Abstract
In addition to honeybee workers, drones also contribute to colonial thermoregulation. We show the drones’ contribution to thermoregulation at 5 different experimental temperatures ranging from 15–34 °C. The frequency and the degree of endothermy depended on the drones’ local ambient temperature and age. Location on brood or non-brood areas had no influence. The frequency of endothermic drones and the intensity of endothermy increased with decreasing temperature. 30% of drones of 8 days and older heated their thorax by more than 1 °C above the abdomen. The youngest drones (0–2 days) did not exceed this level of endothermy. Though young drones were less often engaged in active heat production, their contribution to brood warming was not insignificant because their abundance on the brood nest was 3.5 times higher than that of the oldest drones (≥13 days). Results suggest that the stimulus for the drones’ increased frequency of heating at low experimental temperatures was their low local ambient air and/or comb temperature.
Keywords: Apis mellifera, drone, thermoregulation, thermography
1. INTRODUCTION
Honeybees are insects well known for their distinct ability of social thermoregulation. This essential property allowed the originally tropical insect to expand its range into cold temperate environments and to survive there as a whole colony. They regulate the brood nest temperature within a temperature range of 32–36 °C (e.g., Gates, 1914; Hess, 1926; Himmer, 1932; Büdel, 1955, 1960; Simpson, 1961; Seeley, 1985; Fahrenholz et al., 1989; Bujok et al., 2002; Kleinhenz et al., 2003; Jones et al., 2005). For optimal brood development it is essential that the brood nest within a colony is maintained within this temperature range (Himmer, 1932; Koeniger, 1978; Tautz et al., 2003; Groh et al., 2004). While eggs and larvae (in open brood cells) can tolerate lower temperatures for some time, the pupae (in capped brood cells) are very sensitive to cooling (Himmer, 1932; Tautz et al., 2003; Groh et al., 2004, 2006).
Incubation of the brood has to the greater part to be accomplished by the worker bees (Koeniger, 1978) which react to brood (comb) temperature rather than to air temperature (Kronenberg and Heller, 1982). The individual role of worker bees on colony-level thermoregulation was investigated in hive bees by Harrison (1987), Bujok et al. (2002), Stabentheiner and Kovac (2002) and Kleinhenz et al. (2003).
Honeybee drones are often called “lazy Willi” (Bonsels, 1912) and are often assumed to merely function as “flying sperm”, necessary to inseminate virgin queens. This view, however, is not correct. In preparation for flight and during take off and landing drones always show a high thermogenic capacity with higher thorax temperatures than workers (Kovac and Stabentheiner, 2004; Stabentheiner et al., 2007). With their great body mass they are well positioned for contributing to the thermogenic needs of the colony. Harrison (1987) has shown that they take part in colony heat production under extreme thermal stress conditions. In this study we extended Harrison’s (1987) experiments by investigating the drones’ contribution to colonial thermogenesis under varying thermal stress in a breeding colony.
In young worker bees the ability of active heat production by means of the thoracic muscles develops in their first two days of life (Vollmann et al., 2004). Adult drones exhibit a much higher metabolic rate than juveniles (Fahrenholz et al., 1992), which indicates that endothermy of juvenile drones is also not completely developed after emergence. This suggested that older drones contribute more to colonial heat production. Therefore, we investigated the age dependence of the thermogenic behaviour of drones in a breeding colony.
2. MATERIALS AND METHODS
2.1. Experimental colonies, bee treatment and experimental procedure
Experiments were carried out in two observation hives with two vertically aligned combs, which were covered by plastic foils transparent to infrared radiation. The bees (Apis mellifera carnica Pollmann) could leave and enter the hive via an about 1.5 m long transparent plastic tube with 5 cm inner diameter. Experiments were carried out at 5 different experimental room temperatures (Texp). The thermal stress of the test colonies was varied by placing them in an air stream at temperatures of 15 to 34 °C (= high to low thermal stress). The (ambient) air temperature near the bees (Tair) was measured by a mesh of 20 thermocouples on each side of the colony, mounted at a height of 5–9 mm above the combs. Actual bee position on the combs was determined relative to a wire mesh with 3.1 × 3.4 cm rectangles mounted at a height of 10 mm above the combs. By dividing each mesh rectangle into 5 subsections (a–d and centre) the bee position could be determined with a resolution of about ± 10 mm. Together with experimental room temperature and humidity, air stream and outside temperature, thermocouple data were stored every 10 s on a laptop computer via a network of one ALMEMO 5590-2 (40-channels) and two ALMEMO 2290-8 (5-channels each) data loggers (AHLBORN).
For the first experimental set an observation colony was established in April with a queen and about 4000–5000 workers from a standard bee colony. In May and June 50–100 drones were introduced in the observation colony twice a week. For this purpose, capped drone brood combs from several standard colonies had been brought to an incubator and kept there at 34 °C. The drones emerged during the night and were marked in the morning. Marking was done with a colour code for age identification by small paint dots (EDDING 751 paint marker) on the margin of abdomen and/or thorax so not to interfere with thermographic body temperature measurements. After marking they were added gently from the top of the hive to the upper comb. In the colony they were allowed to acclimatize to colony conditions and distribute for at least one day before the measurement procedure started. All marked drones (of all ages) found on one side of the colony were thermographed by scanning the combs with an infrared (IR) thermographic camera (for details see below). The thermographed comb side was changed regularly between measurement days. For other experiments (Stabentheiner and Kovac, unpubl. data) freshly emerged worker bees had been marked in the same way and introduced in the same observation hive as the drones. The abundance of these workers in different hive regions was counted simultaneously and in the same way as the drones on the days of drone measurement, and used for comparison with the drones.
