Abstract
Women with systemic lupus erythematosus (SLE) have increased risk for coronary heart disease (CHD) which is underestimated by the Framingham risk score (FRS). We hypothesized that new risk scores that include inflammation or vascular age in the risk calculation would better identify women with SLE at risk for CHD, particularly in those with subclinical coronary atherosclerosis. We calculated the FRS and Reynolds risk score (RRS) in 121 women with SLE and 65 age-matched female controls; coronary age-modified risk scores (camFRS, camRRS) were calculated using coronary age derived from the coronary artery calcium (CAC) score. Risk scores were compared in SLE and controls, and in SLE patients with and without CAC. Although CAC was present in 21 SLE patients (17%) and 4 controls (6%) (P=0.033); the FRS, camFRS, RRS, and camRRS, did not differ significantly among SLE and controls (P>0.05), but were all significantly higher in SLE patients with CAC compared to those without (P< 0.001 for all). The cam-FRS (8%, P=0.016) but not cam-RRS (5%, P=0.221) assigned significantly more SLE patients to a category of ≥10% risk than conventional FRS (1%) and RRS (2%). The RRS was of limited use but coronary age may improve CHD risk prediction in SLE.
Keywords: SLE, cardiovascular risk, atherosclerosis
Introduction
Systemic lupus erythematosus is associated with premature atherosclerosis1,2 and an increased prevalence of coronary heart disease.3 Lupus affects predominantly young women, and the risk of CHD relative to age-matched control subjects is higher in younger women with SLE. For example, the risk of CHD was increased 52-fold in women with lupus who were 35 to 44 years old and 2–4 fold in those 45 to 64 years old, compared to women of similar age without SLE.4 Nevertheless, although the relative risk is high, because coronary events are rare in healthy young women, the average absolute risk in women with SLE remains low. Therefore strategies are needed to identify those patients at greatest risk for risk factor modification or other therapy.
Large epidemiologic studies of cardiovascular outcomes in the general population have defined the predictive contribution of individual and combined cardiovascular risk factors and provided composite measures to assess future cardiovascular risk.5,6 For example, the Framingham risk score (FRS) is widely used to stratify asymptomatic patients into different cardiovascular risk categories in order to target the intensity of primary medical intervention.7,8 The National Cholesterol Education Program (NCEP) has recommended that a FRS score of ≥10% be considered as a threshold for potential drug therapy initiation.7
The FRS, which is strongly influenced by age, has limited usefulness in patients with SLE who are primarily relatively young.9 We have reported that the FRS categorized 99% of women in our cohort of lupus patients as being at low-risk, with a median 10-year predicted risk of 1%; furthermore, the FRS was not significantly different in women with SLE and control subjects, despite more prevalent and severe coronary artery atherosclerosis in lupus.10
The limitations of the FRS in predicting cardiovascular risk, particularly in women and in younger populations, have also been recognized in the general population.11–14 Efforts to improve cardiovascular risk prediction have centered on two major modifications to existing models: 1) the incorporation of biomarkers, particularly markers of inflammation such as C-reactive protein (CRP) into models that predict cardiovascular risk, and 2) the incorporation of information provided by non-invasive measures of coronary atherosclerosis such as coronary artery calcification (CAC).
Accordingly, a new cardiovascular risk score, the Reynolds Risk score (RRS), incorporates CRP concentration in the risk model and reclassifies approximately 50% of women in the 10-year FRS 5–20% risk category into different risk categories.15 A second novel approach is to measure CAC, calculate the age associated with this CAC score, and use this “coronary age” in place of the patient’s chronological age in the risk score calculations.16–18
These new approaches developed to predict CHD risk in the general population are particularly attractive in application to SLE because most patients are young women, and the illness is associated with inflammation and increased CAC.1 Therefore, we examined two hypotheses: 1) that the RRS is higher in patients with SLE than controls and increases the proportion of lupus patients assigned to moderate and high risk categories compared to the traditional FRS, and 2) that substituting coronary age in the place of chronological age in the conventional FRS and RRS risk score calculations alters risk stratification in patients with SLE.
Methods
Design and study participants
A cohort of patients with SLE and control subjects, frequency-matched for age, race and sex, are participating in an ongoing study of the mechanisms of atherosclerosis in SLE; detailed methods describing recruitment and measurement of clinical and biochemical variables have been described.10 For the present analysis we included women with SLE and female controls ≥18 years old who had no history of diabetes (defined as the use of anti-diabetic medications or a fasting blood glucose ≥ 126 mg/dL) and no previous cardiovascular event (myocardial infarction, angina, or stroke) or cardiovascular procedure (coronary artery bypass or graft). The presence of any of these conditions automatically places a patient into a high risk group (10-year risk >20%) and our objective was to evaluate if the RRS performed better than the FRS in identifying patients with subclinical atherosclerosis who were not already known to have high CHD risk. Patients met the classification criteria for SLE19 with disease duration of at least one year, and controls had no inflammatory rheumatic disease. The study was approved by the Institutional Review Board at Vanderbilt University and all subjects provided written informed consent.
