Abstract
Myeloproliferative neoplasms (MPNs) are characterized by overproduction of myeloid lineage cells with frequent acquisition of oncogenic JAK2V617F kinase mutations. The molecular mechanisms that regulate energy requirements in these diseases are poorly understood. Transformed cells tend to rely on fermentation instead of more efficient oxidative phosphorylation for energy production. Our data in JAK2V617F-transformed cells show that growth and metabolic activity were strictly dependent on the presence of glucose. Uptake of glucose and cell surface expression of the glucose transporter Glut1 required the oncogenic tyrosine kinase. Importantly, JAK2V617F as well as active STAT5 increased the expression of the inducible rate-limiting enzyme 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase 3 (PFKFB3), which controls glycolytic flux through 6-phosphofructo-1-kinase. PFKFB3 was required for JAK2V617F-dependent lactate production, oxidative metabolic activity and glucose uptake. Targeted knockdown of PFKFB3 also limited cell growth under normoxic and hypoxic conditions and blocked in vivo tumor formation in mice. Overall, these data suggest that inducible PFKFB3 is required for increased growth, metabolic activity and is regulated through active JAK2 and STAT5. Novel therapies that specifically block PFKFB3 activity or expression would therefore be expected to inhibit JAK2/STAT5-dependent malignancies and related cancers.
Keywords: Myeloid neoplasia, tyrosine kinase oncogene, signal transduction, PFKFB3, JAK2
INTRODUCTION
The activating JAK2V617F point mutation is present in the majority of patients with myeloproliferative neoplasms (MPNs), including polycythemia vera, essential thrombocythemia, idiopathic myelofibrosis as well as in other myeloid malignancies including acute myeloid leukemia and infrequently in myelodysplastic syndromes1. JAK2 can also be activated through point mutations at additional sites, such as JAK2R683 mutations in Down syndrome children with acute lymphoblastc leukemias2 or as part of an oncogenic fusion, such as the PCM1-JAK2 fusion, as a consequence of a recurrent t(8;9)(p21;p24)3. There may be substantial overlap in signaling mechanism between these oncogenic forms of JAK2. In particular the STAT5 (STAT5A/5B, signal transducer and activator of transcription 5A/5B) transcription factor is a known target of JAK2 that can participate in normal signaling as well as neoplastic growth and expansion of myeloid progenitors and may have a functional role in other diseases as well4, 5. Little is known about the molecular mechanisms required for expansion of JAK2V617F transformed cells. Proliferating cells have an increased need for energy, required to supply anabolic reactions. Most, if not all cancer cells, are associated with increased glucose consumption and glycolysis for energy production, even under normoxic conditions (Warburg effect)6. The significance and regulation of this energy inefficient mechanism is not well understood.
Under aerobic conditions, the major energy-producing pathway from glucose in normal cells is mitochondrial oxidative phosphorylation. The increased demand for energy in proliferating cancer cells is in contrast to reduced number and function of mitochondria7 as well as energy inefficient fermentation6. Even though the signaling mechanisms that lead to increased glycolysis in transformed cells are poorly understood, the enzymes and their regulation of catalytic activities within these pathways are fairly well characterized. A key rate-limiting enzyme is the bifunctional 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase (PFKFB), which catalyzes the phosphorylation of fructose-6-phosphate to fructose-2,6-bisphosphate, as well as the reverse reaction at a different catalytic site8. Fructose 2,6-bisphosphate itself is not a glycolysis intermediate but a key allosteric activator of 6-phosphofructo-1-kinase (phosphofructokinase-1; PFK-1)9. The conversion of fructose-6-phosphate to fructose-1,6-bisphosphate by PFK-1 is essentially an irreversible step within glycolysis. High cellular ATP or citrate levels block PFK-1 activity and this can be overcome by fructose 2,6-bisphosphate10, 11. The PFKFB family itself consists of four members (PFKFB1–4) and increased levels of isoforms 3 and 4 have been associated with solid and liquid tumors. Also, normal stimuli that induce cell growth have been shown to transiently elevate PFKFB3 levels, the significance of this is not known8.
The signaling mechanisms that regulate the Warburg effect are not well characterized and may differ between types of cancers. Our data suggest that JAK2V617F is sufficient to induce glycolytic lactate production in cell line models. This metabolic change may be in part induced by activation of the STAT5 transcription factor, a direct target of JAK2V617F. We present data demonstrating that the JAK2V617F/STAT5 pathway is sufficient to induce expression of PFKFB3. Targeting of PFKFB3 leads to reduced proliferation and tumor growth in vivo, demonstrating an important role in transformation by active forms of JAK2. PFKFB3 was also required for elevated levels of reactive oxygen species (ROS) under normoxic conditions, hinting at additional regulation of the mitochondrial electron transport chain activity through this metabolic pathway. Overall, our results suggest small molecules that target PFKFB3 may represent a novel therapeutic strategy in diseases associated with activating JAK mutations.
MATERIALS AND METHODS
Cells
Human cell lines KU812 (Ph+; CML) and Molm13 (FLT3-ITD+; AML) were grown in RPMI 1640 (Mediatech, Manassas, VA) containing 10% fetal bovine serum (FBS; Lonza, Walkersville, MD); HEL cells (JAK2V617F+; erythroleukemia) were grown in medium containing additional sodium pyruvate (1mM; Invitrogen, Carlsbad, CA). The murine BaF3 pre-B cell line, BaF3 cells expressing BCR-ABL, JAK2V617F or FLT3-ITD and BaF3 cells with doxycycline-inducible constitutive active STAT5 were maintained as described before12, 13. STAT5 expression was induced by treatment with 1 µg/mL doxycycline (Sigma, St. Louis, MO) in the absence of murine interleukin-3. Unless otherwise indicated, cells were maintained under a 20% O2 atmosphere. In some experiments, cells were treated with kinase inhibitors, including imatinib (1µM; Gleevec, Novartis, Basel, Switzerland), Jak inhibitor I (1µM; Calbiochem, Gibbstown, NJ), and midostaurin (50nM; Tocris Bioscience, Ellisville, MO) or grown in medium without glutamine or glucose (Mediatech; Gibco/Invitrogen; Teknova, Hollister, CA). HEK293T cells were grown in Dulbecco’s Modified Eagle Medium (DMEM; Mediatech) supplemented with 10% FBS. Cell growth was measured by trypan blue (Sigma) exclusion. Human polycythemia vera specimens were obtained with informed consent and approval by the Institutional Review Board of the Memorial Sloan-Kettering Cancer Center. Healthy donor specimens were isolated from discarded platelet apheresis collars (Kraft Family Blood Donor Center/Dana-Farber Cancer Institute).
