Abstract
Objective
To define and contrast multiple joint radiographic osteoarthritis (rOA) phenotypes describing hand and whole-body rOA among African Americans and Caucasians.
Methods
We conducted a cross-sectional analysis in the Johnston County Osteoarthritis Project, using radiographic data for the hands, tibiofemoral (TFJ) and patellofemoral joints, hips, and lumbosacral spine (LS). Films were read for rOA by a single radiologist using standard atlases. Sixteen mutually exclusive hand (n=2083) and 32 whole-body rOA phenotypes (n=1419) were identified. Fisher’s exact tests, corrected for multiple comparisons, were used to compare phenotype frequencies by race and gender. Logistic regression was used to provide odds ratios adjusted for gender, age, and body mass index (BMI).
Results
Hand rOA phenotypes: African Americans compared with Caucasians had significantly less frequent rOA of the distal interphalangeal joints, in isolation and in combination with other hand joint sites, but comparable frequencies of rOA for other hand joint sites. Whole-body rOA phenotypes: African Americans compared with Caucasians had less frequent Hand rOA, in isolation and in combination with other joint sites. In contrast, African Americans compared with Caucasians had more than twice the odds of isolated TFJ rOA and 77% higher odds of TFJ and LS rOA together.
Conclusions
Even after adjustment for gender, age, and BMI, African Americans compared to Caucasians were less likely to have hand rOA phenotypes, but more likely to have knee rOA phenotypes involving the TFJ. African Americans may have a higher burden of multiple large joint OA involvement not captured by most definitions of “generalized OA.”
Osteoarthritis (OA) commonly affects multiple joints, although a universally accepted definition of “generalized” osteoarthritis has yet to be established. A variety of methods for defining generalized OA, such as counting the number of affected joints or summing radiographic grades across multiple joints have been utilized in various studies (1–6). These definitions often result in a sum score or cut-off point that defines individuals with generalized OA. While this is useful to determine case status and overall OA burden, it does not provide information about the full multi-joint OA phenotype of the individual. For prognosis and treatment planning, it may be of use to know which joints are involved most often together, and which joints are rarely simultaneously involved in a given individual.
A few studies have reported on patterns of radiographic or symptomatic OA involvement using various combinations of joint sites (5, 7–9). Clinically identified nodal changes in the hands have been associated with an increased risk of undergoing knee or hip joint replacement (9), increased predisposition to knee OA after meniscectomy (6, 10), and have been variably associated with hip OA in other studies (11, 12). OA at one site has also been found to predict development and/or progression at distant sites (13, 14). The Genetics of Generalized Osteoarthritis study, which enrolled affected sibling pairs based on clinical evidence of hand OA, found that a substantial proportion of individuals had radiographic OA (rOA) of the knee, hip, or knee and hip in addition to hand OA (4).
Multiple joint involvement in OA has been most frequently studied among women, and almost exclusively in Caucasians. Among African populations, lower frequencies of nodal hand OA have been seen compared with Caucasian populations (15). Approximately 10% of men and women in a South African population had rOA in 3 or more joint groups, less than in contemporary Caucasian studies (15). A comparison study of individuals in Jamaica showed a higher frequency of distal interphalangeal joint and knee rOA, but a lower frequency of nodes and of 1st metatarsophalangeal rOA compared to UK subjects, and a reduced frequency of lumbar spine rOA among Jamaican men (16). Sowers, et al, in the only study of rOA patterns among African Americans, found an increased frequency of both hand and knee rOA among African American women, along with an increased frequency of knee rOA alone and of metacarpophalangeal rOA, compared to Caucasian women (17).
Our group has evaluated differences between African Americans and Caucasians at the knee and the hip in the Johnston County Osteoarthritis Project (JoCo OA), a prospective, community-based cohort of individuals in rural North Carolina. Compared to Caucasians, African Americans in this population have a higher prevalence of radiographic and symptomatic knee OA, and a similar to slightly increased prevalence of radiographic and symptomatic hip OA (18, 19). The purpose of the current analysis was to examine potential differences in mutually exclusive multi-joint rOA phenotypes among African American and Caucasian men and women at the joint sites most frequently affected by OA (hands, hips, tibiofemoral and patellofemoral joints, and lumbosacral spine).
Patients and Methods
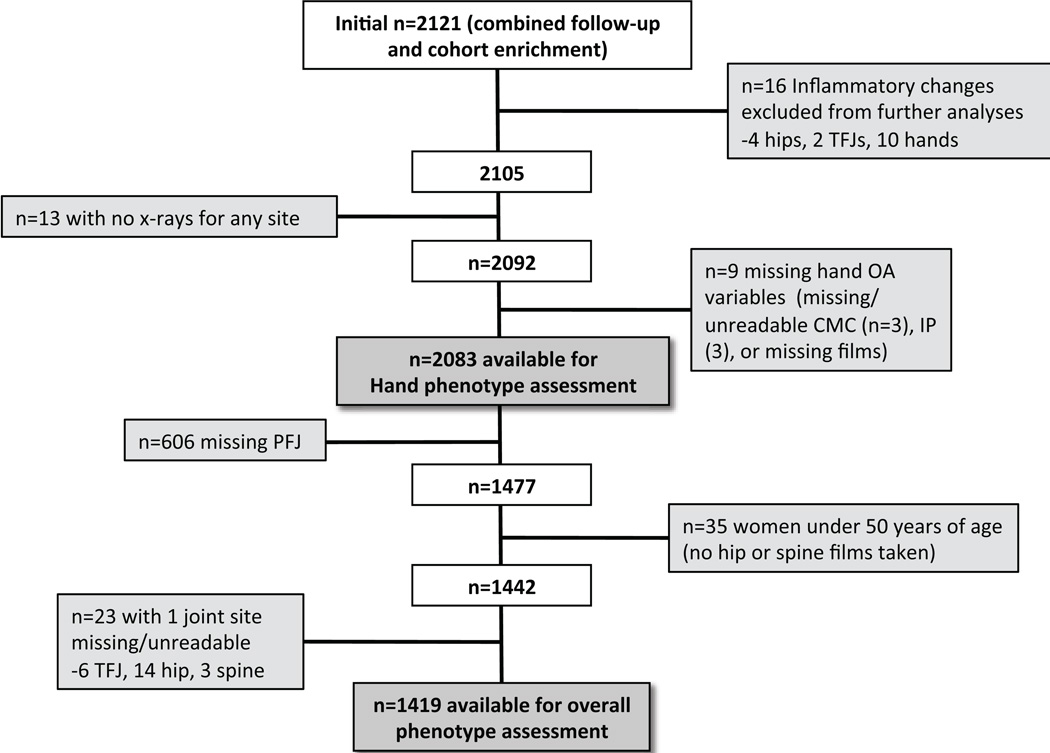
The analyses in this report used data from the JoCo OA, a community-based prospective cohort study of non-institutionalized African American and Caucasian men and women, aged 45 years and older, living in rural North Carolina, both with and without OA, as previously described (18). All participants signed informed consent and completed 2 home interviews and one clinic visit with physical examination, including functional measures and radiographs, administered by trained study personnel. Hand and spine radiographs were added to the study (previously collecting only knee and hip radiographs) at the cohort enrichment (2003–4) and 2nd follow up (2006–10), so data from these time points were used for the current analysis. Starting from a combined total n=2121, two subsamples were analyzed: 2083 individuals with complete data for all hand joints (the hand rOA phenotypes group), and a subset of 1419 who also had complete data for the other four joint sites (the whole-body rOA phenotypes group) (Figure 1).
