Summary
The effects of botulinum neurotoxin A on the passive mechanical properties of skeletal muscle have not been investigated, but may have significant impact in the treatment of neuromuscular disorders including spasticity. Single fiber and fiber bundle passive mechanical testing was performed on rat muscles treated with botulinum neurotoxin A. Myosin heavy chain and titin composition of single fibers was determined by gel electrophoresis. Muscle collagen content was determined using a hydroxyproline assay. Neurotoxin-treated single fiber passive elastic modulus was reduced compared to control fibers (53.00 kPa versus 63.43 kPa). Fiber stiffness and slack sarcomere length were also reduced compared to control fibers and myosin heavy chain composition shifted from faster to slower isoforms. Average titin molecular weight increased 1.77% after treatment. Fiber bundle passive elastic modulus increased following treatment (168.83 kPa versus 75.14 kPa). Bundle stiffness also increased while collagen content per mass of muscle tissue increased 38%. Injection of botulinum neurotoxin A produces an effect on the passive mechanical properties of normal muscle that is opposite to the changes observed in spastic muscles.
Keywords: botulinum neurotoxin A, passive muscle mechanics, titin, extracellular matrix, collagen
INTRODUCTION
The botulinum neurotoxins are produced by Clostridium botulinum and cause botulism. These toxins target neuromuscular junctions, the link between the nervous system and the muscle, preventing the release of acetylcholine through selective cleavage of synaptic proteins.1 This results in muscle paralysis and has been described as chemodenervation. Small doses of the toxin have been used effectively to treat a variety of neuromuscular disorders2, including spasticity secondary to cerebral palsy, stroke, brain trauma, or multiple sclerosis. Benefits of successful botulinum neurotoxin therapy in patients with spasticity include improved gait3, decreased pain4, and improved range of motion.5
Single fibers from spastic muscles have a passive elastic modulus nearly twice that of fibers obtained from normal patients.6 Unlike stiffness, which is not normalized for fiber cross sectional area, elastic modulus represents the intrinsic mechanical properties of the muscle fiber. Therefore, a change in modulus represents a fundamental reorganization of a muscle’s passive load-bearing proteins in response to spasticity, which is independent of muscle size. The sarcomeric protein titin is elastic and bears passive tension in both the fiber7 and, in conjunction with extracellular matrix, the whole muscle.8 Since it is the major source of intracellular passive tension9, a change in titin isoform, concentration, or organization could explain the spasticity-induced passive fiber elasticity change. However, when titin isoforms in spastic muscle were measured, no difference was found in titin size between spastic and normal muscle.10
The extracellular matrix also plays a role in the passive mechanical properties of bundles of fibers and the whole muscle. Skeletal muscle extracellular matrix forms a continuous structure throughout the muscle that provides structural support for individual fibers and plays an important role in force transmission between fibers and the tendon.11 The major structural protein of the extracellular matrix is fibrous collagen, which can change in response to altered use patterns or pathologies. Previous research on spastic muscle fiber bundles showed that the extracellular matrix was hypertrophic but mechanically compromised.12 The reason for decreased passive elastic modulus was unclear, although spastic extracellular matrix appeared morphologically disorganized. A related study demonstrated increased collagen content in vastus lateralis biopsies from spastic cerebral palsy patients and increased collagen content that was positively correlated with spasticity severity.13
Many reports exist regarding the clinical outcomes of neurotoxin therapy, but relatively little is known about the effect of the toxin on the muscle tissue itself. Spasticity results in increased passive elastic modulus of single muscle fibers and reduced passive modulus of the extracellular matrix.6; 12 Since neurotoxin treatment alleviates the symptoms of spasticity, we hypothesized that neurotoxin injection would also mitigate changes in passive mechanical properties of fibers and bundles observed in spastic muscles. Given that no good animal model exists for spasticity, we observed the changes in passive mechanical properties following injection of botulinum neurotoxin A in normal rat muscle.
METHODS
Untrained, mature, male Sprague-Dawley rats (n = 24, Harlan, Indianapolis, IN), with a mean weight 386 ± 5g (mean ± SEM), were used. Rats were housed 2/cage at 20 to 23°C with a 12:12hr dark-light cycle. All procedures were approved by the University of California and the VA Medical Center Committees on the Use of Animal Subjects in Research. Each rat was anesthetized (2% isoflurane, 2.0L/min) and injected with botulinum neurotoxin A (Botox®, Allergan, Irvine, CA) in the right tibialis anterior (TA). Each dose was 6U/kg, usually in a 100µL volume although 2 rats each received this dose in 4 and 20µL volumes. The TA was located by palpation, and the dose was administered by injection at two sites of the TA midbelly.
Two injection sites were used to facilitate diffusion of the toxin throughout the tissue and mimic the treatment of patients who receive neurotoxin in multiple injections. The contralateral TA was left untreated as a control. Titin and hydroxylproline on untreated, age-matched animals were not different than contralateral limbs on treated animals, suggesting that no compensatory effect exists in contralateral limbs (data not shown). Animals were maintained for 1 mo after which both botulinum neurotoxin-injected (BTX) and contralateral (CONTRA) TA muscles were harvested under anesthesia. Previous data from our laboratory demonstrated an 80% reduction in ankle dorsiflexion torque 1 mo post-injection.14 Animals were then euthanized with an intracardiac injection of pentobarbital sodium (0.5ml of 390 mg/ml solution). Harvested muscles were stored in storage solution at −20°C for up to 4 wks.6
Passive mechanical properties were determined for single muscle fibers and fiber bundles as described previously.6; 12; 15 Whole muscle or muscle strips were bathed in chilled relaxing solution, and dissection and fiber manipulation were performed under a stereomicroscope (Leica Microsystems MZ16). For single fiber testing, a single fiber segment 2.0 to 4.5mm in length was teased away from the muscle using microsurgical forceps. Single fibers were randomly sampled from multiple regions of each TA to avoid regional biases. Fibers with visible defects such as tears or kinks were discarded. The fiber segment was transferred to a testing chamber and secured between a force transducer (Aurora Scientific Inc., Ontario, Canada, Model Number 405A, Force Range 10.0mN) and a micromanipulator using 10-0 nylon suture.
