Abstract
Objective
To identify biomarkers that distinguish between active ANCA-associated vasculitis (AAV) and remission in a manner superior or complementary to established markers of systemic inflammation.
Methods
Markers of vascular injury and angiogenesis were measured before and after treatment in a large clinical trial in AAV. 163 subjects enrolled in the Rituximab in ANCA-Associated Vasculitis (RAVE) trial were studied. Serum levels of E-selectin, ICAM-3, MMP1, MMP3, MMP9, P-selectin, thrombomodulin, and VEGF were measured at study screening (time of active disease) and at month 6. ESR and CRP levels had been measured at the time of the clinical visit. The primary outcome was the difference in marker level between screening and month 6 among patients in remission (BVAS/WG score of 0) at month 6.
Results
All subjects had severe active vasculitis (mean BVAS/WG score 8.6 +/− 3.2 SD) at screening. Among the 123 subjects clinically in remission at month 6, levels of all markers except E-selectin showed significant declines. MMP3 levels were also higher among the 23 subjects with active disease at month 6 than among the 123 subjects in remission. MMP3 levels correlated weakly with ESR and CRP.
Conclusion
Many markers of vascular injury and angiogenesis are elevated in severe active AAV and decline with treatment, but MMP3 appears to distinguish active AAV from remission better than the other markers studied. Further study of MMP3 is warranted to determine its clinical utility in combination with conventional markers of inflammation and ANCA titers.
Keywords: biomarkers, vasculitis, ANCA
The disease group of ANCA-associated vasculitis (AAV) includes granulomatosis with polyangiitis (GPA, Wegener’s granulomatosis) and microscopic polyangiitis (MPA), entities that share the features of necrotizing vasculitis of small blood vessels in multiple organ systems, and anti-neutrophil cytoplasmic antibodies (ANCA). Before effective treatments were discovered, AAV was usually fatal after a monophasic illness. Aggressive immunosuppressive therapy has not led to cure, but instead has converted GPA and MPA into chronic diseases. Relapse is common but not universal, unpredictable in its timing, and highly variable in severity. Most patients require chronic immunosuppressive therapy to reduce the risk of severe relapse or to control musculoskeletal, constitutional, or upper airway symptoms.
Because of the highly variable course of disease, long-term management of AAV is challenging. Changes in ANCA titers correlate with changes in disease activity, but discordance between ANCA status and clinical status is high (1–5); in one large study, changes in PR3-ANCA titers explained only 8% of the observed changes in disease activity (5). Generic markers of inflammation [erythrocyte sedimentation rate (ESR) and C reactive protein (CRP)] are typically elevated in active AAV (6–8) but in addition to being non-specific with regard to other inflammatory conditions, these markers do not distinguish active AAV from remission as well as one might think (6, 8) (and data to be shown in this paper). Additional markers are needed to guide therapy and help distinguish highly active disease, mildly active disease, and remission.
Markers of vascular injury and the linked process of angiogenesis are of particular interest in vasculitis and have been investigated as biomarkers in AAV. For example, thrombomodulin is released by damaged endothelial cells; P-selectin is released by platelets activated by damaged microvessels; vascular endothelial growth factor (VEGF) is an inducible mediator of vascular permeability and of angiogenesis following tissue damage; and multiple matrix metalloproteinases (MMPs) are induced during angiogenesis and tissue remodeling. Several of these markers have been reported to be elevated in patients with active AAV, either in comparison to healthy controls, or in comparison to patients in remission, or both (9–14). Evaluation of these markers in a larger, independent cohort is needed.
Utilizing serum specimens collected during the conduct of randomized, controlled, clinical trial of AAV, we performed a study to determine if markers of vascular injury and angiogenesis distinguish between active AAV and remission. We also assessed whether these new markers distinguish important subsets of active AAV.
PATIENTS AND METHODS
Study Design
Subjects for this study were enrolled in the Rituximab in ANCA-Associated Vasculitis (RAVE) trial. Samples from all subjects who completed 6 months in their original treatment groups and had adequate volumes of serum obtained at both the screening and month 6 visits (n=146) were used in this study. Additional samples from screening visits were used from subjects who completed only 4 months in the trial (n=6) and from subjects who were crossed over to the other treatment group during the first 6 months (n=11).
The primary outcome of this study was the difference in marker level between active AAV (at screening) and remission (at month 6) in the same patients, as determined by analysis of the absolute changes in marker levels and analysis of receiver operating characteristic (ROC) curves (see below, Statistical Analysis). Secondary outcomes included differences in marker levels between subjects in remission and subjects with persistent or recurrent active AAV at month 6, differences in marker levels among pre-defined clinical subsets at screening, and association of marker levels with the clinically apparent extent of disease (total BVAS/WG score) at screening.
Summary of Clinical Trial and Clinical Outcome Measures
RAVE was a randomized, double-blinded, multi-center clinical trial that compared standard remission-induction therapy using oral cyclophosphamide (CYC) and glucocorticoids to experimental treatment using the B-cell-depleting agent rituximab (RTX) and glucocorticoids, in 197 patients with new or recurrent, severe AAV (GPA or MPA) (15). All patients tested positive for antibodies to either proteinase 3 (PR3) or myeloperoxidase (MPO). Subjects randomized to receive CYC were switched to maintenance therapy with azathioprine (AZA) if they were clinically in remission between months 4 and 6. Subjects in the RTX arm were not placed on a maintenance agent, but the great majority still had no detectable B cells at month 6 and were therefore still considered to be “on treatment” at this time point. Ongoing or recurrent severe disease during the first six months led to blinded cross-over to the other treatment arm or withdrawal from blinded treatment, and such patients were regarded as treatment failures in determining the clinical endpoints. Per protocol, glucocorticoid (prednisone) treatment was completely withdrawn before six months. Investigators had the option to restart prednisone at no more than 10 mg/day to control recurrent symptoms of mild disease. The rates of achievement of remission were equivalent in the two treatment arms (15).
Activity of vasculitis, in RAVE and in this ancillary study, was assessed using the Birmingham Vasculitis Activity Score for Wegener’s Granulomatosis BVAS/WG (16), in which each severe disease manifestation is given 3 points and each non-severe (mild)manifestation is given 1 point. Remission is defined as BVAS/WG=0; severe disease is defined as the presence of one or more severe manifestations. Every patient had at least one severe manifestation at screening and, therefore, a BVAS/WG score of at least 3. The primary clinical endpoint in RAVE was the proportion of patients in each group who were in remission at month 6 and off prednisone. An important secondary endpoint was the proportion of patients in remission at month 6 with or without prednisone ≤10 mg/day.
