Abstract
Rationale
Insulin-like growth factor binding protein-1 (IGFBP-1) has metabolic effects throughout the body and its expression is regulated in part by insulin. Circulating IGFBP-1 predicts development of cardiometabolic diseases in longitudinal studies and low IGFBP-1 concentrations are associated with insulin resistance and consumption of a high-fat diet. Because of the favorable metabolic effects of regular aerobic exercise, we hypothesized that aerobic exercise training would increase plasma IGFBP-1 concentrations and attenuate the reduction in IGFBP-1 after a high-fat meal.
Methods
Ten overweight (BMI=28.7±0.9kg/m2), older (61±2yr) men and women underwent high-fat feeding and oral glucose tolerance tests (OGTT) at baseline and after 6 months of aerobic exercise training.
Results
In response to aerobic exercise training, subjects increased cardiorespiratory fitness 13% (p<0.05) and insulin sensitivity index 28% (p<0.05). Basal plasma concentrations of IGFBP-1 increased 41% after aerobic exercise training (p<0.05). The insulin response to an OGTT was a significant predictor of fasting plasma IGFBP-1 concentrations at baseline and after exercise training (p=0.02). In response to the high-fat meal at baseline, plasma IGFBP-1 concentrations decreased 58% (p<0.001); a 61% decrease to similar postprandial concentrations was observed after exercise training (p<0.001). Plasma insulin response to the high-fat meal was inversely associated with postprandial IGFBP-1 concentrations at baseline and after exercise training (p=0.06 and p<0.05, respectively).
Conclusion
While aerobic exercise training did not attenuate the response to a high-fat meal, the increase in IGFBP-1 concentrations after exercise training may be one mechanism by which exercise reduces risk for cardiometabolic diseases in older adults.
Keywords: insulin, glucose tolerance, lipemia, diet
1. Introduction
The insulin-like growth factor binding proteins (IGFBP) are a family of proteins that bind insulin-like growth factors (IGF) [1] and affect metabolism through both IGF-dependent and IGF-independent actions [2]. Circulating IGFBP-1 concentration has been proposed as a marker of insulin resistance and circulating IGFBP-1 concentrations directly correlate with measures of insulin sensitivity in older subjects with a range of glucose tolerance and diabetes [3–6]. Furthermore, low IGFBP-1 concentrations are also associated with increased prevalence of cardiovascular disease risk factors and metabolic syndrome [6–10]; therefore, IGFBP-1 may be a marker for cardiometabolic disease risk.
In older adults, circulating IGFBP-1 concentrations may be influenced by lifestyle habits. A sedentary lifestyle is an established risk factor for obesity and cardiometabolic diseases, and initiating a program of exercise training in older, previously sedentary adults can reduce this risk. Aerobic exercise training has been shown to improve insulin sensitivity and reduce insulin levels [11,12] and, therefore, may increase circulating IGFBP-1 concentration. Acute aerobic exercise has been shown to increase circulating IGFBP-1 concentrations [13–16], but to date, few studies have examined the effects of an exercise training intervention on IGFBP-1. One study shows increases in circulating IGFBP-1 after intensive training in previously trained competitive cyclists [17], while another study showed no change in IGFBP-1 concentrations after eight weeks of combined aerobic and resistance training in young women [18]. Consumption of a high-fat diet is also associated with a heightened risk for cardiometabolic diseases, and two cross-sectional studies reported that high dietary fat consumption is associated with low circulating IGFBP-1 concentrations in a range of young to older adults [7,19]. To our knowledge, the acute effect of a high-fat meal on IGFBP-1 concentration has not been reported.
While IGFBP-1 may serve as a marker for insulin resistance, it may have more direct effects on insulin resistance and glucose metabolism through IGF-independent mechanisms. Because of the IGF-independent actions of IGFBP-1 on glucose metabolism and the potential effects of chronic exercise and high-fat feeding on IGFBP-1, we sought to determine the effects of six-month aerobic exercise training and an acute high-fat meal on circulating IGFBP-1 concentrations in older men and women. We hypothesized that plasma IGFBP-1 concentrations would decrease in response to the high-fat meal, and that regular aerobic exercise training would increase basal plasma IGFBP-1 concentrations and attenuate the response to the high-fat meal.
