Abstract
The etiology of neurodegenerative disorders like Parkinson’s disease remains unknown, although many genetic and environmental factors are suggested as likely causes. Neuronal oxidative stress and mitochondrial dysfunction have been implicated as possible triggers for the onset and progression of Parkinson’s neurodegeneration. We have recently shown that long-term treadmill exercise prevented neurological, mitochondrial and locomotor deficits in a chronic 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine and probenecid-induced mouse model of Parkinson’s disease that was originally established in our laboratory. In the present study, we further demonstrated that long-term exercise attenuated both cytochrome c release and elevated levels of p53, which are known to be associated with mitochondrial dysfunction in the striatum of this chronic model. On the other hand, the expressions of mitochondrial transcription factor A and peroxisome proliferator-activated receptor gamma coactivator 1α were unexpectedly upregulated in the striatum of this chronic model, but long-term exercise training brought their levels down closer to normal. Our findings suggest that maintaining normal mitochondrial function is essential for preventing the process of Parkinson’s disease-like neurodegeneration, whereas stimulating the mitochondrial transcription factors for biogenesis is not obligatory.
Keywords: chronic MPTP model, cytochrome c, mitochondrial DNA defect, neurodegeneration, p53, Parkinson’s disease, PGC-1α, TFAM, treadmill exercise
INTRODUCTION
Sporadic Parkinson’s disease (PD) is characterized by a progressive degeneration of nigrostriatal neurons and loss of dopaminergic transmission. The causes and mechanisms underlying the neurodegenerative process in PD remain elusive. Much evidence suggests a role for mitochondrial dysfunction in the pathogenesis of PD. Mitochondrial disorders could be brought on by chronic exposure to neurotoxic xenobiotics or by inherent or induced defects of mitochondrial DNA (mtDNA).
A clear link between mitochondrial dysfunction and PD has been demonstrated in animal models of PD with neurotoxic agents such as 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) and rotenone that inhibit mitochondrial NADH dehydrogenase (complex I) and increase the production of cytotoxic reactive oxygen species (ROS) [4, 12]. Complex I deficiency has been detected in tissues derived from PD patients [28]. We have reported that parkinsonian symptoms and mitochondrial dysfunction can be concomitantly demonstrated in an aged, chronic MPTP/probenecid-induced mouse model of PD (MPD), which provides a suitable experimental replica for concurrently studying neurological and mitochondrial deficits relevant to PD [14, 23].
In addition, a number of studies suggest that point mutations of mtDNA also contribute to mitochondrial respiratory chain deficiency and neuronal cell death associated with PD pathogenesis [3, 11]. Deletion or downregulation of mitochondrial target genes that are essential for upholding mitochondrial biogenesis, such as mitochondrial transcription factor A (TFAM) and peroxisome proliferator-activated receptor gamma coactivator 1α (PGC-1α), has led to mitochondrial dysfunction and contributed to PD-associated loss of DA neurons [7, 10].
Based on experimental and clinical investigations, there is growing evidence implicating that exercise and routine physical activities could potentially reduce the risk of further neurological impairment due to stroke, PD and other degenerative diseases [19, 29]. In a recent study [14], we detected that 18 weeks of treadmill exercise effectively reduced MPTP-induced protein oxidation, mitochondrial dysfunction, loss of dopaminergic neurons and transmission, and locomotor impairment in the chronic MPD, suggesting that long-term endurance exercise is neuronal and mitochondrial protective (see summary in Table 1). While most studies involving exercise on the regulation and expression of mitochondrial gene transcription factors are carried out in tissues with high metabolic activity, such as brown fat, heart and skeletal muscle, similar studies in brain tissue are limited. In this follow-up study, we investigated the impact of long-term exercise on selected mtDNA gene expression in the striatum of the chronic MPD.
Table 1.