All subjects were enrolled using IRB-approved protocols at all participating sites.
Clinical Subgroups
The following subgroups were defined per data at the screening visit: GPA vs. MPA; PR3-ANCA vs. MPO-ANCA; active vs. inactive renal disease; newly diagnosed vs. relapsing disease; already taking vs. not taking glucocorticoids; already taking vs. not taking any immune-suppressive drug (including glucocorticoids).
Selection and Processing of Serum Samples
Serum was collected, processed, and stored at each study site at time of study visits, subsequently shipped to a central repository, and then shipped to the study laboratory. All samples remained frozen at −80 C° until the day the assays were performed.
Biomarker Assays
Levels of E-selectin, intercellcular adhesion molecule (ICAM)-3, P-selectin, and thrombomodulin (as a 4-plex); MMP1, MMP3, and MMP9 (as a 3-plex); and VEGF as a single assay were measured in serum using commercial electrochemiluminescence assays (Meso Scale Discovery, Gaithersburg, MD) (17–19)per the manufacturer’s protocols. Standard curves were included on each plate. Lab personnel who obtained and processed the assay data were blinded to the clinical data associated with the samples.
The precision of these assays has not been reported for serum. Therefore, in an independent set of 200 serum samples from patients with various vasculitides, levels of markers were measured in duplicate in order to estimate coefficients of variation(CV). CV’s were low for E-selectin (7.7%), P-selectin (9.4), MMP9 (6.1), thrombomodulin (6.3), and VEGF (7.0), but were higher for ICAM-3 (12.7), MMP1 (18.3), and MMP3 (39.8; see Discussion).
Healthy Controls
Sera from 20 persons self-identified as being in good health (9 males and 11 females, median age 57) were collected at Boston University under an IRB-approved protocol and were assayed for all 8 experimental markers. This number of subjects was considered too low to draw strong conclusions in comparison to values obtained from patients with AAV. No reference values for healthy persons have been established for the experimental markers, and values often differ among different assays. More information was therefore gathered about reported “normal” values from the literature and from websites of manufacturers of immunoassays. Data from several relatively large (n≥50) studies and from manufacturers reporting values for at least 4 of the markers of interest are shown in Supplementary Table 1.
Additional Laboratory Data
Westergren erythrocyte sedimentation rate (ESR), C reactive protein (CRP), and serum creatinine were assayed at the participating sites. Glomerular filtration rate (GFR, in ml/min per 1.73 m2 body surface area) was estimated from serum creatinine (Cr, in mg/dL) using the MDRD formula (20).
Statistical Analysis
Distributions, Correlations, and Adjustment for Multiple Testing
Distributions of marker values were evaluated for normality using Shapiro-Wilk and Kolmogorov-Smirnov tests, as well as visual inspection of histograms. Since none of the levels of any of the markers was normally distributed among subjects with active vasculitis, data are reported as medians and interquartile ranges and were analyzed using non-parametric statistics. Correlations between pairs of markers were measured using Spearman correlation coefficients. Findings in all analyses were considered significant at P=0.05 after adjustment for multiple comparisons (10 markers tested in each analysis) by calculating the false discovery rate per Benjamini and Hochberg (21). All analyses were performed using SAS 9.1 (SAS Institute, Cary, NC).
Distinguishing Active AAV from Remission
The primary analysis was change in marker level from screening to month 6 among patients in remission at month 6. The Signed Rank test was used to compare the distribution of the absolute changes in marker levels to the predicted null distribution. ROC curves were constructed using logistic regression, with marker level as the independent variable and AAV activity as the dichotomous (active or remission) dependent variable, and areas under these ROC curves (AUC) were interpreted as indices of the markers’ ability to distinguish active AAV from remission. An optimal cut-point for distinguishing active AAV from remission was determined using the Youden index (22), which is the maximum sum of sensitivity and specificity.
Clinical Subsets, Renal Function, and Extent of Active Disease
Marker levels in clinical subsets (see above) were compared by Wilcoxon tests. The effects of GFR on marker levels, performed on samples from patients in remission at month 6 in order to be independent of the presence of active glomerulonephritis, were assessed by linear regression with GFR as the independent variable and marker level as the dependent variable. Association of marker levels with apparent extent of disease at screening was measured using the Spearman correlation coefficient between marker level and total BVAS/WG score. Because it is not clear that BVAS/WG is a fully scalable linear measure, this analysis was considered supplemental to the primary analysis of active AAV vs. remission.
RESULTS
Patient Characteristics at Screening and Follow-up
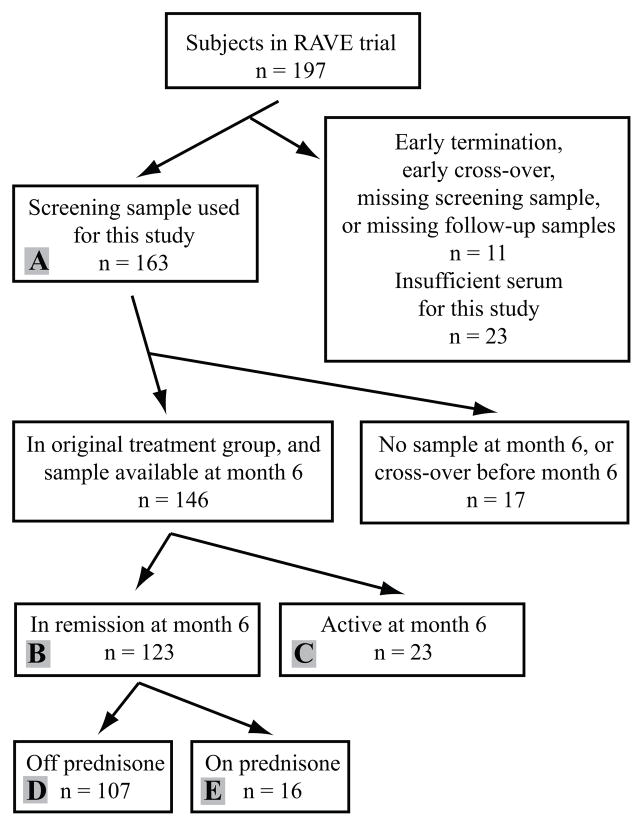
As shown in Figure 1, among 197 patients in the RAVE trial, 163 subjects (80 male and 83 female; median age 52, interquartile range 44–66) were studied based on having sufficient serum samples and follow-up time (see Methods); 146 patients also had available samples from month 6 and had not undergone blinded cross-over for re-induction therapy. Of those 146 subjects, 123 were in remission and 23 had active disease at month 6. Among the 123 subjects in remission, 16 were receiving prednisone ≤10 mg/day, and the remaining subjects were off prednisone. Median BVAS/WG score at screening was 8 (interquartile range 6–10, range 3–16). Among the 23 subjects with active disease at month 6, 4 had severe disease (BVAS/WG scores 4–8) and 19 had non-severe disease(BVAS/WG scores 1–2).