Measurement of glucose uptake
The fluorescent glucose analogue 2-(N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)amino)-2-deoxyglucose (2-NBD-glucose) (Invitrogen) was used to measure the relative uptake of glucose by flow cytometry. Cells (1×106) were incubated with 2-NBD-glucose (30 µM) in PBS (Mediatech) for 20 min at 37°C, subsequently washed twice in cold PBS and analyzed by flow cytometry. Changes in glucose uptake after inhibitor treatment were calculated as differences in fluorescence, relative to unstained cells.
Measurement of lactate production
Lactate production was measured using the Lactate Assay Fluorometric Kit (BioVision, Mountain View, CA) according to the manufacturer’s directions. Briefly, cells (1×106) were incubated for 3h in serum free medium and the amount of lactate in the supernatant was determined. Changes in lactate production were calculated relative to control cells.
Glut1 flow cytometry
Cell surface expression of Glut1 using specific antibodies for human (Abcam, Cambridge, MA) and murine (R&D Systems, Minneapolis, MN) cells was determined. Cells were analyzed by flow cytometry using a FACSCanto II flow cytometer and the FACSDiva cytometry analysis software (BD Biosciences, San Jose, CA). Changes in glucose uptake after inhibitor treatment were calculated as differences in fluorescence, relative to unstained cells.
Immunoblotting
Immunoblotting was performed as described previously using a standard chemiluminescence technique14. Rabbit polyclonal antibodies against PFKFB3 (Abgent, San Diego, CA; Epitomics, Burlingame, CA), HIF1α (Cell Signaling, Danvers, MA), HIF2α (kindly provided by Dr. Kaelin, DFCI), MYC (Roche Applied Science, Indianapolis, IN), STAT5 (Santa Cruz Biotech, Santa Cruz, CA), phospho-STAT5 (Y694 - Cell Signaling), and mouse monoclonal antibodies against β-actin (AC-15; Sigma) were used to detect protein expression.
Targeted knockdown using lentiviral approaches
Knockdown of PFKFB3 was done using lentiviruses containing shRNA against PFKFB3 and a scrambled control. Two different constructs (RNAi Screening Facility, Dana-Farber Cancer Institute) were used (C and D) out of a pool of six. Lentiviruses were generated by co-transfecting HEK293T cells with viral packaging vectors, pMD2.GVSV-G and pCMVΔ8.91 (RNAi Screening Facility) and shRNAs using the TransIT (Mirus, Madison, WI) reagent. HEL cells were infected in the presence of polybrene (5µg/mL; Millipore, Temecula, CA) and selected for one week in medium containing puromycin (3µg/mL; Sigma). For long-term in vivo experiments, three distinct clonal populations with targeted knockdown were combined. The efficiency of knockdown was confirmed by immunoblotting.
Measurement of intracellular reactive oxygen species
The relative intracellular levels of ROS were determined by flow cytometry using the cell-permeable redox-sensitive fluorochrome DCF-DA (2’,7’-dichlorofluorescein diacetate; Calbiochem) as described previously13. Briefly, cells (1×106) were incubated with DCF-DA (20 µM) in phosphate-buffered saline (PBS) for 5 min at 37°C, and subsequently washed twice in cold PBS. Changes in ROS were calculated relative to control cells.
Semi-quantitative real-time PCR
PFKFB3 gene expression was measured by semi-quantitative real-time PCR using specific primers (forward 5’-GGGGAGTTGGTCAGCTTTG-3’; reverse 5’-AAGATGCCGTTGGAACTGAC-3’) and human 60S acidic ribosomal phosphoprotein P0 (hRPLPO) control primers (forward 5′-GTGATGTGCAGCTGATCAAGACT-3′; reverse 5’-GATGACCAGCCCAAAGGAGA-3’). The PCR products were confirmed by DNA sequencing (not shown). Total RNA was extracted (RNeasy kit, Qiagen, Valencia, CA) to synthesize cDNA (Taqman Reverse Transcription Reagents, Applied Biosystems, Foster City, CA) for semi-quantitative real-time PCR (Power SYBR green PCR master mix, Applied Biosystems) using a 7500 Real-Time PCR System (Applied Biosystems).
Assay of fructose 2,6-bisphosphate
The intracellular levels of fructose 2,6-bisphosphate were measured in heated alkaline extracts of cells by the stimulation of potato pyrophosphate-dependent fructose-6-phosphate kinase, as described previously.15
In vivo mouse studies
HEL cells with targeted knockdown of PFKFB3 were used and compared to cells containing scrambled shRNA. Animal studies were performed at the Lurie Family Imaging Center (Dana-Farber Cancer Institute) on protocols approved by the Dana-Farber Cancer Institute Animal Care and Use Committee. For in vivo administration, 5×106 cells were washed, resuspended in 70µL PBS (Mediatech) and combined with 30 µL BD Matrigel Matrix (BD Biosciences, Bedford, MA). The cell suspensions were injected subcutaneously in the right flank of female SCID/Beige mice (8 mice/group; 5–6 weeks of age; Charles River Laboratories, Wilmington, MA). Tumor volumes were measured every 3–4 days after tumors became palpable. Mice were sacrificed when tumors reached 2 cm in any dimension or became ulcerated.
Statistical analysis
For statistical comparison between groups, the Student’s t-test was used. In vivo experiments were analyzed using one-way analysis of variance (ANOVA)/Tukey's multiple comparison test. p values of less than 0.05 were considered significant. Error bars represent SEM (standard error of the mean) of at least three independent experiments.
