Abstract
Objective
The study aims to examine the threshold in disease activity associated with switching biologic treatment regimens in RA patients in real-world clinical practice.
Methods
Using data from a prospective observational North American cohort of RA patients through 12/30/2009, patients who initiated a new anti-TNF agent with ≥ 6 months of follow-up were identified. Patients were classified as switchers or maintainers depending on whether they continued their anti-TNF treatment or switched (including discontinuation) within 12 months. Level of disease activity measured by CDAI and DAS28 at time of switch (corresponding follow-up visit for maintainers) was examined and random effect multivariable logistic regression was used to adjust for covariates.
Results
Mean age and RA duration among 1,549 eligible patients were 56.1 and 9.6 years, 80% were women, 62% were initiating their 1st biologic and 30% 2nd. At time of switch, the median DAS28 and CDAI were 3.1 and 8.4 among maintainers, and 4.0 and 15.2 among switchers. Maintainers also experienced a greater amount of reduction in disease activity compared with switchers (CDAI: −7.7 vs. −2.3; DAS28: −1.1 vs. −0.3). The threshold to switch decreased over calendar time, with the greatest amount of reduction observed among patients with moderate disease activity.
Conclusion
On average, physicians and patients were willing to continue biologic treatment for patients who are at or near low disease activity. The threshold to switch decreased over time, especially among partial responders.
Keywords: Rheumatoid arthritis, switching, anti-TNF drugs, calendar year, physician preference
Introduction
Tumor necrosis factor α inhibitors (anti-TNF therapies) and other biologic agents represent a significant advance in the treatment of patients with rheumatoid arthritis (RA). In clinical trials, these agents have been shown to reduce joint inflammation and radiographic damage and to produce clinical response in up to 70–80% of RA patients (1). Additionally, based upon available data, a number of trials and observational studies have suggested that among those who failed to respond to the first or second anti-TNF agent, switching to yet another anti-TNF agent or a biologic agent with a different mechanism of action may result in an improved clinical response without an apparent increase in the risk of adverse events/toxicity (2–10).
Despite the favorable profile of anti-TNF agents and other biologics, considerable proportions of patients may discontinue their initial treatment and/or switch to a different agent due to a number of reasons. Within one year of treatment initiation, up to 30% of RA patients discontinue their treatment, and the percentage increases to up to 50% at two years (4, 7, 11–13). The most common reasons for switching are lack of efficacy and adverse event/toxicity; together they account for over 80% of all such cases (4, 7, 12, 14).
Currently, the absence of a well-accepted threshold for “lack of efficacy” presents a major gap in the evidence base to help clinicians to decide whether or not to change therapy, although some data and recommendations exist. For example, the BeSt study, a randomized trial mandated adjustment of treatment if the target of DAS44 ≤ 2.4 was not met (15). A task force of rheumatologists conducted a systematic literature review of treat to target (T2T) studies and recommended remission as an optimal treatment target, but it acknowledged that low disease activity was a reasonable target for patients with long-standing RA. The task force also acknowledged that other factors, such as co-morbidities, may impact target disease activity, and that individualized treatment plans remain important (16). Despite the data and the recent recommendations described above, understanding the current practices of rheumatologists in their threshold for switching remains an evidence gap that is particularly important in light of growing interest in quantifying disease activity and adopting T2T strategies which mandate treatment acceleration if a pre-defined level of disease activity has not been reached (17). The key issue is that if the level of disease activity for a T2T protocol is lower than the usual threshold for clinicians and patients to switch patients’ biologic treatments, physicians may find such T2T protocols unacceptable.
In addition to levels of disease activity, other factors also play a role in the decision making process. Yazici and colleagues showed that that the median durations of etanercept and infliximab treatment decreased from 454 days to 237 days once adalimumab became available in the US (13). Likewise, an inverse association between time on treatment and calendar year of treatment initiation was observed based on data from a population-based RA registry containing over 2,300 courses of anti-TNF treatment (11). The observation of increased switching among biologic agents suggests that the threshold to switch may have lowered over time, a temporal trend that has not been examined in these studies.
We used data from a large North American cohort of RA patients to assess the level of disease activity associated with switching the biologic treatment regimen and to evaluate the impact of calendar time on the threshold in disease activity to switch. In addition, we explored factors associated with continuing the biologic treatment regimen despite a sub-optimal treatment response.
Patients and Methods
Study Population
Study participants consisted of RA patients enrolled in the Consortium of Rheumatology Researchers of North America (CORRONA) Registry from 10/01/2001 to 12/30/2009. Details of the CORRONA registry have been published previously (18, 19). Briefly, RA patients satisfying the 1987 ACR diagnosis criteria were enrolled from academic and community rheumatology practices. Data from both physicians and patients were prospectively collected at enrollment and follow-up clinical encounters at approximately 3–4 months intervals; at each visit, common measures of RA disease activity, functional status, and use of RA medications were collected. When changes in treatment were made, physicians were encouraged but not mandated to report the reason (e.g. lack of efficacy, adverse event). All subjects gave written informed consent. The study protocol was approved both by central and local Institutional Review Boards.