Figure 1.
Disposition of subjects in the RAVE trial in relation to selection and analysis of samples. B(with subset of A) = primary analysis: comparison of marker levels in active ANCA-associated vasculitis at screening with marker levels in remission in the same 123 subjects. Group B was also used to evaluate for differential effects of treatment with cyclophosphamide/azathioprin or rituximab. B with C= comparison of remission to relatively mild recurrent or persistent disease at month 6. A= all samples available at screening, for analysis of clinical subsets of ANCA-associated vasculitis and early effects of treatment. D with E = comparison of effects of low-dose prednisone among subjects in remission at month 6.
Of the 163 subjects evaluated at screening, 127 had been diagnosed with GPA and 36 with MPA; 113 were positive for anti-PR3 and 50 for anti-MPO; 80 had active glomerulonephritis; and 77 had a new diagnosis of AAV and 86 had established diagnoses with a severe relapse. At screening, 79 patients were receiving glucocorticoids, and 90 were receiving some immune-ressive drug (glucocorticoids, other drugs, or both); per protocol, initiation or escalation of immune-suppressive drugs to treat the current episode of AAV occurred less than 14 days before initiation of randomized treatment with CYC or RTX.
Marker Levels in Severe AAV and Remission
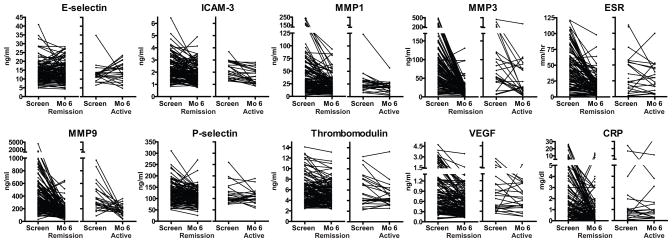
Table 1 and Figure 2 show results of marker measurements at screening (when all patients had severe active disease) and month 6. Serum levels of CRP, ESR, ICAM-3, MMP1, MMP3, MMP9, P-selectin, thrombomodulin, and VEGF were all significantly higher (p<0.05 after adjustment for 10 simultaneous comparisons) at screening than at month 6 in the 123 subjects who were in remission at 6 months (Table 1). Only E-selectin showed no significant change.
Table 1.
Change in marker levels in patients with AAV with transitioning from severe active disease (screening) to remission at month 6.
| Marker | Screening Median (25%,75%) (n=123) |
Remission (month 6) Median (25%,75%) (n=123) |
Difference, Screening minus Remission Median (25%,75%) (n=123) |
P* | Active (month 6) Median (25%,75%) (n=23) |
P† |
|---|---|---|---|---|---|---|
| VEGF | 0.666 (0.391;1.14) | 0.477 (0.283,0.721) | 0.139 (−0.007;0.512) | <0.0001* | 0.612 (0.411,1.120) | 0.11 |
| E-selectin | 13.4 (10.1;16.4) | 12.7 (9.59,17.3) | 0.13 (−2.40;2.80) | 0.63 | 13.8 (10.4,18.1) | 0.45 |
| ICAM3 | 2.13 (1.61;2.84) | 1.70 (1.35,2.29) | 0.34 (−0.09;0.95) | <0.0001* | 1.65 (1.11,2.14) | 0.24 |
| P-selectin | 120 (95.0;150) | 109 (83.7,125) | 16.2 (−4.84;37.9) | <0.0001* | 108 (92.9,121) | 1.0 |
| Thrombomodulin | 5.27 (3.76;6.95) | 4.71 (3.80,6.42) | 0.43 (−0.67;1.38) | 0.0005* | 4.41 (3.32,5.93) | 0.47 |
| MMP1 | 27.5 (18.8;50.4) | 18.2 (11.6,29.1) | 9.20 (1.90;21.9) | <0.0001* | 18.5 (12.3,23) | 0.64 |
| MMP3 | 71.6 (35.1;127) | 13.5 (9.69,21.8) | 54.1 (16.5;103) | <0.0001* | 24.5 (13.6,76.7) | 0.005† |
| MMP9 | 332 (212;554) | 130 (87.5,190) | 196 (76.3;383) | <0.0001* | 161 (122,222) | 0.046 |
| ESR(mm/hr) | 39 (18;61) | 13 (7,24) | 20 (3;39) | <0.0001* | 23 (10,44) | 0.038 |
| CRP (mg/dl) | 1.2 (0.5;3.9) | 0.5 (0.3,1.2) | 0.7 (0;3.1) | <0.0001* | 0.65 (0.3,1.5) | 0.60 |
All values in ng/ml except as noted for ESR and CRP.
Comparing Difference to null hypothesis by Signed rank test, still significant after adjustment for multiple comparisons.
Comparing Active (month 6) to Remission (month 6) by Wilcoxon test, still significant after adjustment for multiple comparisons.
VEGF = vascular endothelial growth factor; ICAM = intercellular adhesion molecule; MMP = matrix metalloproteinase; ESR = erythrocyte sedimentation rate; CRP = C reactive protein.
Figure 2.
Marker levels at screening and at month 6 stratified by remission (n=123) or recurrent disease (n=23) at month 6. Each line connects the data obtained for one patient. VEGF = vascular endothelial growth factor; ICAM = intercellular adhesion molecule; MMP = matrix metalloproteinase; ESR = erythrocyte sedimentation rate; CRP = C reactive protein.