Impact of exercise on protection against nigrostriatal neuronal, mitochondrial, and locomotor deficits in the moderate chronic mouse model of Parkinson’s disease (MPD) [14].
| Deficit Parameter | Sedentary MPD (% of control) | Exercised MPD (% of control) |
|---|---|---|
| Neuronal | ||
| Number of substantia nigra pars compacta tyrosine hydroxylase-positive neurons | Moderate loss (−61%) | Significantly less loss (−12%) |
| Substantia nigra tyrosine hydroxylase-immunoreactivity | Moderate loss (−55%) | Significantly less loss (−18%) |
| Striatal dopamine content | Moderate depletion (−60%) | Less depletion (−35%) |
| Striatal dopamine uptake transporter level | Moderate loss (−35%) | Less loss (−15%) |
| Mitochondrial | ||
| State 3 respiration | Moderate inhibition (−46%) | Normal (−3%) |
| State 4 respiration | Mild inhibition (−26%) | Normal (+3%) |
| ATP production | Moderate inhibition (−47%) | Less inhibition (−22%) |
| Mn superoxide dismutase level | Moderate depletion (−48%) | Less depletion (−17%) |
| Cu-Zn superoxide dismutase level | Mild depletion (−27%) | Near normal (−5%) |
| Locomotor | ||
| Balance beam performance | More foot-slip errors (+31%) | Less foot-slip errors (−35%) |
| Balance beam task latency | Longer latency (+94%) | Near normal latency (+20%) |
MATERIALS AND METHODS
Animals
Male C57BL/6 mice (6–10 months old, weighing 35–40 g) (Harlan Sprague Dawley, Inc., Indianapolis, IN, USA) were housed in single cages with food pellets and water available ad libitum. All animal treatments were carried out strictly in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80-23, revised 1996) and were approved by the Institutional Animal Care and Use Committee from the University of Houston. A total of 42 mice were used in the present study.
Chronic mouse model of Parkinson’s Disease
To prepare the chronic MPD with moderate neurodegeneration, mice were injected with a total of 10 doses of MPTP hydrochloride (15 mg/kg/injection in saline, s.c.) in combination with an adjuvant drug, probenecid (250 mg/kg/injection dissolved in dimethyl sulfoxide, i.p.) on a 5-week schedule, which was originally established in our laboratory [15]. Age-matched, control mice in this study were treated with probenecid only. Probenecid was used to inhibit the rapid clearance and excretion of MPTP and its metabolites from the brain and kidney. Alone, it did not produce any significant neurotoxic effect, but in combination it potentiated the neurotoxicity of MPTP [13].
Treadmill exercise in mice
A six-lane motorized rodent treadmill (Columbus Instruments, Columbus, OH, USA) was utilized for exercise training. For 1 week before, 5 weeks during, and 12 weeks after the completion of chronic MPTP/probenecid treatment (a total of 18 weeks), the exercised group of animals was trained on the treadmill running at 5 days/week, 40 min/day with a speed up to 15 m/min (5 min at 6 m/min, 5 min at 9 m/min, 20 min at 12 m/min, 5 min at 15 m/min, and 5 min at 12 m/min) with 0º of inclination as previously described [1, 14]. All biochemical measurements were performed with isolated striata at least 48 hours after the last session of exercise training to avoid any data misinterpretation due to acute exercise residual effects.
Analysis of striatal cytochrome c
The striatal content of cytochrome c was determined by Western blot analysis using glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as loading control according to the procedures as previously described [14, 23]. A monoclonal mouse anti-cytochrome c (1:750, Chemicon, Temecula, CA, USA) and a monoclonal mouse anti-GAPDH (1:3000, Chemicon) were used as primary antibodies. A goat anti-mouse IgG (1:2000, Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA) was used as a secondary antibody.
RNA extraction and quantitative real-time polymerase chain reaction (qRT-PCR)
Total RNA was extracted from the striatal tissue using the RNeasy Mini Kit (Qiagen Inc., Valencia, CA, USA) according to the manufacturer’s instructions. The integrity of isolated RNA was checked by formaldehyde agarose gel electrophoresis before use. The RNA concentration was determined with a U-2910 double beam spectrophotometer at an absorbance of 260 nm (Hitachi High-Tech, Schaumburg, IL, USA).
Total RNA (1 μg) was reverse transcribed using random hexamers and a multi-scribe reverse transcriptase supplied in the iScript cDNA synthesis kit according to the manufacturer’s instructions (Bio-Rad Laboratories, Hercules, CA, USA). The gene sequence was obtained from the GenBank, and primers for qRT-PCR were designed to span the intron/exon junctions with the Primer Express software (Applied Biosystems, Foster City, CA, USA) to minimize any amplification of residual genomic DNA. The primer sequences for mouse p53, TFAM, PGC-1α, and GAPDH are listed in Table 2. Quantitative RT-PCR was performed using the SYBR Green dye binding method on an ABI Prism 7300 sequence detection system (Applied Biosystems). Thermal cycling conditions included pre-incubation at 50°C for 2 min, DNA polymerase activation at 95°C for 1 min, and 40 PCR cycles for 15 s at 95°C and for 1 min at 60°C. The transcript level for each selected gene sequence was calculated at a cycle threshold value at which each fluorescent signal was first detected above the background. The amplification for each selected gene was normalized relative to the expression of GAPDH, which was used as a reference.