RESULTS
JAK2V617F kinase activity is required for increased glucose uptake and metabolism
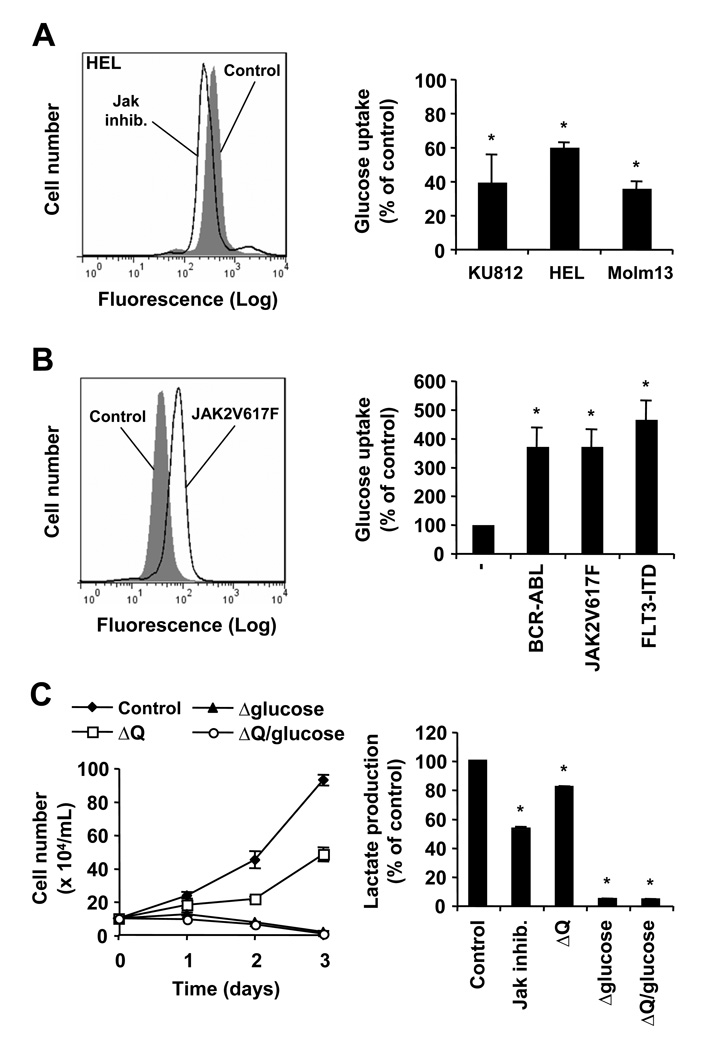
Myeloproliferative neoplasms are frequently associated with the activating JAK2V617F mutation, leading to increased growth and thus elevated energy requirement. In general, transformed cells are thought to cover their need for cellular energy through increased glycolysis and energy inefficient fermentation. The mechanisms that regulate metabolic changes in cells transformed by JAK2V617F are not well understood. Using JAK2V617F-transformed HEL cells, we found that inhibition of the JAK2 kinase activity significantly reduced glucose uptake (60.6±2.7% of control) (Figure 1A, left panel). Similar changes in glucose uptake were also observed in the BCR-ABL transformed cell line KU812 treated with imatinib (40.1±16.0% of control) and the FLT3-ITD-containing Molm13 cells treated with midostaurin (36.3±4.0% of control) (Figure 1A, right panel), as well as BaF3 cells transformed by BCR-ABL (34.1±7.4% of control), JAK2V617F (54.5±2.5% of control) or FLT3-ITD (53.7±4.1% of control) in response to their respective tyrosine kinase inhibitors (Supplementary Fig. S1). In addition, increased glucose uptake was found in murine BaF3.EpoR cells expressing JAK2V617F (371.2% increase) as well as BaF3 cells expressing BCR-ABL (371.7% increase) or FLT3-ITD (465.7% increase), when compared to parental BaF3 cells (Figure 1B). The tyrosine kinase mediated increase in glucose uptake is likely to be shared amongst these oncogenes. Next, we examined the growth requirements for glucose in HEL cells and compared it to glutamine, another potential source of energy. Cells were grown in the presence and absence of glucose, glutamine or combinations thereof. Dialyzed serum by itself, required for these experiments, did not significantly alter cell growth compared to normal serum (not shown). Our data indicate that lack of glucose in the culture medium completely blocked cell growth and led to a loss in viability, whereas absence of glutamine reduced growth to 48.3±4.1% of control, under these experimental conditions (Figure 1C, left panel). A similar dependency on glucose was also observed in the BCR-ABL-expressing K562 cell line (not shown) and BaF3 cells expressing JAK2V617F, BCR-ABL or FLT3-ITD (Supplementary Fig. S2). Importantly, HEL cells also required JAK2V617F kinase activity for increased lactate levels, a sign for increased glycolysis. In the presence of Jak inhibitor, lactate accumulation decreased to 53.2±1.0% of control (Figure 1C, right panel). Also, similar to glucose-dependent growth, lactate production was almost completely abolished in the absence of glucose (4.6±0.3% of control) and the additional absence of glutamine did not further change lactate levels (4.0±0.5% of control). Comparable results were found in BaF3 cells expressing JAK2V617F, BCR-ABL and FLT3-ITD (Supplementary Fig. S3). Increased glucose uptake, lactate production and a dependency on glucose would be consistent with an important role for glycolysis in JAK2V617F transformation.
Figure 1. Glucose metabolism is stimulated in JAK2V617F expressing cells.
A and B, changes in 2-NBD-glucose uptake were measured by flow cytometry in KU812, HEL and Molm13 cells in response to inhibitors of their respective oncogenic tyrosine kinases (A) and in BaF3 cells compared to cells containing BCR-ABL, JAK2V617F or FLT3-ITD (B) (n=3). Typical experiments are shown in the left panel. C, Growth of HEL cells was determined by trypan blue exclusion (left panel) and changes in lactate production were measured (right panel) in response to control medium, Jak inhibitor (1 µM) or medium lacking (△) glutamine (Q) and/or glucose, as indicated (n=3). *, indicates that significant differences (p<0.05) were observed between control and treated cells (A, C) or parental BaF3 cells and their transformed counterparts containing oncogenic tyrosine kinases (B).
JAK2V617F increases expression of Glut1 and PFKFB3
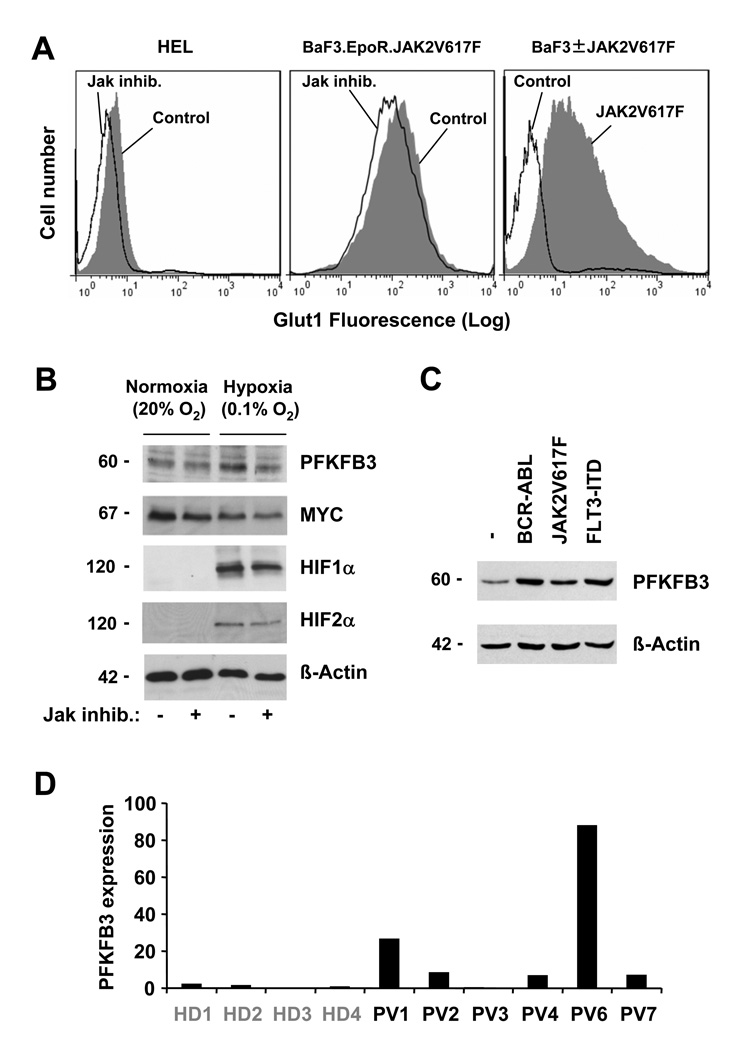