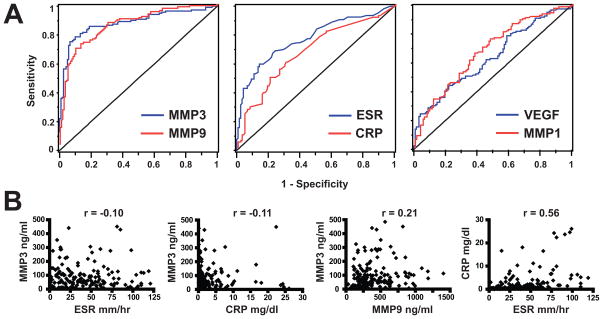
ROC analysis was used to better define how well the different markers were able to distinguish active AAV from remission in these 123 subjects. As shown in Table 2 and Figure 3A, MMP3 and MMP9 distinguished disease states well (AUC > 0.8), whereas performance of the other experimental markers was modest (AUC < 0.7). Using a cut-off value of 33.2 ng/ml (see Methods), MMP3 had a sensitivity of 0.79 for active AAV and a specificity (referring to AAV in remission, not to persons in good health or with other diseases) of 0.9. For MMP9, sensitivity was 0.73 and specificity was 0.87 at a cut-off value of 234 ng/ml.
Table 2.
Discrimination between active vasculitis and remission by different markers in patients who transitioned from active disease at screening to remission at month 6
| Marker | AUC of ROC curve | Optimal Cut-point* | Sensitivity for Active AAV (%)† | Specificity for Active AAV (%)‡ |
|---|---|---|---|---|
| VEGF | 0.64 | 0.747 | 44 | 78 |
| ICAM3 | 0.63 | 1.64 | 74 | 49 |
| P-selectin | 0.62 | 129 | 42 | 80 |
| Thrombomodulin | 0.56 | 5.07 | 55 | 59 |
| MMP1 | 0.68 | 18.7 | 76 | 52 |
| MMP3 | 0.89 | 33.2 | 79 | 90 |
| MMP9 | 0.86 | 234 | 73 | 87 |
| ESR (mm/hr) | 0.77 | 31 | 60 | 86 |
| CRP (mg/dl) | 0.68 | 0.85 | 60 | 69 |
VEGF = vascular endothelial growth factor; ICAM = intercellular adhesion molecule; MMP = matrix metalloproteinase; ESR = erythrocyte sedimentation rate; CRP = C reactive protein.
Youden index = the maximum sum of sensitivity and specificity. Values in ng/ml except as noted for ESR and CRP.
Percentages of subjects at screening in whom marker was higher than the optimal cut-point. N=119 for ESR and CRP, 123 for all other markers.
Percentages of subjects in remission at month 6 in whom marker was lower than the optimal cut-point. N=119 for ESR and CRP, 123 for all other markers.
Figure 3.
Comparison of markers’ ability to distinguish active AAV from remission in a complementary way. A. Receiver operating characteristic (ROC) curves of selected markers, constructed using the values obtained in active disease and remission in 123 subjects. B. Comparison of values of selected pairs of markers in patients with active AAV (at screening). The Spearman correlation coefficient is shown at the top of each panel. MMP = matrix metalloproteinase; ESR = erythrocyte sedimentation rate; CRP = C reactive protein.
Review of data on marker levels in sera from healthy persons, either using the same assay platform (our own 20 controls, and data from the manufacturer) or different assays, was informative for further comparison of MMP-3 and MMP-9 as biomarkers (see Supplementary Table 1). Concentrations of MMP3 are similar in different assay systems and the majority of values seen in subjects with active AAV in this study appeared to be above the normal range. In contrast, levels of MMP9 vary widely with different assays, and it appears likely that most of the values we obtained from subjects with active AAV were within the normal range.
Marker Levels in AAV at Different Levels of Severity
At screening, at which point each subject had at least one severe manifestation of AAV but total BVAS/WG ranged from 3–16, levels of several markers correlated with total BVAS/WG, but correlation was modest, with coefficients never exceeding 0.3(Supplementary Figure 1). MMP3, which showed the strongest association with active AAV relative to remission, showed no correlation with BVAS/WG.
Twenty-three subjects had active disease at month 6. After adjustment for multiple comparisons, only MMP3 showed significantly higher levels in patients with recurrent or persistent, usually mild disease, compared with patients in remission at month 6 (Table 1). Levels of ICAM3, MMP1, MMP3, and MMP9 declined significantly compared to screening among the 23 subjects who had active disease at month 6(analysis not shown, but see Figure 2). Neither P-selectin nor thrombomodulin showed a significant decline. However, these latter findings can probably be attributed to the smaller numbers of patients in this analysis, since the median values of P-selectin and thrombomodulin were similar among the 23 patients with active disease and the 123 patients in remission (see Table 1).
Marker Levels in Clinical Subgroups
Among subjects with active AAV at screening, those with GPA had higher levels of MMP-9, and lower levels of ICAM-3 and thrombomodulin, than did those with MPA. Not surprisingly, these trends were similar in groups defined by PR3 or MPO ANCA specificity (Table 3 and Supplementary Table 2).
Table 3.
Markers differing significantly inpatients stratified by clinical subtype or treatment. Medians and interquartile ranges are shown.
| Marker | AAV Type | ANCA Specificity | ||||
|---|---|---|---|---|---|---|
| GPA(n = 127) | MPA (n= 36) | P* | PR3 (n=113) | MPO (n=50) | P* | |
| ICAM3 | 1.98 (1.47,2.71) | 2.79 (2.17,3.35) | 0.0001 | 1.98 (1.47,2.72) | 2.36 (1.89,2.96) | 0.003 |
| Thrombomodulin | 4.96 (3.54,6.53) | 6.97 (4.16,8.69) | 0.003 | 4.78 (3.49,5.97) | 6.94 (4.18,8.83) | <0.0001 |
| MMP9 | 368 (235,569) | 280 (180,370) | 0.014 | |||
|
| ||||||
| New or Established (Relapsing) AAV | Active Renal Disease | |||||
| New (n=77) | Relapsing (n=86) | P* | Yes (n=80) | No (n=83) | P* | |
|
| ||||||
| VEGF | 0.914 (0.592,1.33) | 0.430 (0.316,0.720) | <0.0001 | |||
| ICAM3 | 2.49 (2.04,2.98) | 1.78 (1.39,2.36) | <0.0001 | |||
| Thrombomodulin | 5.81 (4.44,7.86) | 4.41 (3.43,6.15) | <0.0001 | 6.94 (5.36,8.79) | 3.96 (3.32,4.78) | <0.0001 |
| MMP3 | 95.4 (52.2,163) | 50.9 (29.3,93.7) | <0.0001 | |||
| ESR(mm/hr) | 49 (26,79.5) | 34 (13,52) | 0.001 | |||
|
| ||||||
| Treatment at Screening | Glucocorticoids at Screening | |||||
| Yes (n=90) | No (n=73) | P* | Yes (n=79) | No (n=84) | P* | |
|
| ||||||
| VEGF | 0.461 (0.367,1.46) | 0.812 (0.539,1.28) | 0.001 | |||
| ICAM3 | 1.84 (1.43,2.42) | 2.43 (1.83,2.97) | 0.001 | 1.83 (1.43,2.42) | 2.37 (1.79,2.96) | 0.0003 |
| Thrombomodulin | 4.44 (3.43,6.18) | 5.87 (4.51,7.78) | 0.0003 | 4.41 (3.42,6.53) | 5.73 (4.18,7.27) | 0.001 |
| MMP1 | 23.0 (17.4,31.5) | 35.8 (22.6,60.4) | 0.0008 | 22.9 (18.1,30.4) | 32.3 (21.8,55.7) | 0.002 |
| ESR(mm/hr) | 31 (13,54) | 50 (25,75) | 0.006 | 29 (13,52) | 49 (25,70) | 0.008 |
Data are shown for markers showing significant differences (Wilcoxon tests) after adjustment for multiple comparisons. Data for all markers are shown in Supplementary Tables 2–4. All values in ng/ml except as noted for ESR. VEGF = vascular endothelial growth factor; ICAM = intercellular adhesion molecule; MMP = matrix metalloproteinase; ESR = erythrocyte sedimentation rate.