Patients who started a new anti-TNF agent and had at least one follow-up visit within one year of treatment initiation were eligible to be included in this analysis. A ‘new’ anti-TNF treatment episode required that they had not previously been on that particular anti-TNF agent but did not necessarily mean that they were biologic naïve. Patients could contribute more than one treatment episode if they sequentially initiated different anti-TNF agents. The visit at which treatment initiation was recorded was considered the “index” visit and baseline covariates of interest were assessed at that visit.
Because response to treatment consists of two components: absolute disease activity and the amount of reduction in disease activity since treatment initiation, we defined patients with ‘sub-optimal clinical response’ as those whose CDAI at time of follow-up visit was > 10 (moderate disease activity) and who did not have at least a 10 point improvement in their CDAI compared to baseline, using validated cut points to measure treatment response (20, 21). Among these patients who had neither achieved low disease activity nor had a substantial improvement in their disease activity, we explored factors associated with maintaining (vs. switching) their anti-TNF treatment regimen.
Outcomes of interest
The outcome of the study was whether a patient switched the biologic treatment regimen within 1–6, and 6–12 months after initiation. In the main analysis, switching included either discontinuation of the anti-TNF agent and/or changing to a different biologic agent for reasons other than safety/tolerability. Outcome was evaluated for the two time periods separately: patients who switched were classified as early switchers (switched within 6 months) or late switchers (switched between 6 and 12 months) and patients who continued on the initial anti-TNF agents during the 6 and 12 month time periods were classified as maintainers. Thus, a patient may contribute two treatment episodes (e.g., maintainer for the < 6 months period and the 6–12 months period; maintainer for the < 6 months period and switcher for the 6–12 months period). For patients with multiple visits within 1–6, and 6–12 months after initiation (most of the eligible patients), the latest visit in each time frame was used. Because the observations were not independent, the statistical analyses accounted for the clustered nature of the data. Treatment episodes for patients who discontinued and/or changed biologic therapies for reasons of safety/tolerability were excluded from this analysis because switching for these reasons would likely be less discretionary.
Covariates
Covariates of interest included age, body mass index (BMI), RA disease duration, number of prior disease modifying anti-rheumatic drugs (DMARDs), measures of RA disease activity including Clinical Disease Activity Index (CDAI)(21), disease activity score (DAS28), comorbidities (dichotomized variable: if the patients had at least one of the medical conditions specified in table 4), physician global assessment of disease activity (100 mm visual analog scale), and laboratory tests (C-Reactive Protein, CRP; erythrocyte sedimentation rate, ESR). Since laboratory data were not required to be collected within CORRONA and were present for only approximately 50% of all visits represented in this analysis, results for CRP, ESR, and the DAS28 reflect only the observations for which these data were available. The ratio of swollen joint count to tender joint count (SJC/TJC) was calculated, and patients were categorized into two groups (SJC/TJC < 0.4 or ≥ 0.4) (22). If TJC was 0, the ratio was assigned the value of 1. Calendar year was grouped to reflect the availability of new biologic agents as follows: 2002–2005 (etanercept, infliximab, adalimumab available), 2006–2007 (abatacept and rituximab available) and 2008–2009 (golimumab and certolizumab available).
Table 4.
Multivariable adjusted association between CDAI, MD global, SJC/TJC, and calendar year at follow-up visit with switching (vs. maintaining) biologic treatment regiment among patients with a sub-optimal response‡ (n total = 648, 140 switched and 508 maintained treatment)
| Covariates* | Odds Ratio and 95% Confidence Interval |
|---|---|
| MD Global (rescaled, in unit of 5 points)† | 0.93 (0.88 – 0.99) |
| SJC/TJC ratio | |
| ≥ 0.4 | Referent |
| < 0.4 | 0.56 (0.33 – 0.96) |
| CDAI (in unit of 10 points) | 1.02 (1.00 – 1.04) |
| Calendar Year (grouped to reflect the availability of newer biologics) | |
| 02 – 05 | Referent |
| 06 – 07 | 3.11 (1.70 – 5.68) |
| 08 – 09 | 6.29 (3.54 – 11.18) |
| Comorbidities§ | |
| No | Referent |
| Yes | 1.32 (0.87 – 2.00) |
Patients with sub-optimal response were defined as patients whose CDAI at time of switch was > 10 and reduction in CDAI since treatment initiation was < 10.
measured at the visit where the patient either switched or was maintained on therapy
Scaled from 0 to 100, with higher numbers representing better disease control
comorbidities evaluated included: hypertension, unstable angina/MI/CAD procedure, congestive heart failure, stroke/TIA, cancer, ulcer/GERD/dyspepsia, liver disorder, depression/psychiatric disorder, pulmonary fibrosis/asthma/COPD, diabetes, DVT, elevated creatinine, psoriasis (not arthritis), anemia/hematologic disorder.
Statistical Analysis
For each calendar year, the point prevalence of biologic agent use on April 1st and October 1st, and the average CDAI at time of switching, were calculated. We then compared the descriptive statistics of covariates of interest at baseline visit (the index visit) and the follow-up visit when the patients were categorized as a ‘maintainer’ or a ‘switcher’. The same descriptive statistics were calculated in patient sub-groups stratified by disease duration (2 years or less versus more than 2 years) and disease activity level measured by CDAI at baseline: Low (CDAI < 10), Medium (10≤CDAI<22), High (CDAI≥22) (20).