2. Methods
Subjects
Men and postmenopausal women were originally recruited to participate in studies examining the effects of gene polymorphisms on responses to aerobic exercise training. Subjects were required to: 1) be sedentary (exercise less than 20 min, 2×/week), 2) be 50–75 years of age, 3) not be taking glucose-lowering medication, 4) have no recent history of smoking tobacco, 5) have no previous diagnosis of diabetes mellitus or cardiovascular disease, and 6) not have any other medical condition that would preclude aerobic exercise. Five men and five women (mean age = 60 ± 2 years) from the larger studies who participated in the high-fat meal test and for whom plasma samples were available were randomly chosen for inclusion in this report. The research protocols were approved by the Institutional Review Boards at the University of Maryland College Park and the University of Maryland School of Medicine. All subjects provided written informed consent.
Aerobic Exercise Training Intervention
After baseline testing, subjects underwent 24 weeks of standardized aerobic exercise training, supervised by exercise physiologists and conducted on treadmills, elliptical trainers, and cycle ergometers. Subjects began at a training volume of 3 weekly sessions of 20 minutes at 50% of heart rate reserve and gradually increased to 3 weekly sessions of 40 minutes at 70% of heart rate reserve, a level maintained for the final 14 weeks of the intervention. Subjects also added one weekly, independent walking session during this time. For inclusion in the analyses, subjects were required to have completed at least 80% of the scheduled exercise sessions.
Prior to baseline testing, all subjects underwent six weeks of instruction on the American Heart Association dietary guidelines [20]. Subjects were counseled to consume an isocaloric diet, following the dietary guidelines (<30% total energy intake derived from fat, <10% from saturated fat, and <300mg cholesterol per day) and were weight stable for at least three weeks prior to baseline testing. Throughout the study, subjects maintained this isocaloric diet and remained weight stable to eliminate potentially confounding effects of weight loss and differing dietary intake.
Oral Glucose Tolerance Test
Subjects underwent a 2-hour oral glucose tolerance test (OGTT) after a 12-hour overnight fast. OGTTs were started between 6:30 and 9:00am and subjects consumed more than 250g of dietary carbohydrate for 3 days prior to the test. During final testing, the OGTT was conducted 24–36 hours after the last bout of exercise. A catheter was placed in an antecubital vein and blood samples were drawn into tubes containing 15% potassium EDTA before, and 30, 60, 90, and 120 minutes after the ingestion of a 75-gram glucose solution for measurement of plasma glucose and insulin. Glucose and insulin incremental area under the curve (AUC) were calculated using the trapezoidal method. The insulin sensitivity index (ISIM) was calculated using the method of Matsuda and DeFronzo [21], and the homeostatic model assessments for insulin resistance (HOMA-IR) and β-cell function (HOMA-β) were calculated as described by Matthews et al. [22].
High-fat Meal
Subjects reported to our laboratory between 6:30am and 9:00am after a 12-hour overnight fast with no ethanol consumption for 24 hours. During final testing, the high-fat meal test was conducted 24–36 hours after the last bout of exercise. Subjects consumed a
standard liquid high-fat meal [23] within 3 minutes and blood samples were drawn just prior to consumption of the meal (0 minutes) and every 30 minutes thereafter until 4 hours after consumption (240 minutes). This abbreviated 4-hour high-fat meal test is a valid and reproducible surrogate for the more common 8-hour tests [24]. The size of the fat meal was adjusted for body size and administered at a dose of 386 grams (1,362 kcal) per 2 m2 of body surface area. Of the 1,362 kcal, ~84% were derived from fat (53% of which was saturated fat),13.7% from carbohydrates, and 2.7% from protein. Triglyceride and insulin incremental AUC were calculated using the trapezoidal method.