Table 2.
List of primers used for quantitative real time PCR (qRT-PCR)
| Gene Name | Gene ID # | Forward Primer | Reverse Primer |
|---|---|---|---|
| p53 | NM_011640.3 | 5′-TTAAAAGAGTGCGCCGATAGG-3′ | 5′-GAATGCGTTAAGCAAGGGAAT-3′ |
| TFAM | NM_009360.4 | 5′-GCACCCTGCAGAGTGTTCAA-3′ | 5′-CGCCCAGGCCTCTACCTT-3′ |
| PGC-1α | NM_008904.2 | 5′-TGCGGGATGATGGAGACA-3′ | 5′-GCGAAAGCGTCACAGGTGTA-3′ |
| GAPDH | NM_008084.2 | 5′-CTCATGACCACAGTCCATGC-3′ | 5′-CACATTGGGGGTAGGAACAC-3′ |
Statistical Analysis
Statistical comparisons of values between two animal groups were carried out by unpaired Student’s t-test, and multiple group comparisons were conducted by one-way analysis of variance (ANOVA) with Tukey’s post-hoc test with the Prism software (GraphPad Software, Inc., La Jolla, CA, USA). Data are represented as mean ± S.E.M. In all cases, a P value of <0.05 was considered to be significantly different.
RESULTS
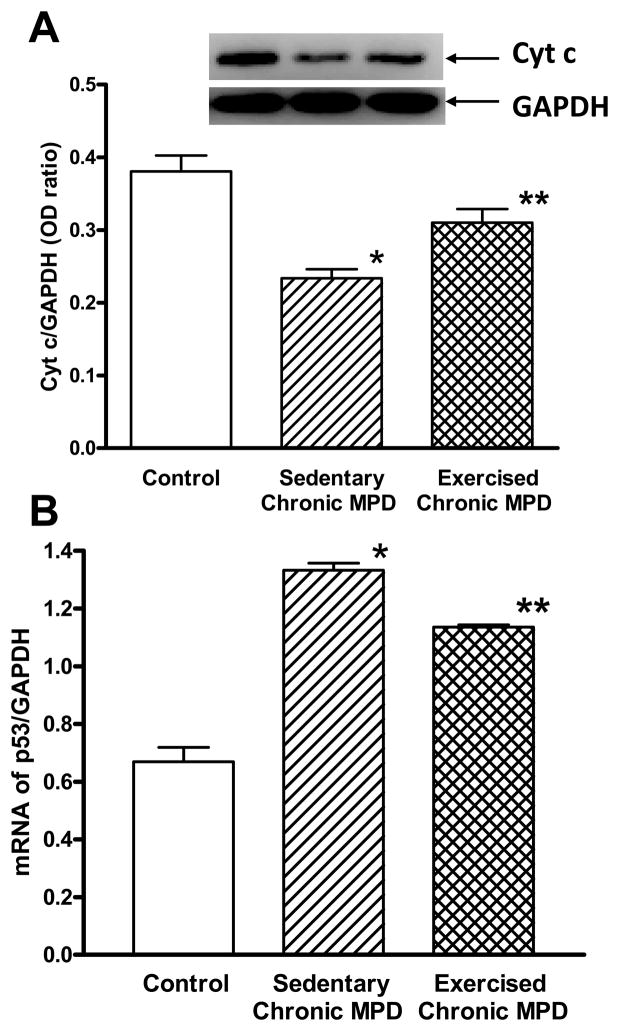
Cytochrome c is an essential component of the electron transport chain associated with the inner membrane of the mitochondria. Its release is believed to trigger apoptotic cell death in neurodegenerative disorders [8]. In this study, the sedentary chronic MPD exhibited a significant loss of striatal cytochrome c levels when compared with the normal control animals (Fig. 1A). After the chronic MPD were exercised for 18 weeks, the striatal cytochrome c level was partially depleted when compared with the control mice but to a lesser extent when compared with the sedentary chronic MPD (Fig. 1A). A significant difference in cytochrome c levels due to exercise was also evident when the interaction between all three experimental groups was analyzed by a one-way ANOVA (F2,15 = 20.25, P < 0.0001). These data indicate that persistent release of mitochondrial cytochrome c in the striatum of the chronic MPD can be demonstrated 12 weeks following repetitive MPTP/probenecid treatment. Long-term treadmill exercise in the chronic MPD reduces the loss of mitochondrial cytochrome c level, thus it could retain some antioxidant protection against neuronal loss.