After the fiber was mounted in the chamber, the fiber was lengthened until the force transducer output was ~2µN. Fiber diameter was measured from both a top and side view in 3 locations, allowing cross sectional area of an ellipse to be calculated. Fiber length was measured from suture loop to suture loop using a stage mounted micrometer and cross-hair eyepiece, and slack sarcomere length (at ~2µN) was recorded using laser diffraction. The fiber was extended in a stepwise fashion, in 250µm increments, with a 2-min stress relaxation period between each extension.6 Sarcomere length and fiber tension were recorded at the end of each stress relaxation period.
Fiber bundles were dissected by cutting clusters of intact fibers away from whole muscle tissue and were tested in the same fashion as single fibers except that a 3-min stress relaxation period was used between bundle extensions to permit complete stress-relaxation. The average diameter of a bundle was <300µm, suggesting that bundles from CONTRA muscles contained ~7 fibers/bundle. Since BTX fibers have smaller cross-sectional areas, bundles of the same diameter from BTX muscle contained ~17 fibers. After testing, fibers and bundles were transferred to microcentrifuge tubes and suspended in sodium dodecyl sulfate-vertical agarose gel electrophoresis (SDS-VAGE) sample buffer.16 Samples were stored at −80°C until analyzed by gel electrophoresis. Passive stress and strain were calculated for each fiber and bundle, and passive elastic modulus was calculated as the slope of the stress-strain curve. Passive stiffness was calculated as the slope of the tension-sarcomere length curve. Since slack and failure sarcomere lengths were variable, modulus and stiffness calculations were limited to sarcomere lengths between 2.5 and 4.0µm, corresponding to the descending limb of the rat sarcomere length-tension curve.17
The myosin heavy chain composition of single fibers was determined using sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE).18 Standards for myosin heavy chain gels were prepared by homogenizing adult rat TA muscle, which contains the 4 adult skeletal muscle myosin isoforms, types 1, 2A, 2X, and 2B. Single fiber samples were prepared by boiling for 3 min in sample buffer. Gels were run at 4°C for 1 hr at 10mA followed by 18 to 22 hrs at 275V and were fixed and stained following the Silver Stain Plus procedure (Bio-Rad, Hercules, CA). Bands were categorized by isoform type; the denisty of each band was quantified using a GS-800 Calibrated Densitometer and Quantity One 1-D Analysis software (Bio-Rad).
The molecular mass of titin in single fibers was determined using SDS-VAGE.16 Titin standards were obtained by homogenizing human cadaver soleus and rat cardiac muscle with molecular weights of ~3700kDa and 2992kDa, respectively.19 Single fiber samples were prepared by boiling for 3 min. Gels were run at 4°C for 5 hrs at 15mA constant current. Agarose gels were fixed and stained according to the Silver Stain Plus procedure, except that gels were dried for ~20 hrs at 40°C immediately after fixation. Relative mobility and intensity of each band was quantified using Quantity One (Bio-Rad). The relative mobilities of proteins on the SDS-VAGE gel were linearly related to the log of their molecular weights.16 For fibers containing dual titin isoforms, an average molecular weight was also calculated by weighting the contribution of each isoform by the relative intensity of its band as determined by densitometry.
A hydroxyproline assay was performed to estimate the collagen content of BTX and CONTRA muscles.20 About 100mg of tissue was dissected from whole muscle and hydrolyzed in 6N HCl at 100°C for 18 hrs. Samples were then neutralized to about pH 7 using NaOH and diluted 15× to <1µg/mL hydroxyproline. A Chloramine-T solution was added to the diluted sample, followed by the addition of p-dimethylaminobenzaldehyde in solution. After incubation at 60°C for 30 mins, the concentration of hydroxyproline in the samples was determined by spectrophotometry at 550nm and was normalized to the mass of the original tissue sample. Hydroxyproline standards provided a standard curve for spectrophotometry.
Dependent variables of fiber diameter, slack sarcomere length, modulus, stiffness, myosin heavy chain composition, and titin composition were screened for normality and homogeneity of variances. Mean values were compared between BTX and CONTRA groups by one-way ANOVA and post-hoc Tukey tests. Linear regressions were performed to determine correlations between measured parameters. Significance was set at p < 0.05, and all values are reported as mean ± SEM. Since dependent variable variance among single fibers was greater than variance among animals, each single fiber and bundle was treated as an individual sample.
RESULTS
One month post-injection, rat weight had increased to 424.1 ± 5.4g. After harvesting, the CONTRA TA had more than twice the mass of the BTX TA (Table 1, p < 0.001). The cross sectional area of single fibers from the BTX TA (n = 64) was significantly reduced from the CONTRA TA (n = 66, Table 1, p < 0.001). Since the smallest BTX fibers were too small to produce detectable diffraction patterns, single fiber dissection on the BTX TA was biased toward larger fibers. Therefore, the mean diameter of fibers from the BTX TA was likely overestimated. Mean slack sarcomere length for BTX and CONTRA fibers was 2.194 ± 0.025µm and 2.295 ± 0.016µm, respectively (Table 1, p < 0.005).
TABLE 1.