Figure 1. Flow chart of inclusion of individuals with radiographic data for hand and whole-body rOA phenotype assessments.
Self-reported age, gender, and race were obtained from interviewer-administered questionnaires, while body mass index (BMI) was calculated in kg/m2 from height (cm) and weight (kg) measured during clinic examination by trained study examiners. This cross-sectional analysis included demographic, clinical, and radiographic data collected at the same time for each participant (either during the 2003–4 or 2006–10 time period). The JoCo OA study has been continuously approved by the Institutional Review Boards of the University of North Carolina and of the Centers for Disease Control and Prevention in Atlanta, GA.
Radiographs
Radiographs were obtained at a single clinic visit for each participant and interpreted for the hands, knees, hips, and spine as follows. Hands: Posteroanterior radiographs were read for Kellgren-Lawrence (KL) grade (20) at each of 30 joints for each hand (distal interphalangeal [DIP], proximal interphalangeal [PIP], metacarpophalangeal [MCP], carpometacarpal [CMC], thumb IP and MCP). Knees: Fixed flexion, weight-bearing posteroanterior views of the tibiofemoral joint (TFJ) using the Synaflexer™device (CCBR-Synarc, San Francisco, CA) were read for KL grade. Patellofemoral joints (PFJ) were assessed using sunrise views and were graded for osteophytes (OST) using the Burnett atlas (21). PFJ films were added later in the study and only ~70% had been read at the time of this analysis. Hips: Anteroposterior supine pelvis films were used to determine hip KL grade. Spine: Lateral lumbosacral spine (LS) films (taken with the participant lying on his/her left side) were graded for OST and disc narrowing (DN) at 5 levels (L1/2 through L5/S1) again using the Burnett atlas. LS and hip films were not obtained on women under the age of 50 years. All films were read by a single experienced musculoskeletal radiologist (JBR) previously shown to have high intra- and inter-rater reliability (κ=0.89 and 0.86, respectively) (22).
We defined rOA of the TFJ or hip as a KL grade ≥ 2 in any TFJ or hip joint, respectively. Replaced knees were categorized as having OA if the participant reported OA as the reason for the replacement or if rOA was present in the contralateral knee. Replaced hips were categorized as OA only if the participant reported OA as the reason for the joint replacement, as hips are more often subject to replacement due to fracture. For the PFJ, any OST ≥ 2 was considered to indicate PFJ rOA. For hand rOA, we used a composite definition requiring bilateral involvement, at least one DIP with KL grade ≥ 2, and at least 3 joints (DIP, PIP, or CMC) involved (4). Hand joint group OA (e.g. DIP OA) was defined if any joint in the group had a KL grade ≥ 2. LS rOA was defined if both OST and DN graded ≥ 1 were simultaneously present at least one vertebral level.
Statistical analysis
We did not use population-based weightings, as this analysis used a sample from the JoCo OA that included both the population-based original follow up sample and the cohort enrichment sample. The unit of analysis is the person throughout. Descriptive statistics were calculated for age, BMI, race, and gender for the sample with complete data for hand phenotypes (n=2083) and for the sample with complete data for whole-body phenotypes (n=1419). Frequencies of rOA were determined using the above definitions for each subsample. Then, 16 mutually exclusive phenotypes were defined for hand joint groups and 32 mutually exclusive phenotypes for whole-body phenotypes, representing all possible combinations of multi-joint rOA. As each phenotype was mutually exclusive, the referent group for a given phenotype was the combination of all others, so the sample size remained constant. Frequencies were calculated for each phenotype by race and by gender, and comparisons were made using Fisher’s exact tests due to small cell sizes in the contingency tables. We used the Hochberg method (23) of correction for multiple comparisons via the multproc procedure in Stata (24).
To allow for adjusted analysis using 4 explanatory variables (race, gender, age, and BMI), only those phenotypes seen in at least 40 individuals (approximately 10 events per covariate) were assessed using logistic regression models (25). For the hand phenotypes sample (n=2083), this represented 8 of 16 phenotypes (those occurring in at least 2% of this sample): No hand OA, DIP only, PIP only, CMC only, DIP and PIP only, DIP and CMC only, DIP/PIP/CMC, and DIP/PIP/MCP/CMC. For whole-body rOA phenotypes (n=1419), this comprised 12 of 32 phenotypes (those in at least 3% of this sample): No OA, Hand only, LS only, TFJ only, Hip only, Hand and LS, TFJ and LS, Hip and LS, Hand/TFJ/LS, Hip/TFJ/LS, Hand/LS/Hip, and Hand/TFJ/Hip/LS. Race by gender interactions were determined to be significant at a p-value of <0.1; analyses stratified by race and gender were performed where there were significant interactions. The regression models without interactions included terms for race, gender, age and BMI.
Results
Two subsamples were used in the analysis as above and as shown in Table 1 and Figure 1. Of the n=2121 in the total sample with multi-joint radiographic data, 16 had evidence of inflammatory arthritis and were excluded. Another 22 were missing radiographic data on at least one hand joint, leaving 2083 for analysis of hand rOA phenotypes. For whole-body rOA phenotypes, 606 individuals were missing PFJ reads (due to knee replacement or interpretation not available), 35 were women under age 50 who did not undergo hip or spine radiography, and 23 were missing data on at least one joint site, leaving 1419 for the analysis (Figure 1). Selected characteristics of each sample are detailed in Table 1; comparable to many OA samples, the mean age is over 65 years, and the mean BMI is obese (approximately 31 kg/m2). About one third of the sample was male, and one third African American. Overall, about 42% had TFJ rOA, 12% had PFJ rOA, 36% had hip rOA, and 32% had hand OA. LS rOA was very common, occurring in 62% of the sample (Table 1).
Table 1.