Clinical assessment
Patients with SLE and controls were evaluated using a standardized clinical interview, physical examination, laboratory tests, and review of medical records as described in detail previously.1,10 Parental history of cardiovascular disease (myocardial infarction or stroke) before the age of 65 was recorded. We measured height and weight, and body mass index (BMI) was calculated by dividing the weight in kilograms by the square of height in meters. Blood pressure was recorded as the average of two consecutive measurements performed 5 minutes apart after subjects had rested supine for 10 minutes.
Coronary artery calcium assessment
Electron beam tomography (EBT) was performed as described previously.1 An Imatron C-150 scanner (GE Imatron, South San Francisco, CA) was used in all the controls and in 96 patients with SLE; a 64-row multidetector CT (LightSpeed VCT, GE, Milwaukee, WI) was used in 25 patients with SLE. All the scans were scored, as described by Agatston et al.20 by a single experienced investigator (PR) unaware of the subjects’ clinical status. Agatston scores above zero were considered positive for CAC.
Laboratory tests
A fasting blood sample was collected and total cholesterol, high-density and low-density lipoprotein cholesterol, and triglyceride concentrations measured in the hospital clinical laboratory. C-reactive protein (CRP) was measured by the hospital clinical laboratory in patients with SLE. For 41 patients with SLE who had CRP concentrations below 3 mg/dl, and for all the controls, a high sensitivity CRP (hsCRP) assay was performed using ELISA (Millipore) with a lower sensitivity limit of 0.125 mg/dl.
Cardiovascular risk scores
Framingham Risk Score (FRS)
The 10-year risk (%) of having a hard coronary event (myocardial infarction or coronary death) was calculated using the ATP III algorithm.7 The model includes age, sex, smoking status, systolic blood pressure, treatment for hypertension, and total and HDL cholesterol concentrations. The FRS classifies individuals as having low (<10%), intermediate (≥10% to <20%) or high (≥20%) 10-year risk. The risk score for a 19 year-old subject was calculated using the values for a 20-year old woman.
Reynolds Risk Score (RRS)
The RRS calculates the 10-year risk (%) of having myocardial infarction, ischemic stroke, coronary revascularization or cardiovascular death.15 The model includes age, sex, systolic blood pressure, total and HDL cholesterol concentrations, smoking status, hsCRP concentration, and parental history of myocardial infarction before the age of 60 years. The RRS classifies individuals as having low (<10%), intermediate (≥10% to <20%) or high (≥20%) 10-year predicted risk. The RRS was calculated using the published formula for women.15
Cardiovascular scores modified according to coronary age
Coronary age was calculated from the CAC score as described by McClelland.17 The prevalence and severity of CAC increases with age. This relationship, defined in large populations, can be used to infer a modified chronological age for an individual that is termed coronary age. For example, a CAC score of 100 Agatston units results in an estimated coronary age of 73 years (95% confidence interval 71–76).17 Therefore, if a 40 year old woman (who would be expected to have a CAC of 0 Agatson units) has a score of 100 units, her coronary age would be 73 years17. The calculated coronary age can also be younger than the chronological age in individuals who have a CAC score that is lower than expected. For example, a CAC score of 10 Agatston units results in an estimated coronary age of 56 years (95CI % 53–60), and if a 73 year old woman had a CAC score of 10, her coronary age would be 56 years.17
Coronary calcification is not usually present in healthy women younger than 39 years of age.21–24 If a subject was ≤39 years of age and had no coronary calcium, the chronological age was used as the coronary age. If a woman with a chronological age >39 years had no coronary calcium, the assigned coronary age was 39 years.17 When the coronary age was >79 years, we assigned the highest number of points in the Framingham model (16 points) for the age component.
The coronary age-modified FRS (camFRS) and RRS (camRRS) were calculated by replacing the chronological age with coronary age rounded to the next integer. Other risk factors that are scored according to age in the FRS (lipid profile and smoking status) were scored using the chronological age.
In order to compare the performance of the 10-year risk scores (FRS, RRS, camFRS and camRRS), we assigned a risk score of 30% to individuals who reached or exceeded this risk level since the FRS risk estimates above this level are reported as ≥30%.
Statistical Analysis
Demographic and clinical characteristics were described as frequencies and proportion of categorical variables, or median with interquartile ranges [IQR] for continuous variables. Clinical characteristics were compared using Wilcoxon rank sum tests for continuous variables and Pearson Chi-square test for categorical variables. Participants were classified as <10% risk and ≥10% risk using the 10-year cardiovascular scores; comparisons between conventional risk scores and corresponding coronary-age modified risk scores were assessed by McNemar’s test. To assess whether having SLE or not (disease status) was an effect modifier of the association between the 10-year risk scores and the severity of coronary calcification, we used the proportional odds model with cross-product term of disease status and the 10-year risk scores (FRS or RRS). Statistical analysis was performed using R 2.10.0 and a two-sided 5% significance level was considered significant.
Results
The clinical characteristics of the study population are described in Table 1. As described previously in this cohort,10 women with SLE had lower total and LDL-cholesterol and higher triglyceride concentrations compared to controls. We show in Table 1 that 8% (10/121) patients with SLE and 8% (5/65) controls were receiving statins. Statin use was more common in lupus patients with atherosclerosis than those without (19% vs. 6%, P value=0.05). When we excluded patients and controls who were receiving statins from the analysis, the median level (IQR) of LDL cholesterol was 95.0 (77.5–124.5 mg/dL) in patients with SLE and 108.0 (88.8–137.3 mg/dL) in controls (P=0.02); total cholesterol and HDL cholesterol were not statistically significant (P=0.10 and P=0.64, respectively). Women with SLE were more likely to use antihypertensive medications, and concentrations of CRP and CAC scores were higher than in controls (P=0.002, P<0.001 and P=0.027 respectively).