Body Composition and Cardiorespiratory Fitness
Fat mass and lean body mass were measured using dual-energy X-ray absorptiometry (DPX-L; Lunar, Madison, WI). Computed tomography (CT: General Electric Hi-Light Scanner, Fairfield, CT) was used to measure cross-sectional areas of visceral adipose tissue (VAT) and subcutaneous abdominal adipose tissue (SAT) at L4–L5. Cardiorespiratory fitness was measured as maximal oxygen consumption (VO2max) during a graded exercise test on a treadmill to maximal effort as determined by standard physiological criteria. VO2 was measured continuously using a validated indirect calorimetry system consisting of a mixing chamber, bi-directional turbine, and mass spectrometer. The test consisted of a continuous series of two-minute stages, where speed was fixed and treadmill grade was increased 2% in each stage. Values of VO2 were averaged in 30-second increments and VO2max was defined as the highest oxygen consumption value obtained for a full 30-second increment.
Blood analyses
Blood samples were collected in tubes containing 15% potassium EDTA and centrifuged. Plasma was removed and stored at −80°C so that all samples from a given subject were analyzed in the same assay. IGFBP-1 concentrations were measured in samples from the high-fat meal test (0 and 240 minutes) using enzyme-linked immunosorbent assay plates from MesoScale Discovery (Gaithersburg, MD). All samples were run in duplicate in the same assay; the intra-assay coefficient of variation was < 9%. Plasma triglyceride levels were determined by a 2-step colorometric assay (kit 337-B; Sigma Diagnostics Inc, St Louis, MO). Plasma insulin levels were determined by radioimmunoassay (Linco Research, St. Charles, MO). Plasma glucose levels were analyzed with a glucose analyzer (2300 STAT Plus, YSI, Yellow Springs, OH).
Statistical Analyses
Data are presented as mean ± SEM. Repeated measures ANOVA was used to detect differences in plasma IGFBP-1 concentrations in response to a high-fat meal at baseline and after aerobic exercise training. Paired Student's t-tests were used to detect changes in weight, lean body mass, fat mass, VO2max, insulin, glucose, and triglycerides at baseline and after aerobic exercise training. Data for HOMA-IR and ISIM were not normally distributed and were therefore analyzed using the Wilcoxon signed-rank test and Spearman correlation analyses. Bivariate Pearson correlation analyses were used to test for correlations between plasma IGFBP-1 concentrations and glucose, insulin, and triglyceride levels.
3. Results
Effects of the intervention on cardiorespiratory fitness and body composition
All subjects completed 6 months of aerobic exercise training with >80% compliance. After aerobic exercise training, VO2max increased 13% (2.03 ± 0.19 vs. 2.31 ± 0.19 L/min, p=0.001). There was no significant change in body weight (81.3 ± 3.2 vs. 80.3 ± 3.4 kg, p = 0.21); however, we did detect small changes in body composition as lean body mass increased (47.2 ± 2.9 vs. 48.2 ± 2.9 kg, p = 0.008) and fat mass decreased (37.2 ± 3.0 vs. 35.5 ± 3.1 kg, p = 0.01) after the intervention. There were no significant changes in VAT (131 ± 8 vs. 128 ± 10 cm2, p = 0.33) or SAT (346 ± 36 vs. 328 ± 34 cm2, p = 0.85) after aerobic exercise training.
Effects of the intervention on metabolic variables (Table 1)
Table 1.
Metabolic characteristics of subjects at baseline and after 6-months of aerobic exercise training (n=10).
