The Sleep Institute, CEDIP/FAPESP (#998/14303-3), CEPE, UNIFESP, FADA, CAPES, CEMSA and CNPQ. The authors thank all volunteers and researchers involved in this study.
REFERENCES
- 1.Rokholm B, Baker JL, Sørensen TI. The levelling off of the obesity epidemic since the year 1999 - a review of evidence and perspectives. Obes Rev. 2010;11:835–46. doi: 10.1111/j.1467-789X.2010.00810.x. [DOI] [PubMed] [Google Scholar]
- 2.Duffey KJ, Gordon-Larsen P, Jacobs DR, Jr, Williams OD, Popkin BM. Differential associations of fast food and restaurant food consumption with 3-y change in body mass index: the Coronary Artery Risk Development in Young Adults Study. Am J Clin Nutr. 2007;85:201–8. doi: 10.1093/ajcn/85.1.201. [DOI] [PubMed] [Google Scholar]
- 3.Waller CE, Du S, Popkin BM. Patterns of overweight, inactivity, and snacking in Chinese children. Obes Res. 2003;11:957–61. doi: 10.1038/oby.2003.132. [DOI] [PubMed] [Google Scholar]
- 4.Campión J, Milagro F, Martínez JA. Epigenetics and obesity. Prog Mol Biol Transl Sci. 2010;94:291–347. doi: 10.1016/B978-0-12-375003-7.00011-X. [DOI] [PubMed] [Google Scholar]
- 5.Herrera BM, Lindgren CM. The genetics of obesity. Curr Diab Rep. 2010;10:498–505. doi: 10.1007/s11892-010-0153-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Flatt JP. Differences in basal energy expenditure and obesity. Obesity. 2007;15:2546–8. doi: 10.1038/oby.2007.304. [DOI] [PubMed] [Google Scholar]
- 7.Bose M, Oliván B, Laferrère B. Stress and obesity: the role of the hypothalamic-pituitary-adrenal axis in metabolic disease. Curr Opin Endocrinol Diabetes Obes. 2009;16:340–6. doi: 10.1097/MED.0b013e32832fa137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bjorvatn B, Sagen IM, Oyane N, et al. The association between sleep duration, body mass index and metabolic measures in the Hordaland Health Study. J Sleep Res. 2007;16:66–76. doi: 10.1111/j.1365-2869.2007.00569.x. [DOI] [PubMed] [Google Scholar]
- 9.Hasler G, Buysse DJ, Klaghofer R, et al. The association between short sleep duration and obesity in young adults: a 13-year prospective study. Sleep. 2004;27:661–6. doi: 10.1093/sleep/27.4.661. [DOI] [PubMed] [Google Scholar]
- 10.Spiegel K, Tasali E, Penev P, Van Cauter E. Brief communication: sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann Intern Med. 2004;141:846–50. doi: 10.7326/0003-4819-141-11-200412070-00008. [DOI] [PubMed] [Google Scholar]
- 11.Taheri S, Lin L, Austin D, Young T, Mignot E. Short sleep duration is associated with reduced leptin, elevated ghrelin, and increased body mass index. PLoS Med. 2004;1:210–7. doi: 10.1371/journal.pmed.0010062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.National Sleep Foundation. 2002 “Sleep in America” Poll. Washington, DC: National Sleep Foundation; 2002. [Google Scholar]
- 13.Holmback U, Forslund A, Forslund J, et al. Metabolic responses to nocturnal eating in men are affected by sources of dietary energy. J Nutr. 2002;132:1892–9. doi: 10.1093/jn/132.7.1892. [DOI] [PubMed] [Google Scholar]
- 14.Spiegel K, Knutson K, Leproult R, Tasali E, Van Cauter E. Sleep loss: a novel risk factor for insulin resistance and type 2 diabetes. J Appl Physiol. 2005;99:2008–19. doi: 10.1152/japplphysiol.00660.2005. [DOI] [PubMed] [Google Scholar]
- 15.Van Cauter E, Polonsky KS, Scheen AJ. Roles of circadian rhythmicity and sleep in human glucose regulation. Endocrine Rev. 1997;18:716–38. doi: 10.1210/edrv.18.5.0317. [DOI] [PubMed] [Google Scholar]
- 16.Knutsson A. Relationships between serum triglycerides and g-glutamyltransferase among shift and day workers. J Intern Med. 1989;226:337–9. doi: 10.1111/j.1365-2796.1989.tb01405.x. [DOI] [PubMed] [Google Scholar]
- 17.Afaghi A, O'Connor H, Chow CM. High-glycemic-index carbohydrate meals shorten sleep onset. Am J Clin Nutr. 2007;85:426–30. doi: 10.1093/ajcn/85.2.426. [DOI] [PubMed] [Google Scholar]
- 18.Barclay L. High-glycemic-index carbohydrate meals may shorten sleep onset. Am J Clin Nutr. 2007;85:426–30. doi: 10.1093/ajcn/85.2.426. [DOI] [PubMed] [Google Scholar]
- 19.Driver HS, Shulman I, Baker FC, Buffenstein R. Energy content of the evening meal alters nocturnal body temperature but not sleep. Physiol Behav. 1999;68:17–23. doi: 10.1016/s0031-9384(99)00145-6. [DOI] [PubMed] [Google Scholar]