In an effort to further understand the biochemical changes associated with increased glucose uptake and glycolysis, we initially looked for abnormalities in expression of genes involved in this metabolic pathway. The expression of Glut1 was determined by flow cytometry in HEL cells in response to Jak inhibitor. Whereas HEL cells showed positive staining for Glut1, the cell surface expression was reduced by inhibition of the JAK2 kinase activity (Figure 2A, left panel), similar to the changes observed in Jak inhibitor treated BaF3.EpoR cells that express JAK2V617F (Figure 2A, middle panel). In BaF3.EpoR cells, expression of JAK2V617F led to a dramatic increase in cell surface expression of Glut1 compared to growth factor deprived parental cells (Figure 2A, right panel). Additional real-time PCR data showed that in particular the rate-limiting HK2 (hexokinase 2) and PFKFB3 (phosphofructokinase 3) were regulated in HEL cells in response to Jak inhibitor (not shown). Since glycolysis is expected to be elevated under hypoxia (0.1% O2) compared to normoxia (20 % O2), we measured PFKFB3 expression under normal and low oxygen pressure. The protein expression of PFKFB3 was found to be increased under hypoxic conditions and reduced in response to Jak inhibitor. (Figure 2B). It is thought that Myc as well as the hypoxia inducible proteins HIF1α and HIF2α participate in the regulation of genes involved in glucose metabolism16, 17. All three proteins are partially inhibited by Jak inhibitor treatment, whereas only Myc is expressed under normoxic conditions and its expression is decreased under hypoxia (Figure 2B). This would indicate that JAK2V617F induced expression of PFKFB3 does not depend on HIF1α or HIF2α under normoxic condition and MYC would not be expected to be sufficient for PFKFB3 expression under hypoxia, due to the divergent expression of both proteins. In BaF3 cells, expression of JAK2V617F is sufficient to induce PFKFB3 levels and this is comparable to BCR-ABL and FLT3-ITD expressing cells (Figure 2C). To further confirm these data, we also looked at changes in PFKFB3 mRNA expression by real-time PCR in granulocytes from JAK2V617F+ polycythemia vera specimens (n=6) compared to healthy donors (n=4). PFKFB3 expression was found to be significantly increased (9.1-fold increase, p<0.05), compared to the mean expression levels in healthy donors (Figure 2D). Thus, these data suggest a potential link between oncogenic tyrosine kinase activity and expression of PFKFB3.
Figure 2. JAK2V617F is associated with increased expression of Glut1 and PFKFB3.
A, cell surface expression of Glut1 was determined by flow cytometry. BaF3 cells were compared to cells expressing JAK2V617F or HEL and BaF3.EpoR.JAK2V617F cells were left untreated or treated with Jak inhibitor (1µM, 18 h), as indicated (typical experiment shown). B, expression of PFKFB3, MYC, HIF1α, HIF2α and β-actin was determined by immunoblotting in HEL cells maintained under normoxic (20% O2) and hypoxic (0.1% O2) conditions. C, expression of PFKFB3 and β-actin was determined by immunoblotting in parental BaF3 and cells containing BCR-ABL, JAK2V617F or FLT3-ITD. D, mRNA expression of PFKFB3 was compared between healthy donors (HD) and polycythemia vera patient (PV) specimens. Changes were calculated relative to the average expression of PFKFB3 in healthy donors.
Active STAT5 is sufficient for induced PFKFB3 expression
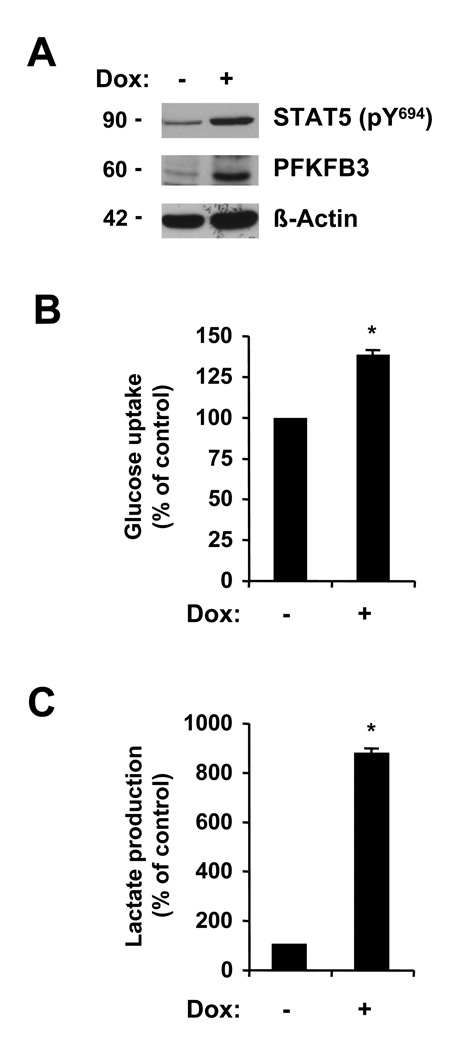
The STAT5 transcription factor is activated by phosphorylation through JAK2V617F and required for transformation. The effects of active STAT5 on PFKFB3 are not known. We studied the potential role of STAT5 in PFKFB3 expression and the regulation of metabolic functions, using previously generated BaF3 cells with doxycycline inducible, active transforming STAT5. Induced expression of this active STAT5 by doxycycline treatment, increased the levels of phospho-STAT5 (phospho-Tyr694) as well as the expression of PFKFB3 (Figure 3A). The induction of PFKFB3 also correlated with increased metabolic activity. We observed elevated glucose uptake in active STAT5 expressing cells (38.6±2.0% increase) (Figure 3B), but the changes were smaller than those observed in JAK2V617F expressing BaF3 cells (Figure 1B). Also, active STAT5 led to a 8.7-fold increase in lactate production (Figure 3C), which would be consistent with increased glycolysis. Thus, these data would imply that active STAT5 is sufficient to induce the expression of PFKFB3 and increased metabolic activity, but it is likely that in vivo additional factors may play a role in the regulation of glycolysis by JAK2V617F.
Figure 3. Activation of the STAT5 pathway is sufficient for increased metabolism.
Untreated BaF3 cells with doxycycline inducible constitutively active STAT5 (−) were used and compared to cells treated (+) with 1 µg/mL doxycycline (DOX). Expression of active STAT5, PFKFB3 and β-actin was determined by immunoblotting (A). Changes in glucose uptake (B) and lactate production (C) were determined in response to expression of active STAT5 (n=3). *, indicates that significant differences (p<0.05) were observed between active STAT5-expressing and control cells.