Random effect logistic regression was used to model the multivariable association between CDAI at follow-up visit, change in CDAI since treatment initiation, calendar year, previous anti-TNF agent use, age at RA onset, number of prior non-biologic DMARDs, BMI, and time to switch (1–6 months, or 6–12 months) with switching (vs. maintaining) biologic treatment regimen. Physician preference was estimated by assessing the extent to which switching clustered at the physician level. To examine the interaction between calendar year of treatment initiation and the threshold to switch, a separate multivariable random effect logistic regression model was built consisting solely of calendar year, disease activity (patients with moderate disease activity (10≤CDAI<22) versus patients with high or low disease activity), and the interaction term between the two. Finally, random effect logistic regression was used to model the multivariable association between CDAI, MD global, SJC/TJC, and calendar year at follow-up visit with switching (vs. maintaining) biologic treatment regiment among the subgroup of patients with a sub-optimal response (CDAI > 10 and change in CDAI < 10). Standard errors were adjusted appropriately to account for the clustered nature of the data.
Sensitivity analysis
Because reporting a reason for switching was optional, a sensitivity analysis was performed that included only patients who switched explicitly for the reason of lack of efficacy, designated by the treating physician. Also, because results might differ for patients switching to a new biologic versus simply discontinuing the previous biologic, a second sensitivity analysis was performed that defined switching only as changing to a new agent; discontinuations where the patient did not initiate a new biologic within 1 year of starting the previous biologic were excluded.
Results
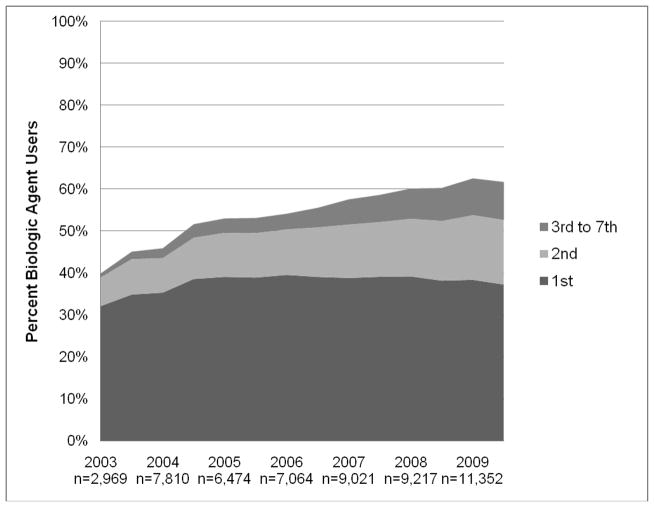
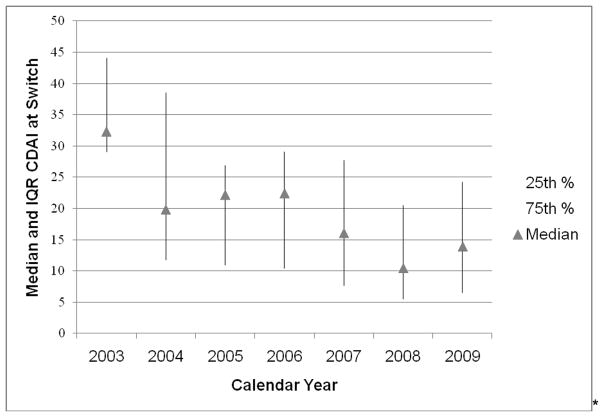
Based on CORRONA data through 12/30/2009, a total of 22,966 RA patients were enrolled with 136,738 visits accrued. Overall, the prevalence of biologic agent use within CORRONA increased from 40% in 2003 to 62% in 2009 (Figure 1). When subjects were categorized based on whether they are on their 1st, 2nd, 3rd or more biologic agents, the greatest amount of increase in the prevalence was observed among subjects receiving their 2nd–7th biologic agents. The level of disease activity at which patients switched gradually decreased over time (Figure 2). In 2008 – 2009, patients who switched had a median CDAI between 10 and 15, slightly above the CDAI threshold for low disease activity.
Figure 1.
Prevalence of biologic agent use in a large North American cohort of RA patients by calendar year and history of previous biologic agent use
Figure 2.
Level of disease activity* at which RA patients switched their biologic treatment regimen, by calendar year
* measured by the clinical disease activity index (CDAI); the thresholds for low, moderate, and high disease activity for CDAI are <= 10, between 10 and 22, and >= 22
We identified 3,351 RA patients who initiated a new anti-TNF agent, and after applying the inclusion and exclusion criteria, 1,549 patients were eligible and they contributed 2,302 treatment episodes that were included in this analysis. Among these patients, 1,247 continued on the same treatment and 302 discontinued and/or switched to a different biologic agent within one year. Most (62%) of these patients were on their first anti-TNF treatment, 30% were on the second anti-TNF treatment, and 8% were on the third. At the time of treatment initiation, the average age was 56.1 (standard deviation, 12.9) and 80% were women.