Figure 1. Effect of 18 weeks of exercise on striatal cytochrome c and p53 levels in the chronic MPD.
(A) The level of striatal cytochrome c was measured by Western immunoblotting using a GAPDH loading control. In sedentary chronic MPD, the striatal cytochrome c level was considerably lower than the control animals (*P<0.0001). After the chronic MPD were exercised for 18 weeks, the level of cytochrome c in the striatum of chronic MPD was partially but significantly retained (**P=0.041 when compared with the control animals, **P=0.0043 when compared with the sedentary chronic MPD). N=5–8 per group. (B) The level of striatal p53 mRNA was measured by qRT-PCR and expressed over that of GAPDH. In the sedentary chronic MPD, the striatal p53 gene expression was markedly higher than the control animals (*P<0.0001). In chronic MPD that had been exercised for 18 weeks, the striatal p53 mRNA expression was still higher when compared with the control animals (**P<0.0001), but it was significantly lower than the sedentary chronic MPD (**P<0.0001). N=6 per group. Statistical comparisons were performed with unpaired Student’s t-test.
Originally identified as a tumor suppressor gene, p53 is also known to play a role in neuronal cell death [5]. Its activation through mitochondrial release of cytochrome c is indicative of cellular oxidative stress or DNA damage [18]. Here, we demonstrated that striatal p53 mRNA was considerably elevated in the sedentary chronic MPD (Fig. 1B). Its expression in exercised chronic MPD was significantly lower than the sedentary counterparts, although its level was still significantly higher than the control mice (Fig. 1B). A significant difference in p53 mRNA levels due to exercise was also shown when the interaction between all three experimental groups was analyzed by a one-way ANOVA (F2,15 = 112.7, P < 0.0001). These results suggest that p53 activation is associated with neurodegenerative process in the chronic MPD, and 18 weeks of exercise partially attenuates p53 levels suggesting a condition of lesser DNA damage.
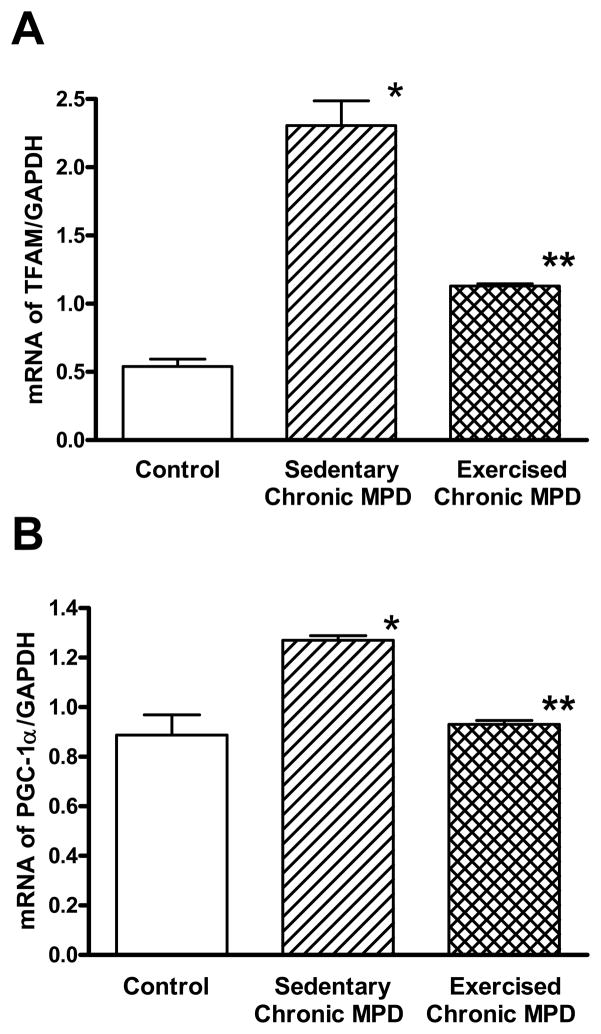
TFAM and PGC-1α are respectively considered as an important transcription factor and a coactivator for maintaining mitochondrial homeostasis and biogenesis [27]. However, our results were contrary to our initial expectation. We observed that striatal expression of TFAM (Fig. 2A) and PGC-1α (Fig. 2B) mRNAs were elevated in the sedentary chronic MPD. The increase of TFAM level was apparently more robust than that of PGC-1α. After 18 weeks of exercise, although the striatal TFAM mRNA was still significantly higher than the control animals, its level was substantially lower than that of the sedentary chronic MPD group (Fig. 2A). A significant difference in TFAM mRNA due to exercise was also demonstrated when the interaction between all three experimental groups was analyzed by a one-way ANOVA (F2,15 = 67.42, P < 0.0001). On the other hand, the PGC-1α mRNA level in the exercised chronic MPD was equivalent to the control group and was significantly lower than the sedentary chronic MPD group (Fig. 2B). A significant difference in PGC-1α mRNA due to exercise was also confirmed when the interaction between all three experimental groups was analyzed by a one-way ANOVA (F2,15 = 18.33, P < 0.0001). These findings indicate that TFAM and PGC-1α are upregulated in response to repetitive MPTP/probenecid treatment, whereas their levels approach normal values when the chronic MPD is exercise-trained for 18 weeks.