Sample Dimensions
| CONTRA | BTX | |
|---|---|---|
| TA Mass* | 0.892±0.014g | 0.418±0.022g |
| Fiber Cross Sectional Area* | 9160±7µm2 | 3523±3µm2 |
| Bundle Diameter | 0.370±0.024mm | 0.357±0.027mm |
| Fiber Slack Sarcomere Length* | 2.30±0.02µm | 2.19±0.03µm |
| Bundle Slack Sarcomere Length | 2.25±0.03µm | 2.27±0.06µm |
Values are mean ± SEM,
indicates statistically significant difference between CONTRA and BTX (p < 0.01)
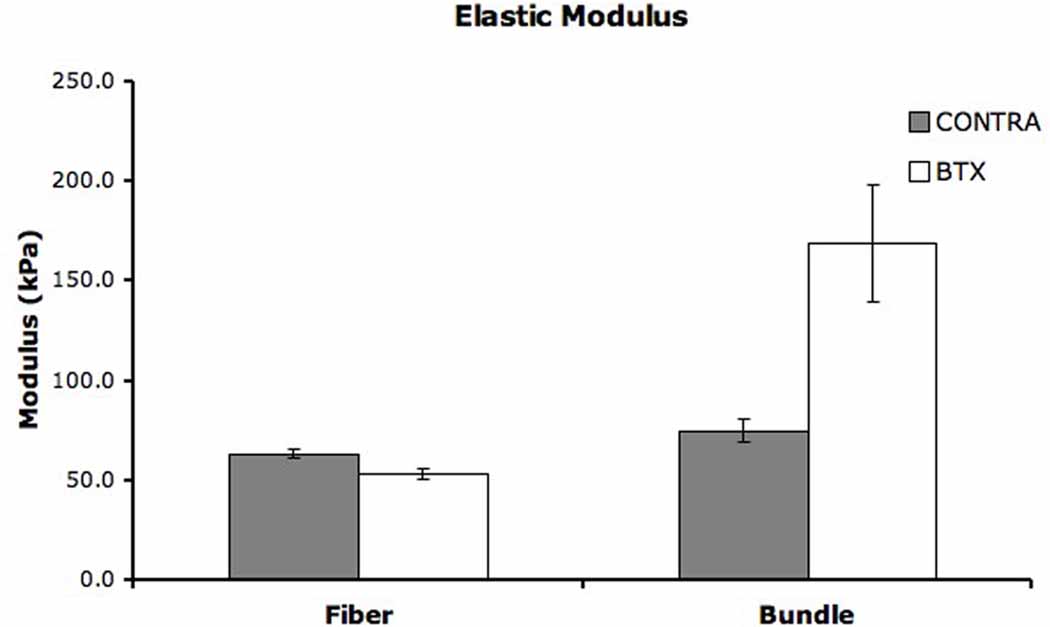
Mean stiffness of CONTRA fibers was 0.258 ± 0.014N/µm, representing nearly a 3-fold increase over the mean stiffness of BTX fibers (0.085 ± 0.005N/µm). Since stiffness is not normalized by cross sectional area, the difference between groups was largely based on difference in fiber size and reflects fiber structural differences. This is supported by linear regression of fiber diameter and stiffness, which revealed a significant correlation for both BTX (r2 = 0.368, p < 0.001) and CONTRA sides (data not shown, r2 = 0.577, p < 0.001). Elastic modulus represents a normalized value and, therefore, reflects the intrinsic passive mechanical properties of the fiber. Average elastic modulus of CONTRA fibers was 63.43 ± 2.40kPa while the modulus of BTX fibers was 53.00 ± 2.63kPa (Fig. 1, p < 0.005).
Figure 1. Elastic Modulus.
Mean elastic modulus of single fibers and fiber bundles. Error bars indicate SEM. A significant difference was found between BTX and CONTRA sides for both single fibers (p < 0.005) and fiber bundles (p < 0.01).
Fiber bundle diameters were not significantly different between BTX and CONTRA legs (Table 1, p = 0.726). Bundle slack sarcomere lengths were 2.27 ± 0.06µm on the BTX leg and 2.25 ± 0.03µm on the CONTRA leg (Table 1, p = 0.865). Bundle stiffness and modulus were both significantly different between BTX and CONTRA sides. Stiffness values for BTX injected and CONTRA bundles were 7.47 ± 0.81kPa and 4.17 ± 0.70kPa, respectively (p < 0.005). Since diameters were not different between BTX and CONTRA bundles, the difference in stiffness represents a change in intrinsic mechanical properties. Mean modulus values for BTX and CONTRA bundles were 168.83 ± 29.60kPa and 75.14 ± 6.03kPa, respectively (Fig. 1, p < 0.01).
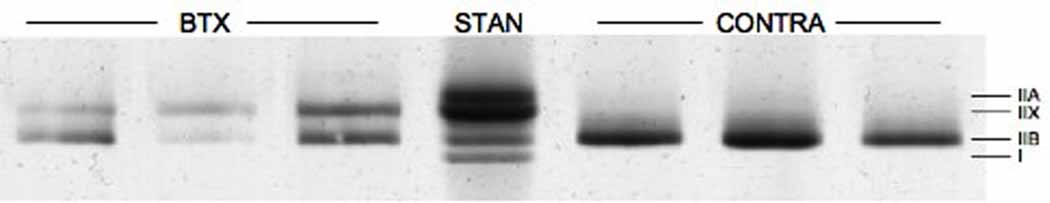
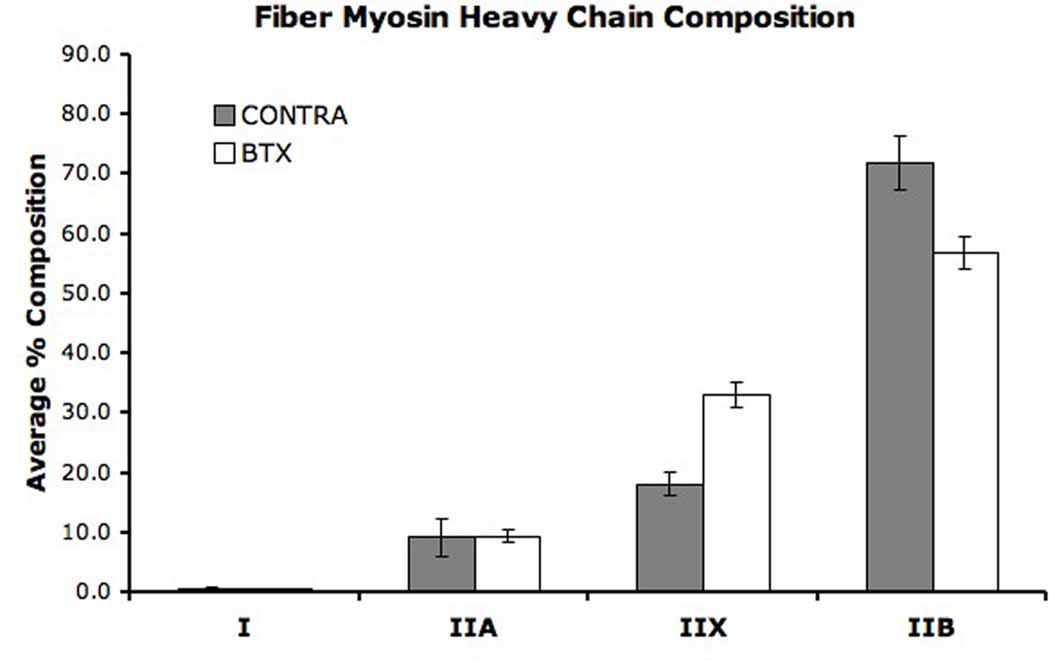
SDS-PAGE for myosin heavy chain composition was performed on 19 CONTRA and 24 BTX fibers (Fig. 2). While the relative amount of type IIA myosin was unchanged by neurotoxin injection, a significant shift occurred in mean fiber composition from type IIB to IIX indicating a slower fiber type (Fig. 2, p < 0.01). This shift is consistent with a previously published report.21
Figure 2. SDS-PAGE for Fiber Myosin Heavy Chain Composition.