For a second series of trials, 400 drones, emerged within about 10 hours, were introduced unmarked in a similar observation hive as described above. It was checked before that this colony had no other drones. For measurement, the thermographic camera was focused on the centre of a comb. Body temperature of all drones which were visible within about two hours of recording was measured later during evaluation from the thermograms of the infrared video sequences. Measurements were done 3–5 hours after introducing the drones, and repeated on days 1, 2, 5, 6, 12, and 13.
2.2. Body and comb temperature measurement
Dorsal honeybee body and comb surface temperature was measured thermographically with an infrared camera (ThermaCam SC2000 NTS; FLIR, Inc.) equipped with a close-up lens (320×240 pixel sensor, thermal resolution < 0.1 °C). The infrared video sequences allowed measurement of body surface temperature without behavioural impairment. The infrared camera was calibrated for offset errors against an AGA1010 reference radiator visible in the IR picture via a highly reflective alumina mirror, or against a custom-made peltier-driven miniature reference source of known temperature and emissivity. Non-uniformity within thermographic pictures was corrected between the camera’s internal shutter calibrations by a VBA macro which took into account differential within-picture drift in dependence on the bees’ position in the image. Attenuation of the IR radiation by the plastic foils covering the colonies was compensated for by changing the atmospheric transmission value accordingly during evaluation. Using an infrared emissivity of 0.97 of the insect cuticle and of 0.95 of the comb wax, surface temperature was measured to the nearest 0.7 °C by this procedure (Stabentheiner and Schmaranzer, 1987; Kovac and Stabentheiner, 1999).
Thermographic data were stored digitally with 14 bit resolution on a DOLCH FlexPac-400-XG portable computer at a rate of 2 frames s−1 for the bees on the combs. In addition, thermographic scenes were stored in real time (25 frames s−1) on a SONY Hi8 Video Walkman, together with the spoken commentary on bee marking (age code), position in the wire mesh grid, behaviour, and the prevailing type of comb cells at the drones’ position (open and closed honey or brood cells, pollen cells and empty cells). At the same time we pointed at the drone of interest with a needle which was visible in the video. After the measurements, once per day, the exact ranges of brood cells and honey (open and sealed where applicable), pollen stores and empty cells were drawn on a transparent plastic film laid over the sides of the combs where the bee temperatures had been measured.
2.3. Data evaluation
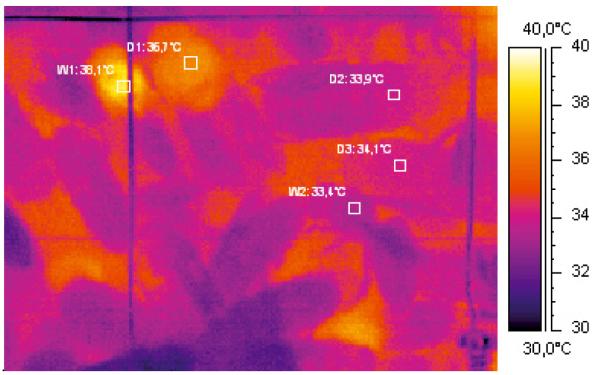
First, the infrared video tapes were searched for points of time when drones had been indicated in the thermographic pictures with a needle. The drones’ behaviour was judged from the thermographic video sequences and the spoken commentary on the tapes. In total, 2588 thermograms of drones (Fig. 1) were evaluated in this way.
Figure 1.
Thermogram of endothermic and ectothermic drones (D) and workers (W) on a comb. Values beside squares indicate the mean thorax temperature of the insects (D1: 36.7 °C, D2: 33.9 °C, D3: 34.1 °C, W1: 38.1 °C, W2: 33.4 °C). Ambient air temperature: ~33.0 °C.
The surface temperatures of head (Thead), thorax (Tthorax) and abdomen (Tabdomen), and of cell rims were calculated from the IR-files after the measurements, with AGEMA Research (FLIR) software controlled by a custom programmed Excel VBA macro (Microsoft Corporation). Temperature data necessary for exact temperature calculation at the thermographic measurement points were automatically extracted from the logger files and interpolated temporally by this macro. Ambient air temperature at the actual positions of the drones was calculated by triangular interpolation between adjacent thermocouples with a different Excel macro (Microsoft Corporation), which extracted the necessary temperature data from the logger files.
Statistical analyses were conducted using the Statgraphics package (Statistical Graphics Corporation) or with self written Excel sheets (Microsoft Corporation) according to Sachs (1997). Correlations and ANCOVA were calculated with Statgraphics or ORIGIN (OriginLab Corporation). For Chi2 statistics the significance level was adjusted according to the Bonferroni correction for multiple comparisons wherever applicable (Sachs, 1997).