Comparing GPA to MPA, anti-PR3 to anti-MPO ANCA, new to relapsing AAV, presence or absence of active renal disease, and treatment (any immune-suppressive treatment, or specifically glucocorticoids), at screening.
MMP3 and thrombomodulin were higher in patients with active renal disease than in those without (Table 3 and Supplementary Table 3). Since the effects of active renal disease are not easily distinguishable from effects of GFR with these data, the effect of GFR on levels of these markers among subjects in remission at month 6 was also measured. GFR affected levels of both thrombomodulin (0.54 ng/ml increase per 10 ml/min decrease in GFR) and MMP3 (2.1 ng/ml increase per 10 ml/min decrease in GFR)among subjects in remission. However, when these values were compared to the ranges of values seen in active AAV (see Table 1), GFR appeared to be a minor contributor to levels of MMP3.
Effects of Treatment
Subjects already being treated with immune-suppressive drugs at screening had lower levels of ESR, ICAM1, MMP1, thrombomodulin, and VEGF than did untreated subjects (Table 3 and Supplementary Table 4). Four of these markers (all except MMP1) were also higher in subjects with newly diagnosed AAV than in subjects with relapsing disease (Table 3 and Supplementary Table 3). Effects of treatment during the first 6 months were also evaluated. Among subjects in remission at month 6, only MMP1 differed between subjects who had received CYC/AZA and those who had received RTX (Supplementary Table 5). Subjects who had been placed back on prednisone (≤ 10 mg) had somewhat higher levels of MMP3 and MMP9 than subjects who remained off glucocorticoids (Supplementary Table 5). The most important conclusion to be drawn from these analyses is that the findings regarding MMP3 cannot be explained by direct effects of particular medications.
Correlations between Marker Levels
The correlation coefficients between levels of the marker that best distinguished between severe active disease and remission based on ROC analysis(MMP3) and established markers of inflammation were quite low (with ESR, r=−0.10 at screening; with CRP, r=−0.11 at screening)(Figure 3B). In contrast, ESR and CRP correlated relatively well with each other (r=0.56 at screening). No other correlations between pairs of markers exceeded r=0.4 (data not shown). Overall, these results suggest that MMP3 provides additional information about active AAV beyond what is obtained with ESR or CRP.
DISCUSSION
This investigation of a series of biomarkers related to microvascular damage and/or angiogenesis in a large group of patients with AAV demonstrated that many of the markers were elevated in patients with severe active AAV and declined significantly with treatment. Levels of MMP3 distinguished active AAV from remission better than did ESR and CRP, and levels of MMP3 correlated poorly with these two markers. MMP3 was also the only marker that appeared to be higher among the small number of patients with milder active disease at month 6 than among those patients in remission. These results indicate that serum MMP3 is particularly worthy of further study as an additional laboratory test to assist in the evaluation and management of patients with AAV.
Two markers differed significantly in patients stratified by the presence of active renal disease (MMP3 and thrombomodulin). However, since MMP3 was also highly associated with active AAV in general, and since thrombomodulin levels remained elevated in many patients who achieved remission, prospects for using these markers to help screen for active renal disease in AAV are limited. Since markers of damage to microvascular beds were chosen for study rather than markers of granulomatous disease, it was not surprising that levels of most markers were similar in patients with GPA or MPA. Among those that did differ, caution in interpretation may still be indicated (e.g., for thrombomodulin), since the prevalence of renal disease is higher in MPA than GPA.
The main strengths of this study derive from the use of a large cohort of patients with clinical data collected in a standardized manner at defined times. The study was large enough to allow comparison of the performance of experimental markers to each other and to that of established markers of inflammation.
A limitation of this study is the absence of detailed information about treatment, particularly the dosing of glucocorticoids at and shortly before the screening visit. However, it was encouraging that treatment (yes or no, either limited to glucocorticoids or including all immune-suppressive drugs) at the time of screening was not associated with higher levels of any marker. Association of treatment with lower levels of several markers indicated that if early treatment had any influence on the results, it was to bias the study against finding significant differences between active AAV and remission. Strictly speaking, this study (like most others) cannot distinguish between effects of successful treatment and direct effects of treatment independent of their success; only longitudinal studies, with repeated measurements from patients who either experience flares or remain in remission on various levels of treatment (as will be possible using future samples from RAVE), would allow for analysis of such distinctions. Finally, the study had limited power to assess marker levels during mild relapses and was not designed to assess prediction of future relapses, nor to distinguish AAV from other disease states that might elevate marker levels.
Two technical details merit discussion. First, in a separate study, the CV of measurement error for the MMP3 assay was 39.8%. However, large measurement errors bias against finding significant differences between groups or significant changes within a subject over time (23). More importantly MMP3 (using reagents developed for a commercial capture ELISA, R&D Systems) was included in a multiplex assay platform that was applied to the same set of samples (Monach, R. Warner, K. Johnson, et al., manuscript in preparation), and MMP3 levels obtained with these two different assays correlated highly with each other (R-squared = 0.91). Secondly, serum levels of MMP1, P-selectin, and VEGF are reported to be higher than plasma levels (24), probably because these proteins can be released from platelets; it is not clear whether serum or plasma levels of these markers will serve as better biomarkers of inflammatory microvascular diseases.