At the follow-up visit within one year, maintainers had lower disease activity compared to switchers (median CDAI 8.4 vs. 15.2; mean DAS28 3.1 vs. 4.0) (Table 1). Maintainers also experienced a greater amount of improvement (reduction) in disease activity since treatment initiation (median change in CDAI −7.7 vs. −2.3, median change in DAS28 −1.1 vs. −0.3). Significant differences were also observed in tender and swollen joint counts, patient and physician global, pain, and mHAQ. On average, maintainers used a greater number of prior DMARDS compared to switchers. None of the laboratory measures (i.e. CRP and ESR) were significantly different between maintainers and switchers (Table 1).
Table 1.
Comparison of patient characteristics measured at time of biologic treatment regimen switch or the corresponding visit for those maintained on therapy
| Patient Characteristics | Maintainers (n=2,000) | Switchers (n=302) | P-value | ||
|---|---|---|---|---|---|
| N | Median (IQR) | N | Median (IQR) | ||
| At time of switch | |||||
| Age at onset of RA | 1983 | 46.0 (19.0) | 297 | 48.0 (19.0) | 0.08 |
| BMI | 1992 | 27.8 (8.8) | 301 | 29.1 (9.2) | 0.01 |
| Duration of RA | 1992 | 7.0 (11.0) | 300 | 6.0 (11.0) | 0.91 |
| Number of Prior | |||||
| DMARDS used | 2000 | 1.0 (2.0) | 302 | 2.0 (2.0) | < 0.001 |
| CDAI | 1901 | 8.4 (14.1) | 290 | 15.2 (19.2) | < 0.001 |
| DAS28 | 925 | 3.1 (2.0) | 118 | 4.0 (1.7) | < 0.001 |
| Tender joints count (TJC) | 1991 | 1.0 (4.0) | 299 | 2.0 (8.0) | < 0.001 |
| Swollen joint count (SJC) | 1992 | 2.0 (6.0) | 299 | 4.0 (10.0) | < 0.001 |
| Patient global | 1910 | 23.0 (42.0) | 294 | 42.5 (44.0) | < 0.001 |
| MD global | 1996 | 13.0 (20.0) | 300 | 23.0 (33.5) | < 0.001 |
| Subject pain VAS | 1941 | 25.0 (40.0) | 298 | 40.0 (52.0) | < 0.001 |
| mHAQ | 1960 | 0.3 (0.6) | 298 | 0.5 (0.9) | < 0.001 |
| CRP | 788 | 0.5 (1.5) | 102 | 0.7 (2.3) | 0.90 |
| ESR | 976 | 16.0 (25.0) | 122 | 17.5 (21) | 0.93 |
| Change from Baseline | |||||
| Change in CDAI | 1798 | −7.7 (17.6) | 272 | −2.3 (14.0) | < 0.001 |
| Change in DAS28 | 570 | −1.1 (2.0) | 66 | −0.3 (1.9) | < 0.001 |
| Change in TJC | 1963 | −2.0 (7.0) | 296 | 0.0 (6.0) | < 0.001 |
| Change in SJC | 1964 | −2.0 (6.0) | 296 | 0.0 (4.0) | < 0.001 |
| Change in patient global | 1817 | −8.0 (33.0) | 277 | −2.0 (33.0) | < 0.001 |
| Change in MD global | 1980 | −13 (26.0) | 298 | −5.0 (25.0) | < 0.001 |
| Change in subject pain | |||||
| VAS | 1866 | −9 (32.0) | 283 | −2.0 (29.0) | < 0.001 |
| Change in mHAQ | 1896 | 0.0 (0.3) | 286 | 0.0 (0.3) | < 0.001 |
| Change in CRP | 461 | −0.1 (1.5) | 58 | 0.0 (0.5) | 0.19 |
| Change in ESR | 609 | −3.0 (15.0) | 69 | −1.0 (15) | 0.31 |
Stratifying patients by their baseline level of disease activity and RA disease duration, descriptive statistics measured at the follow-up visit suggested that the level of disease activity and the amount of improvement acceptable enough to continue patients on biologic treatment regimen was dependent on patients’ initial disease activity (Table 2). For example, for patients with disease duration ≥ 2 years, the median change in CDAI measured at the follow-up visit for patients who switched was 1.7 units (i.e. slight worsening), −1.3 units (slight improvement), and −9.6 units (some improvement) for those who started in low, moderate, and high baseline disease activity, respectively. Among those who remained on therapy, the median change in CDAI at the corresponding visits were −1.9, −7.1, and −20.0 units, respectively, demonstrating that physicians required differing levels of response in order to continue patients on therapy, conditional on where the patient started. Similar trends were observed with respect to patient and physician global and pain. Results were similar for those with RA disease duration < 2 years (data not shown).
Table 2.