3. RESULTS
3.1. Location on combs
Bees were classified according to age in the following way: 0–2 d, 3–7 d, 8–12 d and ≥13 d. The distribution of investigated drones on brood (open and sealed) and non-brood areas on the combs is shown in Figure 2. The distribution of drones differed from that of worker bees investigated at the same time. Drones of all ages preferred the brood area significantly less often than workers of the same age. The maximum number of drones on brood areas was observed at an age of 3–7 days with 35.1%, and for workers at the age of 0–2 days with 72.6% (comparison between drones and workers, of all age classes on brood: χ2 = 182.63, on open brood: χ2 = 172.42, on sealed brood: χ2 = 50.09, threshold: 11.345, P < 0.01; comparison between age classes of drones and workers sorted for age: χ2 = 99.72, 7.66, 43.97, 40.59, threshold: 3.841, P < 0.05).
Figure 2.
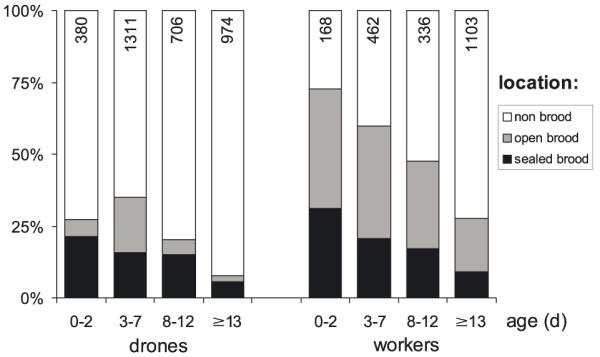
Comparison of the distribution of drones and worker bees of different age on different areas on the comb. The frequency is given in percent of total number of investigated bees (n in column).
In general, young drones were more often observed on the brood nest area than the old ones. The drones’ relative abundance on brood and non-brood areas was 1:2.7, 1:1.9, 1:3.9 and 1:11.8 from the youngest to the oldest drones. The relative abundance of workers on brood versus non-brood areas was higher than in drones and reversed with age (1:0.4, 1:0.7, 1:1.1 and 1:2.6 from the youngest to the oldest workers). With one exception the relative abundance of different age classes of both drones and workers on brood and non-brood areas was significantly different (Tab. I). The number of drones, as well as of workers found on sealed brood decreased with increasing age of the insects. On open brood the same trend was visible for the worker bees. For the drones the trend was not so clear, because the 3-7 days old drones preferred the open brood nest disproportionately more frequently (Fig. 2; see Tab. I for statistics). The relative abundance of drones of different ages on sealed versus open brood was 1:0.3, 1:1.2, 1:0.4 and 1:0.4 (youngest to oldest). By contrast, the relative abundance of workers on sealed versus open brood was higher than in drones (1:1.3, 1:1.9, 1:1.8 and 1:2.0).
Table I.
Chi2 values for the distribution of drones and workers of 4 different age classes on open and sealed brood, and non-brood areas (Fig. 2). Upper right half of tables: brood vs. non-brood, lower left half of the tables: open brood vs. sealed brood (*, threshold = 6.960 for P < 0.05 and **, threshold = 9.885 for P < 0.01; df = 6; N: drones = 20–898; workers = 46–798).
| Drones | 0–2 d | 3–7 d | 8–12 d | ≥ 13 d |
|---|---|---|---|---|
| 0–2 d | x | 8.45* | 6.32 | 88.77** |
| 3–7 d | 38.6** | x | 47.21** | 231.7** |
| 8–12 d | 0.82 | 36.4** | x | 57.04** |
| ≥ 13 d | 0.59 | 21.8** | 0.00 | x |
|
| ||||
| Workers | 0–2 d | 3–7 d | 8–12 d | ≥ 13 d |
|
| ||||
| 0–2 d | x | 8.78* | 28.40** | 132.15** |
| 3–7 d | 2.44 | x | 11.53** | 143.63** |
| 8–12 d | 1.43 | 0.06 | x | 46.95** |
| ≥ 13 d | 3.19 | 0.06 | 0.22 | x |
The frequency of drones on brood areas was 35.5% at 15 °C, 32.6% at 20 °C, 17.9% at 25 °C, 31.4% at 30 °C, and 35.6% at 34 °C experimental temperature. Significant differences were detected only between 25 °C and the other experimental temperatures (χ2 = 25.9–43.34 for significant and χ2 = 0.00–1.99 for insignificant comparisons; threshold: 7.879, P < 0.05).