(A) Each lane contains protein from a single fiber except for the middle lane, which contains a standard made from whole TA homogenate. Most fibers are composed of one or two types of myosin heavy chain. (B) Average myosin heavy chain composition for single fibers. Error bars indicate SEM. A significant difference exists between BTX and CONTRA sides in types IIX and IIB content (p < 0.01). Myosin heavy chain type I was rarely expressed in tested single fibers.
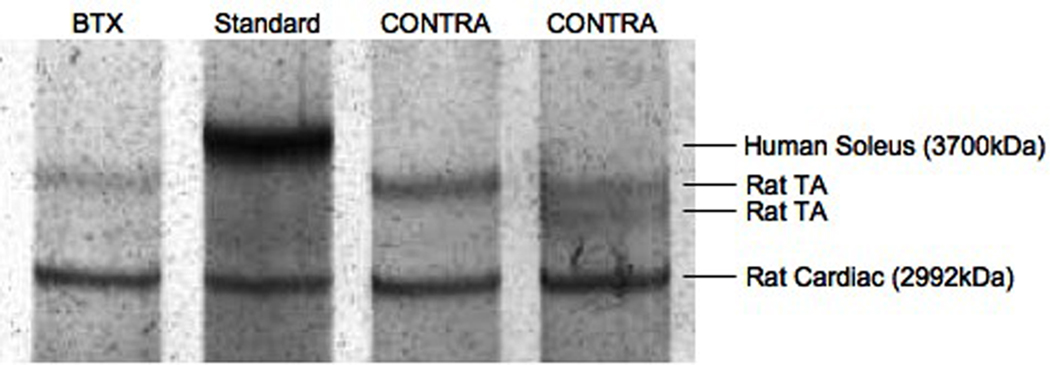
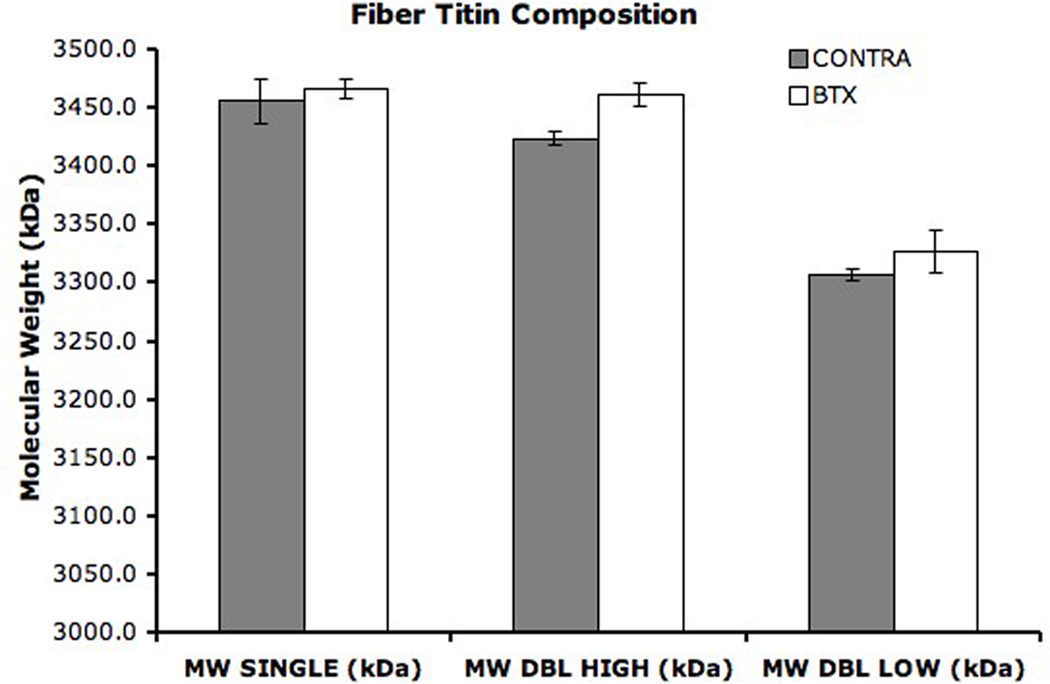
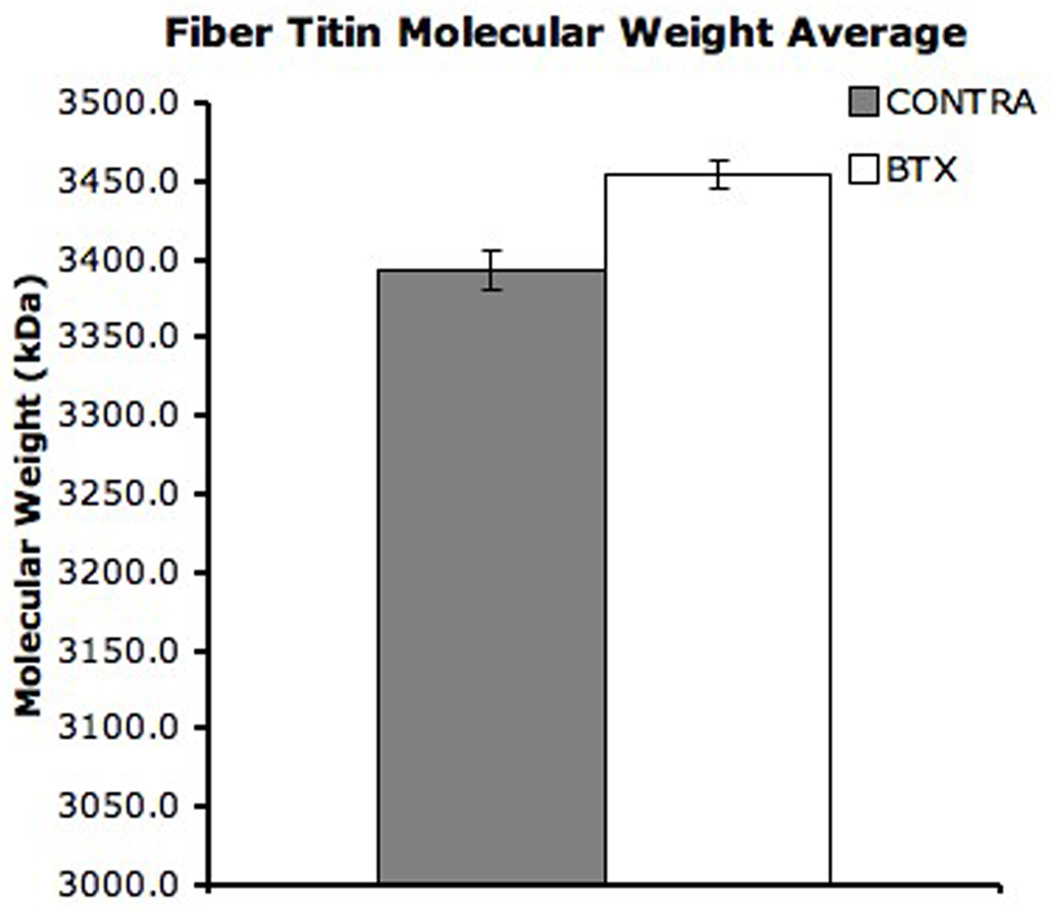
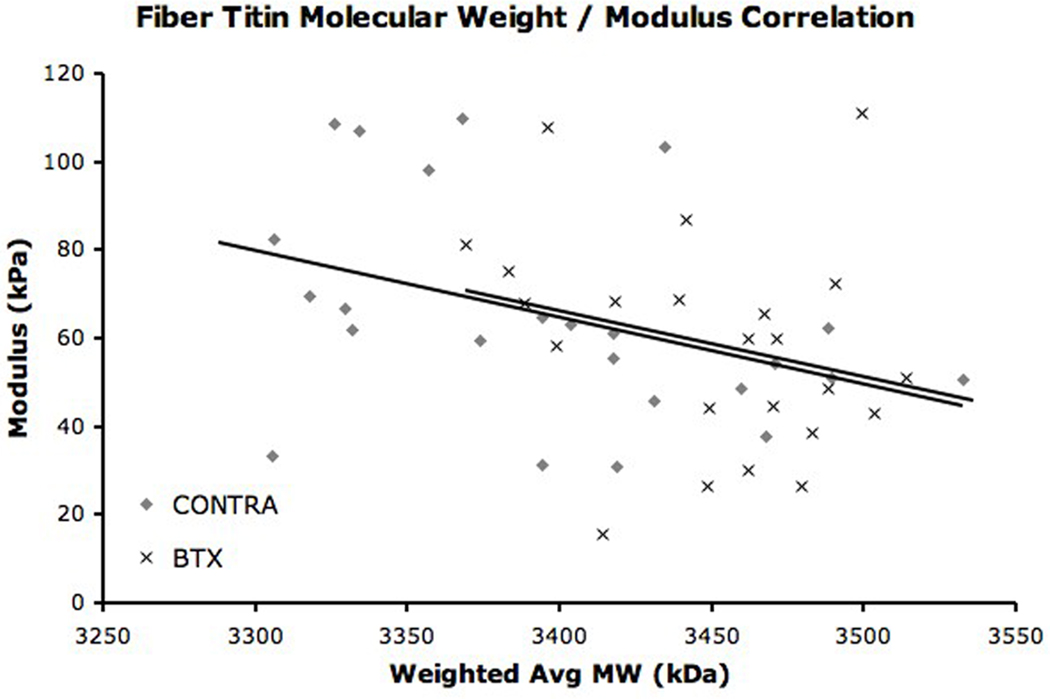
The titin composition of single fibers was also determined by gel electrophoresis (Fig. 3). Most BTX (n = 20) and some CONTRA (n = 10) fibers expressed only one titin isoform of average molecular weights 3466± 8kDa and 3456±19kDa, respectively. These molecular weights were not different from each other (p > 0.6). Five BTX fibers and eighteen CONTRA fibers also expressed a secondary titin isoform of average molecular weight 3327±18kDa and 3310±5kDa, respectively. The molecular weight of the secondary titin isoforms was not different between groups (p = 0.156) but was significantly different than the primary titin isoform (p < 0.01). A weighted average molecular weight was calculated for each fiber with dual titin bands. Using this weighted average, the mean molecular weight of titin in all fibers from CONTRA TA was 3393.5 ± 12.8kDa (Fig. 4). This molecular weight was significantly lower than the mean molecular weight of fibers from BTX TA, which was 3454.4 ± 8.9kDa (p < 0.001). Of all sarcomeric proteins, titin is the greatest determinant of passive mechanical properties of the fiber and is proposed as responsible for setting slack sarcomere length and passive elastic modulus.7; 22 However, in our study, only a weak correlation was found when the single fiber mean titin molecular weight was regressed with single fiber modulus (Fig. 5, r2 = 0.127, p < 0.05); no correlation was found for slack sarcomere length (r2 = 0.026, p = 0.277).