| OGTT | Baseline | After Training | P-values |
|---|---|---|---|
| Fasting plasma glucose (mmol/L) | 5.1 ± 0.2 | 5.2 ± 0.2 | 0.58 |
| 2-hr OGTT glucose (mmol/L) | 6.9 ± 0.6 | 6.8 ± 06 | 0.90 |
| Glucose incremental AUC (mmol/L/120 min) | 172 ± 57 | 160 ± 53 | 0.51 |
| Fasting plasma insulin (pmol/L) | 88 ± 7 | 77 ± 6 | 0.047 |
| 2-hr plasma insulin (pmol/L) | 523 ± 75 | 344 ± 47 | 0.010 |
| Insulin incremental AUC (pmol/L/120 min) | 53238 ± 7429 | 44356 ± 4027 | 0.10 |
| HOMA-β | 166 ± 17 | 154 ± 34 | 0.75 |
| HOMA-IR | 3.0 ± 0.3 | 2.3 ± 0.2 | 0.038 |
| ISIM | 2.5 ± 0.2 | 3.2 ± 0.3 | 0.025 |
| High-fat Meal | Baseline | After Training | P-values |
|---|---|---|---|
| Fasting TG (mmol/L) (n=8*) | 1.50 ± 0.29 | 1.26 ± 27 | 0.45 |
| TG incremental AUC (mg/dl/240 min) (n=8*) | 156 ± 30 | 156 ± 37 | 0.99 |
| Insulin incremental AUC (pmol/L/240 min) (n=8*) | 21566 ± 7625 | 14411 ± 5095 | 0.09 |
Data are means ± SEM.
TG and insulin data during the oral fat load were unavailable for 2 subjects.
TG: triglycerides, OGTT: oral glucose tolerance test, HOMA-IR: homeostatic model assessment for insulin resistance, HOMA-β: homeostatic model assessment for β-cell function, IISIM: Insulin sensitivity index of Matsuda and DeFronzo.
At baseline, subjects had normal fasting plasma glucose levels and normal glucose tolerance in response to an OGTT. After aerobic exercise training, there were no statistically significant improvements in plasma glucose variables. Fasting plasma insulin decreased significantly after 6-months of aerobic exercise training (Table 1, p < 0.05), as did 2-hour plasma insulin levels during the OGTT (Table 1, p = 0.01). HOMA-IR and ISIM improved significantly after aerobic exercise training in these subjects (Table 1, p < 0.05); HOMA-β was not significantly different after aerobic exercise training (Table 1). During the high-fat meal, plasma triglycerides and insulin increased significantly as expected at baseline and after aerobic exercise training (Table 1, p ≤ 0.01 for all). No statistically significant decrease was observed in the response of triglycerides to a high-fat meal after aerobic exercise training. The insulin response to the high-fat meal tended to decrease ~33% after aerobic exercise training (Table 1, p = 0.09). Aerobic exercise-training induced changes in glucose and insulin responses to the OGTT or high-fat meal were not associated with changes in fat mass or lean body mass.
Effects on plasma IGFBP-1 concentrations (Figure 1)
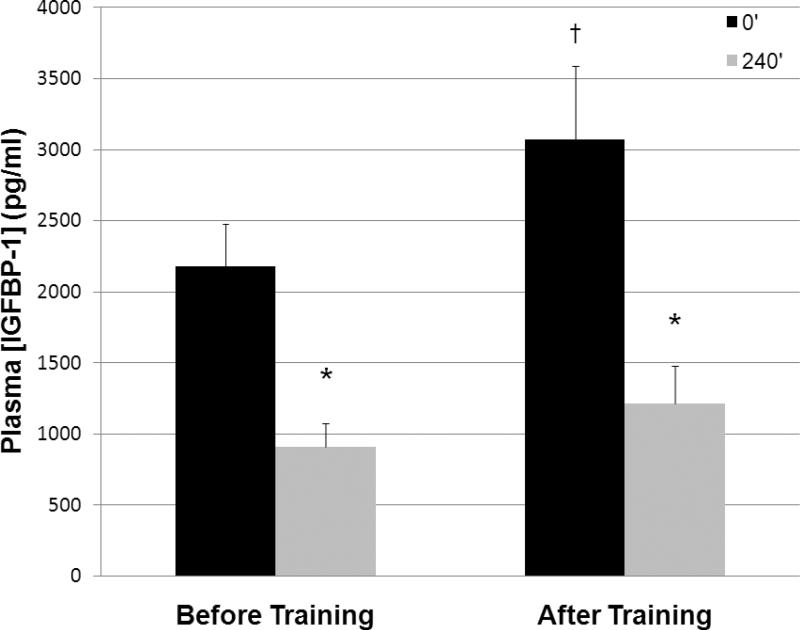
Figure 1.