Comparison of selected patient characteristics at time of biologic treatment regimen switch or the corresponding visit for those who were maintained on therapy among those with ≥ 2 years disease duration, by baseline disease activity level
| Baseline Disease Activity | ||||||
|---|---|---|---|---|---|---|
| CDAI < 10 | 10≤CDAI<22 | CDAI≥22 | ||||
| N | Median (IQR) | N | Median (IQR) | N | Median (IQR) | |
| CDAI | ||||||
| Switchers | 44 | 8.0 (8.5) | 76 | 13.8 (13.1) | 110 | 23.8 (19.6) |
| Maintainers | 293 | 4.3 (6.3) | 551 | 8.9 (11.5) | 679 | 12.8 (18.1) |
| Change in CDAI | ||||||
| Switchers | 44 | 1.7 (7.8) | 76 | −1.3 (13.4) | 110 | −9.6 (17.6) |
| Maintainers | 293 | −1.9 (5.8) | 551 | −7.1 (10.6) | 679 | −20.0 (17.5) |
| Patient global | ||||||
| Switchers | 45 | 35 (45) | 77 | 45 (40) | 110 | 50 (45) |
| Maintainers | 295 | 17 (29) | 554 | 25 (40) | 683 | 30 (42) |
| MD global | ||||||
| Switchers | 48 | 15 (20) | 77 | 24 (22) | 113 | 30 (30) |
| Maintainers | 305 | 9 (15) | 568 | 14 (19) | 716 | 16 (28) |
| Subject pain | ||||||
| VAS | ||||||
| Switchers | 45 | 35 (35) | 78 | 40 (51) | 113 | 50 (45) |
| Maintainers | 298 | 20 (28) | 556 | 25 (40) | 697 | 32 (42) |
After controlling for both the level of disease activity and the amount of improvement from baseline, calendar year was significantly associated with switching (Table 3). Compared to patients who initiated anti-TNF treatment between 2002 and 2005, the likelihood of switching was more than two folder greater in 2005–2006 (odds ratio [OR] = 2.62, 95% CI 1.64–4.19); this effect was even greater in 2008–2009 (OR = 6.05, 95% CI 3.72–9.86). There was a significant clustering effect by physician in the decision to switch (OR=1.32, 95%CI 1.16–1.95). Additionally, a significant interaction between calendar year at treatment initiation and disease activity was observed: the likelihood to switch among patients with moderate disease activity (CDAI between 10 and 22) was greater over calendar time compared to those with low or high disease activity (OR = 2.4, 95% CI 1.0 – 6.0).
Table 3.
Multivariable Adjusted Association between Absolute Disease Activity, Change in Disease Activity, and Calendar Time with Switching the Biologic Treatment Regimen within the first year (A total of 2,070 treatment episodes; 272 switched and 1,798 maintained treatment)
| Covariates* | Odds Ratio and 95% Confidence Interval |
|---|---|
| Disease Activity (measured as Clinical | |
| Disease Activity Index, or CDAI) | |
| < 10 (mild) | Referent |
| 10 – 22 (moderate) | 1.94 (1.36 – 2.75) |
| ≥ 22 (high) | 3.39 (2.27 – 5.06) |
| Improvement in Disease Activity (change in CDAI from baseline) | 1.02 (1.01–1.04) |
| Calendar Year | |
| 02 – 05 | Referent |
| 06 – 07 | 2.62 (1.64 – 4.19) |
| 08 – 09 | 6.05 (3.72 – 9.86) |
| Number of Previous Anti-TNF agents used | |
| 0 | Referent |
| 1 or more | 1.43 (1.08–1.89) |
| Timing of the follow-up visit | |
| ≤ 6 months | Referent |
| 6–12 months | 1.26 (0.93–1.69) |
measured at the visit where the patient either switched or was maintained on therapy
There was a significant clustering effect at physician level in the decision to switch (OR=1.32, 95% CI 1.16–1.95)
To examine whether the observed association between calendar time and switching biologics may be explained by changing patient characteristics over time, we have compared baseline patient characteristics across the three cohorts of patients including age, age at RA onset, disease duration, mHAQ, DAS28, CDAI, patient and physician global, and glucocorticoid use. The distributions of these characteristics were comparable (data not shown).
The sensitivity analysis that restricted the study population to only those who switched for physician designated reasons of lack of efficacy yielded a similar effect of calendar year (data not shown). The interaction between moderate disease activity and calendar year at treatment initiation was likewise stronger (OR=3.4, 95% CI 1.1 – 10.2). When those did not initiate another biologic agent after discontinuing the initial anti-TNF agent were excluded, the results were consistent with those from the main analysis, and the interaction between calendar time and moderate disease activity was similar (OR=5.7, 95% CI 1.1 – 30.4).
We identified a subgroup of 667 treatment episodes (159 switched and 508 maintained therapy) with sub-optimal response to their anti-TNF treatment (CDAI > 10 and change in CDAI > −10). After adjusting for comorbidities, level of disease activity, and calendar year, patients with a SJC/TJC ratio < 0.4 and higher (better) physician global were more likely to be maintained on the same therapy (Table 4).
Discussion
We found that within one year of starting new anti-TNF therapy, patients who were continued on their initial anti-TNF treatment regimen had, on average, 3–4 tender/swollen joints, a median DAS28 of 3.1, and a median CDAI of 8.4. The results suggest that in this large, North American RA cohort, a disease state close to low disease activity is considered ‘good enough’ to continue anti-TNF therapy. Additionally, the results of our study support the hypothesis that, in addition to level of disease activity and the amount of improvement since treatment initiation, more recent calendar time is strongly and significantly associated with a three to six-fold increase in the likelihood to switch among biologic agents. We found a significant clustering effect at physician level, suggesting that physicians have quantifiable differences in their proclivity to switch patients. Finally, we observed that among partial responders, physician perception of disease activity, as measured by physician global and the SJC/TJC ratio, played a role in the decision to switch, independent of the measurement of disease activity quantified by the CDAI.