Figure 3. SDS-VAGE for Fiber Titin Composition.
(A) Each lane contains protein from a single fiber except the standard lane, which is loaded with homogenate from whole human soleus. Each lane is also loaded with the rat cardiac standard. Fibers contain either 1 (n=20 BTX, n = 10 CONTRA) or 2 (n = 5 BTX, n = 18 CONTRA) titin isoforms. (B) Molecular weight is calculated for unknown single fibers using the position relative to the standards. Average molecular weight is shown for titin from BTX and CONTRA single fibers. Error bars indicate SEM. For both BTX and CONTRA single fibers, the lower double band is significantly different from the higher double band and the single band (p < 0.01). The single band and the higher double band were not significantly different for BTX (p = 0.959) or CONTRA (p = 0.054) fibers.
Figure 4. Fiber Titin Molecular Weight Average.
The contribution of each of the double bands was weighted by the relative intensity of the band on the gel. The average titin molecular weight is significantly different between BTX and CONTRA limbs (p < 0.001). Error bars indicate SEM.
Figure 5. Fiber Titin Molecular Weight / Modulus Correlation.
Linear regression of fiber average titin molecular weight and fiber modulus. This relationship approaches significance for both BTX (r2 = 0.234, p = 0.067) and CONTRA fibers (r2 = 0.157, p = 0.055).
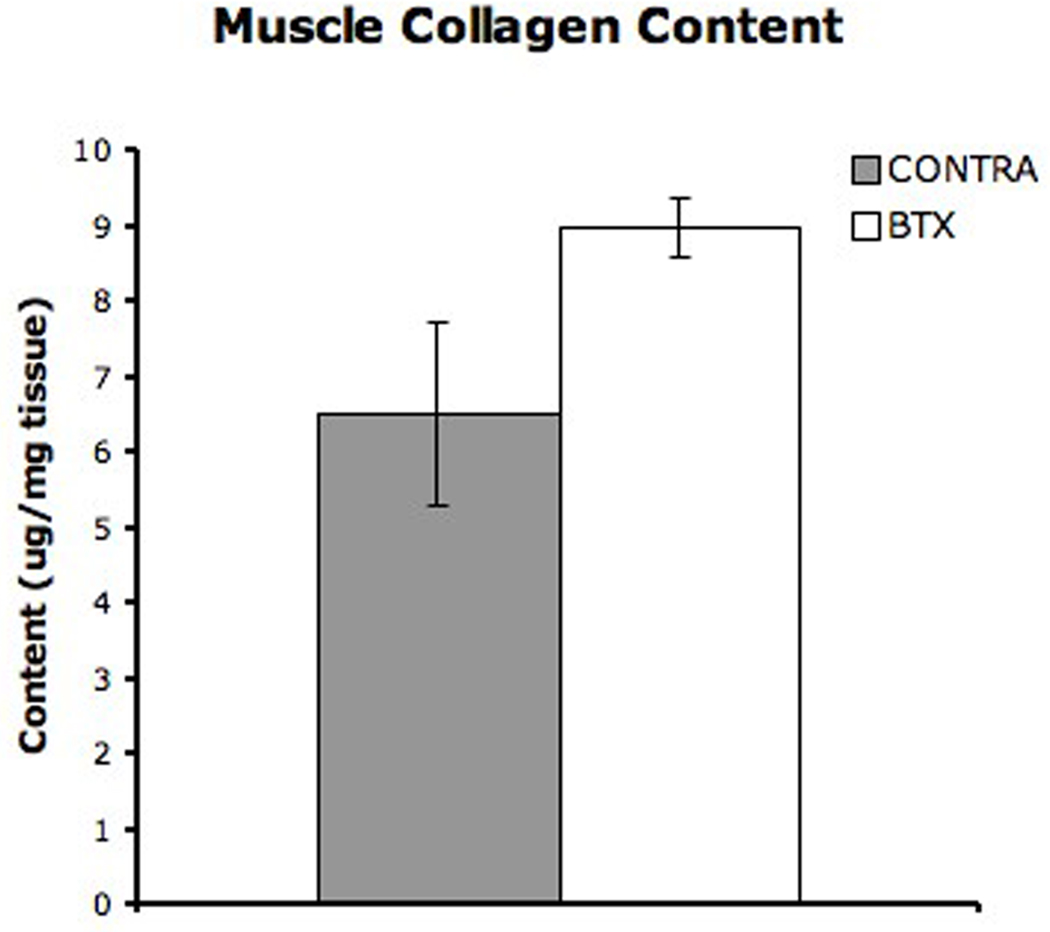
Total collagen content in whole tissue was determined in ten CONTRA muscles and eight BTX muscles. Average collagen content in CONTRA and BTX muscles was 6.51 ± 1.23 and 8.97 ± 0.39µg/mg respectively (Fig. 6, p = 0.204). Therefore, on average, BTX muscle had ~40% more collagen per unit mass compared to CONTRA muscle.
Figure 6. Muscle Collagen Content.