3.2. Thorax temperature and thorax temperature excess
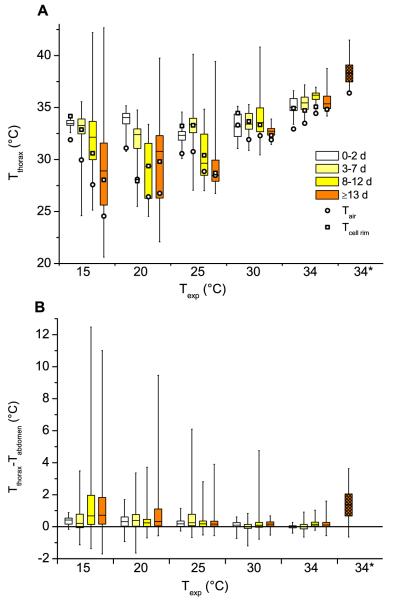
The median thorax temperatures of the drones decreased with increasing experimental temperature stress (range of medians: 28.5–38.3 °C, Fig. 3A). At experimental room temperatures of up to 25 °C younger drones had a warmer thorax than older ones. At room temperatures above 25 °C the thoracic temperatures of different age classes were quite similar. There, the warmest individuals were drones of the oldest group (≥ 13 days, median Tthorax = 38.3 °C) observed near the entrance running actively in preparation for leaving the hive. The variation (range) of the thorax temperatures increased with the age (especially at 15 °C, 20 °C and 25 °C experimental room temperature), but decreased with increasing experimental temperature. Also indicated in Figure 3A is the temperature of the ambient air (circles) and of the cell rim of the comb (squares) near the drones. Both temperatures were usually within the range of the body temperature. The temperature of the cell rims was always above the air temperature except in one age class at 20 °C.
Figure 3.
Thorax surface temperature (A), and thorax surface temperature excess compared with abdomen (B), comb (C) and ambient air (D) of drones of different ages in dependence on thermal stress (Texp). Number of measurements with increasing age: 15 °C: 17, 80, 158, 187; 20 °C: 107, 294, 44, 163; 25 °C: 60, 136, 122, 241; 30 °C: 64, 155, 207, 48; 34 °C: 28, 186, 31, 148; 34* °C: 111. 34*: drones near the entrance running busy in preparation for leaving the hive. Shown are box plots (median, Q1, Q3, minimum, maximum), and mean values for Tair and Tcell rim. X: no data.
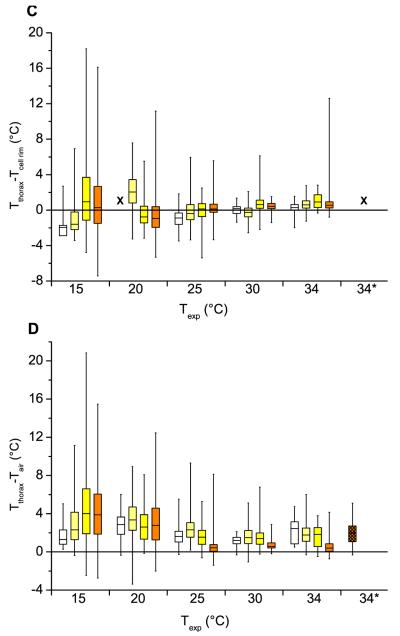
The median thorax temperature excess against the abdomen may be used as a unit of measurement for the endothermy of insects (Fig. 3B). From moderate to strong thermal stress (25-15 °C experimental room temperature), it was elevated above zero. The highest temperature excess of one individual was >12 °C at the highest thermal stress (15 °C). The median level of thorax temperature excess decreased with increasing experimental room temperature and was about zero at 30 and 34 °C. At 15 °C the two older age classes (8–12 d, ≥ 13 d) showed an obviously higher thorax temperature excess compared to younger drones. However, at the other experimental room temperatures this was not visible. The highest median temperature excess (1.4 °C) was observed in drones in preparation for leaving the hive (≥ 13 days, Fig. 3B). As the distribution of data changed strongly with experimental conditions, however, standard statistical methods were not very useful in evaluating the effect of thermal stress and age on the degree of endothermy. The variation was highest in the older and smallest in the youngest age classes, and decreased for all classes with increasing experimental temperature. The Kolmogorov-Smirnov test showed that distributions differed between age classes within the thermal treatments (P < 0.05), with the exception of the two youngest and two oldest age classes at 15 °C and the two youngest classes at 34 °C.
The median thorax temperature excess against the cell rim of the comb (Fig. 3C) showed no obviously uniform trend for age classes and experimental room temperatures. At 15 and 25 °C it increased discernibly with drone age from negative to positive values. However, at 20 °C the cell rim data of the youngest age class was missing and no trend could be observed. At lower thermal stress (30 °C, 34 °C) the temperature excess was around zero or only slightly elevated. Again, the variation decreased with increasing experimental room temperature.
The median thorax temperature excess against the ambient air was always elevated above the zero level (Fig. 3D). The median of the thorax was typically more than 1 °C higher than the surrounding air in the hive. It was highest at 15 °C and increased with increasing age at this temperature. However, this trend was not visible in the other trials (20–34 °C) where it was quite similar between age classes. The variation decreased at higher experimental room temperatures.
3.3. Frequency of heat production (endothermy)
Another possibility to describe the drones’ contribution to colony heat production is to count the number of drones heating their thorax above a certain level. We classified the drones according to the following scheme (numbers are °C):
(a) ectothermic: Thead ± 0.19 = Tthorax = Tabdomen ± 0.19 (= difference to 100% in Fig. 4A),
three levels of endothermy: weak: Thead + 0.2 ≤ Tthorax ≥ Tabdomen + 0.2 (Fig. 4A), moderate: Thead + 0.2 ≤ Tthorax ≥ Tabdomen + 0.5 (Fig. 4B), sizable: Thead + 0.2 ≤ Tthorax ≥ Tabdomen + 1.0 (Fig. 4C).