Characteristics for the sample with data for hand rOA phenotypes and the subsample with data for whole-body rOA phenotypes.
| Characteristics | Hand rOA Phenotypes Sample (n=2083)* | Whole Body rOA Phenotypes Subsample (n=1419)† |
|---|---|---|
| Mean (SD) or n (%) | Mean (SD) or n (%) | |
| Age | 65.1 (10.9) | 67.4 (9.7) |
| BMI | 31.3 (7.1) | 30.9 (6.3) |
| African American | 705 (33.9) | 456 (32.1) |
| Men | 689 (33.1) | 490 (34.5) |
| Any TFJ rOA | -- | 589 (41.5) |
| Any PFJ Ost>=2 | -- | 172 (12.1) |
| Any Hip rOA | -- | 503 (35.5) |
| Any LS rOA | -- | 877 (61.8) |
| Any Hand rOA‡ | 600 (28.8) | 453 (31.9) |
| Any DIP rOA | 899 (43.2) | 671 (47.3) |
| Any PIP rOA | 593 (28.5) | 447 (31.5) |
| Any MCP rOA | 195 (9.4) | 150 (10.6) |
| Any CMC rOA | 576 (27.7) | 446 (31.5) |
Hand rOA sample size for age is 2081 and for BMI is 2079
Whole body rOA sample size for BMI is 1418, and for PIP, MCP, and CMC n=1418
For Hand rOA, we used a composite definition requiring bilateral involvement, at least one DIP with KL grade ≥2, and at least 3 joints (DIP, PIP, or CMC) involved (4)
rOA: radiographic osteoarthritis; BMI: body mass index; TFJ: tibiofemoral joint; PFJ: patellofemoral joint; LS: lumbosacral spine; DIP: distal interphalangeal joint; PIP: proximal interphalangeal joint; MCP: metacarpophalangeal joint; CMC: carpometacarpal joint
Hand rOA Phenotypes (n=2083)
The frequency of each of the 16 mutually exclusive phenotypes by race and by gender is shown in Table 2. Many of the phenotypes were uncommon in the sample, occurring in less than 30 individuals (MCP only, DIP and MCP only, PIP and MCP only, PIP and CMC only, MCP and CMC only, DIP/PIP/MCP, DIP/MCP/CMC, PIP/MCP/CMC). The remainder of the phenotypes occurred in at least 40 individuals and were included in adjusted analyses, below. The most common outcome in this community-based sample was no hand rOA (45%, n=929), followed by DIP only (13%, n=269), DIP/PIP/CMC (9%, n=189), and DIP and PIP only (8%, n=166).
Table 2.
Frequencies and Adjusted Odds Ratios* for the 16 mutually exclusive hand rOA phenotypes by race and gender
| Comparison by Race | Comparison by Gender | |||||||
|---|---|---|---|---|---|---|---|---|
| Hand rOA phenotype |
Caucasian (n=1378) |
AA (n=705) |
Unadjusted Fisher’s exact |
Adjusted Odds Ratio for AA* |
Women (n=1394) |
Men (n=689) |
Unadjusted Fisher’s exact |
Adjusted Odds Ratio for men* |
| n(%) | n(%) | p value† | (95% CI)§ | n(%) | n(%) | p value† | (95% CI)§ | |
| No Hand rOA | 495 (36) | 434 (62) | <0.001 | See text‡ | 590 (42) | 339 (49) | 0.003 | See text‡ |
| DIP only | 200 (15) | 69 (10) | 0.002 | 0.66 (0.49–0.89) | 187 (13) | 82 (12) | 0.367 | 0.86 (0.65–1.13) |
| PIP only | 45 (3) | 31 (4) | 0.217 | 1.35 (0.84–2.18) | 46 (3) | 30 (4) | 0.263 | 1.39 (0.87–2.23) |
| MCP only | 10 (<1) | 8 (1) | 0.330 | -- | 5 (<1) | 13 (2) | 0.001 | -- |
| CMC only | 81 (6) | 41 (6) | 1.000 | 0.99 (0.67–1.47) | 82 (6) | 40 (6) | 1.000 | 1.01 (0.68–1.49) |
| DIP and PIP | 138 (10) | 28 (4) | <0.001 | 0.42 (0.27–0.64) | 122 (9) | 44 (6) | 0.071 | 0.69 (0.48–1.00) |
| DIP and MCP | 9 (<1) | 7 (1) | 0.431 | -- | 10 (1) | 6 (1) | 0.791 | -- |
| PIP and MCP | 2 (<1) | 2 (<1) | 0.608 | 3 (<1) | 1 (<1) | 1.000 | -- | |
| DIP and CMC | 97 (7) | 19 (3) | <0.001 | 0.40 (0.24–0.66) | 80 (6) | 36 (5) | 0.685 | 0.89 (0.59–1.34) |
| PIP and CMC | 11 (<1) | 10 (1) | 0.245 | -- | 13 (1) | 8 (1) | 0.644 | -- |
| MCP and CMC | 6 (<1) | 3 (<1) | 1.000 | -- | 0 (0) | 9 (1) | <0.001 | -- |
| DIP, PIP, MCP | 19 (1) | 10 (1) | 1.000 | -- | 14 (1) | 15 (2) | 0.045 | -- |
| DIP, MCP, CMC | 10 (<1) | 1 (<1) | 0.111 | -- | 6 (<1) | 5 (1) | 0.521 | -- |
| PIP, MCP, CMC | 1 (<1) | 4 (<1) | 0.048 | -- | 4 (<1) | 1 (<1) | 1.000 | -- |
| DIP, PIP, CMC | 167 (12) | 22 (3) | <0.001 | 0.24 (0.15–0.39) | 151 (11) | 38 (6) | <0.001 | 0.44 (0.30–0.65) |