Table 1.
Prevalence of traditional cardiovascular risk factors and biologic measurements in women with SLE and female controls
| Controls (n=65) |
SLEs (n=121) |
P* | |
|---|---|---|---|
| Demographics | |||
| Age (years) | 41 (32–46) | 39 (30–46) | 0.470 |
| Caucasian (%) | 48 (74%) | 81 (67%) | 0.330 |
| Disease duration (years) | NA | 6.0 (3.0–11.0) | --- |
| Lipid profile | |||
| Total cholesterol (mg/dL) | 184 (161–206) | 163 (138–203) | 0.031 |
| High-density lipoprotein cholesterol (mg/dL) | 47 (40–61) | 49 (37–56) | 0.776 |
| Low-density lipoprotein cholesterol (mg/dL) | 112 (89–137) | 92 (76–124) | 0.005 |
| Triglycerides (mg/dL) | 77 (61–108) | 96 (71–146) | 0.008 |
| Current use of statins | 5 (8%) | 10 (8%) | 0.879 |
| Other cardiovascular risk factors | |||
| Systolic blood pressure (mmHg) | 114 (106–127) | 115 (106–124) | 0.724 |
| Diastolic blood pressure (mmHg) | 70.0 (65.0–78.0) | 71.5 (65.0–78.5) | 0.606 |
| Body mass index (kg/m2) | 24.9 (22.0–30.3) | 26.6 (23.2–32.5) | 0.110 |
| Use of anti-hypertensive drugs | 7 (11%) | 37 (31%) | 0.002 |
| Current smoking status | 11 (17%) | 25 (21%) | 0.538 |
| Family history of coronary heart disease | 9 (14%) | 21 (17%) | 0.535 |
| C-reactive protein (mg/L) | 0.57 (0.17–2.48) | 3.00 (0.68–6.00) | <0.001 |
| Presence of subclinical atherosclerosis¶ | 4 (6%) | 21 (17%) | 0.033 |
Data are presented as median (interquartile range) for continuous variables, count (percentage) for categorical variables.
Subclinical atherosclerosis present if coronary artery calcium >0 Agatston units
Wilcoxon’s rank sum test was used for comparing continuous variables, and percentages were compared using the chi-square test.
Comparison of Risk Scores in Patients with SLE and Controls
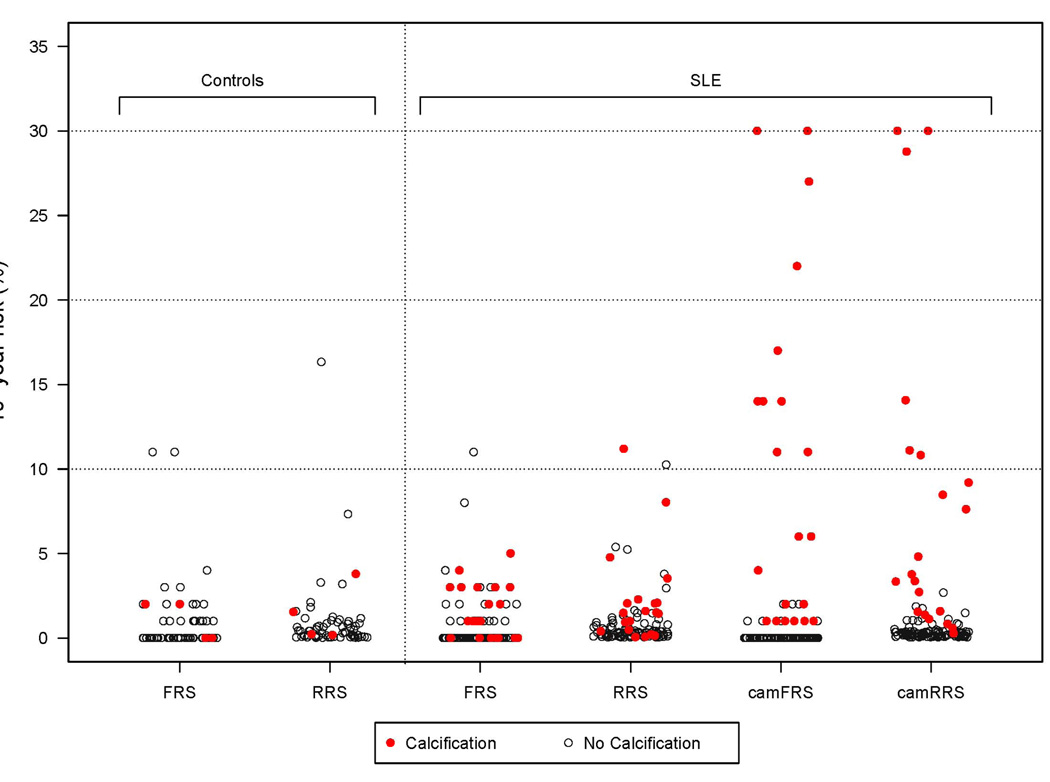
None of the risk scores differed significantly among women with SLE and controls (P>0.05 for all). As we have reported previously,10 the FRS assigned almost all controls and women with SLE to the low risk category (<10%) (Figure 1); only 3% (2/65) of controls and 1% (1/121) of women with SLE fell into the intermediate risk (≥10% to <20%) (Table 2). The RRS assigned 1.5% (1/65) of controls and 2% (2/121) of women with SLE to intermediate risk (≥10% to <20%) (Table 2). Neither the FRS nor the RRS score assigned any control or lupus patient to high risk (≥20%) (Figure 1).