PFKFB3 is required for transformation by JAK2V617F
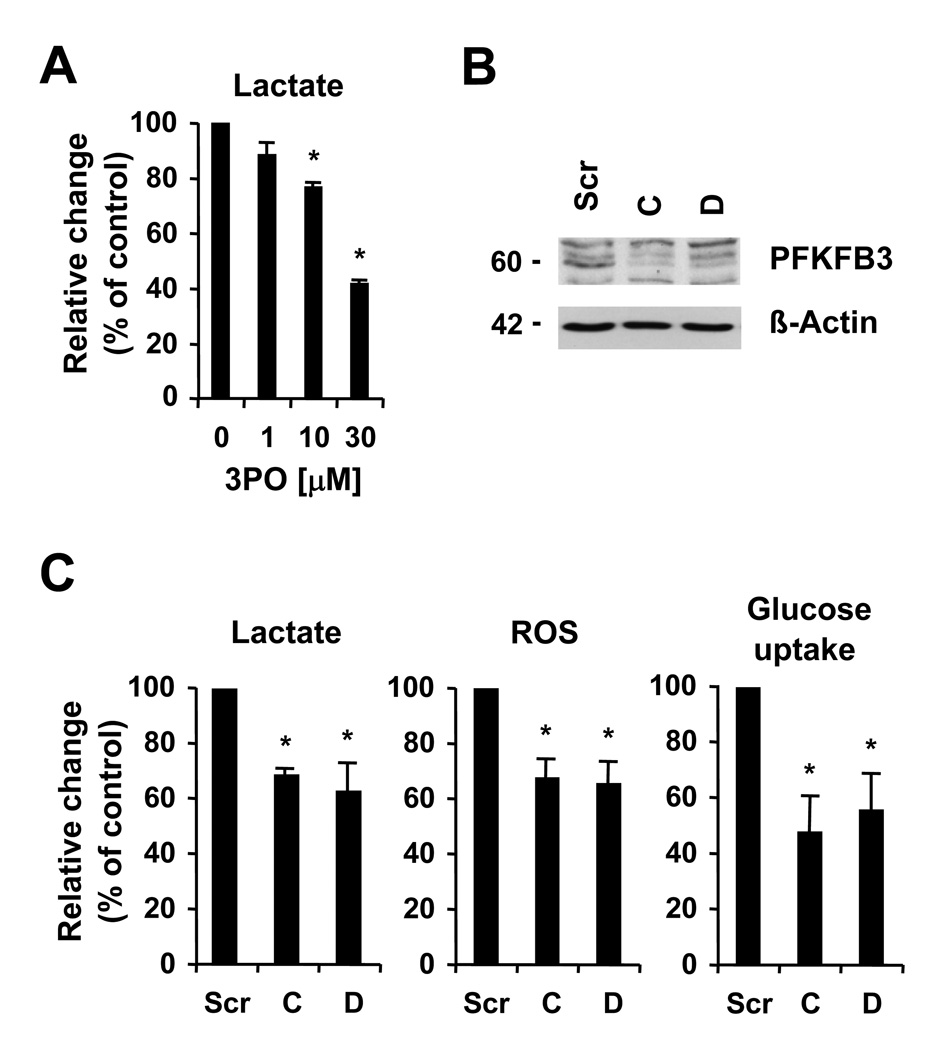
In order to confirm the role of PFKFB3 in increased metabolism and glycolysis, we used targeted approaches to inhibit its enzymatic activity. Clem et al.18 recently identified several small molecule drugs with activity against PFKFB3, including 3-(3-pyridinyl)-1-(4-pyridinyl)-2-propen-1-one (3PO). We found that 3PO efficiently reduced lactate production in HEL cells in a dose-dependent manner, with significant inhibition of the relative production of lactate starting at 10µM 3PO (76.7±1.0% of control, n=3, p<0.05) after 18h of treatment (Figure 4A). Small molecule drugs are known to frequently inhibit additional targets and we therefore also used a lentiviral-based shRNA approach to knockdown PFKFB3 expression. Out of the six shRNA constructs tested, two were found to significantly knockdown the protein levels of PFKFB3 in HEL cells, compared to a construct containing a scrambled sequence (Figure 4B). The efficacy of PFKFB3 knockdown was also confirmed by real-time PCR (not shown). Further, the levels of fructose 2,6-bisphosphate were found to be significantly reduced in HEL cells with PFKFB3-targeting constructs C (7.8±2.5 % of control, n=3, p<0.005) and D (9.9±2.5 % of control, n=3, p<0.005). Next, the effect of PFKFB3 knockdown on glucose metabolism was determined by measuring changes in glucose uptake and intracellular ROS, as an indicator for changes in mitochondrial electron transport. Consistent with an important role for PFKFB3 in glucose and energy metabolism, knockdown reduced lactate production (62.9%–68.7% of control) as well as intracellular ROS (65.9%–67.8% of control) and glucose uptake (47.7%–55.6% of control) in HEL cells (Figure 4C). These data would imply a crucial role for PFKFB3 in the hyperactive glucose metabolism in HEL cells.
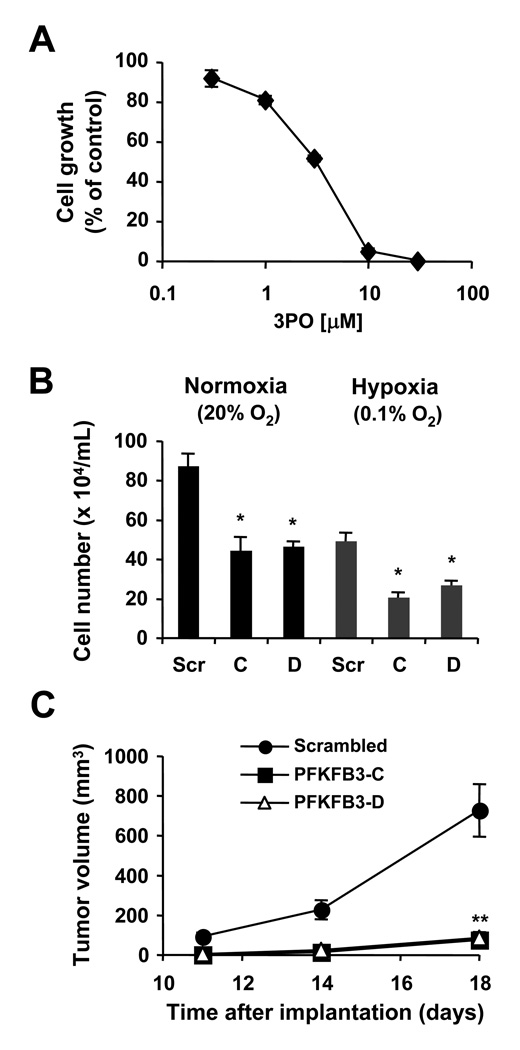
Figure 4. PFKFB3 is associated with increased metabolism.