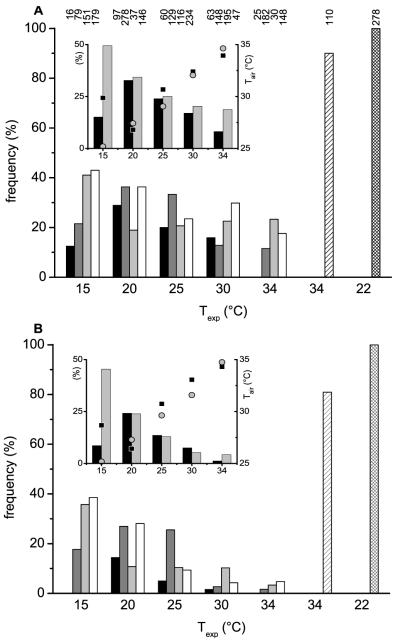
Figure 4.
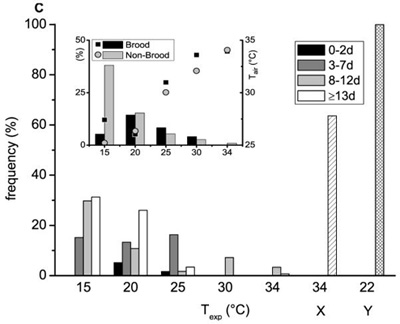
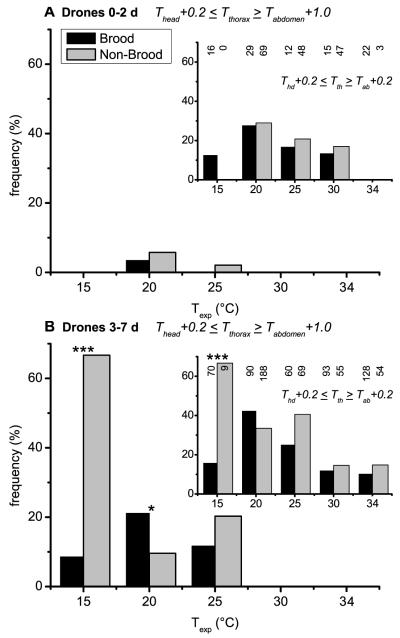
Percentage of heating drones of different ages in dependence on thermal stress (Texp) at different levels of endothermy: (A) Thead + 0.2 ≤ Tthorax ≥ Tabdomen + 0.2; (B) Thead + 0.2 ≤ Tthorax ≥ Tabdomen + 0.5; (C) Thead + 0.2 ≤ Tthorax ≥ Tabdomen + 1.0. X: drones at age ≥13 days inside the hive at the entrance running busy in preparation for flight. Y: adult drones at the hive entrance outside the hive during pre-flight warm-up (Kovac and Stabentheiner, 2004). Number of investigated drones for each age class is written above each column.
Insert: Percentage of heating of drones on brood and non-brood areas in dependence on thermal stress (Texp) at different levels of endothermy (independent of age, left y-axis) and temperature of the ambient air near the drones (Tair, right y-axis; squares for brood, circles for non-brood).
The first level contains drones with a minimal to strong endothermic reaction, whereas the third level includes only drones with a sizable heat production (Fig. 4A–C). The number of actively heat producing drones decreased in most cases in all age classes with increasing experimental temperature. This trend was visible at all 3 levels of endothermy. The very strong temperature stress at Texp = 15 °C demonstrated that the ability of endothermy significantly depends on the age of the investigated drones. The oldest drones (≥ 13 d) were at all 3 levels more frequently endothermic than the youngest ones (0–2 days) (P < 0.05; χ2 = 5.67; χ2 = 9.54; χ2 = 7.02; threshold = 3.841, df = 1). At higher experimental room temperatures an age dependence was less clearly noticeable. The last two columns represent old drones (≥ 13 d) inside (X) and outside (Y) the hive in preparation for leaving the colony and flight. More than 60% and 100% of them were still endothermic at the third (‘sizable’) level, respectively (Fig. 4C).
The inserts in Figure 4A–C show the portion of heating drones of all age classes on brood and non-brood areas and their mean ambient hive air temperature (Tair). On the non-brood area a continuous increase of the frequency of heating drones with decreasing experimental room temperature was detected. This trend was also present in the brood area but at 15 °C the number of heating drones decreased strongly. The mean Tair near the measured drones, as shown in the inserts of Figure 4, decreased nearly linearly with decreasing experimental room temperature in both brood (squares) and non-brood areas (circles). The one exception from this observation in the brood area at 15 °C (increased Tair) concurred with the observed decrease of heating drones there.
In trials with medium and high experimental room temperatures (20–34 °C) the portion of heating drones did not differ between brood and non-brood areas (P > 0.05; χ2 < 3.18; threshold = 3.841, df = 1) except at 34 °C (Fig. 4A; P < 0.05; χ2 = 8.82; threshold = 3.841, df = 1). At a very low experimental room temperature of 15 °C significantly less heating drones were observed on the brood area at all 3 levels of endothermy (P < 0.01; χ2 = 49.23, 60.75, 54.22, respectively; threshold = 6.635, df = 1). Figure 5 presents the frequency of heating drones of 3 age classes (Fig. 5A: 0–2 d, B: 3–7 d, C: ≥ 8 d) at two different levels of endothermy, sizeable and weak (insert), on brood and non-brood areas. With decreasing Texp the frequency of heating drones increased in the 3–7 day and older (≥ 8 days) drones especially in the non-brood area. Differences between the two areas were highly significant for the two older classes (P < 0.001; 3-7 d: χ2 = 20.89, 14.66; ≥ 8 d: χ2 = 29.05, 24.61; threshold = 10.83, df = 1) at a Texp of 15 °C.