Figure 1. 10-year risk estimates (%) in women with SLE and age-matched controls.
Subjects with coronary calcium are represented by red filled circles, and those without coronary calcium are represented by black empty circles. FRS= 10-year Framingham risk score; RRS: 10-year Reynolds risk score; camFRS: coronary age-modified FRS; cam-RRS: coronary age-modified RRS.
Table 2.
Cardiovascular risk estimates in women with SLE and female controls
| Cardiovascular risk scores | Controls (n=65) |
SLE (n=121) |
P- value* |
||||
|---|---|---|---|---|---|---|---|
| Median risk (IQR) |
<10% risk n (%) |
≥10% risk n (%) |
Median risk (IQR) |
<10% risk n (%) |
≥10% risk n (%) |
||
| 10 year Framingham risk (%) | 0 (0–1) | 63 (97%) | 2 (3%) | 0 (0–1) | 120 (99%) | 1 (1%) | 0.245 |
| 10 year Framingham coronary age-modified risk (%) | 0 (0–0) | 63 (97%) | 2 (3%) | 0 (0–0) | 111 (92%) | 10 (8%) | 0.170 |
| 10 year Reynolds risk (%) | 0.3 (0.1–0.9) | 64 (98.5%) | 1 (1.5%) | 0.3 (0.1–0.9) | 119 (98%) | 2 (2%) | 0.953 |
| 10 year Reynolds coronary age-modified risk (%) | 0.3 (0.1–0.5) | 64 (98.5%) | 1 (1.5%) | 0.3 (0.1–0.8) | 115 (95%) | 6 (5%) | 0.243 |
Data are presented as median and interquartile range (IQR) for 10-year risk.
Pearson Chi-square test was used to compare the proportions of subjects with risk <10% and ≥10% in SLE vs. Controls
The coronary age modified risk scores tended to be higher in women with SLE than controls (camFRS P=0.072; camRRS P=0.091) but these differences were not significant. Eight percent (10/121) of women with SLE and 3% (2/65) of controls had a camFRS score of ≥10% (p=0.170) (Table 2). Compared to the conventional FRS (1/121; 1%), the camFRS assigned more patients with SLE (10/121; 8%) to a risk category of ≥10% (P=0.016). Five percent (6/121) of women with SLE and 1.5% (1/65) of controls had a camRRS score ≥10% (P=0.243) (Table 2). Compared to the conventional RRS (2/121; 2%), the camRRS assigned 6/121 (5%) patients with SLE to a risk category of ≥10% (P=0.221).
The interaction between disease status, conventional 10-year risk scores and CAC severity was not significant for FRS (P=0.237) and RRS (P=0.156), likely due to the small number of participants with CAC.
Coronary Age versus Chronological Age
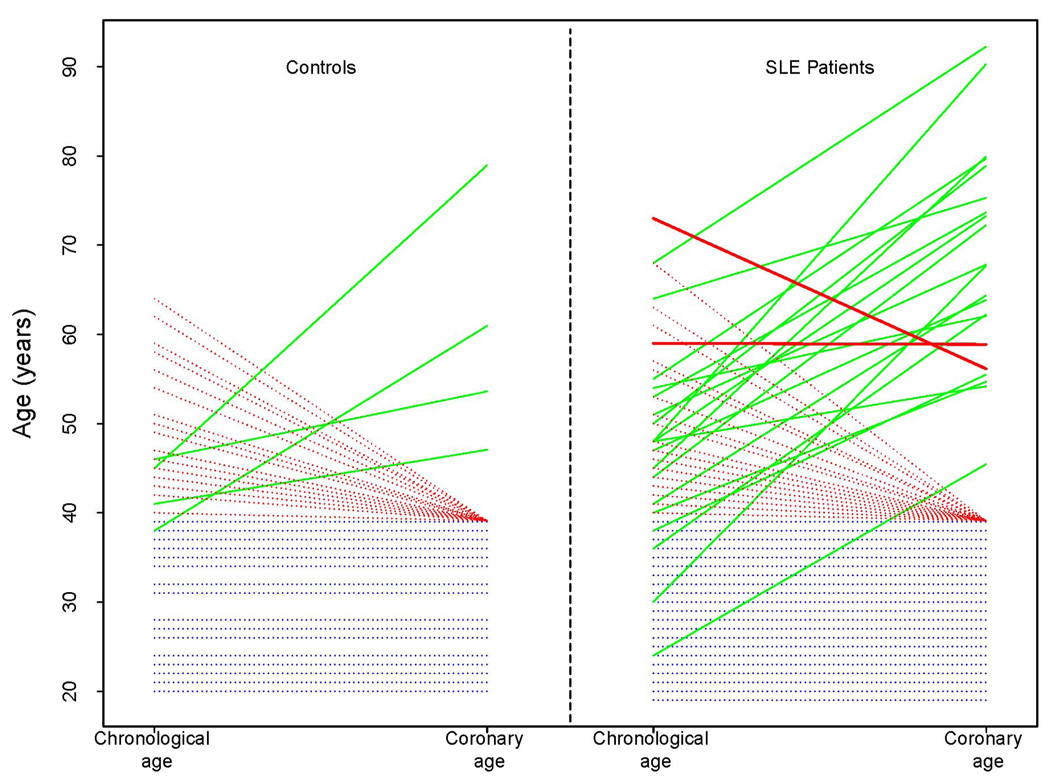
Subclinical atherosclerosis, characterized by the detection of CAC, was present in 21/121(17%) of women with SLE and in 4/65 (6%) of the controls (Table 1). One patient with SLE had a CAC score that was appropriate for her chronological age, and another had a CAC score that corresponded to a lower coronary age compare to her chronological age (17 years of difference). In all the remaining women with subclinical atherosclerosis, the CAC scores were higher than expected for their age range which resulted in higher coronary ages compared to their chronological ages (Figure 2).