Figure 2. Effect of 18 weeks of exercise on striatal TFAM and PGC-1α mRNA expression in the chronic MPD.
The mRNA expressions of TFAM (A) and PGC-1α (B) in the striatum were measured by qRT-PCR using GAPDH as a reference. In the sedentary chronic MPD, the striatal TFAM and PGC-1α mRNA levels were significantly elevated (A, *P<0.0001; B, *P=0.001, respectively) when compared with the level in the control animals. Following 18 weeks of treadmill exercise, although the TFAM mRNA level in the striatum of chronic MPD was still higher than the control animals (A, **P<0.0001), it was substantially lower than that in the sedentary chronic MPD (A, **P<0.0001). The striatal PGC-1α mRNA level in the exercised chronic MPD was normal when compared with the control animals (B, **P=0.615) but it was significantly lower than that in the sedentary chronic MPD (B, **P<0.0001). N=6 per group. Statistical comparisons were performed with unpaired Student’s t-test.
DISCUSSION
The chronic MPD used in this study was originally developed and has been well characterized in our laboratory. In contrast to the most commonly used acute and subacute MPTP mouse models of PD, in which neurological and behavioral deficits are short-lived and spontaneously reversed soon after treatment, the chronic MPD has long-term neurological deficits showing many features resembling PD lasting for at least 6 months, long after MPTP has completely dissipated systemically from the animal [13]. The observed phenotypic features in the chronic MPD include marked depletion of DA content and terminal DA uptake in association with significant behavioral deficits and loss of DA cells in the substantia nigra pars compacta (SNpc) [15, 24, 26]. Early neuronal apoptosis and delayed appearance of α-synuclein-positive inclusion bodies along with ultrastructural neuronal damage in the SNpc have also been demonstrated [17, 21].
The mechanisms underlying the progressive nigrostriatal neurodegeneration in PD are not fully understood, although ROS-induced neuronal oxidative stress, mitochondrial dysfunction, neuronal apoptosis due to p53 accumulation, and mitochondrial cytochrome c release have been demonstrated in acute in vivo and in vitro experimental models of PD [6, 20]. Recently, we have demonstrated a persistent loss of SNpc neurons and striatal DA functions in association with mitochondrial and behavioral deficits in the chronic MPD, suggesting an intimate relationship between neurodegeneration and mitochondrial dysfunction [14, 23]. This finding is further supported by our discoveries showing concurrent neurological, mitochondrial and behavioral protection following long-term exercise or melatonin treatment in the chronic MPD [14, 22]. In this report, our results not only validated the mechanistic role of p53 activation and cytochrome c release involved in a chronic in vivo experimental model of PD, they also implied the protective role of exercise in attenuating the defective p53 and cytochrome c systems leading to a reduction of neuronal, mitochondrial and locomotor deficits as previously seen in the chronic MPD [14].