Figure 5.
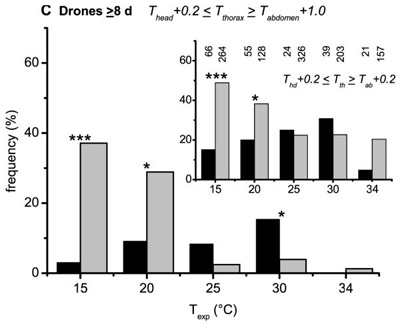
Percentage of sizably (Thead + 0.2 ≤ Tthorax ≥ Tabdomen + 1.0) and weakly (Thead + 0.2 ≤ Tthorax ≥ Tabdomen + 0.2, inserts) heating drones of different ages (A: 0–2 d, B: 3–7 d, C: ≥ 8 d) on brood and non-brood areas in dependence on thermal stress (Texp). Number of investigated drones for each age class and Texp is written above each column in the inserts.
With an ANCOVA we tested whether location on brood or non-brood area, temperature of ambient air (Tair) or comb surface (Tcell rim) had an influence on the frequency of heating drones (inserts in Fig. 4, data for Tcell rim not shown). Results clearly revealed that the location on the comb (brood or non-brood) had no influence on the frequency of heating (P = 0.097, P = 0.866, P = 0.912 for the weak, moderate and sizable level of endothermy, respectively). Further analysis with the brood and non-brood data combined showed that the frequency of heating drones was negatively correlated with Tair (P < 0.01; R2 = 0.7609, 0.7521, 0.5967 at the 3 levels, respectively; n = 10), and it was also negatively correlated with Tcell rim (P < 0.05; R2 = 0.76973, 0.68097, 0.56665; n = 10).
The temperature of the comb surface correlated significantly with the temperature of the ambient air beside the ectothermic drones (Tcell rim = 0.7388 × Tair + 9.6975, R2 = 0.6073, P < 0.0001, n = 1291), and the heating drones (at least weakly endothermic; Tcell rim = 0.8550 × Tair + 5.9413, R2 = 0.7293, P < 0.0001, n = 463).
In conclusion our results suggest that ambient air and comb temperature near the measured drones had most influence on their frequency of heating and level of endothermy, and no influence of location (brood or non-brood areas) was found.
4. DISCUSSION
In a breeding honeybee colony, many of the individual worker bees contribute actively to colonial thermogenesis (Harrison, 1987; Bujok et al., 2002; Stabentheiner and Kovac, 2002; Kleinhenz et al., 2003). However, what about the drones? They do not over-winter, they are present only in a small number in the colonies during the breeding season (about 5–8%, from the own experience as beekeeper), and they are not so frequently found in the brood nest where thermoregulation is most important (Fig. 2). However, their ability to produce heat is undeniable, because for flight they increase their thorax temperature up to 39.6–43.1 °C which is higher than in workers (Kovac and Stabentheiner, 2004; Stabentheiner et al., 2007). This study demonstrates that drones contribute to thermoregulation in a breeding colony, and sorts out the contribution of different ages. Especially the trials at low experimental room temperature of 15 °C, which means a strong thermal stress for an observation hive (comparable with outdoor ambient temperatures of about 5–8 °C for a standard colony) provided clear evidence. All investigated drones older than 0–2 d exhibited a median thorax temperature which was obviously elevated above that of the abdomen (Fig. 3). With decreasing thermal stress heat production decreased as expected. However, individual drones did not take part equally in heat production. Many showed no or only weak endothermy, while others exhibited a thoracic temperature excess (against the abdomen) of more than 12 °C (Fig. 3B). The task threshold model of task allocation in polyandrous social insect colonies (e.g. Graham et al., 2006) suggests that the differential decisions of individual bees to heat or not is determined by temperature threshold differences. This probably applies to drones too.
In worker honeybees it was shown that endothermy develops within the first 36–96 hours after emergence (Vollmann et al., 2004). Our results indicate that the development of the drones’ endothermy takes a similar course. The 0-2 days old drones showed a considerably smaller reaction to low experimental temperature than the older ones (Fig. 5). This is in accordance with Fahrenholz et al. (1992), who observed a strong increase in heat production with increasing drone age. Heat production of drone groups as measured by direct calorimetry was significantly higher in adult fertile drones (≥ 14 d) than in juvenile drones (~ 7 d) (19.7, 2.7 and 3.4 times at 20 °C, 25 °C and 30 °C ambient temperature, respectively).
In contrast to the results of Harrison (1987) and in accordance with Currie (1987) our drones of all age classes were found for the lager part on peripheral non-brood areas (Fig. 2). The younger drones preferred the (warmer) brood area more than the older ones. This explains the warmer thorax of the young drones at experimental room temperatures of up to 25 °C, though they are less often endothermic (Fig. 3A). Worker bees of all age classes, however, were generally found more frequently on the brood area than drones, building up two or three insulation layers above the brood at low experimental temperatures. However, this did not lead to an active crowding out of the drones from this location by the higher number of workers. We never observed an active attempt of workers to force a drone away from an area (3371 observations). Drone proportion between brood and non-brood areas did not change with increasing thermal stress.