Figure 2. Relationship between chronological age and coronary age among controls and patients with SLE.
Each line represents a single subject. Blue dotted lines represent subjects with a chronological age ≤ 39 years old who did not have coronary calcium and therefore chronological age = coronary age. Red dotted lines represent subjects with a chronologic age >39 years without coronary calcium. Solid lines represent subjects with coronary calcium; solid green lines represent those with chronological age < coronary age, and solid red lines those with chronological age ≥ coronary age.
Risk Scores in Patients with SLE with and without Coronary Artery Calcification Chronological Age
Among the 21 SLE patients with CAC, twenty (95%) had a CAC score above the 75th age-adjusted percentile, and 13/20 (65%) patients had at least one major cardiovascular risk factor (cigarette smoking, blood pressure ≥140/90 mmHg or on antihypertensive medication, HDL cholesterol <40 mg/dL, coronary heart disease in female first-degree relative <65 years, women ≥55 years). All cardiovascular risk scores were significantly higher among patients with CAC compared to those without (P<0.001 for all) (Table 3). However, the FRS assigned none of the 21 patients with SLE who had CAC to the intermediate risk (≥10% to <20%) category; 1/100 (1%) of patients without CAC was assigned to the intermediate risk category (Table 3) (Figure 1). The RRS assigned 1/21 (5%) patients with CAC and 1/100 (1%) without CAC to the intermediate risk category (Table 3) (Figure 1). Moreover, the FRS and RRS did not assign the same patients to the intermediate risk category.
Table 3.
Cardiovascular risk scores in SLE patients with CAC and without
| Cardiovascular risk scores | SLE without CAC (n=100) |
SLE with CAC (n=21) |
P- value* |
||||
|---|---|---|---|---|---|---|---|
| Median 10-year risk (%) |
<10% risk n (%) |
≥10% risk n (%) |
Median 10-year risk (%) |
<10% risk n (%) |
≥10% risk n (%) |
||
| • 10 year Framingham risk | 0 (0–0) | 99 (99%) | 1 (1%) | 1 (0–3) | 21 (100%) | 0 (0%) | 0.645 |
| • 10 year Framingham coronary age-modified risk | 0 (0–0)¶ | 100 (100%) | 0 (0%) | 6 (1–14)¶ | 11 (52%) | 10 (48%) | <0.0001 |
| • 10 year Reynolds risk | 0.3 (0.1–0.6) | 99 (99%) | 1 (1%) | 1.5 (0.4–2.1) | 20 (95%) | 1 (5%) | 0.219 |
| • 10 year Reynolds coronary age-modified risk | 0.2 (0.1–0.4) ¶ | 100 (100%) | 0 (0%) | 3.8 (1.6–10.8) ¶ | 15 (71%) | 6 (29%) | <0.0001 |
Data are presented as median (interquartile range) for 10-year risk.
Pearson Chi-square test was used to compare proportions of patients with SLE with risk <10% and ≥10% in those with CAC and without CAC.
P < 0.001 for Wilcoxon signed rank test for comparisons between conventional risk scores and coronary age-modified risk scores (e.g. FRS vs. camFRS, etc)
Coronary Age Modified Risk Scores in Patients with SLE with and without Coronary Artery Calcification
Compared to the conventional FRS and RRS, coronary age-modified risk scores decreased significantly in lupus patients without CAC, but increased significantly in those with CAC (Table 3). The camFRS reassigned 6/21 (29%) SLE patients with CAC from FRS low risk (<10%) to intermediate risk (10% to <20%) and 4/21 (19%) from FRS low risk to high risk (≥20%) (Figure 1). All of the patients assigned to a higher risk had an original FRS between 0% and 5%. The one patient that was categorized as intermediate risk with the original FRS (FRS=11%) had no CAC and shifted to low risk with a camFRS of 2%. In total, the camFRS assigned 10/21 (48%) patients with CAC and 0/100 (0%) without CAC to a risk category of ≥10% (Table 3) (Figure 1).