- 20.American Academy of Sleep Medicine. Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. Sleep. 1999;22:667–89. [PubMed] [Google Scholar]
- 21.American Sleep Disorders Association. Recording and scoring leg movements - The atlas task force. Sleep. 1993;16:748–59. [PubMed] [Google Scholar]
- 22.Jasper HH. The ten-twenty electrode system of the international federation. Electroencephalogr Clin Neurophysiol. 1958;10:371–5. [PubMed] [Google Scholar]
- 23.Iber C, Ancoli-Israel S, Chesson A, Jr, Quan S. American Academy of Sleep Medicine: Westchester, IL; 2007. The AASM manual for the scoring of sleep and associated events: rules, terminology, and technical specification, 1st. [Google Scholar]
- 24.Carskadon MA, Dement WC. Krieger MH, Roth T, Dement WC. Principles and practice of sleep medicine. 4th edition. Philadelphia: WB Saunders; 2005. Normal human sleep: an overview; pp. 13–23. [Google Scholar]
- 25.Lohman TG, Roche AF, Martorell R. Anthropometric standardization reference manual. Champaign, IL: Human Kinetics; 1988. [Google Scholar]
- 26.Armitage R, Hoffmann RF. Sleep EEG, depression and gender. Sleep Med Rev. 2001;5:237–46. doi: 10.1053/smrv.2000.0144. [DOI] [PubMed] [Google Scholar]
- 27.Dzaja A, Arber S, Hislop J, et al. Women's sleep in health and disease. J Psychiatr Res. 2005;39:55–76. doi: 10.1016/j.jpsychires.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 28.Hasler G, Buysse DJ, Klaghofer R, et al. The association between short sleep duration and obesity in young adults: a 13-year prospective study. Sleep. 2004;27:661–6. doi: 10.1093/sleep/27.4.661. [DOI] [PubMed] [Google Scholar]
- 29.Vorona R, Winn M, Babineau T, Eng B, Feldman H, Ware J. Overweight and obese patients in a primary care population report less sleep than patients with a normal body mass index. Arch Intern Med. 2005;165:25–30. doi: 10.1001/archinte.165.1.25. [DOI] [PubMed] [Google Scholar]
- 30.Mikuni E, Ohoshi T, Hayashi K, et al. Glucose intolerance in an employed population. Tohoku J Exp Med. 1983;141(suppl):251–6. doi: 10.1620/tjem.141.suppl_251. [DOI] [PubMed] [Google Scholar]
- 31.Padilha, HG, Crispim, CA, Zimberg, IZ, Folkard S, Tufik S, de Mello MT. Metabolic Responses on the Early Shift. Chronobiol Int. 2010;27:1–13. doi: 10.3109/07420528.2010.489883. [DOI] [PubMed] [Google Scholar]
- 32.Grandner MA, Kripke DF, Naidoo N, Langer RD. Relationships among dietary nutrients and subjective sleep, objective sleep, and napping in women. Sleep Med. 2010;11:180–4. doi: 10.1016/j.sleep.2009.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dantas RO, Aben-Athar CG. Aspectos dos efeitos do sono no aparelho digestório. Arq Gastroenterol. 2002;39:55–9. doi: 10.1590/s0004-28032002000100010. [DOI] [PubMed] [Google Scholar]
- 34.Spring B, Maller O, Wurtman J, Digman L, Cozolino L. Effects of protein and carbohydrate meals on mood and performance: interactions with sex and age. J Psychiatr Res. 1983;17:155–67. doi: 10.1016/0022-3956(82)90017-6. [DOI] [PubMed] [Google Scholar]
- 35.Spring B. Recent research on the behavioral effects of tryptophan and carbohydrate. Nutr Health. 1984;3:55–68. doi: 10.1177/026010608400300204. [DOI] [PubMed] [Google Scholar]
- 36.Dattilo M, Crispim CA, Zimberg IZ, Tufik S, de Mello MT. Meal distribution across the day and its relationship with body composition. Biol Rhythm Res. 2011;42:119–29. [Google Scholar]
- 37.Bell EA, Rolls BJ. Energy density of foods affects energy intake across multiple levels of fat content in lean and obese women. Am J Clin Nutr. 2001;73:1010–8. doi: 10.1093/ajcn/73.6.1010. [DOI] [PubMed] [Google Scholar]
- 38.Rolls BJ, Drewnowski A, Ledikwe JH. Changing the energy density of the diet as a strategy for weight management. J Am Diet Assoc. 2005;105:98–103. doi: 10.1016/j.jada.2005.02.033. [DOI] [PubMed] [Google Scholar]
- 39.Hebert JR, Ma Y, Clemow L, et al. Gender differences in social desirability and social approval bias in dietary self-report. Am J Epidemiol. 1997;146:1046–55. doi: 10.1093/oxfordjournals.aje.a009233. [DOI] [PubMed] [Google Scholar]