Stimulus for the drones’ increased heating was their low ambient air and/or comb temperature (see inserts in Fig. 4, and Fig. 5). This is supported by the following findings. An ANCOVA showed that location of the drones on brood or non-brood area had no influence on their frequency of heating (compare also Fig. 5). These results indicate that heating by drones did not occur in response to the brood and brood pheromone(s), as has been demonstrated for worker bees (Koeniger, 1978). This is supported by the observation that even the oldest drones, where we assume full development of flight muscle function and thus endothermic capacity (≥8 d, Fig. 5C), reacted to low experimental temperature only in the non-brood areas where changes of Ta near the bees were most pronounced (Fig. 3). Second, we found good correlations between the frequency of heating and ambient air and comb temperature. At 15 °C experimental temperature, where the general trend to a higher portion of heating drones was reversed (inserts of Fig. 4), the higher air and comb temperatures on the brood nest locations near the measured drones demonstrates the dominant role of these two parameters. We do not yet know, however, whether the drones react to comb temperature rather than to air temperature like workers do (Kronenberg and Heller, 1982).
One might argue that drones do not really actively contribute to colonial thermoregulation because their main area of abundance is not the brood nest where the heat is most urgently needed (Fig. 2), and because active heating is not more frequent there (Fig. 4, inserts). However, a certain number of them is heating on the brood nest, and those drones which heat outside the brood area reduce the thermal gradient and in this way reduce the heat flow from the brood area, which will allow the workers to reduce endothermic activity. Each source inside a colony that contributes to heat production is involved in colony temperature homeostasis at low outside temperatures. As individual endothermy is always an active process in bees (activation of flight muscles, e.g. Goller and Esch, 1990, 1991) endothermic drones contribute actively to social thermoregulation.
In spite of their small number the contribution of drones to total colonial heat production is not insignificant. In the endothermic state they will produce more heat than a worker bee because of their larger body mass (worker bee: 78.3 mg, drone: 206.0 mg, with empty honey crop and intestine; Gmeinbauer and Crailsheim, 1993). Harrison (1987) calculated that each drone produces 1.5 times as much heat as a worker bee, mainly because they are larger. Based on the empty mass relation of 2.6 we expect an even higher value. Even when the drones do not perform active heat production by means of their thoracic muscles (in the ectothermic state), their 2.6 times greater body mass will result in an about 2.6 times higher resting metabolism than in a resting worker bee (see Kovac et al., 2007 for workers).
Kovac et al. (2007) showed that resting worker bees are hardly really ectothermic at ambient temperatures between 15–30 °C. At an air temperature of 27 °C a mean difference of about 0.8 °C between thorax and abdomen was observed. Future investigations will have to show whether the same behaviour becomes operative in drones at low ambient temperatures.
In conclusion, our results show that drones contribute to colonial thermoregulation, mainly at high thermal stress caused by low ambient temperature, and in reaction to their local ambient temperature. Older drones contribute more to colony thermoregulation than younger ones.
ACKNOWLEDGEMENTS
Supported by the Austrian Fonds zur Förderung der Wissenschaftlichen Forschung (FWF, P13916). Thanks are also due to Bettina Germann, Lisbeth Postl and Jutta Vollmann for help with data evaluation, and to two anonymous referees for helpful comments.
REFERENCES
- Bonsels W. Ein Roman für Kinder, 1.-3. Auflage, Berlin, Leipzig: 1912. Die Biene Maja und ihre Abenteuer; p. 178 S. 1912. 8°. [Google Scholar]
- Büdel A. Schwankungen der Lufttemperatur in der Wabengasse eines brütenden Bienenvolkes. Z. Bienenforsch. 1955;3:88–92. [Google Scholar]
- Büdel A. Bienenphysik. In: Büdel A, Herold E, editors. Biene und Bienenzucht. Ehrenwirth; Munich: 1960. [Google Scholar]
- Bujok B, Kleinhenz M, Fuchs S, Tautz J. Hot spots in the bee hive. Naturwissenschaften. 2002;89:299–301. doi: 10.1007/s00114-002-0338-7. [DOI] [PubMed] [Google Scholar]
- Currie RW. The biology and behaviour of drones. Bee World. 1987;68:129–143. [Google Scholar]
- Fahrenholz L, Lamprecht I, Schricker B. Thermal investigations of a honey bee colony: thermoregulation of the hive during summer and winter and heat production of members of different bee castes. J. Comp. Physiol. B. 1989;159:551–560. [Google Scholar]