The camRRS reassigned 3/21 (14%) of patients with CAC from RRS low risk to intermediate risk, 2/21 (10%) from low risk to high risk, and 1/21 (5%) from intermediate risk to high risk (Figure 1). One (1%) patient with a RRS of 10% shifted to low risk with a camRRS of 2%; this patient did not have CAC. In total, the camRRS assigned 6/21 (29%) with CAC and 0/100 (0%) without CAC, to a risk category of ≥10% (Table 3) (Figure 1).
Discussion
Our major findings regarding the use of novel cardiovascular risk prediction models in SLE are: 1) despite having increased cardiovascular risk4,25 and accelerated atherosclerosis,1,2 women with SLE and age-matched controls have similar risk scores irrespective of the cardiovascular risk model used; 2) in SLE patients, the 10-year risk estimates were significantly higher in those with CAC compared to those without, but in almost all patients these were below the 10% threshold for 10-year risk often used to guide treatment intervention; 3) risk scores that used coronary age in place of chronological age assigned 30–50% of patients with CAC to the ≥10 % 10-year predicted risk category.
As we have reported previously,10 the FRS performed poorly, assigning low risk estimates to almost all patients with SLE, even those with CAC. Thus, new risk models such as the Reynolds risk score (RRS) that include a measure of inflammation (CRP), are attractive alternatives in predicting cardiovascular risk in SLE patients. We hypothesized that the RRS would be higher in women with lupus than control subjects. However, the RSS was similar in patients with lupus and controls and assigned low risk estimates to almost all subjects.
The reason that the RRS does not differ markedly in patients with lupus and controls is that elevated CRP concentrations do not have a large effect on the risk estimate in the absence of major risk factors because risk is calculated from the interaction between multiple risk factors.6,8 For example, the median values for risk factors in our patients with SLE (40 year old non-smoking woman, SBP 115 mmHg, total cholesterol 163 mg/dL, HDL cholesterol 49 mg/dL, and no parental history of myocardial infarction) result in a RRS between 0.1% and 0.3% for a CRP concentration between 0.5 mg/L and 20 mg/L. If instead we consider a 73 year old woman with the same measurements, the RRS ranges from 10% to 18% for a CRP concentration between 0.5 mg/L and 20 mg/L. In the general population, the RRS improved risk stratification most in individuals with FRS in the 5–20% range;15 however, in our population only 2.5% of patients with SLE fell into this category.
The analysis examining the effect modification role of disease status on the 10-year risk and coronary calcification suggested a differential relationship with SLE patients having more severe calcium for any given score compare to controls; however the low number of participants with CAC, especially among controls, limited the power of our analysis (P interaction, 0.237 and 0.156 for FRS and RRS, respectively).
The FRS and RRS 10-year risk models are useful to identify individuals that require therapy but these short-term models neglect the cumulative damage of modifiable risk factors26 and underestimate risk in young patients.26,27 In order to improve risk prediction in the general population, measures of vascular age have been examined for prediction efficacy.
Increased atherosclerosis, detected as CAC, is a robust finding in lupus,1 suggesting that risk scores that incorporate this information may be used to improve risk stratification. The amount of CAC increases with age and is correlated with the amount of atherosclerosis and cardiovascular risk. 28,29 CAC and traditional risk scores, such as the FRS, provide independent information about cardiovascular risk.7,30 One approach to incorporating the information provided by both CAC and FRS into risk prediction is to calculate coronary age from the CAC score, and to use it, rather than chronological age, in risk score calculations. As we have reported previously, this approach increased 10-year risk estimates in SLE patients with CAC.10 In the present study we used a model that was derived from a population-based sample from six communities in the United States, the Multi-Ethnic Study of Atherosclerosis (MESA) study.17 In that population replacing chronological age with coronary age in the risk estimation model improved the ability of the FRS score to predict future cases of cardiovascular events with the area under the receiver operating characteristic curve increasing from 0.75 to 0.79 (P<0.001)17.
We found that the coronary age- modified FRS assigned 8% of patients with SLE, and 48% of those with CAC, to a 10-year risk category of >10%. This suggests that strategies that use coronary age rather than chronological age in risk score calculations may identify patients with higher cardiovascular risk more accurately. One could argue that using coronary age, a variable that is derived from CAC scores, in risk models is a circular process. However, it is important to recognize (as shown in Figure 2) that the presence of CAC does not automatically increase coronary age and therefore predicted risk. A CAC score higher than expected for a particular age would indeed result in a coronary age higher than the chronological age. However, CAC scores equal or lower than predicted for age would result in coronary age equal or lower than the chronological age, as happened in two patients with SLE who had subclinical atherosclerosis.
Another way to use CAC to assess cardiovascular risk is to determine the severity of CAC relative to age and sex-appropriate values. The National Cholesterol Education Program (NCEP) ATP III guidelines suggest that a CAC score ≥75th sex-age-related percentile is an indication of advanced coronary atherosclerosis and requires preventive therapy if other risk factors are present.7 In our study 95% of lupus patients with CAC (20/21) had scores ≥75th sex-age-related percentile, and 65% of these patients (13/20) had at least one major CHD risk factor. The use of the 75th percentile CAC score as a risk threshold would identify more patients than the coronary age modified risk scores; however the ability of these different approaches to predict hard cardiovascular outcomes is unknown.