Plasma IGFBP-1 response to a high-fat meal at baseline and after 6-months of aerobic exercise training (n=10). Data are means ± SEM. *Significant difference compared to 0' (p < 0.001), †Significant difference compared to baseline (p < 0.05).
We found significant main effects of aerobic exercise training and a high-fat meal on plasma IGFBP-1 concentrations. After 6-month aerobic exercise training, fasting plasma IGFBP-1 concentrations increased 41% (Figure 1, p < 0.05). At baseline, IGFBP-1 concentrations decreased 58% four hours after a high-fat meal (Figure 1, p < 0.001). This effect persisted after 6 months of aerobic exercise training, when IGFBP-1 concentration decreased 61% in response to the high-fat meal (Figure 1, p < 0.001).
Relationships between plasma IGFBP-1 and insulin concentrations
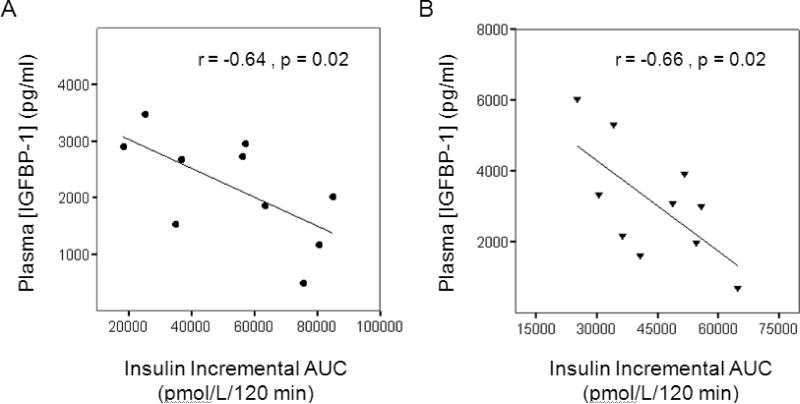
Plasma insulin levels were associated with plasma IGFBP-1 concentrations at baseline and after aerobic exercise training. Plasma insulin responses to an OGTT (insulin incremental AUC) inversely correlated with fasting plasma IGFBP-1 concentrations at baseline and after aerobic exercise training (Figure 2, p = 0.02 for both). Neither HOMA-IR nor ISIM correlated with IGFBP-1 concentrations at baseline or after aerobic exercise training.
Figure 2.
Scatterplots depicting the correlation between insulin incremental area under the curve (AUC) during the oral glucose tolerance test and plasma [IGFBP-1] at baseline (A) and after six months of aerobic exercise training (B).
The plasma insulin response to a high-fat meal (insulin incremental AUC) tended to be inversely associated with plasma IGFBP-1 concentration after the high-fat meal at baseline (r = −0.58, p = 0.06) and was significantly and inversely associated with plasma IGFBP-1 concentrations after the high-fat meal after aerobic exercise training (r = −0.71, p = 0.02). Plasma triglycerides did not correlate with plasma IGFBP-1 concentrations in the fasting state (r = 0.22–.24, p > 0.57) or in response to the high-fat meal (r = −0.12–0.22, p > 0.6).
4. Discussion
The major findings of the present report are that a) a six-month aerobic training program significantly increases circulating IGFBP-1 concentrations in overweight, normoglycemic (and normal glucose tolerant) middle-aged to older adults, and b) a high-fat meal induces a large, acute reduction in circulating IGFBP-1 concentrations at baseline and after aerobic exercise training. The increase in IGFBP-1 after aerobic exercise training may be related to improvements in fasting and postprandial insulin levels in older adults. Interestingly, despite the observed metabolic improvements and lower insulinemia after aerobic exercise training, the response of IGFBP-1 to a high-fat meal was not attenuated by regular aerobic exercise training.