The amount residual RA disease activity that is sufficient to continue therapy in our study is similar to that from a cross-sectional survey of over 200 RA patients that found that a DAS28 of 3.5 was satisfactory enough to stay on their current treatment regimen (23). The threshold for switching in our study is higher than those mandated in T2T studies and proposed by the task force, suggesting that a singular, non-flexible target for T2T guidelines regardless of individual patient characteristics, e.g., level of disease activity at treatment initiation, may not be feasible or widely accepted in routine practice. As we have hypothesized, the threshold in disease activity at time of switch, rather than staying static, has lowered over calendar years. Perhaps most important is the finding of a significant interaction between disease activity and calendar year; we found that the effect of calendar time was greater for those with moderate disease activity compared with those with low or high disease activity at time of switch, suggesting that the availability and penetration of newer biologic agents makes the greatest impact on the treatment of these patients for whom the threshold to switch is perhaps more subjective.
The finding that calendar time was significantly associated with switching is consistent with those reported in two separate studies that the time patients stayed on their anti-TNF agents became shorter over time (11, 13). Although discordant results have reported that no difference in one year drug survival rate and threshold to switch was observed over time, those findings may not be comparable to ours because they looked at an earlier and shorter calendar time (Hyrich et al, 2002–2005; Gomez-Reino et al., 2000–2003) while our study data spanned a longer and more recent time period where the number of alternative biologic agents was greater (7, 12). This discrepancy suggests a fast-changing pattern in the utilization of biologic agents among RA patients and that in future studies examining biologic agent use, the time frame should be clearly defined and calendar time adjusted for.
We observed a significant clustering effect by physician associated with switching, consistent with the idea that there are individual physician-specific factors that affect treatment choices. Physician preference to switch may be one of these factors. Other physician or practice-associated factors may also contribute to this effect (e.g. insurance status, formulary status of particular biologics, case mix of the physician’s RA patients). This ‘natural variability’ allows for examination of outcomes associated with switching vs. maintaining in future comparative effectiveness research analyses.
Previous data from a large population-based RA cohort showed that a sizable proportion of RA patients were maintained on the same treatment despite sub-optimal treatment response (3). We observed the same finding; among 667 treatment episodes with sub-optimal response, 508 were not changed to a new therapy. A higher (better) physician global, and a SJC/TJC ratio < 0.4 were significantly associated with not switching. These two measures are helpful in as much as they may assist in identifying limitations of the CDAI, which in certain patients may be influenced (through the patient global score) by factors other than RA (e.g. low back pain, fibromyalgia). A low SJC/TJC ratio has been suggested to reflect a discrepancy between a low level of disease activity perceived by the physician and a higher level of disease activity perceived by the patient that may be influenced by chronic pain syndromes (22). Even after multivariable adjustment for CDAI and other covariates, an SJC/TJC ratio of < 0.4 was associated with maintaining therapy among patients who continued to have moderate or high disease activity and had not had a substantial improvement measured by CDAI.
Among this subgroup of patients, it is possible that other factors that we did not assess may contribute to a reluctance to switch. For example, Wolfe and colleagues found that many RA patients were satisfied with their treatment despite continued moderate disease activity (24). Another limitation of our study is that we did not have the physician-designated reason for treatment switching for all treatment episodes, as this question was optional and only started being collected in 2007. To address this issue, we conducted sensitivity analysis including only those who switched with a physician-designated reason related to lack of efficacy. The results from the sensitivity analysis were similar to those from the main analysis. Additionally, our findings were further supported by similar results from the sensitivity analysis in which switching referred to only when the patient changed to a different biologic. Another limitation is that our results may not be generalizable to patient populations outside the United States, e.g., countries that do not allow for biologic treatment for RA patients with low or moderate disease activity or where other restrictions (e.g. insurance) strongly affect biologic use.
Our study has a number of strengths. We used data collected through the end of 2009 and therefore were able to study the treatment patterns in recent years. Anti-TNF agents and other biologics in general are quickly evolving to more ubiquitous use, and patients who fail to respond to anti-TNF agents now have a number of alternatives to try. Additionally, the CORRONA cohort collects data at routine clinical encounters from large number of physicians and patients drawn from both community and academic practices and has few inclusion criteria other than established RA. Therefore, the data represents diverse patient populations and treatment patterns from many practices in the U.S.
In conclusion, the results of our study suggest that low disease activity is a sufficient target for most clinicians and patients to be satisfied enough to continue biologic therapy. Moreover, we observed increased switching and likely a lowered threshold to switch in recent years. This temporal trend is significantly more pronounced among those with moderate disease activity, which account for a large proportion of all RA patients (25). The discrepancy between levels of disease activity as measured and perceived by physicians and patients may partially explain why treatment was not accelerated in patients with a seemingly sub-optimal treatment response measured by the CDAI. Additional research is needed to determine the optimal level of disease activity above which switching is justified, and the best sequence of biologic agents to use in order to achieve favorable long-term outcomes. Finally, given large disparities in the access and rate of utilization of biologic agents throughout the world, it is likely that observational data from country-specific registries will be needed to characterize and generate data useful to inform more optimal switching regimens (26).