A, HEL cells were treated for 18 hours with 3PO and lactate production was measured. B and C, HEL cells containing scrambled shRNA (Scr) or two different PFKFB3-targeting constructs C and D were used, as indicated. B, expression of PFKFB3 and β-actin was determined by immunoblotting. C, relative changes in lactate production (left panel), levels of intracellular ROS (middle panel) and 2-NBD-glucose uptake (right panel) were measured in response to PFKFB3 knockdown (n=3). *, indicates that significant differences (p<0.05) were observed between control and treated cells.
PFKFB3 is essential for tumor growth in vivo in a xenotransplant mouse model
Further, the effect of the PFKFB3 inhibitor 3PO was tested on cell growth. 48h treatment of HEL cells led to a dose-dependent reduction in cell growth with an EC50 of 3 µM (Figure 5A). It is not clear from these data if the result is a specific effect of 3PO on PFKFB3 activity or whether there are additional targets that lead to reduced cell growth and loss in viability. To clarify this, HEL cells with PFKFB3 knockdown were used and cell growth was measured under normoxic and hypoxic conditions. An overall 44% reduction in growth in cells containing scrambled shRNA under hypoxia compared to normoxia was found (Figure 5B). Cells with PFKFB3 knockdown showed similar further reduction under hypoxia in cell growth (43%–53% reduction), relative to their respective controls under normoxia. Also, PFKFB3 knockdown significantly reduced cell growth, independent of the oxygen pressure. Changes in growth were due in part to increased apoptosis in cells with PFKFB3 knockdown. We found that both constructs significantly increased the fraction of annexin V/propidiumiodide positive cells by 8.2–15.2 % (n=3; p<0.05) (Supplementary Fig. S4). In contrast, we observed only small, but not significant differences in cell cycle distribution or transwell migration in vitro (not shown). Finally, HEL cells containing scrambled shRNA were injected s.c. into SCID/Beige mice (n=8) and tumor growth compared to cells containing either of the two different shRNA constructs targeting PFKFB3. Surprisingly, HEL cells with PFKFB3 knockdown did not support in vivo tumor growth to the same extent as in vitro cell growth. Palpable tumor volume was significantly reduced from cells containing either the PFKFB3-C (76.3±40.6 mm3, n=8, p<0.0001) or the PFKFB3-D (87.2±22.7 mm3, n=8, p<0.0001) shRNA construct, as compared to palpable tumor volume from cells containing scrambled shRNA (728.4±132.1 mm3, n=8; day 18) (Figure 5C). Overall, these data implicate PFKFB3 as an important regulator of cellular transformation in cells with an active JAK2/STAT5 pathway.
Figure 5. PFKFB3 is required for increased growth.
A, HEL cells were treated for 48h with different concentrations of 3PO and cell growth was determined. B, HEL cells containing scrambled shRNA or PFKFB3-targeting constructs C and D were used to measure changes in growth of cells maintained under normoxic (20% O2) or hypoxic (0.1% O2) conditions (n=3). *, indicates that significant differences (p<0.05) were observed between control and treated cells. C, in vivo tumor formation of HEL cells with targeted knockdown of PFKFB3 (■, △) or scrambled shRNA (●) was determined in SCID/Beige mice (n=8). The volumes of subcutaneous tumors were measured and compared 18 days after initial injection. **, indicates that significant differences (p<0.0001) were observed between scrambled and PFKFB3-targeting constructs C and D (11, 14 and 18 days after tumor cell injection).
DISCUSSION
Transformed cells are commonly thought to be associated with increased glycolytic flux and tend to direct the majority of glucose towards lactate fermentation, thus covering part or most of the cellular energy (ATP) requirement. This apparently inefficient way of energy production, normally activated under hypoxia, occurs even under normoxic conditions (Warburg effect)6. The molecular switches involved in these metabolic changes and the significance of these events have just begun to be elucidated. One surprising observation of our cell line data is the complete dependency on glucose for cell growth and viability, hinting at glycolysis as a crucial pathway in transformation of cells with an active JAK2/STAT5 pathway. Our results indicate that metabolic changes are not simply intrinsic activities of cancer cells but coordinated events that can be specifically induced by the JAK2V617F oncogenic tyrosine kinase. Signaling mechanisms that regulate changes in the expression of glycolytic enzymes have been mainly associated with transcription factors that have a rather global effect on expression, including Myc and Hif1α/Hif2α16. In our model the STAT5 transcription factor was found to be a strong inducer of PFKFB3. It is not known whether STAT5 can directly enhance expression of PFKFB3, or if this occurs through other mechanisms, such as upregulation of MYC or HIF2α by active STAT512,19. Both, MYC and HIF proteins are thought to be key regulators of metabolic reprogramming in transformed cells16,17. An additional possibility could be the involvement of downstream effectors of STAT5 that regulate PFKFB3 expression. It should be emphasized that PFKFB3 can not only be regulated at the transcriptional level but it is also a target of ubiquitination and proteasomal degradation20, alternative splicing21, 22 as well as activation by serine phosphorylation21, which all may have additional effects on the functional expression of this enzyme.
STAT5 is frequently but not exclusively activated in hematologic malignancies with tyrosine kinase oncogenes and can also be found, for example in lung, prostate and breast cancer23–25. Consistent with this observation, high levels of PFKFB3 have also been frequently observed in a variety of solid tumors, including brain, breast, colon, kidney, lung, ovary, prostate, stomach and thyroid cancer26, 27. It is therefore possible that STAT5 participates in the regulation of PFKFB3 in these diseases. Even though our studies focused on cells with active JAK2/STAT5, it cannot be excluded that mechanisms independent of this pathway may be involved in the regulation of PFKFB3 as well. As pointed out before, a major function of PFKFB3 is to allow high glycolytic flux through PFK1, even in the presence of elevated levels of ATP. Transformed cells already have an intrinsically high demand for energy, thus limiting excess ATP levels. This may be in particular true for cells transformed by tyrosine kinase oncogenes, which lead to abundant phosphorylation of cellular proteins. Recently, Fang et al.28 described an ATP-regulating mechanism in cancer cells that is associated with a deregulated PI3K pathway. Overexpression of the UTPase ENTPD5 (ectonucleoside triphosphate diphosphohydrolase 5), localized to the endoplasmic reticulum, was associated with ATP consumption and increased glycolysis. ENTPD5 promotes protein N-glycosylation and folding, and acts in concert with cytidine monophosphate kinase1 and adenylate kinase 1 to hydrolyze ATP to AMP. Thus, this pathway may constitute an alternative mechanism to the regulation of PFK-1 by PFKFB3 in the absence of sufficient JAK2/STAT5 activation or it may complement the regulation of PFK-1 by PFKFB3 through lowering of cellular ATP levels.