| DIP, PIP, MCP, CMC | 87 (6) | 16 (2) | <0.001 | 0.35 (0.20–0.62) | 81 (6) | 22 (3) | 0.010 | 0.51 (0.31–0.85) |
only estimated for those phenotypes occurring in at least 40 persons, models included dichotomous race (referent=Caucasian) and gender (referent=female) and continuous age (years) and BMI (kg/m2))
Bold indicates significance after adjustment for multiple comparisons using the Hochberg method
See text: significant interaction by race and gender
Bold indicates a significant adjusted result (95% CI does not include 1)
rOA: radiographic osteoarthritis; AA: African American; DIP: distal interphalangeal joint; PIP: proximal interphalangeal joint; MCP: metacarpophalangeal joint; CMC: carpometacarpal joint
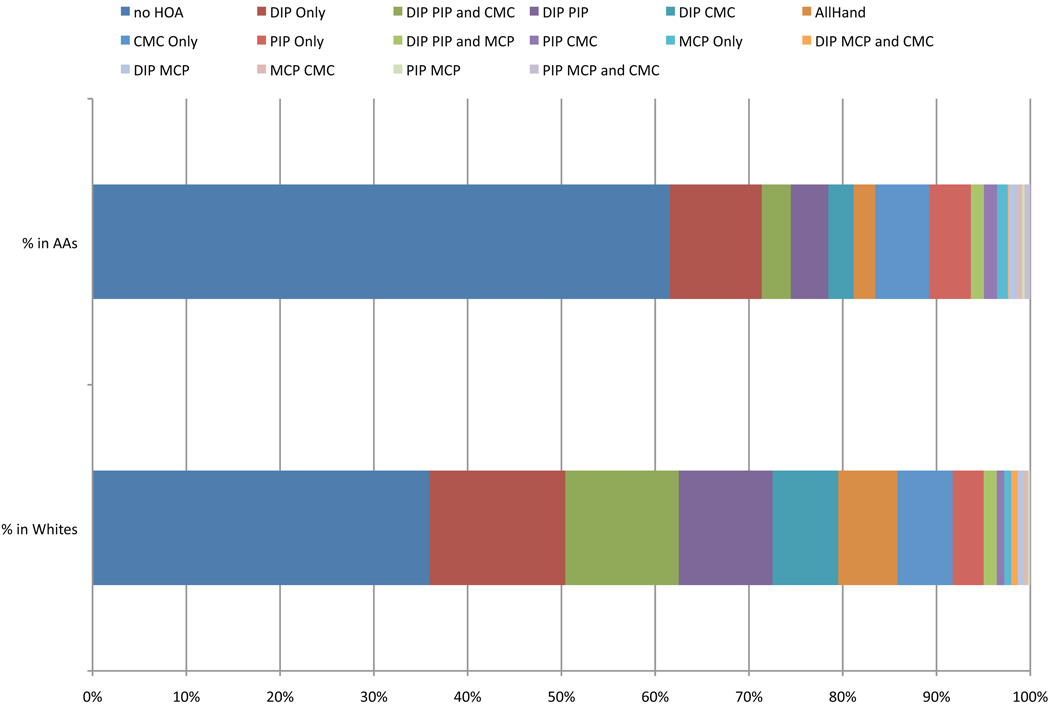
Significant differences in the unadjusted frequencies of hand rOA phenotypes by race and gender are shown in Table 2, and for race in Figure 2. African Americans were more likely than Caucasians to have no rOA in the hands, and were, in general, less likely to have any of the phenotypes that involved the DIPs. This difference was statistically significant (after adjusting for multiple comparisons) for DIP only, DIP and PIP, DIP and CMC, DIP/PIP/CMC, and DIP/PIP/MCP/CMC (p-values all ≤ 0.002). Involvement of the PIPs or CMCs alone was similar among African Americans and Caucasians. Fewer significant differences were seen by gender, with men more likely to have no hand rOA, slightly more likely to have MCP only or MCP and CMC alone (but these were very infrequent), and less likely to have DIP/PIP/CMC rOA (p<0.001) or involvement of all the hand joints (p=0.01).
Figure 2. Distribution of mutually exclusive hand rOA phenotypes by race.
The frequency of each joint combination is shown, for African Americans (AAs) at the top and Caucasians at the bottome, sorted by frequency among Caucasians, to describe the overall shift in pattern for hand joint involvement among AAs.
Adjusted results are shown in Table 2, with the exception of the no Hand rOA outcome, which demonstrated an interaction between race and gender (p for interaction=0.007) and is summarized here. Compared to Caucasian women, Caucasian men had 76% higher odds of having no hand rOA (aOR 1.76, 95% CI [1.36–2.29]), while African American men and women had more than 2 times higher odds of having no hand rOA (AA men aOR 3.11 [2.18–4.43]; AA women aOR 3.36 [2.57–4.41]). The results after adjustment for age and BMI again show that African Americans were less likely to have any phenotype including the DIPs (aORs 0.24 to 0.66, Table 2). Also after adjustment, the odds of men having multiple hand joint involvement (DIP/PIP/CMC or DIP/PIP/MCP/CMC rOA) were reduced by about 50% compared to women. As expected, increasing age was associated most strongly with outcomes including multiple joints, and not with single joint involvement except at the CMC; increasing BMI was significantly associated with higher odds of DIP/PIP/CMC and DIP/PIP/MCP/CMC rOA (covariate aORs not shown).
Whole Body rOA Phenotypes (n=1419)
The frequencies of the 32 mutually exclusive whole body rOA phenotypes are shown in Table 3. As noted for the hand rOA phenotypes, several of the phenotypes were very infrequent, occurring in ≤ 20 individuals (14 of 32 phenotypes, see Table 3). Six of the combinations were seen in 20–40 individuals each (Table 3). The remaining 12 phenotypes (no OA, Hand only, LS only, TFJ only, Hip only, Hand and LS, TFJ and LS, Hip and LS, Hand/TFJ/LS, Hip/TFJ/LS, Hand/LS/Hip, and Hand/TFJ/Hip/LS) were seen in at least 40 individuals and are included in the adjusted results, below. Again, the most common outcome in the sample was no rOA at any site (17%, n=237), followed by LS only (16%, n=231) and TFJ and LS (7%, n=104).
Table 3.
Frequencies and Adjusted Odds Ratios (aOR)* for the 32 mutually exclusive whole-body rOA phenotypes by race and gender.