Average collagen content in whole tissue as determined using a hydroxyproline assay. Collagen content in the BTX tissue was 38% higher than collagen content in the CONTRA tissue (p = 0.204)
DISCUSSION
Passive mechanical properties and protein expression of the rat TA changed 1 mo after treatment with botulinum neurotoxin. TA mass and fiber diameter both decreased, as did single fiber elastic modulus. Fiber bundle passive elastic modulus and stiffness increased after treatment. These mechanical changes were accompanied by a shift in single fibers to a slower myosin heavy chain isoform and larger average titin molecular weight. Titin molecular weight was significantly correlated with passive elastic modulus but not slack sarcomere length. Collagen content was increased in BTX muscle. These single fiber and fiber bundle changes in passive elastic modulus are opposite those observed in spastic muscles, while single fiber diameter and slack sarcomere length also decreased in spastic muscle.6; 12
After treatment with botulinum neurotoxin, the passive elastic modulus of single fibers was decreased ~15% compared to the CONTRA side. This is a modest effect considering that the elastic modulus of spastic single fibers is nearly twice that of normal single fibers.6 An average spastic muscle fiber also has less than 1/3 the cross-sectional area of a normal fiber. Therefore, in spastic muscle, increased modulus, a material property, is offset by decreased stiffness, a structural property. Botulinum neurotoxin injection did, however, have a large effect on single fiber passive stiffness. Passive stiffness in BTX single fibers dropped ~70% and was significantly correlated with fiber diameter. Selection of BTX fibers was biased toward larger fibers, which likely results in an overestimate of the passive stiffness. If this is true, BTX average fiber stiffness may actually be much less than is reported here. Thus, simply decreasing fiber size secondary to botulinum neurotoxin treatment may have important clinical implications in attenuating the effects of increased single fiber passive elastic modulus in spastic patients. While spastic muscle fibers have reduced single fiber cross-sectional area compared to normal fibers, it is unknown whether treatment with botulinum neurotoxin further decreases fiber diameter in spastic patients. If fiber diameter is reduced further, then passive stiffness of those fibers would likely also decrease.
Although titin has been characterized as the major intracellular determinant of passive elasticity,9 the average titin molecular weight of a single fiber was not strongly correlated with fiber passive elastic modulus. Among BTX and CONTRA fibers, average titin molecular weight accounted for only 23 and 16%, respectively, of the variability in modulus. These weak correlations are similar to results in other studies demonstrating that the molecular weight of titin found in spastic muscle is no different than the molecular weight of titin found in normal muscle10 even though the modulus of spastic muscle fibers is nearly twice that of normal fibers.6 In a separate study using fiber bundles from several different muscles, the contribution of titin to the total passive load bearing of the bundle ranged from 24 to 57%.9 Other extra- and intra-cellular passive load bearing structures accounted for as much at 39% of total passive tension in a bundle.9 Thus, great diversity exists in the contribution of each passive load bearing structure among different muscle types. Factors other than titin influence single fiber passive elastic modulus, including intracellular microtubules and intermediate filaments.23
The mean passive elastic modulus of BTX bundles was more than twice the mean modulus of CONTRA bundles. Since the treatment effect on a single fiber modulus was relatively small, a change of this magnitude in the bundle suggests a change in the passive mechanical properties of extracellular structural material. Collagen content in whole BTX tissue was 38% higher compared to CONTRA tissue and may account for the increased bundle modulus. Previous research showed that fiber bundles obtained from normal muscle have a passive elastic modulus more than 4 times the modulus of bundles taken from spastic muscle.12 A hydroxyproline assay of muscle biopsies from patients with spasticity demonstrated increased collagen content compared to normal muscle.13 Taken together, these results suggest that the accumulated collagen in spastic muscle is not properly organized for passive load bearing like it is in normal muscle. Injection with botulinum neurotoxin also results in increased collagen content and may partially reverse the mechanical deficiency found in spastic muscle if the additional collagen is properly oriented and crosslinked for passive load-bearing. Whether or not well-organized collagen is accumulated in spastic muscle after neurotoxin treatment remains to be determined. Also, it is unknown how neurotoxin injection affects the expression of various isoforms of collagen and what role alternate isoform expression profiles might play in the muscle’s passive mechanical properties. Indeed, shifts in collagen isoform expression were observed in rat hindlimb immobilization and unloading models.24; 25
Our experimental model has limitations. Our results were derived from a single treatment of normal rat muscle with botulinum neurotoxin A. It is unclear if the response of human spastic muscle differs from the response of normal rat muscle. Furthermore, patients receiving botulinum neurotoxin treatment often receive multiple doses over an extended period of time. Our results do not indicate how muscle may continue to change in response to long-term therapy. Finally, we chose to study the effect of neurotoxin treatment on the TA, which is not an anti-gravity muscle; it is unclear if anti-gravity muscles would respond differently to neurotoxin treatment. Further work is needed to resolve these limitations.
In conclusion, single fiber diameter and passive stiffness decreased after treatment with botulinum neurotoxin. Small decreases also occurred in slack sarcomere length and passive elastic modulus. The passive elastic modulus of fiber bundles increased after treatment. These changes were paralleled by changes in titin molecular weight and collagen content. These results suggest that botulinum neurotoxin treatment may mitigate the passive mechanical changes observed in spastic muscle.
ACKNOWLEDGEMENTS
This work was supported by NIH grants AR057013 and 14 HD050837. Richard L. Lieber, PhD is an unpaid consultant for Allergan, Inc.