The source of circulating MMP3 in AAV is not apparent from review of the literature. MMP3 can be made by a wide range of cell types, including microvascular endothelial cells stimulated in vitro (25, 26). Study of MMP3 in the vasculature has focused on diseases of large arteries. Genetic polymorphisms in the MMP3 gene have been associated with multiple cardiovascular outcomes related to atherosclerosis (27), and with risk of Kawasaki disease or with aneurysm formation in that disease (28–30). Circulating MMP3 levels were elevated in a cohort of patients with Takayasu’s arteritis (31). Indeed, in one of the two studies in which MMP3 levels were measured and found to be elevated in patients with WG, the marker was chosen to address the hypothesis that WG leads to accelerated atherosclerosis (13, 14). Study of MMP3 in other autoimmune diseases has focused on its stronger association with inflammatory arthritis than with involvement of other organ systems (32). The latter study also highlighted the concern that glucocorticoid treatment may elevate levels of MMP3 (32). However, our data argue against glucocorticoids as the major driver of MMP3 levels in AAV.
Studies of patients with severe active disease are an appropriate initial screen for markers of interest in pursuing clinically relevant goals. Study of MMP3 and other promising biomarkers in samples collected during long-term follow-up in the RAVE trial and other cohorts will allow for additional evaluation of the clinical utility of these markers in combination with other clinical and laboratory data.
Supplementary Material
Supplementary Figure 1. Correlations between marker levels and apparent extent of active ANCA-associated vasculitis as measured by the BVAS/WG. Data are shown from the screening visit, at which each subject had at least one severe active manifestation and thus a score of at least 3. Spearman correlation coefficients and associated p-values are shown above each panel.
Supplementary Table 1. Marker levels in controls in this study, in literature from assay manufacturers, or a selection of relatively large studies in the scientific literature.
Supplementary Table 2. Marker levels inpatients stratified by AAV subtype or ANCA specificity. Medians and interquartile ranges are shown.
Supplementary Table 3. Marker levels in patients with a new or an established diagnosis of AAV, and in patients stratified by the presence or absence of active renal disease at screening. Medians and interquartile ranges are shown.
Supplementary Table 4. Marker levels in patients stratified by treatment status at screening. Medians and interquartile ranges are shown.
Supplementary Table 5. Marker levels in patients in remission at month 6, stratified by treatment.
Acknowledgments
The authors thank all of the members of the RAVE-ITN research group (see Appendix), and Mr. Roger Smith (Merck) for technical assistance.
Financial supports or conflicts disclosure:
This work was sponsored by the Vasculitis Clinical Research Consortium which has received support from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (U54AR057319 and 1RC1 AR058303), the National Center for Research Resources (U54 RR019497), the National Institute of Neurological Disorders and Stroke (NS064808), and the Office of Rare Diseases Research.
The Rituximab in ANCA-Associated Vasculitis(RAVE) trial was performed with the support of the Immune Tolerance Network (NIH Contract #N01 AI15416), an international clinical research consortium supported by the National Institute of Allergy and Infectious Diseases and the Juvenile Diabetes Research Foundation.. Genentech and Biogen Idec provided the study medications and partial funding. At the Mayo Clinic and Foundation, the trial was supported by a Clinical and Translational Science Award from the National Center for Research Resources (NCRR) (RR024150-01); at Johns Hopkins University, by grants from the NCRR (RR025005) and career development awards (K24 AR049185 to Dr. Stone, and K23 AR052820 to Dr. Seo); and at Boston University, by a Clinical and Translational Science Award (RR 025771), grants from the National Institutes of Health (M01 RR00533) and a career development award (K24 AR02224 to Dr. Merkel).
Dr. Monach was also supported by an Arthritis Investigator Award from the Arthritis
APPENDIX
Members of the RAVE-ITN Research Group are as follows: Protocol Co-chairs – U. Specks (Mayo Clinic), J.H. Stone (Massachusetts General Hospital); Mayo Clinic – U. Specks, S.R. Ytterberg, F.C. Fervenza, K.A. Keogh, T. Peikert, J.M. Golbin, L. Klein, K. Mieras, C. Beinhorn, S. Fisher, M.L. Clawson, S. Bendel, A.M. Hummel (Mayo Clinic Eisenberg Research Pharmacy); Boston University – P.A. Merkel, E.Y. Kissin, P.A. Monach, M.R. Clark-Cotton, C.A. McAlear, J.L. Pettit, M.B. Sutton, R.L. Widom, G.A. Farina, M.J. DiMarzio, S.P. Johnson, A. Schiller Patel; Johns Hopkins University – P. Seo, J.H. Stone, D. Hellmann, D. Geetha, A. Saleh, P. Wung, L.P. Sejismundo, C. Humphrey, M. Marriott, Y. Goldsborough, A. Pinachos, K. Gauss, L. King; Cleveland Clinic Foundation – C.A. Langford, G.S. Hoffman, R.A. Hajj-Ali, J.J. Carey, E.S. Molloy, C.L. Koening, D. Bork, T.M. Clark, K.A. Tuthill, T. Markle, J. Petrich; Hospital for Special Surgery – R. Spiera, D.R. Alpert, S.J. DiMartino, J.K. Gordon, N.K. Moskowitz, K.A. Kirou, J. Samuels, S.A. Kloiber, E. Julevic, M. O’Donohue, A. Patel; University of Groningen – C.G.M. Kallenberg, C. Stegeman, P. Rasker, K. Mulder, P. Limburg, J. Kosterink; Duke University – E.W. St. Clair, N.B. Allen, E. Scarlett, M. Tochacek; University of Alabama–Birmingham – A. Turkiewicz, B. Fessler, W. Chatham, A. Turner; Coordinating Centers: Rho – D. Ikle, D. Weitzenkamp, W. Wu, T. D’Lugin, C. Jacob; National Institute of Allergy and Infectious Diseases – L. Webber, L. Ding, S. Adah; Immune Tolerance Network – N.K. Tchao, M. Mueller, K. Bourcier, A. Asare, V. Seyfert-Margolis, P. Tosta, N.B. Skeeter, C.L. Anderson, A.N. Archampong.