| Comparison by Race | Comparison by Gender | |||||||
|---|---|---|---|---|---|---|---|---|
| Phenotype | Caucasian (n=963) | AA (n=456) | Fisher’s exact | aOR for AA* | Women (n=929) | Men (n=490) | Fisher’s exact | aOR for men* |
| n(%) | n(%) | p value† | (95% CI)§ | n(%) | n(%) | p value† | (95% CI)§ | |
| No rOA at any site | 140 (15) | 97 (21) | 0.002 | See text | 145 (16) | 92 (19) | 0.135 | See text |
| Hand only‖ | 37 (4) | 5 (1) | 0.004 | 0.31 (0.12–0.79) | 36 (4) | 6 (1) | 0.005 | 0.30 (0.13–0.73) |
| LS only | 160 (17) | 71 (16) | 0.645 | 0.88 (0.65–1.21) | 137 (15) | 94 (19) | 0.034 | 1.29 (0.96–1.72) |
| PFJ only | 2 (<1) | 1 (<1) | 1.000 | -- | 2 (<1) | 1 (<1) | 1.000 | -- |
| TFJ only | 27 (3) | 34 (7) | <0.001 | 2.51 (1.48–4.25) | 40 (4) | 21 (4) | 1.000 | 1.14 (0.65–1.99) |
| Hip only | 43 (4) | 28 (6) | 0.193 | 1.41 (0.86–2.31) | 50 (5) | 21 (4) | 0.442 | 0.73 (0.43–1.24) |
| TFJ and PFJ Only | 6 (<1) | 3 (<1) | 1.000 | -- | 7 (1) | 2 (<1) | 0.727 | -- |
| Hand and TFJ Only | 15 (2) | 5 (1) | 0.632 | -- | 16 (2) | 4 (1) | 0.236 | -- |
| Hand and PFJ Only | 1 (<1) | 0 (0) | 1.000 | -- | 0 (0) | 1 (<1) | 0.345 | -- |
| Hip and TFJ Only | 17 (2) | 12 (3) | 0.316 | -- | 17 (2) | 12 (2) | 0.435 | -- |
| Hip and PFJ Only | 0 (0) | 1 (<1) | 0.321 | -- | 0 (0) | 1 (<1) | 0.345 | -- |
| Hand and Hip Only | 17 (2) | 3 (<1) | 0.146 | -- | 17 (2) | 3 (1) | 0.095 | -- |
| Hand and LS Only | 55 (6) | 8 (2) | <0.001 | 0.31 (0.14–0.66) | 46 (5) | 17 (3) | 0.224 | 0.65 (0.37–1.15) |
| TFJ and LS Only | 59 (6) | 45 (10) | 0.016 | 1.77 (1.17–2.67) | 59 (6) | 45 (9) | 0.054 | 1.61 (1.07–2.43) |
| PFJ and LS Only | 2 (<1) | 2 (<1) | 0.598 | -- | 3 (<1) | 1 (<1) | 1.000 | -- |
| Hip and LS Only | 54 (6) | 27 (6) | 0.807 | 1.13 (0.70–1.82) | 50 (5) | 31 (6) | 0.472 | 1.20 (0.75–1.91) |
| Hand, TFJ, and Hip Only | 23 (2) | 2 (<1) | 0.008 | -- | 14 (2) | 11 (2) | 0.396 | -- |
| Hand, PFJ, and Hip Only | 1 (<1) | 1 (<1) | 0.540 | -- | 2 (<1) | 0 (0) | 0.548 | -- |
| Hand, TFJ, and LS Only | 63 (7) | 10 (2) | <0.001 | 0.32 (0.16–0.63) | 50 (5) | 23 (5) | 0.615 | 0.82 (0.49–1.37) |
| Hand, PFJ, and LS Only | 1 (<1) | 0 (0) | 1.000 | -- | 0 (0) | 1 (<1) | 0.345 | -- |
| Hand, TFJ, and PFJ Only | 4 (<1) | 3 (<1) | 0.687 | -- | 4 (<1) | 3 (1) | 0.698 | -- |
| PFJ, TFJ, and LS Only | 15 (2) | 19 (4) | 0.005 | -- | 24 (3) | 10 (2) | 0.588 | -- |
| PFJ, TFJ, and Hip Only | 3 (<1) | 4 (<1) | 0.221 | -- | 7 (1) | 0 (0) | 0.103 | -- |
| Hip, TFJ, and LS Only | 40 (4) | 24 (5) | 0.341 | 1.32 (0.78–2.24) | 40 (4) | 24 (5) | 0.594 | 1.23 (0.73–2.08) |
| Hand, LS, and Hip Only | 58 (6) | 5 (1) | <0.001 | 0.18 (0.07–0.46) | 45 (5) | 18 (4) | 0.345 | 0.69 (0.39–1.22) |
| PFJ, LS and Hip Only | 4 (<1) | 2 (<1) | 1.000 | -- | 3 (<1) | 3 (1) | 0.422 | -- |
| Hand, TFJ, Hip, and LS Only | 50 (5) | 13 (3) | 0.053 | 0.53 (0.28–1.01) | 43 (5) | 20 (4) | 0.686 | 0.87 (0.50–1.53) |
| Hand, PFJ, Hip and LS Only | 3 (<1) | 1 (<1) | 1.000 | -- | 3 (<1) | 1 (<1) | 1.000 | -- |
| Hand, PFJ, TFJ and LS Only | 23 (2) | 3 (<1) | 0.032 | -- | 20 (2) | 6 (1) | 0.298 | -- |
| PFJ, TFJ, Hip and LS Only | 10 (1) | 14 (3) | 0.008 | -- | 13 (1) | 11 (2) | 0.280 | -- |
| PFJ, TFJ, Hip and Hand | 5 (<1) | 2 (<1) | 1.000 | -- | 6 (1) | 1 (<1) | 0.433 | -- |
| Hand, TFJ, PFJ, Hip, and LS | 25 (3) | 11 (2) | 1.000 | -- | 30 (3) | 6 (1) | 0.021 | -- |
only estimated for those phenotypes occurring in at least 40 persons, models included dichotomous race (referent=Caucasian) and gender (referent=female) and continuous age (years) and BMI (kg/m2))
Bold indicates significance after adjustment for multiple comparisons using the Hochberg method
See text: significant interaction by race and gender
Bold indicates a significant adjusted result (95% CI does not include 1)
For Hand rOA, we used a composite definition requiring bilateral involvement, at least one DIP with KL grade ≥2, and at least 3 joints (DIP, PIP, or CMC) involved (4)
aOR: adjusted odds ratio; rOA: radiographic osteoarthritis; AA: African American; LS: lumbosacral spine; PFJ: patellofemoral joint; TFJ: tibiofemoral joint
Differences in frequencies by race and gender are shown in Table 3. No gender differences were significant after adjustment for multiple comparisons. However, several of the differences by race were statistically significant. African Americans were more likely to have TFJ rOA in isolation (p<0.001). Caucasians were more likely to have phenotypes that included hand rOA, with significant differences for Hand and LS rOA, Hand/TFJ/LS, and Hand/Hip/LS (p ≤ 0.001).