The role of coronary artery calcium (CAC) screening for stratification of cardiovascular risk in patients with SLE is not known. Because CAC screening is expensive and exposes patients to radiation, current consensus statements consider CAC measurement reasonable only in asymptomatic patients at intermediate risk (10-year risk >10% and <20%).31 This guidance would mean that CAC measurement would not be indicated in most women with lupus, since the vast majority are at low risk (<10% 10-year risk) by conventional cardiovascular risk prediction models. However, conventional risk prediction models perform poorly in lupus and additional studies to define methods for better risk stratification in SLE, including the use of CAC, will be important.
One of the limitations of our study is that we cannot define the true predictive value of the risk scores. To do so would require prospective information about both baseline risk factors and future cardiovascular events; however, lupus is an uncommon disease, and although coronary events are more frequent than in the general population, they occur in only a few patients. Thus, prospective studies of cardiovascular risk prediction models in SLE will be logistically difficult. Instead, we have examined the association between risk scores and the presence of coronary calcification, a marker of subclinical atherosclerosis, in a cross-sectional study. Nevertheless, because an increased prevalence of CAC is one of the most reproducible differences in cardiovascular risk markers in patients with lupus compared to controls1, we anticipate that the most useful CHD risk scores in SLE will be those that incorporate CAC. Another limitation is that the risk models we applied were not derived in lupus populations.
In conclusion, our findings suggest that the RRS, although it includes C-reactive protein in the risk calculation, is of limited use in women with SLE. Coronary age, calculated from CAC scores, may improve CHD risk prediction women with SLE.
Acknowledgments
Financial support: grants HL65082, 5P60AR56116, 5T32GM007569-33 and 1UL1RR024975 from the National Institutes of Health
Reference List
- 1.Asanuma Y, Oeser A, Shintani AK, et al. Premature coronary-artery atherosclerosis in systemic lupus erythematosus. N Engl J Med. 2003;349:2407–2415. doi: 10.1056/NEJMoa035611. [DOI] [PubMed] [Google Scholar]
- 2.Roman MJ, Shanker BA, Davis A, et al. Prevalence and correlates of accelerated atherosclerosis in systemic lupus erythematosus. N Engl J Med. 2003;349:2399–2406. doi: 10.1056/NEJMoa035471. [DOI] [PubMed] [Google Scholar]
- 3.Aranow C, Ginzler EM. Epidemiology of cardiovascular disease in systemic lupus erythematosus. Lupus. 2000;9:166–169. doi: 10.1191/096120300678828208. [DOI] [PubMed] [Google Scholar]
- 4.Manzi S, Meilahn EN, Rairie JE, et al. Age-specific Incidence Rates of Myocardial Infarction and Angina in Women with Systemic Lupus Erythematosus: Comparison with the Framingham Study. Am J Epidemiol. 1997;145:408–415. doi: 10.1093/oxfordjournals.aje.a009122. [DOI] [PubMed] [Google Scholar]
- 5.Wilson PW, D'Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837–1847. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 6.Grundy SM, Pasternak R, Greenland P, Smith S, Jr, Fuster V. Assessment of cardiovascular risk by use of multiple-risk-factor assessment equations: a statement for healthcare professionals from the American Heart Association and the American College of Cardiology. Circulation. 1999;100:1481–1492. doi: 10.1161/01.cir.100.13.1481. [DOI] [PubMed] [Google Scholar]
- 7.Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Final Report. Circulation. 2002;106:3143. [PubMed] [Google Scholar]
- 8.Persell SD, Lloyd-Jones DM, Baker DW. National Cholesterol Education Program risk assessment and potential for risk misclassification. Prev Med. 2006;43:368–371. doi: 10.1016/j.ypmed.2006.06.017. [DOI] [PubMed] [Google Scholar]
- 9.Goldberg RJ, Urowitz MB, Ibanez D, Nikpour M, Gladman DD. Risk factors for development of coronary artery disease in women with systemic lupus erythematosus. J Rheumatol. 2009;36:2454–2461. doi: 10.3899/jrheum.090011. [DOI] [PubMed] [Google Scholar]
- 10.Chung CP, Oeser A, Avalos I, Raggi P, Stein CM. Cardiovascular risk scores and the presence of subclinical coronary artery atherosclerosis in women with systemic lupus erythematosus. Lupus. 2006;15:562–569. doi: 10.1177/0961203306071870. [DOI] [PubMed] [Google Scholar]
- 11.Berry JD, Lloyd-Jones DM, Garside DB, Greenland P. Framingham risk score and prediction of coronary heart disease death in young men. Am Heart J. 2007;154:80–86. doi: 10.1016/j.ahj.2007.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cavanaugh-Hussey MW, Berry JD, Lloyd-Jones DM. Who exceeds ATP-III risk thresholds? Systematic examination of the effect of varying age and risk factor levels in the ATP-III risk assessment tool. Prev Med. 2008;47:619–623. doi: 10.1016/j.ypmed.2008.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pollin IS, Kral BG, Shattuck T, et al. High prevalence of cardiometabolic risk factors in women considered low risk by traditional risk assessment. J Womens Health (Larchmt) 2008;17:947–953. doi: 10.1089/jwh.2007.0640. [DOI] [PubMed] [Google Scholar]