References
- 1.Kerr GS, Fleisher TA, Hallahan CW, Leavitt RY, Fauci AS, Hoffman GS. Limited prognostic value of changes in antineutrophil cytoplasmic antibody titer in patients with Wegener’s granulomatosis. Arthritis Rheum. 1993;36(3):365–71. doi: 10.1002/art.1780360312. [DOI] [PubMed] [Google Scholar]
- 2.Kyndt X, Reumaux D, Bridoux F, Tribout B, Bataille P, Hachulla E, et al. Serial measurements of antineutrophil cytoplasmic autoantibodies in patients with systemic vasculitis. Am J Med. 1999;106(5):527–33. doi: 10.1016/s0002-9343(99)00064-9. [DOI] [PubMed] [Google Scholar]
- 3.Boomsma MM, Stegeman CA, van der Leij MJ, Oost W, Hermans J, Kallenberg CG, et al. Prediction of relapses in Wegener’s granulomatosis by measurement of antineutrophil cytoplasmic antibody levels: a prospective study. Arthritis Rheum. 2000;43(9):2025–33. doi: 10.1002/1529-0131(200009)43:9<2025::AID-ANR13>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 4.Girard T, Mahr A, Noel LH, Cordier JF, Lesavre P, Andre MH, et al. Are antineutrophil cytoplasmic antibodies a marker predictive of relapse in Wegener’s granulomatosis? A prospective study. Rheumatology (Oxford) 2001;40(2):147–51. doi: 10.1093/rheumatology/40.2.147. [DOI] [PubMed] [Google Scholar]
- 5.Finkielman JD, Merkel PA, Schroeder D, Hoffman GS, Spiera R, St Clair EW, et al. Antiproteinase 3 antineutrophil cytoplasmic antibodies and disease activity in Wegener granulomatosis. Ann Intern Med. 2007;147(9):611–9. doi: 10.7326/0003-4819-147-9-200711060-00005. [DOI] [PubMed] [Google Scholar]
- 6.Kalsch AI, Csernok E, Munch D, Birck R, Yard BA, Gross W, et al. Use of highly sensitive C-reactive protein for followup of Wegener’s granulomatosis. J Rheumatol. 2010;37(11):2319–25. doi: 10.3899/jrheum.100302. [DOI] [PubMed] [Google Scholar]
- 7.Hind CR, Winearls CG, Lockwood CM, Rees AJ, Pepys MB. Objective monitoring of activity in Wegener’s granulomatosis by measurement of serum C-reactive protein concentration. Clin Nephrol. 1984;21(6):341–5. [PubMed] [Google Scholar]
- 8.Sproson EL, Jones NS, Al-Deiri M, Lanyon P. Lessons learnt in the management of Wegener’s Granulomatosis: long-term follow-up of 60 patients. Rhinology. 2007;45(1):63–7. [PubMed] [Google Scholar]
- 9.Li CG, Reynolds I, Ponting JM, Holt PJ, Hillarby MC, Kumar S. Serum levels of vascular endothelial growth factor (VEGF) are markedly elevated in patients with Wegener’s granulomatosis. Br J Rheumatol. 1998;37(12):1303–6. doi: 10.1093/rheumatology/37.12.1303. [DOI] [PubMed] [Google Scholar]
- 10.Hergesell O, Andrassy K, Nawroth P. Elevated levels of markers of endothelial cell damage and markers of activated coagulation in patients with systemic necrotizing vasculitis. Thromb Haemost. 1996;75(6):892–8. [PubMed] [Google Scholar]
- 11.Boehme MW, Schmitt WH, Youinou P, Stremmel WR, Gross WL. Clinical relevance of elevated serum thrombomodulin and soluble E-selectin in patients with Wegener’s granulomatosis and other systemic vasculitides. Am J Med. 1996;101(4):387–94. doi: 10.1016/S0002-9343(96)00230-6. [DOI] [PubMed] [Google Scholar]
- 12.Iwakawa J, Matsuyama W, Kubota S, Mitsuyama H, Suetsugu T, Watanabe M, et al. Increased serum vascular endothelial growth factor levels in microscopic poly angiitis with pulmonary involvement. Respir Med. 2006;100(10):1724–33. doi: 10.1016/j.rmed.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 13.Bjerkeli V, Halvorsen B, Damas JK, Nordoy I, Yndestad A, Aukrust P, et al. Expressio’n of matrix metalloproteinases in patients with Wegener’s granulomatosis. Ann Rheum Dis. 2004;63(12):1659–63. doi: 10.1136/ard.2003.017954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Leeuw K, Sanders JS, Stegeman C, Smit A, Kallenberg CG, Bijl M. Accelerated atherosclerosis in patients with Wegener’s granulomatosis. Ann Rheum Dis. 2005;64(5):753–9. doi: 10.1136/ard.2004.029033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stone JH, Merkel PA, Spiera R, Seo P, Langford CA, Hoffman GS, et al. Rituximab versus cyclophosphamide for ANCA-associated vasculitis. N Engl J Med. 2010;363(3):221–32. doi: 10.1056/NEJMoa0909905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stone JH, Hoffman GS, Merkel PA, Min YI, Uhlfelder ML, Hellmann DB, et al. A disease-specific activity index for Wegener’s granulomatosis: modification of the Birmingham Vasculitis Activity Score. International Network for the Study of the Systemic Vasculitides (INSSYS) Arthritis Rheum. 2001;44(4):912–20. doi: 10.1002/1529-0131(200104)44:4<912::AID-ANR148>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 17.Kokkotou E, Conboy LA, Ziogas DC, Quilty MT, Kelley JM, Davis RB, et al. Serum correlates of the placebo effect in irritable bowel syndrome. Neurogastroenterol Motil. 2010;22(3):285–e81. doi: 10.1111/j.1365-2982.2009.01440.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grip O, Janciauskiene S. Atorvastatin reduces plasma levels of chemokine (CXCL10) in patients with Crohn’s disease. PLoS One. 2009;4(5):e5263. doi: 10.1371/journal.pone.0005263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Friese RS, Rao F, Khandrika S, Thomas B, Ziegler MG, Schmid-Schonbein GW, et al. Matrix metalloproteinases: discrete elevations in essential hypertension and hypertensive end-stage renal disease. Clin Exp Hypertens. 2009;31(7):521–33. doi: 10.3109/10641960802668730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145(4):247–54. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 21.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Royal Stat Soc B. 1995;57(1):289–300. [Google Scholar]
- 22.Youden WJ. Index for rating diagnostic tests. Cancer. 1950;3(1):32–5. doi: 10.1002/1097-0142(1950)3:1<32::aid-cncr2820030106>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 23.Harris EK. Statistical principles underlying analytic goal-setting in clinical chemistry. Am J Clin Pathol. 1979;72(2 Suppl):374–82. [PubMed] [Google Scholar]
- 24.R&D Systems. www.rndsystems.com.