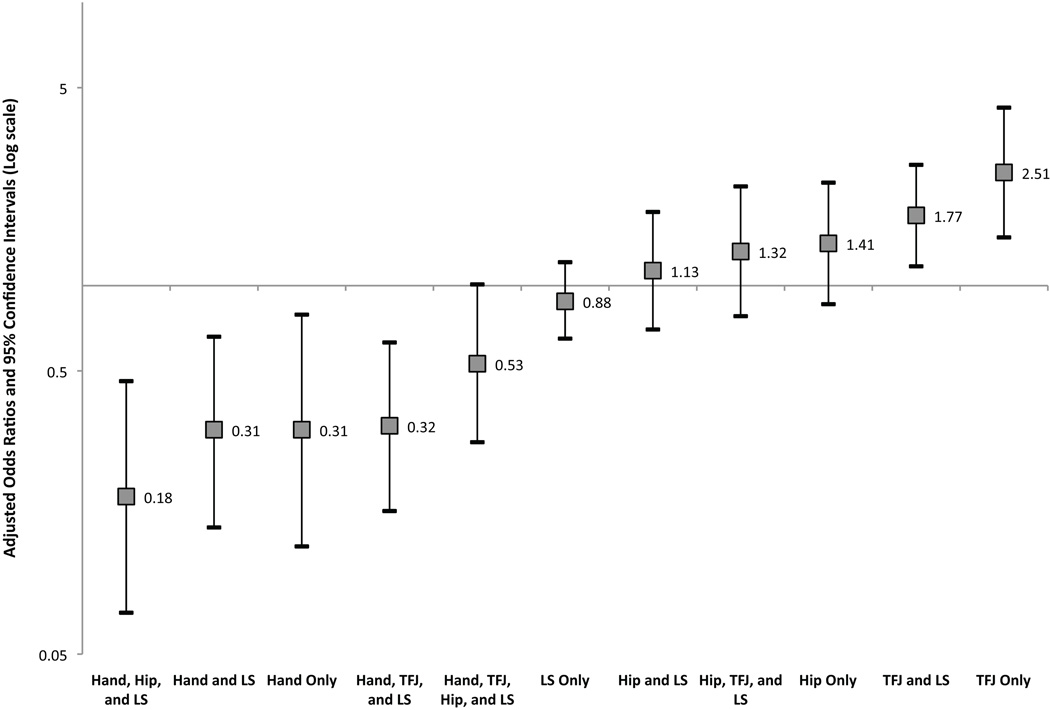
Adjusted results for those phenotypes seen in at least 40 participants are shown in Table 3 and Figure 3. As seen for the hand phenotypes, there was a significant interaction between race and gender only for no rOA (p interaction=0.01). Compared to Caucasian women, African American women had nearly twice the odds of having no OA at any site (aOR 1.93 [1.30–2.88]), while the odds for men were not significantly different (Caucasian men aOR 1.48 [0.99–2.19], AA men aOR 1.19 [0.70–2.00]). The results after adjustment again show that African Americans were less likely to have phenotypes that included Hand rOA (aOR 0.18 to 0.53, Table 3). African Americans were more likely to have phenotypes involving the TFJ, with 2.5 times the odds of TFJ rOA alone and 77% higher odds of TFJ and LS rOA compared to Caucasians. African Americans also had 30–40% higher odds of having only Hip rOA or Hip/TFJ/LS, although these were not statistically significant. Men had 70% reduced odds of Hand rOA only compared to women and 61% increased odds of TFJ and LS rOA. Again, increasing age was associated with higher odds of multiple joint involvement, but not with single joint involvement. Increasing BMI was inversely associated with Hand only, LS only, Hip only, and Hand/Hip/LS outcomes, while BMI was associated with higher odds of TFJ rOA alone (covariate aORs not shown).
Figure 3. Adjusted Odds Ratios and 95% CI for Whole-body rOA Phenotypes among African Americans compared to Caucasians.
The adjusted odds ratios for African Americans (AAs) compared to Caucasians are shown, from those phenotypes least likely in AAs on the left to those most likely on the right. The point estimate of the aOR is shown with 95% CI indicated by the bars. The horizontal line is at an aOR=1 (no difference). A logarithmic scale is used due to the logarithmic nature of the ratio measures.
Discussion
We have identified several differences in mutually exclusive multi-joint rOA phenotypes among African American and Caucasian men and women in this community-based sample. Considering first hand rOA phenotypes, African Americans had less frequent DIP rOA in any combination, but similar involvement of rOA in the PIPs and CMCs and their combinations. For whole-body rOA phenotypes, significant differences by race included more frequent involvement of the TFJ and the combination of TFJ and LS rOA in African Americans, while Caucasians had more frequent hand rOA and its combinations. Interestingly, the racial differences we found in rOA phenotypes were, in general, more significant than the gender differences.
The frequency of rOA in this community-based population was quite high. Prior reports of OA prevalence at the knee and hip in the JoCo OA (18) used the baseline weighted sample; as the current study includes the second follow up (roughly 10 years later) of those individuals, it is reasonable to think that more of them would have developed rOA, resulting in higher rOA frequency in the current analysis. Older estimates of rOA prevalence were lower (26–28) than those reported more recently (18, 19, 29), likely due to increased obesity and aging of the populations under study in addition to methodologic differences.
Patterns of hand joint involvement have been assessed in other studies, primarily in Caucasians, showing that hand joint rOA tends to group by row rather than by ray, and symmetric involvement is common (30–33). Egger, et al, reporting on patterns of hand rOA in the Chingford study, found that although the strongest associations among hand joints were for other joints in the same group (e.g. DIP with DIP), there were also substantial associations between DIP and PIP involvement (33). Kraus, et al, in a family study selected on the basis of clinical hand OA, found that 3 rOA combinations (DIP and PIP [29%], DIP, PIP, MCP, and CMC1 [29%] and DIP, PIP, and CMC1 [35%]) were most common (4). In our community study including individuals with and without evidence of OA, we also found these combinations to be among the most common (8%, 5%, and 9%, respectively), but additionally identified a high frequency of individual joint involvement (particularly of the DIPs[13%], and CMC1 [6%]). Sowers, et al, in the only recent study to assess hand rOA in African Americans, found a similar frequency of DIP, PIP, and CMC1 in African American and Caucasian women, but a greater frequency of MCP OA in African Americans (17). Studies in African descent populations, in comparison to Caucasian populations, have found a reduced frequency of Heberden’s nodes, similar frequency of PIP, MCP, and CMC1 rOA, and a higher prevalence of DIP rOA (16, 34). We also found that PIP and CMC1 rOA occurred at a similar frequency in African Americans and Caucasians, but in contrast to Sowers, et al, found that MCP rOA was also similar between groups, and in contrast to the previous reports in African descent populations (South African and Jamaican), found that DIP rOA was much less frequent among African Americans compared to Caucasians.
There is significant variability among joints assessed, methods of defining OA, and populations studied among the few studies assessing whole-body phenotypes in OA. Riyazi, et al, in a sibling study of Dutch individuals recruited based on the presence of symptomatic OA, found that combinations of hand and spine, knee and spine, and hip and spine were most common among probands and siblings, all of whom had at least 2 joint sites involved (8). In common with these investigators, we found LS OA and combinations of joints including the LS to be common in our population. Several authors have found associations between two joint sites, such as the hand and TFJ (5), hand and hip (35), and TFJ and hip (7). While these phenotypes alone were uncommon in our analysis (2% or less), phenotypes including these pairs of joints along with other joint sites were fairly frequent, such that any combination of hand and TFJ was seen in 18%, hand and hip in 16%, and TFJ and hip in 18%.