REFERENCES
- 1.Blasi J, Chapman ER, Link E, et al. Botulinum neurotoxin A selectively cleaves the synaptic protein SNAP-25. Nature. 1993;365:160–163. doi: 10.1038/365160a0. [DOI] [PubMed] [Google Scholar]
- 2.Jankovic J. Botulinum toxin in clinical practice. Journal of neurology, neurosurgery, and psychiatry. 2004;75:951–957. doi: 10.1136/jnnp.2003.034702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hesse S, Lucke D, Malezic M, et al. Botulinum toxin treatment for lower limb extensor spasticity in chronic hemiparetic patients. Journal of neurology, neurosurgery, and psychiatry. 1994;57:1321–1324. doi: 10.1136/jnnp.57.11.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wissel J, Muller J, Dressnandt J, et al. Management of spasticity associated pain with botulinum toxin A. Journal of pain and symptom management. 2000;20:44–49. doi: 10.1016/s0885-3924(00)00146-9. [DOI] [PubMed] [Google Scholar]
- 5.Love SC, Valentine JP, Blair EM, et al. The effect of botulinum toxin type A on the functional ability of the child with spastic hemiplegia a randomized controlled trial. Eur J Neurol. 2001;8 Suppl 5:50–58. doi: 10.1046/j.1468-1331.2001.00038.x. [DOI] [PubMed] [Google Scholar]
- 6.Fridén J, Lieber RL. Spastic muscle cells are shorter and stiffer than normal cells. Muscle Nerve. 2003;27:157–164. doi: 10.1002/mus.10247. [DOI] [PubMed] [Google Scholar]
- 7.Bartoo ML, Linke WA, Pollack GH. Basis of passive tension and stiffness in isolated rabbit myofibrils. American Journal of Physiology. 1997;273:C266–C276. doi: 10.1152/ajpcell.1997.273.1.C266. [DOI] [PubMed] [Google Scholar]
- 8.Linke WA, Popov VI, Pollack GH. Passive and active tension in single cardiac myofibrils. Biophys J. 1994;67:782. doi: 10.1016/S0006-3495(94)80538-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prado LG, Makarenko I, Andresen C, et al. Isoform diversity of giant proteins in relation to passive and active contractile properties of rabbit skeletal muscles. The Journal of general physiology. 2005;126:461–480. doi: 10.1085/jgp.200509364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Olsson MC, Kruger M, Meyer LH, et al. Fibre type-specific increase in passive muscle tension in spinal cord-injured subjects with spasticity. J Physiol. 2006;577:339–352. doi: 10.1113/jphysiol.2006.116749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Trotter JA, Purslow PP. Functional morphology of the endomysium in series fibered muscles. Journal of Morphology. 1992;212:109. doi: 10.1002/jmor.1052120203. [DOI] [PubMed] [Google Scholar]
- 12.Lieber RL, Runesson E, Einarsson F, Fridén J. Inferior mechanical properties of spastic muscle bundles due to hypertrophic but compromised extracellular matrix material. Muscle & Nerve. 2003;28:464–471. doi: 10.1002/mus.10446. [DOI] [PubMed] [Google Scholar]
- 13.Booth CM, Cortina-Borja MJ, Theologis TN. Collagen accumulation in muscles of children with cerebral palsy and correlation with severity of spasticity. Developmental Medicine and Child Neurology. 2001;43:314–320. doi: 10.1017/s0012162201000597. [DOI] [PubMed] [Google Scholar]
- 14.Minamoto VB, Hulst JB, Lim M, et al. Increased efficacy and decreased systemic-effects of botulinum toxin A injection after active or passive muscle manipulation. Dev Med Child Neurol. 2007;49:907–914. doi: 10.1111/j.1469-8749.2007.00907.x. [DOI] [PubMed] [Google Scholar]
- 15.Wood DJ, Zollman J, Reuban JP, Brandt PW. Human skeletal muscle: properties of the "chemically skinned" fiber. Science (Washington D.C.) 1975;187:1075. doi: 10.1126/science.187.4181.1075. [DOI] [PubMed] [Google Scholar]
- 16.Warren CM, Krzesinski PR, Greaser ML. Vertical agarose gel electrophoresis and electroblotting of high-molecular-weight proteins. Electrophoresis. 2003;24:1695–1702. doi: 10.1002/elps.200305392. [DOI] [PubMed] [Google Scholar]
- 17.Burkholder TJ, Lieber RL. Sarcomere length operating range of muscles during movement. Journal of Experimental Biology. 2001;204:1529. doi: 10.1242/jeb.204.9.1529. [DOI] [PubMed] [Google Scholar]
- 18.Talmadge RJ, Roy RR. Elecrophoretic separation of rat skeletal muscle myosin heavy-chain isoforms. J Appl Physiol. 1993;75:2337. doi: 10.1152/jappl.1993.75.5.2337. [DOI] [PubMed] [Google Scholar]
- 19.Freiburg A, Trombitas K, Hell W, et al. Series of exon-skipping events in the elastic spring region of titin as the structural basis for myofibrillar elastic diversity. Circ Res. 2000;86:1114–1121. doi: 10.1161/01.res.86.11.1114. [DOI] [PubMed] [Google Scholar]
- 20.Edwards CA, O'Brien WD., Jr Modified assay for determination of hydroxyproline in a tissue hydrolyzate. Clin Chim Acta. 1980;104:161–167. doi: 10.1016/0009-8981(80)90192-8. [DOI] [PubMed] [Google Scholar]
- 21.Dodd SL, Selsby J, Payne A, et al. Botulinum neurotoxin type A causes shifts in myosin heavy chain composition in muscle. Toxicon. 2005;46:196–203. doi: 10.1016/j.toxicon.2005.03.022. [DOI] [PubMed] [Google Scholar]
- 22.Wang K, McCarter R, Wright J, et al. Viscoelasticity of the sarcomere matrix of skeletal muscles. The titin-myosin composite filament is a dual-stage molecular spring. Biophys J. 1993;64:1161. doi: 10.1016/S0006-3495(93)81482-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Granzier H, Irving T. Passive tension in cardiac muscle: contribution of collagen, titin, microtubules, and intermediate filaments. Biophys J. 1995;68:1027. doi: 10.1016/S0006-3495(95)80278-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miller TA, Lesniewski LA, Muller-Delp JM, et al. Hindlimb unloading induces a collagen isoform shift in the soleus muscle of the rat. Am J Physiol Regul Integr Comp Physiol. 2001;281:R1710–R1717. doi: 10.1152/ajpregu.2001.281.5.R1710. [DOI] [PubMed] [Google Scholar]
- 25.Ahtikoski AM, Koskinen SO, Virtanen P, et al. Regulation of synthesis of fibrillar collagens in rat skeletal muscle during immobilization in shortened and lengthened positions. Acta Physiologica Scandinavica. 2001;172:131–140. doi: 10.1046/j.1365-201X.2001.00848.x. [DOI] [PubMed] [Google Scholar]