- 14.Lakoski SG, Greenland P, Wong ND, et al. Coronary artery calcium scores and risk for cardiovascular events in women classified as "low risk" based on Framingham risk score: the multi-ethnic study of atherosclerosis (MESA) Arch Intern Med. 2007;167:2437–2442. doi: 10.1001/archinte.167.22.2437. [DOI] [PubMed] [Google Scholar]
- 15.Ridker PM, Buring JE, Rifai N, Cook NR. Development and validation of improved algorithms for the assessment of global cardiovascular risk in women: the Reynolds Risk Score. JAMA. 2007;297:611–619. doi: 10.1001/jama.297.6.611. [DOI] [PubMed] [Google Scholar]
- 16.Shaw LJ, Raggi P, Berman DS, Callister TQ. Coronary artery calcium as a measure of biologic age. Atherosclerosis. 2006;188:112–119. doi: 10.1016/j.atherosclerosis.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 17.McClelland RL, Nasir K, Budoff M, Blumenthal RS, Kronmal RA. Arterial age as a function of coronary artery calcium (from the Multi-Ethnic Study of Atherosclerosis [MESA]) Am J Cardiol. 2009;103:59–63. doi: 10.1016/j.amjcard.2008.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schisterman EF, Whitcomb BW. Coronary age as a risk factor in the modified Framingham risk score. BMC Med Imaging. 2004;4:1. doi: 10.1186/1471-2342-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40:1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 20.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–832. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 21.McClelland RL, Chung H, Detrano R, Post W, Kronmal RA. Distribution of coronary artery calcium by race, gender, and age: results from the Multi-Ethnic Study of Atherosclerosis (MESA) Circulation. 2006;113:30–37. doi: 10.1161/CIRCULATIONAHA.105.580696. [DOI] [PubMed] [Google Scholar]
- 22.Hoffmann U, Massaro JM, Fox CS, Manders E, O'Donnell CJ. Defining normal distributions of coronary artery calcium in women and men (from the Framingham Heart Study) Am J Cardiol. 2008;102:1136–1141. doi: 10.1016/j.amjcard.2008.06.038. 1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoff JA, Chomka EV, Krainik AJ, Daviglus M, Rich S, Kondos GT. Age and gender distributions of coronary artery calcium detected by electron beam tomography in 35,246 adults. Am J Cardiol. 2001;87:1335–1339. doi: 10.1016/s0002-9149(01)01548-x. [DOI] [PubMed] [Google Scholar]
- 24.WRITING GROUP. Lloyd-Jones D, Adams R, et al. Heart Disease and Stroke Statistics--2009 Update: A Report From the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119:e21–e181. doi: 10.1161/CIRCULATIONAHA.108.191261. [DOI] [PubMed] [Google Scholar]
- 25.Esdaile JM, Abrahamowicz M, Grodzicky T, et al. Traditional Framingham risk factors fail to fully account for accelerated atherosclerosis in systemic lupus erythematosus. Arthritis Rheum. 2001;44:2331–2337. doi: 10.1002/1529-0131(200110)44:10<2331::aid-art395>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 26.Marma AK, Lloyd-Jones DM. Systematic examination of the updated Framingham heart study general cardiovascular risk profile. Circulation. 2009;120:384–390. doi: 10.1161/CIRCULATIONAHA.108.835470. [DOI] [PubMed] [Google Scholar]
- 27.Marma AK, Berry JD, Ning H, Persell SD, Lloyd-Jones DM. Distribution of 10-year and lifetime predicted risks for cardiovascular disease in US adults: findings from the National Health and Nutrition Examination Survey 2003 to 2006. Circ Cardiovasc Qual Outcomes. 2010;3:8–14. doi: 10.1161/CIRCOUTCOMES.109.869727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Budoff MJ, Shaw LJ, Liu ST, et al. Long-term prognosis associated with coronary calcification: observations from a registry of 25,253 patients. J Am Coll Cardiol. 2007;49:1860–1870. doi: 10.1016/j.jacc.2006.10.079. [DOI] [PubMed] [Google Scholar]
- 29.Budoff MJ, Nasir K, McClelland RL, et al. Coronary calcium predicts events better with absolute calcium scores than age-sex-race/ethnicity percentiles: MESA (Multi-Ethnic Study of Atherosclerosis) J Am Coll Cardiol. 2009;53:345–352. doi: 10.1016/j.jacc.2008.07.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.D'Agostino RB, Sr, Grundy S, Sullivan LM, Wilson P. Validation of the Framingham coronary heart disease prediction scores: results of a multiple ethnic groups investigation. JAMA. 2001;286:180–187. doi: 10.1001/jama.286.2.180. [DOI] [PubMed] [Google Scholar]
- 31.Greenland P, Bonow RO, Brundage BH, et al. ACCF/AHA 2007 clinical expert consensus document on coronary artery calcium scoring by computed tomography in global cardiovascular risk assessment and in evaluation of patients with chest pain: a report of the American College of Cardiology Foundation Clinical Expert Consensus Task Force (ACCF/AHA Writing Committee to Update the 2000 Expert Consensus Document on Electron Beam Computed Tomography) developed in collaboration with the Society of Atherosclerosis Imaging and Prevention and the Society of Cardiovascular Computed Tomography. J Am Coll Cardiol. 2007;49:378–402. doi: 10.1016/j.jacc.2006.10.001. [DOI] [PubMed] [Google Scholar]