Limitations of the current analysis include its cross-sectional nature, which does not allow determination of the timing of joint involvement, although this could be considered in future studies, as the JoCo OA is a longitudinal cohort with ongoing follow up assessments. As our goal was to describe a comprehensive set of mutually exclusive rOA phenotypes by gender and race, we included only a minimal set of key covariables in the models (age, BMI), and did not include other potentially important variables such as symptoms, socioeconomic variables, occupation or physical activity; these data are available and could be used in future analyses.. Some of the phenotypes, as would be expected, had a small cell size, which precluded adjusted analyses for every possible outcome. PFJ films were read only for osteophytes and not for joint space narrowing, and interpretations for a portion of PFJ films were not available at the time of this analysis, limiting our sample size, although it is still quite sizeable. We did not have radiographs of the cervical spine or feet, so these sites could not be included although they are commonly affected by rOA. This study also has substantial strengths, including the large overall sample size, inclusion of African American and Caucasian men and women, and multiple joint standardized radiographs read by a single musculoskeletal radiologist (JBR) with high reliability. Because of the large sample size, we were able to define discrete, mutually exclusive rOA phenotypes for both hand rOA and whole-body rOA which had not been previously reported, as well as differences by both race and gender in these phenotypes.
Conclusions
We have shown, in a large, community-based sample, that multi-joint rOA involvement varies by race in a more significant manner than by gender, and that while African Americans were more likely to have TFJ rOA and combinations including TFJ rOA, they were less likely to have hand rOA (particularly DIP rOA), or combinations including hand rOA, compared to Caucasians. These findings remained significant after adjustment for gender, age, and BMI. Therefore, while “generalized OA” as often defined (hand rOA or nodal changes with other joints) may be less frequent in African Americans, this group may have a higher burden of large joint involvement, particularly TFJ and LS rOA. Such differences in radiographic patterns of OA, if confirmed in other populations and future studies, may impact selection of participants for clinical research, particularly for studies of “generalized OA,” and are suggestive of a substantial clinical and public health burden of large-joint OA among African Americans.
Acknowledgments
We would like to thank the staff and participants of the Johnston County Osteoarthritis Project without whom this work would not have been possible, as well as Philip G. Conaghan MBBS PhD and Yvonne Golightly PT PhD for input on the manuscript drafts.
Funding for this work was provided in part by: Nelson: American College of Rheumatology Clinical Investigator Fellowship Award 2009; NIH/NIAMS Loan Repayment Award L30-AR056604
Jordan/Renner: CDC/Association of Schools of Public Health S043 and S3486
Jordan/Renner/Schwartz: NIAMS P60-AR30701. The funding sources were not involved in study design, data collection or interpretation, writing or approval of the manuscript for publication.
Abbreviations (in order of appearance)
- OA
osteoarthritis
- rOA
radiographic osteoarthritis
- TFJ
tibiofemoral joint
- PFJ
patellofemoral joint
- LS
lumbosacral spine
- BMI
body mass index
- JoCo OA
Johnston County Osteoarthritis Project
- KL
Kellgren-Lawrence
- DIP
distal interphalangeal joint
- PIP
proximal interphalangeal joint
- MCP
metacarpophalangeal joint
- CMC
carpometacarpal joint
- OST
osteophytes
- DN
disc space narrowing
- aOR
adjusted odds ratio
- CI
confidence interval
- AA
African American
Footnotes
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Competing interest statement:
The authors have no financial or personal conflicts of interest in relation to this work.
References
- 1.Lawrence JS. Generalized osteoarthrosis in a population sample. Am J Epidemiol. 1969;90:381–389. doi: 10.1093/oxfordjournals.aje.a121083. [DOI] [PubMed] [Google Scholar]
- 2.Cooper C, Egger P, Coggon D, Hart DJ, Masud T, Cicuttini F, et al. Generalized osteoarthritis in women: Pattern of joint involvement and approaches to definition for epidemiological studies. J Rheumatol. 1996;23:1938–1942. [PubMed] [Google Scholar]
- 3.Felson DT, Couropmitree NN, Chaisson CE, Hannan MT, Zhang Y, McAlindon TE, et al. Evidence for a mendelian gene in a segregation analysis of generalized radiographic osteoarthritis: The framingham study. Arthritis Rheum. 1998;41:1064–1071. doi: 10.1002/1529-0131(199806)41:6<1064::AID-ART13>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 4.Kraus VB, Jordan JM, Doherty M, Wilson AG, Moskowitz R, Hochberg M, et al. The genetics of generalized osteoarthritis (GOGO) study: Study design and evaluation of osteoarthritis phenotypes. Osteoarthritis Cartilage. 2007;15:120–127. doi: 10.1016/j.joca.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 5.Hirsch R, Lethbridge-Cejku M, Scott WW, Jr, Reichle R, Plato CC, Tobin J, et al. Association of hand and knee osteoarthritis: Evidence for a polyarticular disease subset. Ann Rheum Dis. 1996;55:25–29. doi: 10.1136/ard.55.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Doherty M, Watt I, Dieppe P. Influence of primary generalised osteoarthritis on development of secondary osteoarthritis. Lancet. 1983;2:8–11. doi: 10.1016/s0140-6736(83)90003-x. [DOI] [PubMed] [Google Scholar]
- 7.Cvijetic S, Campbell L, Cooper C, Kirwan J, Potocki K. Radiographic osteoarthritis in the elderly population of zagreb: Distribution, correlates, and the pattern of joint involvement. Croat Med J. 2000;41:58–63. [PubMed] [Google Scholar]
- 8.Riyazi N, Meulenbelt I, Kroon HM, Ronday KH, Hellio le Graverand MP, Rosendaal FR, et al. Evidence for familial aggregation of hand, hip, and spine but not knee osteoarthritis in siblings with multiple joint involvement: The GARP study. Ann Rheum Dis. 2005;64:438–443. doi: 10.1136/ard.2004.024661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Valdes AM, McWilliams D, Arden NK, Doherty SA, Wheeler M, Muir KR, et al. Involvement of different risk factors in clinically severe large joint osteoarthritis according to the presence of hand interphalangeal nodes. Arthritis Rheum. 2010;62:2688–2695. doi: 10.1002/art.27574. [DOI] [PubMed] [Google Scholar]