The proposed importance of mtDNA transcription factor TFAM and coactivator PGC-1α in promoting mitochondrial biogenesis and neuronal protection against oxidative stress in PD has been supported mainly by results obtained from specific gene-manipulated animal models. Deletion of TFAM and PGC-1α genes makes these animals more susceptible to MPTP neurotoxicity and more easily inducible to generate PD-like symptoms [7, 30]. It has been postulated that monitoring these mitochondrial gene activities could serve as diagnostic tools, and boosting mitochondrial bioenergetics through the enhancement of these transcription factors could be potential targets for preventing or treating PD [2]. Unfortunately, there is no evidence based on our current study that impairment of TFAM and PGC-1α exists in a chronic non-genetic mouse model of PD. In contrast, the chronic MPD exhibits sustained and upregulated levels of TFAM and PGC-1α even 12 weeks after the PD disorder is induced. This indicates an ongoing high activity of the mitochondrial transcription factor and coactivator that perhaps attempts to compensate for the loss of mitochondrial function and promote its biogenesis for repairing the cellular damage. Our observations further suggest that simply escalating TFAM and PGC-1α gene expression is not sufficient to rescue the ongoing elevation of p53, release of cytochrome c, and impaired mitochondrial and neurobehavioral functions in the chronic MPD. Therefore, the outcome of this study does not appear to support a therapeutic approach for treating PD by targeting TFAM or PGC-1α gene regulation.
Much of the published effects of exercise on TFAM and PGC-1α in activating mitochondrial biogenesis are carried out in the skeletal muscle, in which the transcriptional regulation tends to be transient and not mandatory for producing exercise-induced adaptations [9, 16, 25]. We report here, in the striatal region of the brain, TFAM and PGC-1α are upregulated in response to repeated oxidative insults of MPTP in a manner that may try to compensate for mitochondrial dysfunction, but apparently they are not required to be kept high for maintaining normal mitochondrial function following long-term exercise. Thus, when the chronic MPD is exercised and oxidative stress is reduced, mitochondrial function returns to normal, and these transcription factors do not need to be constantly stimulated.
In summary, in a chronic mouse model of Parkinson’s disease, we have previously reported that long-term treadmill exercise protects against neurological, mitochondrial and locomotor deficits. We now additionally report that exercise reduces cytochrome c release and p53 elevation, which are associated with mitochondrial dysfunction in the striatum of this chronic model. On the other hand, the transcriptional activities of TFAM and PGC-1α are unexpectedly upregulated in the striatum of this chronic model, and exercise training brings their levels down closer to normal ranges. Therefore, our research suggests that maintaining normal mitochondrial function is essential for preventing the process of PD-like neurodegeneration, whereas stimulating the mitochondrial transcription factors for biogenesis is not obligatory.
Highlights.
Cytochrome c is depleted and p53 level is elevated in chronic Parkinson’s mice.
Long-term exercise prevents cytochrome c release and P53 elevation.
TFAM and PGC-1α transcriptional activities are upregulated in Parkinson’s mice.
Long-term exercise brings TFAM and PGC-1α levels down closer to normal ranges.
Exercise reduces mitochondrial damage and prevents neurodegenerative process.
Acknowledgments
This work was supported by a grant from the US National Institute of Neurological Disorders and Stroke (NS 47920 to Y.S.L.). The authors thank Mr. Hubert Lau for providing constructive suggestions and editorial assistance in the preparation of this manuscript.
Abbreviations used
- ANOVA
analysis of variance
- DA
dopamine
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- MPD
mouse model of Parkinson’s disease
- MPTP
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- mtDNA