High glycolytic flux and the Warburg effect do not preclude the possibility that mitochondrial activity can be increased in cancer cells. For example, we have previously demonstrated that elevated glucose metabolism in BCR-ABL transformed cells is associated with elevated levels of intracellular ROS29, 30. The majority of these ROS are a byproduct of reactions from the mitochondrial electron transport chain13. This would support the notion that increased glycolytic flux and pyruvate production do not only elevate lactate levels but fuel mitochondrial electron flux as well. Nevertheless, mitochondria may display reduced functionality in transformed cells and may not be a significant factor in providing cellular energy7, but a causal relationship between increased glucose metabolism and change in these activities has not been established. Our results, demonstrating reduced ROS in cells with PFKFB3 knockdown, would indicate that this enzyme may also be a key molecule in regulating mitochondrial ROS. This pathway is of particular relevance to the disease process since ROS may not simply be a by-product. ROS are involved in regulating cellular signaling events, can be a major cause for DNA damage and have the potential to cause genomic instability31. In chronic myelogenous leukemia, ROS have been implicated in DNA damage and drug resistance, caused by point mutations in the BCR-ABL kinase domain32. It would be expected that this basic mechanism of drug resistance has broad implications to all cancers with high glycolytic activity and elevated intracellular ROS. Limiting PFKFB3 function may therefore help to maintain genomic stability and would be predicted to delay the onset of drug resistance and disease progression.
Our data using a lentiviral shRNA approach suggest a crucial role of PFKFB3 for in vivo tumor growth and expand previous data33 by linking its regulation, at least in part, to activation of the JAK2/STAT5 pathway. The role of JAK/STAT signaling for dysregulated glucose metabolism has not been sufficiently appreciated and targeting mechanism involved in its regulation may open a venue for novel therapeutic approaches. We have used the small molecule drug 3PO, a compound previously identified as a potent inhibitor of PFKFB318, to efficiently inhibit lactate production and longer exposure of cells to this drug led to a drastic loss in cell growth. It is not known whether 3PO has additional off target effects, but these results are consistent with our genetic approaches. Targeting glycolytic mechanisms, as a central pathway in carbon metabolism, is of obvious concern. PFKFB3 knockout mice are known to be embryonically lethal34, suggesting a crucial role for this enzyme not only in transformed cells but also in proliferating or differentiating tissue. The functional role of PFKFB3 in adult mice has not yet been studied and may be quite different. Nevertheless, the PFKFB3 inhibitor 3PO showed efficacy in a mouse model, without causing apparent side effects18, suggesting the feasibility of this approach. In humans, there are three isoforms of the PFKFB3 target PFK-1. Defects in the muscle specific isoform PFKM causes glycogen storage disease type 7 (GSD7 or Tarui disease), characterized by different degrees, of exercise intolerance, myopathy, loss of muscle and red cell PFK activity or congenital nonspherocytic hemolytic anemia35. One would expect that targeting PFKFB3 leads to a somewhat increased risk of side effects, similar to the symptoms observed in this disease. It would now be important to see whether different tumors are similarly dependent on PFKFB3 and whether targeting its expression has similar effects on tumor growth as in our model.
Supplementary Material
Acknowledgments
Financial support: This work is supported in part by National Institutes of Health grant (CA134660-03, M.S.). R.L.L. is a Geoffrey Beene Junior Chair.
REFERENCES
- 1.Levine RL, Gilliland DG. Myeloproliferative disorders. Blood. 2008;112:2190–2198. doi: 10.1182/blood-2008-03-077966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bercovich D, Ganmore I, Scott LM, Wainreb G, Birger Y, Elimelech A, et al. Mutations of JAK2 in acute lymphoblastic leukaemias associated with Down's syndrome. Lancet. 2008;372:1484–1492. doi: 10.1016/S0140-6736(08)61341-0. [DOI] [PubMed] [Google Scholar]
- 3.Reiter A, Walz C, Watmore A, Schoch C, Blau I, Schlegelberger B, et al. The t(8;9)(p22;p24) is a recurrent abnormality in chronic and acute leukemia that fuses PCM1 to JAK2. Cancer Res. 2005;65:2662–2667. doi: 10.1158/0008-5472.CAN-04-4263. [DOI] [PubMed] [Google Scholar]
- 4.Teglund S, McKay C, Schuetz E, van Deursen JM, Stravopodis D, Wang D, et al. Stat5a and Stat5b proteins have essential and nonessential, or redundant, roles in cytokine responses. Cell. 1998;93:841–850. doi: 10.1016/s0092-8674(00)81444-0. [DOI] [PubMed] [Google Scholar]
- 5.Hennighausen L, Robinson GW. Interpretation of cytokine signaling through the transcription factors STAT5A and STAT5B. Genes Dev. 2008;22:711–721. doi: 10.1101/gad.1643908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Warburg O. On respiratory impairment in cancer cells. Science. 1956;124:269–270. [PubMed] [Google Scholar]
- 7.Pedersen PL. Tumor mitochondria and the bioenergetics of cancer cells. Prog Exp Tumor Res. 1978;22:190–274. doi: 10.1159/000401202. [DOI] [PubMed] [Google Scholar]
- 8.Yalcin A, Telang S, Clem B, Chesney J. Regulation of glucose metabolism by 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatases in cancer. Exp Mol Pathol. 2009;86:174–179. doi: 10.1016/j.yexmp.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 9.Van Schaftingen E, Hue L, Hers HG. Fructose 2,6-bisphosphate, the probably structure of the glucose- and glucagon-sensitive stimulator of phosphofructokinase. Biochem J. 1980;192:897–901. doi: 10.1042/bj1920897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Uyeda K, Furuya E, Luby LJ. The effect of natural and synthetic D-fructose 2,6-bisphosphate on the regulatory kinetic properties of liver and muscle phosphofructokinases. J Biol Chem. 1981;256:8394–8399. [PubMed] [Google Scholar]