- 10.Englund M, Paradowski PT, Lohmander LS. Association of radiographic hand osteoarthritis with radiographic knee osteoarthritis after meniscectomy. Arthritis Rheum. 2004;50:469–475. doi: 10.1002/art.20035. [DOI] [PubMed] [Google Scholar]
- 11.Ledingham J, Dawson S, Preston B, Milligan G, Doherty M. Radiographic patterns and associations of osteoarthritis of the hip. Ann Rheum Dis. 1992;51:1111–1116. doi: 10.1136/ard.51.10.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Croft P, Cooper C, Wickham C, Coggon D. Is the hip involved in generalized osteoarthritis? Br J Rheumatol. 1992;31:325–328. doi: 10.1093/rheumatology/31.5.325. [DOI] [PubMed] [Google Scholar]
- 13.Dahaghin S, Bierma-Zeinstra SM, Reijman M, Pols HA, Hazes JM, Koes BW. Does hand osteoarthritis predict future hip or knee osteoarthritis? Arthritis Rheum. 2005;52:3520–3527. doi: 10.1002/art.21375. [DOI] [PubMed] [Google Scholar]
- 14.Hassett G, Hart DJ, Doyle DV, March L, Spector TD. The relation between progressive osteoarthritis of the knee and long term progression of osteoarthritis of the hand, hip, and lumbar spine. Ann Rheum Dis. 2006;65:623–628. doi: 10.1136/ard.2005.038414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Solomon L, Beighton P, Lawrence JS. Osteoarthrosis in a rural south african negro population. Ann Rheum Dis. 1976;35:274–278. doi: 10.1136/ard.35.3.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bremner JM, Lawrence JS, Miall WE. Degenerative joint disease in a jamaican rural population. Ann Rheum Dis. 1968;27:326–332. doi: 10.1136/ard.27.4.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sowers M, Lachance L, Hochberg M, Jamadar D. Radiographically defined osteoarthritis of the hand and knee in young and middle-aged african american and caucasian women. Osteoarthritis Cartilage. 2000;8:69–77. doi: 10.1053/joca.1999.0273. [DOI] [PubMed] [Google Scholar]
- 18.Jordan JM, Helmick CG, Renner JB, Luta G, Dragomir AD, Woodard J, et al. Prevalence of knee symptoms and radiographic and symptomatic knee osteoarthritis in african americans and caucasians: The johnston county osteoarthritis project. J Rheumatol. 2007;34:172–180. [PubMed] [Google Scholar]
- 19.Jordan JM, Helmick CG, Renner JB, Luta G, Dragomir AD, Woodard J, et al. Prevalence of hip symptoms and radiographic and symptomatic hip osteoarthritis in african americans and caucasians: The johnston county osteoarthritis project. J Rheumatol. 2009;36:809–815. doi: 10.3899/jrheum.080677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kellgren JH, Lawrence JS. Atlas of standard radiographs: The epidemiology of chronic rheumatism. 1963;vol. 2 [Google Scholar]
- 21.Burnett S, Hart DJ, Cooper C, Spector TD. A radiographic atlas of osteoarthritis. 1994 [Google Scholar]
- 22.Jordan JM, Linder GF, Renner JB, Fryer JG. The impact of arthritis in rural populations. Arthritis Care Res. 1995;8:242–250. doi: 10.1002/art.1790080407. [DOI] [PubMed] [Google Scholar]
- 23.Hochberg Y. A sharper bonferroni procedure for multiple tests of significance. Biometrika. 1988;75:800–802. [Google Scholar]
- 24.Newson R ALSPAC Study Team. Multiple-test procedures and smile plots. The Stata Journal. 2003;3:109–132. [Google Scholar]
- 25.Peduzzi P, Concato J, Kemper E, Holford TR, Feinstein AR. A simulation study of the number of events per variable in logistic regression analysis. J Clin Epidemiol. 1996;49:1373–1379. doi: 10.1016/s0895-4356(96)00236-3. [DOI] [PubMed] [Google Scholar]
- 26.Tepper S, Hochberg MC. Factors associated with hip osteoarthritis: Data from the first national health and nutrition examination survey (NHANES-I) Am J Epidemiol. 1993;137:1081–1088. doi: 10.1093/oxfordjournals.aje.a116611. [DOI] [PubMed] [Google Scholar]
- 27.Anderson JJ, Felson DT. Factors associated with osteoarthritis of the knee in the first national health and nutrition examination survey (HANES I). evidence for an association with overweight, race, and physical demands of work. Am J Epidemiol. 1988;128:179–189. doi: 10.1093/oxfordjournals.aje.a114939. [DOI] [PubMed] [Google Scholar]
- 28.Felson DT, Naimark A, Anderson J, Kazis L, Castelli W, Meenan RF. The prevalence of knee osteoarthritis in the elderly. the framingham osteoarthritis study. Arthritis Rheum. 1987;30:914–918. doi: 10.1002/art.1780300811. [DOI] [PubMed] [Google Scholar]
- 29.Dillon CF, Rasch EK, Gu Q, Hirsch R. Prevalence of knee osteoarthritis in the united states: Arthritis data from the third national health and nutrition examination survey 1991–94. J Rheumatol. 2006;33:2271–2279. [PubMed] [Google Scholar]
- 30.Kalichman L, Cohen Z, Kobyliansky E, Livshits G. Patterns of joint distribution in hand osteoarthritis: Contribution of age, sex, and handedness. Am J Hum Biol. 2004;16:125–134. doi: 10.1002/ajhb.20007. [DOI] [PubMed] [Google Scholar]
- 31.Poole J, Sayer AA, Hardy R, Wadsworth M, Kuh D, Cooper C. Patterns of interphalangeal hand joint involvement of osteoarthritis among men and women: A british cohort study. Arthritis Rheum. 2003;48:3371–3376. doi: 10.1002/art.11339. [DOI] [PubMed] [Google Scholar]
- 32.Niu J, Zhang Y, LaValley M, Chaisson CE, Aliabadi P, Felson DT. Symmetry and clustering of symptomatic hand osteoarthritis in elderly men and women: The framingham study. Rheumatology (Oxford) 2003;42:343–348. doi: 10.1093/rheumatology/keg110. [DOI] [PubMed] [Google Scholar]
- 33.Egger P, Cooper C, Hart DJ, Doyle DV, Coggon D, Spector TD. Patterns of joint involvement in osteoarthritis of the hand: The chingford study [Chingford] J Rheumatol. 1995;22:1509–1513. [PubMed] [Google Scholar]
- 34.Solomon L, Beighton P, Lawrence JS. Rheumatic disorders in the south african negro. part II. osteo-arthrosis. S Afr Med J. 1975;49:1737–1740. [PubMed] [Google Scholar]
- 35.Hochberg MC, Lane NE, Pressman AR, Genant HK, Scott JC, Nevitt MC. The association of radiographic changes of osteoarthritis of the hand and hip in elderly women. J Rheumatol. 1995;22:2291–2294. [PubMed] [Google Scholar]