Significance and Innovation.
We found that in real world clinical practice, the treatment goal was at or near low disease activity that within one year of starting new anti-TNF therapy, patients who were continued on their initial anti-TNF treatment regimen had a median DAS28 of 3.1 and a media CDAI of 8.4.
We found that the threshold in disease activity to switch from one anti-TNF agent to another biologic agent has lowered over calendar time and that the likelihood to switch has increased 3–6 fold, especially among patients who continued to have moderate disease activity measured by CDAI despite treatment with an anti-TNF agent.
We found a significant clustering effect at physician level with regard to switching.
We found that a low swollen joint count/tender joint count ratio and better physician global was associated with maintaining therapy among patients who continued to have moderate to high levels of disease activity measured by CDAI despite treatment with an anti-TNF agent.
Acknowledgments
Dr. Curtis receives support from NIH (AR053351) and AHRQ (R01HS018517). Dr. Zhang receives support from AHRQ (T32HS013852).
CORRONA receives support from Abbott, Amgen, BMS, Centocor, Genentch, Lilly and Roche in the form of contracted subscriptions to the database. Amgen contributed to the design of this analysis and was involved in the early stage of protocol development as part of its subscription contract. The final analysis plan and interpretation of the results were solely those of the authors.
Footnotes
Disclosures:
Dr. Reed has a research contract with CORRONA. Dr. Kremer receives research support from Amgen, Abbott, Centocor, BMS, Genentech, HGS, Pfizer, Roche and UCB as well as honoraria from Abbott, Centocor, BMS, Roche and Genentech. Dr. Greenberg receives salary support from research grants from the NIH (K23AR054412), the Arthritis Foundation and the National Arthritis Research Foundation. He has received fees as Chief Scientific Officer and restricted stock shares in CORRONA, and has received consulting fees from Genentech and Pfizer. Dr. Curtis receives salary support from NIH (AR053351) and AHRQ (R01 R01HS018517). He is a consultant and has received research support from Amgen, Centocor, CORRONA, Pfizer, Roche/Genentech, Abbott, and UCB.
References
- 1.Yazici Y. Treatment of rheumatoid arthritis: we are getting there. Lancet. 2009;374(9685):178–80. doi: 10.1016/S0140-6736(09)60792-3. [DOI] [PubMed] [Google Scholar]
- 2.Finckh A, Ciurea A, Brulhart L, Moller B, Walker UA, Courvoisier D, et al. Which subgroup of patients with rheumatoid arthritis benefits from switching to rituximab versus alternative anti-tumour necrosis factor (TNF) agents after previous failure of an anti-TNF agent? Ann Rheum Dis. 2010;69(2):387–93. doi: 10.1136/ard.2008.105064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hyrich KL, Lunt M, Dixon WG, Watson KD, Symmons DP. Effects of switching between anti-TNF therapies on HAQ response in patients who do not respond to their first anti-TNF drug. Rheumatology (Oxford) 2008;47(7):1000–5. doi: 10.1093/rheumatology/ken127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hjardem E, Ostergaard M, Podenphant J, Tarp U, Andersen LS, Bing J, et al. Do rheumatoid arthritis patients in clinical practice benefit from switching from infliximab to a second tumor necrosis factor alpha inhibitor? Ann Rheum Dis. 2007;66(9):1184–9. doi: 10.1136/ard.2006.054742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smolen JS, Kay J, Doyle MK, Landewe R, Matteson EL, Wollenhaupt J, et al. Golimumab in patients with active rheumatoid arthritis after treatment with tumour necrosis factor alpha inhibitors (GO-AFTER study): a multicentre, randomised, double-blind, placebo-controlled, phase III trial. Lancet. 2009;374(9685):210–21. doi: 10.1016/S0140-6736(09)60506-7. [DOI] [PubMed] [Google Scholar]
- 6.Genovese MC, Becker JC, Schiff M, Luggen M, Sherrer Y, Kremer J, et al. Abatacept for rheumatoid arthritis refractory to tumor necrosis factor alpha inhibition. N Engl J Med. 2005;353(11):1114–23. doi: 10.1056/NEJMoa050524. [DOI] [PubMed] [Google Scholar]
- 7.Hyrich KL, Lunt M, Watson KD, Symmons DP, Silman AJ. Outcomes after switching from one anti-tumor necrosis factor alpha agent to a second anti-tumor necrosis factor alpha agent in patients with rheumatoid arthritis: results from a large UK national cohort study. Arthritis Rheum. 2007;56(1):13–20. doi: 10.1002/art.22331. [DOI] [PubMed] [Google Scholar]
- 8.Favalli EG, Arreghini M, Arnoldi C, Panni B, Marchesoni A, Tosi S, et al. Anti-tumor necrosis factor alpha switching in rheumatoid arthritis and juvenile chronic arthritis. Arthritis Rheum. 2004;51(2):301–2. doi: 10.1002/art.20242. [DOI] [PubMed] [Google Scholar]