To our knowledge, this is the first study to investigate IGFBP-1 responses to a high-fat meal and to long-term, standardized exercise training; however, there are previous reports of the effects of exercise and diet on IGFBPs. Several studies show increases in circulating IGFBP-1 concentrations after acute aerobic exercise [13–16], but all studies have not reached the same conclusion [25]. Manetta et al. [26] show that serum IGFBP-1 concentrations (as determined ~72 hours after the last exercise bout) are ~ 50% higher in trained middle-aged cyclists compared to their sedentary counterparts. Together with our results, this supports an effect of aerobic exercise training on IGFBP-1 concentration. Studies showing an increase in IGFBP-1 concentrations after acute exercise are in agreement that concentrations return to baseline 12–24 hours after exercise [13,15,16]. As our measurements were obtained 24–36 hours after the last bout of exercise, the increase in IGFBP-1 in our subjects is not likely attributable to the final bout of exercise, but to a chronic effect of exercise training. Future studies will be necessary to address the duration of the effect of chronic aerobic exercise on IGFBP-1 concentrations in older subjects. In contrast to our data, one study involving 8-weeks of aerobic and resistance exercise training in a group of young women showed no change in serum IGFBP-1 concentrations as measured >48 hrs after the most recent exercise bout [18]. While this appears discordant with our findings, differences between this and the present report may be due to the use of a different training regimen or a sample of relatively lean younger women.
In contrast to regular aerobic exercise, Heald et al. [19,27] report that high dietary fat intake is associated with low fasting circulating IGFBP-1 concentrations in cross-sectional studies of two large cohorts. Consistent with these reports, we find that an acute high-fat meal reduced plasma IGFBP-1 concentrations regardless of exercise training status. While the exact duration of this suppression is not yet established, it is possible that the summation of repeated meals high in fat content act to chronically reduce IGFBP-1 concentrations.
Two previous studies reported the effect of combined diet and exercise on IGFBP-1 concentrations [28,29]. Foster et al. [29] reported that acute aerobic exercise increases IGFBP-1 concentration in young men, and that this is partially reduced by post-exercise carbohydrate consumption with a corresponding increase in circulating insulin level; however, the effect of exercise on diet-induced changes in IGFBP-1 was not studied as in the present report. Ngo et al. [28] reported that 11 days of daily aerobic exercise with a low fat (<10%) and high carbohydrate 70–75%) diet increased serum IGFBP-1 concentrations by 53%. It is unclear whether the effects of this intervention are due to aerobic exercise, dietary composition, weight loss, or some combination of these. In the present report, subjects were stabilized on a low-fat diet prior to baseline testing and maintained this isocaloric diet throughout the intervention. Thus, it is quite likely that the effects we observe are due to the aerobic exercise training regimen and not diet or weight loss. We have previously shown that aerobic exercise training independently increases insulin sensitivity and reduces insulin responses to OGTT; however, the addition of weight loss may result in greater benefit [30,31]. Future prospective studies will be necessary to address the interactive effects of regular aerobic exercise and chronic dietary habits (both macronutrient composition and caloric intake) on IGFBP-1 concentrations.
Insulin has been the most investigated regulator of IGFBP-1 expression to date as it suppresses IGFBP-1 expression and secretion by the liver [32]. Changes in insulin levels may be partially responsible for the changes we observe in IGFBP-1 concentrations after aerobic exercise training. Fasting insulin levels were reduced by 12% in our subjects after aerobic exercise training and this may be responsible for the increase in IGFBP-1 concentrations we observed after exercise training. After the high-fat meal, we found that IGFBP-1 concentrations decreased 58% and 61% at baseline and after aerobic exercise training, respectively. Given the relatively short (89-minute) half-life of IGFBP-1 [33], it is possible that insulin suppression of IGFBP-1 expression was primarily responsible for this reduction. Peak insulin levels during the high-fat meal in our subjects were ~250 pmol/L, near the concentration found to elicit half-maximal suppression of IGFBP-1 expression [34]. Since plasma insulin levels were ~33% lower during the high-fat meal test after exercise training, we would expect an attenuated reduction in IGFBP-1 concentrations if insulin were the sole cause of the reduction in IGFBP-1 concentrations. The similar relative reductions in IGFBP-1 at baseline and after aerobic exercise training suggest that factors other than insulin are at least partially responsible for the reduction in IGFBP-1 concentrations. These mechanisms may be unique to the fat meal, but because IGFBP-1 is higher in the fasted state and lower in the fed state, they may also be due to high caloric intake in general.