- Fahrenholz L, Lamprecht I, Schricker B. Calorimetric investigations of the different bee castes of honey bees, Apis mellifera carnica. J. Comp. Physiol. B. 1992;162:119–130. [Google Scholar]
- Gates V. The temperature of the bee-colony. Bull. US. Dept. Agric. 1914:96. [Google Scholar]
- Gmeinbauer R, Crailsheim K. Glucose Utilization During Flight of Honeybee (Apis mellifera) Workers, Drones and Queens. J. Insect. Physiol. 1993;39:959–967. [Google Scholar]
- Goller F, Esch HE. Muscle potentials and temperature acclimation and acclimatization in flight muscles of workers and drones of Apis mellifera. J. Therm. Biol. 1990;15:307–312. [Google Scholar]
- Goller F, Esch HE. Oxygen consumption and flight muscle activity during heating in workers and drones of Apis mellifera. J. Comp. Physiol. B. 1991;161:61–67. [Google Scholar]
- Graham S, Myerscough MR, Jones JC, Oldroyd BP. Modelling the role of intracolonial genetic diversity on regulation of brood temperature in honey bee (Apis mellifera L.) colonies. Insect. Soc. 2006;53:226–232. [Google Scholar]
- Groh C, Tautz J, Rossler W. Synaptic organization in the adult honey-bee brain is influenced by brood-temperature control during pupal development. Proc. Natl. Acad. Sci. USA. 2004;101:4268–4273. doi: 10.1073/pnas.0400773101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groh C, Ahrens D, Rossler W. Environment- and age-dependent plasticity of synaptic complexes in the mushroom bodies of honeybee queens. Brain Behav. Evol. 2006;68:1–14. doi: 10.1159/000092309. [DOI] [PubMed] [Google Scholar]
- Harrison JM. Roles of individual honeybee workers and drones in colonial thermogenesis. J. Exp. Biol. 1987;129:53–61. doi: 10.1242/jeb.129.1.53. [DOI] [PubMed] [Google Scholar]
- Hess WR. Die Temperaturregulation im Bienenvolk. Z. Vgl. Physiol. 1926;4:465–487. [Google Scholar]
- Himmer A. Die Temperaturverhältnisse bei den sozialen Hymenopteren. Biol. Rev. 1932;7:224–253. [Google Scholar]
- Jones CJ, Helliwell P, Beekman M, Maleszka R, Oldroyd BP. The effects of rearing temperature on developmental stability and learning and memory in the honeybee, Apis mellifera. J. Comp. Physiol. A. 2005;191:1121–1129. doi: 10.1007/s00359-005-0035-z. [DOI] [PubMed] [Google Scholar]
- Kleinhenz M, Bujok B, Fuchs S, Tautz J. Hot bees in empty broodnest cells: heating from within. J. Exp. Biol. 2003;206:4217–4231. doi: 10.1242/jeb.00680. [DOI] [PubMed] [Google Scholar]
- Koeniger N. Das Wärmen der Brut bei der Honigbiene (Apis mellifera L.) Apidologie. 1978;9:305–320. [Google Scholar]
- Kovac H, Stabentheiner A. Effect of food quality on the body temperature of wasps (Vespula vulgaris) J. Insect Physiol. 1999;45:183–190. doi: 10.1016/s0022-1910(98)00115-2. [DOI] [PubMed] [Google Scholar]
- Kovac H, Stabentheiner A. Thermografische Messung der Körpertemperatur von abfliegenden und landenden Drohnen und Arbeiterinnen (Apis mellifera carnica Pollm.) am Nesteingang. Mitt. Dtsch. Ges. Allg. Angew. Entomol. 2004;14:463–466. [Google Scholar]
- Kovac H, Stabentheiner A, Hetz SK, Petz M, Crailsheim K. Respiration of resting honeybees. J. Insect Physiol. 2007;53:1250–1261. doi: 10.1016/j.jinsphys.2007.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronenberg F, Heller C. Colonial Thermoregulation in Honey Bees (Apis mellifera) J. Comp. Physiol. 1982;148:65–76. [Google Scholar]
- Sachs L. Angewandte Statistik. Springer; Berlin, Heidelberg, New York: 1997. [Google Scholar]
- Seeley TD. Honeybee Ecology: A Study of Adaptation in Social Life. Princeton Univ. Press; Princeton: 1985. [Google Scholar]
- Simpson J. Nest climate regulation in honey bee colonies. Science. 1961;133:1327–1333. doi: 10.1126/science.133.3461.1327. [DOI] [PubMed] [Google Scholar]
- Stabentheiner A, Schmaranzer S. Thermographic Determination of Body Temperatures in Honey Bees and Hornets: Calibration and Applications. Thermology. 1987;2:563–572. [Google Scholar]
- Stabentheiner A, Kovac H. Beitrag unterschiedlich alter Arbeiterinnen zur Wärmeproduktion im Brutnest von Bienenvölkern. Apidologie. 2002;33:499–500. [Google Scholar]
- Stabentheiner A, Kovac H, Schmaranzer S. Thermal Behaviour of Honeybees During Aggressive Interactions. Ethology. 2007;113:995–1006. doi: 10.1111/j.1439-0310.2007.01403.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tautz J, Maier S, Groh C, Roessler W, Brockmann A. Behavioral performance in adult honey bees is influenced by the temperature experienced during their pupal development. Proc. Natl. Acad. Sci. USA. 2003;100:7343–7347. doi: 10.1073/pnas.1232346100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollmann J, Stabentheiner A, Kovac H. Die Entwicklung der Endothermie bei Honigbienen (Apis mellifera carnica Pollm.) Mitt. Dtsch. Ges. Allg. Angew. Entomol. 2004:467–470. [Google Scholar]