- 25.Hanemaaijer R, Koolwijk P, le Clercq L, de Vree WJ, van Hinsbergh VW. Regulation of matrix metalloproteinase expression in human vein and microvascular endothelial cells. Effects of tumour necrosis factor alpha, interleukin 1 and phorbol ester. Biochem J. 1993;296 ( Pt 3):803–9. doi: 10.1042/bj2960803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cornelius LA, Nehring LC, Roby JD, Parks WC, Welgus HG. Human dermal microvascular endothelial cells produce matrix metalloproteinases in response to angiogenic factors and migration. J Invest Dermatol. 1995;105(2):170–6. doi: 10.1111/1523-1747.ep12317080. [DOI] [PubMed] [Google Scholar]
- 27.Ye S. Influence of matrix metalloproteinase genotype on cardiovascular disease susceptibility and outcome. Cardiovasc Res. 2006;69(3):636–45. doi: 10.1016/j.cardiores.2005.07.015. [DOI] [PubMed] [Google Scholar]
- 28.Park JA, Shin KS, Kim YW. Polymorphism of matrix metalloproteinase-3 promoter gene as a risk factor for coronary artery lesions in Kawasaki disease. J Korean Med Sci. 2005;20(4):607–11. doi: 10.3346/jkms.2005.20.4.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ikeda K, Ihara K, Yamaguchi K, Muneuchi J, Ohno T, Mizuno Y, et al. Genetic analysis of MMP gene polymorphisms inpatients with Kawasaki disease. Pediatr Res. 2008;63(2):182–5. doi: 10.1203/PDR.0b013e31815ef224. [DOI] [PubMed] [Google Scholar]
- 30.Shimizu C, Matsubara T, Onouchi Y, Jain S, Sun S, Nievergelt CM, et al. Matrix metalloproteinase haplotypes associated with coronary artery aneurysm formation in patients with Kawasaki disease. J Hum Genet. 2010;55(12):779–84. doi: 10.1038/jhg.2010.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matsuyama A, Sakai N, Ishigami M, Hiraoka H, Kashine S, Hirata A, et al. Matrix metalloproteinases as novel disease markers in Takayasu arteritis. Circulation. 2003;108(12):1469–73. doi: 10.1161/01.CIR.0000090689.69973.B1. [DOI] [PubMed] [Google Scholar]
- 32.Ribbens C, Martin y Porras M, Franchimont N, Kaiser MJ, Jaspar JM, Damas P, et al. Increased matrix metalloproteinase-3 serum levels in rheumatic diseases: relationship with synovitis and steroid treatment. Ann Rheum Dis. 2002;61(2):161–6. doi: 10.1136/ard.61.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meso Scale Discovery. www.meso-scale.com.
- 34.eBioscience. www.ebioscience.com.
- 35.IBL. www.ibl-america.com.
- 36.Invitrogen. www.invitrogen.com.
- 37.Korybalska K, Pyda M, Kawka E, Grajek S, Breborowicz A, Witowski J. Interpretation of elevated serum VEGF concentrations in patients with myocardial infarction. Cytokine. 2011;54(1):74–8. doi: 10.1016/j.cyto.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 38.Kut C, Mac Gabhann F, Popel AS. Where is VEGF in the body? A meta-analysis of VEGF distribution in cancer. Br J Cancer. 2007;97(7):978–85. doi: 10.1038/sj.bjc.6603923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rho YH, Chung CP, Oeser A, Solus J, Asanuma Y, Sokka T, et al. Inflammatory mediators and premature coronary atherosclerosis in rheumatoid arthritis. Arthritis Rheum. 2009;61(11):1580–5. doi: 10.1002/art.25009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ponthieux A, Herbeth B, Droesch S, Haddy N, Lambert D, Visvikis S. Biological determinants of serum ICAM-1, E-selectin, P-selectin and L-selectin levels in healthy subjects: the Stanislas study. Atherosclerosis. 2004;172(2):299–308. doi: 10.1016/j.atherosclerosis.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 41.Martin S, Rieckmann P, Melchers I, Wagner R, Bertrams J, Voskuyl AE, et al. Circulating forms of ICAM-3 (cICAM-3). Elevated levels in autoimmune diseases and lack of association with cICAM-1. J Immunol. 1995;154(4):1951–5. [PubMed] [Google Scholar]
- 42.Young-Min SA, Beeton C, Laughton R, Plumpton T, Bartram S, Murphy G, et al. Serum TIMP-1, TIMP-2, and MMP-1 in patients with systemic sclerosis, primary Raynaud’s phenomenon, and in normal controls. Ann Rheum Dis. 2001;60(9):846–51. [PMC free article] [PubMed] [Google Scholar]
- 43.Manicourt DH, Fujimoto N, Obata K, Thonar EJ. Serum levels of collagenase, stromelysin-1, and TIMP-1. Age-and sex-related differences in normal subjects and relationship to the extent of joint involvement and serum levels of antigenic keratan sulfate in patients with osteoarthritis. Arthritis Rheum. 1994;37(12):1774–83. doi: 10.1002/art.1780371211. [DOI] [PubMed] [Google Scholar]
- 44.Hlatky MA, Ashley E, Quertermous T, Boothroyd DB, Ridker P, Southwick A, et al. Matrix metalloproteinase circulating levels, genetic polymorphisms, and susceptibility to acute myocardial infarction among patients with coronary artery disease. Am Heart J. 2007;154(6):1043–51. doi: 10.1016/j.ahj.2007.06.042. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Correlations between marker levels and apparent extent of active ANCA-associated vasculitis as measured by the BVAS/WG. Data are shown from the screening visit, at which each subject had at least one severe active manifestation and thus a score of at least 3. Spearman correlation coefficients and associated p-values are shown above each panel.
Supplementary Table 1. Marker levels in controls in this study, in literature from assay manufacturers, or a selection of relatively large studies in the scientific literature.
Supplementary Table 2. Marker levels inpatients stratified by AAV subtype or ANCA specificity. Medians and interquartile ranges are shown.
Supplementary Table 3. Marker levels in patients with a new or an established diagnosis of AAV, and in patients stratified by the presence or absence of active renal disease at screening. Medians and interquartile ranges are shown.
Supplementary Table 4. Marker levels in patients stratified by treatment status at screening. Medians and interquartile ranges are shown.
Supplementary Table 5. Marker levels in patients in remission at month 6, stratified by treatment.