mitochondrial DNA
- PD
Parkinson’s disease
- PGC-1α
peroxisome proliferator-activated receptor gamma coactivator 1α
- ROS
reactive oxygen species
- SNpc
substantia nigra pars compacta
- TFAM
mitochondrial transcription factor A
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Al-Jarrah M, Pothakos K, Novikova L, Smirnova IV, Kurz MJ, Stehno-Bittel L, Lau YS. Endurance exercise promotes cardiorespiratory rehabilitation without neurorestoration in the chronic mouse model of parkinsonism with severe neurodegeneration. Neuroscience. 2007;149:28–37. doi: 10.1016/j.neuroscience.2007.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beal MF. Therapeutic approaches to mitochondrial dysfunction in Parkinson’s disease. Parkinsonism Relat Disord. 2009;15(Suppl 3):S189–194. doi: 10.1016/S1353-8020(09)70812-0. [DOI] [PubMed] [Google Scholar]
- 3.Bender A, Krishnan KJ, Morris CM, Taylor GA, Reeve AK, Perry RH, Jaros E, Hersheson JS, Betts J, Klopstock T, Taylor RW, Turnbull DM. High levels of mitochondrial DNA deletions in substantia nigra neurons in aging and Parkinson disease. Nat Genet. 2006;38:515–517. doi: 10.1038/ng1769. [DOI] [PubMed] [Google Scholar]
- 4.Betarbet R, Sherer TB, MacKenzie G, Garcia-Osuna M, Panov AV, Greenamyre JT. Chronic systemic pesticide exposure reproduces features of Parkinson’s disease. Nat Neurosci. 2000;3:1301–1306. doi: 10.1038/81834. [DOI] [PubMed] [Google Scholar]
- 5.Culmsee C, Mattson MP. p53 in neuronal apoptosis. Biochem Biophys Res Commun. 2005;331:761–777. doi: 10.1016/j.bbrc.2005.03.149. [DOI] [PubMed] [Google Scholar]
- 6.Duan W, Zhu X, Ladenheim B, Yu QS, Guo Z, Oyler J, Cutler RG, Cadet JL, Greig NH, Mattson MP. p53 inhibitors preserve dopamine neurons and motor function in experimental parkinsonism. Ann Neurol. 2002;52:597–606. doi: 10.1002/ana.10350. [DOI] [PubMed] [Google Scholar]
- 7.Ekstrand MI, Terzioglu M, Galter D, Zhu S, Hofstetter C, Lindqvist E, Thams S, Bergstrand A, Hansson FS, Trifunovic A, Hoffer B, Cullheim S, Mohammed AH, Olson L, Larsson NG. Progressive parkinsonism in mice with respiratory-chain-deficient dopamine neurons. Proc Natl Acad Sci U S A. 2007;104:1325–1330. doi: 10.1073/pnas.0605208103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gorman AM, Ceccatelli S, Orrenius S. Role of mitochondria in neuronal apoptosis. Dev Neurosci. 2000;22:348–358. doi: 10.1159/000017460. [DOI] [PubMed] [Google Scholar]
- 9.Hood DA. Mechanisms of exercise-induced mitochondrial biogenesis in skeletal muscle. Appl Physiol Nutr Metab. 2009;34:465–472. doi: 10.1139/H09-045. [DOI] [PubMed] [Google Scholar]
- 10.Keeney PM, Dunham LD, Quigley CK, Morton SL, Bergquist KE, Bennett JP., Jr Cybrid models of Parkinson’s disease show variable mitochondrial biogenesis and genotype-respiration relationships. Exp Neurol. 2009;220:374–382. doi: 10.1016/j.expneurol.2009.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kraytsberg Y, Kudryavtseva E, McKee AC, Geula C, Kowall NW, Khrapko K. Mitochondrial DNA deletions are abundant and cause functional impairment in aged human substantia nigra neurons. Nat Genet. 2006;38:518–520. doi: 10.1038/ng1778. [DOI] [PubMed] [Google Scholar]
- 12.Krueger MJ, Singer TP, Casida JE, Ramsay RR. Evidence that the blockade of mitochondrial respiration by the neurotoxin 1-methyl-4-phenylpyridinium (MPP+) involves binding at the same site as the respiratory inhibitor, rotenone. Biochem Biophys Res Commun. 1990;169:123–128. doi: 10.1016/0006-291x(90)91442-u. [DOI] [PubMed] [Google Scholar]
- 13.Lau YS. Progressive neurodegeneration in the MPTP/probenecid model of Parkinson’s disease. In: Ebadi M, Pfeiffer R, editors. Parkinson’s disease. CRC Press; Boca Raton, FL: 2005. pp. 109–115. [Google Scholar]
- 14.Lau YS, Patki G, Das-Panja K, Le WD, Ahmad SO. Neuroprotective effects and mechanisms of exercise in a chronic mouse model of Parkinson’s disease with moderate neurodegeneration. Eur J Neurosci. 2011;33:1264–1274. doi: 10.1111/j.1460-9568.2011.07626.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lau YS, Trobough KL, Crampton JM, Wilson JA. Effects of probenecid on striatal dopamine depletion in acute and long-term 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-treated mice. Gen Pharmacol. 1990;21:181–187. doi: 10.1016/0306-3623(90)90898-v. [DOI] [PubMed] [Google Scholar]