- 9.Bombardieri S, Ruiz AA, Fardellone P, Geusens P, McKenna F, Unnebrink K, et al. Effectiveness of adalimumab for rheumatoid arthritis in patients with a history of TNF-antagonist therapy in clinical practice. Rheumatology (Oxford) 2007;46(7):1191–9. doi: 10.1093/rheumatology/kem091. [DOI] [PubMed] [Google Scholar]
- 10.van der Bijl AE, Breedveld FC, Antoni CE, Kalden JR, Kary S, Burmester GR, et al. An open-label pilot study of the effectiveness of adalimumab in patients with rheumatoid arthritis and previous infliximab treatment: relationship to reasons for failure and anti-infliximab antibody status. Clin Rheumatol. 2008;27(8):1021–8. doi: 10.1007/s10067-008-0866-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Du Pan SM, Dehler S, Ciurea A, Ziswiler HR, Gabay C, Finckh A. Comparison of drug retention rates and causes of drug discontinuation between anti-tumor necrosis factor agents in rheumatoid arthritis. Arthritis Rheum. 2009;61(5):560–8. doi: 10.1002/art.24463. [DOI] [PubMed] [Google Scholar]
- 12.Gomez-Reino JJ, Carmona L. Switching TNF antagonists in patients with chronic arthritis: an observational study of 488 patients over a four-year period. Arthritis Res Ther. 2006;8(1):R29. doi: 10.1186/ar1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yazici Y, Krasnokutsky S, Barnes JP, Hines PL, Wang J, Rosenblatt L. Changing patterns of tumor necrosis factor inhibitor use in 9074 patients with rheumatoid arthritis. J Rheumatol. 2009;36(5):907–13. doi: 10.3899/jrheum.080592. [DOI] [PubMed] [Google Scholar]
- 14.Oei HB, Hooker RS, Cipher DJ, Reimold A. High rates of stopping or switching biological medications in veterans with rheumatoid arthritis. Clin Exp Rheumatol. 2009;27(6):926–34. [PubMed] [Google Scholar]
- 15.Goekoop-Ruiterman YP, de Vries-Bouwstra JK, Allaart CF, van Zeben D, Kerstens PJ, Hazes JM, et al. Clinical and radiographic outcomes of four different treatment strategies in patients with early rheumatoid arthritis (the BeSt study): a randomized, controlled trial. Arthritis Rheum. 2005;52(11):3381–90. doi: 10.1002/art.21405. [DOI] [PubMed] [Google Scholar]
- 16.Smolen JS, Aletaha D, Bijlsma JW, Breedveld FC, Boumpas D, Burmester G, et al. Treating rheumatoid arthritis to target: recommendations of an international task force. Ann Rheum Dis. 2010;69(4):631–7. doi: 10.1136/ard.2009.123919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bakker MF, Jacobs JW, Verstappen SM, Bijlsma JW. Tight control in the treatment of rheumatoid arthritis: efficacy and feasibility. Ann Rheum Dis. 2007;66(Suppl 3):iii56–60. doi: 10.1136/ard.2007.078360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kremer J. The CORRONA database. Ann Rheum Dis. 2005;64(Suppl 4):iv37–41. doi: 10.1136/ard.2005.043497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kremer JM. The CORRONA database. Autoimmun Rev. 2006;5(1):46–54. doi: 10.1016/j.autrev.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 20.Greenberg JD, Harrold LR, Bentley MJ, Kremer J, Reed G, Strand V. Evaluation of composite measures of treatment response without acute-phase reactants in patients with rheumatoid arthritis. Rheumatology (Oxford) 2009;48(6):686–90. doi: 10.1093/rheumatology/kep054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aletaha D, Nell VP, Stamm T, Uffmann M, Pflugbeil S, Machold K, et al. Acute phase reactants add little to composite disease activity indices for rheumatoid arthritis: validation of a clinical activity score. Arthritis Res Ther. 2005;7(4):R796–806. doi: 10.1186/ar1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Studenic P, Smolen JS, Aletaha D. Perception of RA Disease Activity by Patients and Physicians: Reasons for and Estimators of Discrepancies. Arthritis and Rheumatism. 2010;62 (10 supplement):S79. doi: 10.1002/art.34543. [DOI] [PubMed] [Google Scholar]
- 23.Leeb BF, Andel I, Leder S, Leeb BA, Rintelen B. The patient’s perspective and rheumatoid arthritis disease activity indexes. Rheumatology (Oxford) 2005;44(3):360–5. doi: 10.1093/rheumatology/keh484. [DOI] [PubMed] [Google Scholar]
- 24.Wolfe F, Michaud K. Resistance of rheumatoid arthritis patients to changing therapy: discordance between disease activity and patients’ treatment choices. Arthritis Rheum. 2007;56(7):2135–42. doi: 10.1002/art.22719. [DOI] [PubMed] [Google Scholar]
- 25.van Vollenhoven RF, Klareskog L. Clinical responses to tumor necrosis factor alpha antagonists do not show a bimodal distribution: data from the Stockholm tumor necrosis factor alpha followup registry. Arthritis Rheum. 2003;48(6):1500–3. doi: 10.1002/art.11027. [DOI] [PubMed] [Google Scholar]
- 26.Kremer JM, Greenberg J. Interpreting registry-derived drug studies: does societal context matter? Arthritis Rheum. 2009;60(11):3155–7. doi: 10.1002/art.24880. [DOI] [PubMed] [Google Scholar]