- 11.Van Schaftingen E, Hers HG. Inhibition of fructose-1,6-bisphosphatase by fructose 2,6-biphosphate. Proc Natl Acad Sci U S A. 1981;78:2861–2863. doi: 10.1073/pnas.78.5.2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wernig G, Gonneville JR, Crowley BJ, Rodrigues MS, Reddy MM, Hudon HE, et al. The Jak2V617F oncogene associated with myeloproliferative diseases requires a functional FERM domain for transformation and for expression of the Myc and Pim proto-oncogenes. Blood. 2008;111:3751–3759. doi: 10.1182/blood-2007-07-102186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reddy MM, Fernandes MS, Salgia R, Levine RL, Griffin JD, Sattler M. NADPH oxidases regulate cell growth and migration in myeloid cells transformed by oncogenic tyrosine kinases. Leukemia. 2011;25:281–289. doi: 10.1038/leu.2010.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fernandes MS, Reddy MM, Gonneville JR, DeRoo SC, Podar K, Griffin JD, et al. BCR-ABL promotes the frequency of mutagenic single-strand annealing DNA repair. Blood. 2009;114:1813–1819. doi: 10.1182/blood-2008-07-172148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Van Schaftingen E, Lederer B, Bartrons R, Hers HG. A kinetic study of pyrophosphate: fructose-6-phosphate phosphotransferase from potato tubers. Application to a microassay of fructose 2,6-bisphosphate. Eur J Biochem. 1982;129:191–195. doi: 10.1111/j.1432-1033.1982.tb07039.x. [DOI] [PubMed] [Google Scholar]
- 16.Gordan JD, Thompson CB, Simon MC. HIF and c-Myc: sibling rivals for control of cancer cell metabolism and proliferation. Cancer Cell. 2007;12:108–113. doi: 10.1016/j.ccr.2007.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dang CV, Kim JW, Gao P, Yustein J. The interplay between MYC and HIF in cancer. Nat Rev Cancer. 2008;8:51–56. doi: 10.1038/nrc2274. [DOI] [PubMed] [Google Scholar]
- 18.Clem B, Telang S, Clem A, Yalcin A, Meier J, Simmons A, et al. Small-molecule inhibition of 6-phosphofructo-2-kinase activity suppresses glycolytic flux and tumor growth. Mol Cancer Ther. 2008;7:110–120. doi: 10.1158/1535-7163.MCT-07-0482. [DOI] [PubMed] [Google Scholar]
- 19.Fatrai S, Wierenga AT, Daenen SM, Vellenga E, Schuringa JJ. Identification of HIF2alpha as an important STAT5 target gene in human hematopoietic stem cells. Blood. 2011;117:3320–3330. doi: 10.1182/blood-2010-08-303669. [DOI] [PubMed] [Google Scholar]
- 20.Herrero-Mendez A, Almeida A, Fernandez E, Maestre C, Moncada S, Bolanos JP. The bioenergetic and antioxidant status of neurons is controlled by continuous degradation of a key glycolytic enzyme by APC/C-Cdh1. Nat Cell Biol. 2009;11:747–752. doi: 10.1038/ncb1881. [DOI] [PubMed] [Google Scholar]
- 21.Bando H, Atsumi T, Nishio T, Niwa H, Mishima S, Shimizu C, et al. Phosphorylation of the 6-phosphofructo-2-kinase/fructose 2,6-bisphosphatase/PFKFB3 family of glycolytic regulators in human cancer. Clin Cancer Res. 2005;11:5784–5792. doi: 10.1158/1078-0432.CCR-05-0149. [DOI] [PubMed] [Google Scholar]
- 22.Kessler R, Eschrich K. Splice isoforms of ubiquitous 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase in human brain. Brain Res Mol Brain Res. 2001;87:190–195. doi: 10.1016/s0169-328x(01)00014-6. [DOI] [PubMed] [Google Scholar]
- 23.Ahonen TJ, Xie J, LeBaron MJ, Zhu J, Nurmi M, Alanen K, et al. Inhibition of transcription factor Stat5 induces cell death of human prostate cancer cells. J Biol Chem. 2003;278:27287–27292. doi: 10.1074/jbc.M304307200. [DOI] [PubMed] [Google Scholar]
- 24.Lai SY, Johnson FM. Defining the role of the JAK-STAT pathway in head and neck and thoracic malignancies: implications for future therapeutic approaches. Drug Resist Updat. 2010;13:67–78. doi: 10.1016/j.drup.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 25.Clevenger CV. Roles and regulation of stat family transcription factors in human breast cancer. Am J Pathol. 2004;165:1449–1460. doi: 10.1016/S0002-9440(10)63403-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Atsumi T, Chesney J, Metz C, Leng L, Donnelly S, Makita Z, et al. High expression of inducible 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase (iPFK-2; PFKFB3) in human cancers. Cancer Res. 2002;62:5881–5887. [PubMed] [Google Scholar]
- 27.Kessler R, Bleichert F, Warnke JP, Eschrich K. 6-Phosphofructo-2-kinase/fructose-2,6-bisphosphatase (PFKFB3) is up-regulated in high-grade astrocytomas. J Neurooncol. 2008;86:257–264. doi: 10.1007/s11060-007-9471-7. [DOI] [PubMed] [Google Scholar]
- 28.Fang M, Shen Z, Huang S, Zhao L, Chen S, Mak TW, et al. The ER UDPase ENTPD5 promotes protein N-glycosylation, the Warburg effect, and proliferation in the PTEN pathway. Cell. 2010;143:711–724. doi: 10.1016/j.cell.2010.10.010. [DOI] [PubMed] [Google Scholar]
- 29.Kim JH, Chu SC, Gramlich JL, Pride YB, Babendreier E, Chauhan D, et al. Activation of the PI3K/mTOR pathway by BCR-ABL contributes to increased production of reactive oxygen species. Blood. 2005;105:1717–1723. doi: 10.1182/blood-2004-03-0849. [DOI] [PubMed] [Google Scholar]
- 30.Sattler M, Verma S, Shrikhande G, Byrne CH, Pride YB, Winkler T, et al. The BCR/ABL tyrosine kinase induces production of reactive oxygen species in hematopoietic cells. J Biol Chem. 2000;275:24273–24278. doi: 10.1074/jbc.M002094200. [DOI] [PubMed] [Google Scholar]
- 31.Rodrigues MS, Reddy MM, Sattler M. Cell cycle regulation by oncogenic tyrosine kinases in myeloid neoplasias: from molecular redox mechanisms to health implications. Antioxid Redox Signal. 2008;10:1813–1848. doi: 10.1089/ars.2008.2071. [DOI] [PubMed] [Google Scholar]
- 32.Koptyra M, Falinski R, Nowicki MO, Stoklosa T, Majsterek I, Nieborowska-Skorska M, et al. BCR/ABL kinase induces self-mutagenesis via reactive oxygen species to encode imatinib resistance. Blood. 2006;108:319–327. doi: 10.1182/blood-2005-07-2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chesney J, Mitchell R, Benigni F, Bacher M, Spiegel L, Al-Abed Y, et al. An inducible gene product for 6-phosphofructo-2-kinase with an AU-rich instability element: role in tumor cell glycolysis and the Warburg effect. Proc Natl Acad Sci U S A. 1999;96:3047–3052. doi: 10.1073/pnas.96.6.3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chesney J, Telang S, Yalcin A, Clem A, Wallis N, Bucala R. Targeted disruption of inducible 6-phosphofructo-2-kinase results in embryonic lethality. Biochem Biophys Res Commun. 2005;331:139–146. doi: 10.1016/j.bbrc.2005.02.193. [DOI] [PubMed] [Google Scholar]
- 35.Nakajima H, Raben N, Hamaguchi T, Yamasaki T. Phosphofructokinase deficiency; past, present and future. Curr Mol Med. 2002;2:197–212. doi: 10.2174/1566524024605734. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.