- 16.Leick L, Wojtaszewski JF, Johansen ST, Kiilerich K, Comes G, Hellsten Y, Hidalgo J, Pilegaard H. PGC-1alpha is not mandatory for exercise- and training-induced adaptive gene responses in mouse skeletal muscle. Am J Physiol Endocrinol Metab. 2008;294:E463–474. doi: 10.1152/ajpendo.00666.2007. [DOI] [PubMed] [Google Scholar]
- 17.Meredith GE, Totterdell S, Petroske E, Santa Cruz K, Callison RC, Jr, Lau YS. Lysosomal malfunction accompanies alpha-synuclein aggregation in a progressive mouse model of Parkinson’s disease. Brain Res. 2002;956:156–165. doi: 10.1016/s0006-8993(02)03514-x. [DOI] [PubMed] [Google Scholar]
- 18.Mihara M, Erster S, Zaika A, Petrenko O, Chittenden T, Pancoska P, Moll UM. p53 has a direct apoptogenic role at the mitochondria. Mol Cell. 2003;11:577–590. doi: 10.1016/s1097-2765(03)00050-9. [DOI] [PubMed] [Google Scholar]
- 19.Miyai I, Fujimoto Y, Ueda Y, Yamamoto H, Nozaki S, Saito T, Kang J. Treadmill training with body weight support: its effect on Parkinson’s disease. Arch Phys Med Rehabil. 2000;81:849–852. doi: 10.1053/apmr.2000.4439. [DOI] [PubMed] [Google Scholar]
- 20.Nair VD. Activation of p53 signaling initiates apoptotic death in a cellular model of Parkinson’s disease. Apoptosis. 2006;11:955–966. doi: 10.1007/s10495-006-6316-3. [DOI] [PubMed] [Google Scholar]
- 21.Novikova L, Garris BL, Garris DR, Lau YS. Early signs of neuronal apoptosis in the substantia nigra pars compacta of the progressive neurodegenerative mouse 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine/probenecid model of Parkinson’s disease. Neuroscience. 2006;140:67–76. doi: 10.1016/j.neuroscience.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 22.Patki G, Lau YS. Melatonin protects against neurobehavioral and mitochondrial deficits in a chronic mouse model of Parkinson’s disease, Pharmacology. Biochemistry and Behavior. 2011 doi: 10.1016/j.pbb.2011.06.026. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Patki G, Che Y, Lau YS. Mitochondrial dysfunction in the striatum of aged chronic mouse model of Parkinson’s disease. Front Aging Neurosci. 2009;1:3. doi: 10.3389/neuro.24.003.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Petroske E, Meredith GE, Callen S, Totterdell S, Lau YS. Mouse model of Parkinsonism: a comparison between subacute MPTP and chronic MPTP/probenecid treatment. Neuroscience. 2001;106:589–601. doi: 10.1016/s0306-4522(01)00295-0. [DOI] [PubMed] [Google Scholar]
- 25.Pilegaard H, Saltin B, Neufer PD. Exercise induces transient transcriptional activation of the PGC-1alpha gene in human skeletal muscle. J Physiol. 2003;546:851–858. doi: 10.1113/jphysiol.2002.034850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pothakos K, Kurz MJ, Lau YS. Restorative effect of endurance exercise on behavioral deficits in the chronic mouse model of Parkinson’s disease with severe neurodegeneration. BMC Neurosci. 2009;10:6. doi: 10.1186/1471-2202-10-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scarpulla RC. Nuclear control of respiratory gene expression in mammalian cells. J Cell Biochem. 2006;97:673–683. doi: 10.1002/jcb.20743. [DOI] [PubMed] [Google Scholar]
- 28.Schapira AH, Cooper JM, Dexter D, Clark JB, Jenner P, Marsden CD. Mitochondrial complex I deficiency in Parkinson’s disease. J Neurochem. 1990;54:823–827. doi: 10.1111/j.1471-4159.1990.tb02325.x. [DOI] [PubMed] [Google Scholar]
- 29.Smith AD, Zigmond MJ. Can the brain be protected through exercise? Lessons from an animal model of parkinsonism. Exp Neurol. 2003;184:31–39. doi: 10.1016/j.expneurol.2003.08.017. [DOI] [PubMed] [Google Scholar]
- 30.St-Pierre J, Drori S, Uldry M, Silvaggi JM, Rhee J, Jager S, Handschin C, Zheng K, Lin J, Yang W, Simon DK, Bachoo R, Spiegelman BM. Suppression of reactive oxygen species and neurodegeneration by the PGC-1 transcriptional coactivators. Cell. 2006;127:397–408. doi: 10.1016/j.cell.2006.09.024. [DOI] [PubMed] [Google Scholar]