IGFBP-1 concentrations may simply be a marker of metabolic improvements and reduced insulinemia; however, recent research suggests that IGFBP-1 exerts direct metabolic effects. IGFBP-1 binds integrin receptors in skeletal muscle and other tissue [35,36] and crosstalk between integrin receptor and insulin signaling pathways may, in part, increase insulin sensitivity [37]. In a rodent model, overexpression of IGFBP-1 resulted in a reduction in the development of insulin resistance [2], and longitudinal studies in humans show that low concentrations of IGFBP-1 predict the development of abnormal glucose regulation [38] and heightened cardiovascular disease mortality [6–9]. While fasting IGFBP-1 concentration correlated with insulin incremental AUC during the OGTT in our subjects, improvements in HOMA-IR and ISIM did not correlate with plasma IGFBP-1 concentrations. This may be due to our sample being predominately composed of subjects with normal glucose tolerance, or it is possible that a larger number of subjects is necessary to detect any association. It should also be noted that the concentrations of IGF-1 and -2 were not measured in the present study. Because FBP-1 may exhert IGF-dependent metabolic effects, future studies will be required to determine whether any effect of IGFBP-1 concentration is mediated through IGF-independent or IGF-dependent mechanisms.
Conclusion
We conclude that aerobic exercise training has a potentially beneficial effect to increase fasting plasma IGFBP-1 concentrations in previously sedentary middle-aged to older adults. In contrast, ingestion of a meal high in fat has a large and rapid effect to lower circulating IGFBP-1 concentration. Aerobic exercise training did not attenuate the adverse effect of a high-fat meal on plasma IGFBP-1 concentrations. This study begins to shed light on the interaction between diet and exercise effects on IGFBP-1 and may indicate a mechanism by which chronic exercise and diets low in fat confer protection from cardiometabolic diseases with advancing age.
Acknowledgements
Our appreciation is extended to the men and women who participated in this study and to the nurses, technicians, and students who assisted with this study.
Institutional Approval: This study was approved by the Institutional Review Boards at the University of Maryland College Park and the University of Maryland School of Medicine.
Funding This research was supported by the National Institutes of Health [AG-15389 (J.M.H.), AG-17474 (J.M.H.), T32-AG-000268 (J.M.H. and E.P.W)], the Department of Veterans Affairs [CDA-2-0039 (S.J.P.)], the University of Maryland Claude D. Pepper Older Americans Independence Center (P30-AG-12583), and the Baltimore VA Medical Center Geriatric Research, Education and Clinical Center (GRECC).
Abbreviations
- ANOVA
analysis of variance
- AUC
area under the curve
- EDTA
ethylenediaminetetraacetic acid
- HOMA-β
homeostatic model assessment for β cell function
- HOMA-IR
homeostatic model assessment for insulin resistance
- IGF
insulin-like growth factor
- IGFBP
insulin-like growth factor binding protein
- IGFBP-1
insulin-like growth factor binding protein-1
- ISIM
insulin sensitivity index
- OGTT
oral glucose tolerance test
- SAT
subcutaneous abdominal adipose tissue
- TG
triglyceride
- VAT
visceral adipose tissue
- VO2
oxygen consumption
- VO2max
maximal oxygen consumption
Footnotes
Author Contributions Steven J. Prior: Data collection, analysis, and interpretation; manuscript preparation
Nathan T. Jenkins: Data collection and analysis; manuscript revision
Josef Brandauer: Data collection and analysis; manuscript revision
Edward P. Weiss: Data collection and analysis; manuscript revision
James M. Hagberg: Study design, funding, and supervision; data collection; manuscript revision
Conflict of Interest: The authors have no conflict of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Juul A. Serum levels of insulin-like growth factor I and its binding proteins in health and disease. Growth Horm.IGF.Res. 2003;13:113–170. doi: 10.1016/s1096-6374(03)00038-8. [DOI] [PubMed] [Google Scholar]
- 2.Wheatcroft SB, Kearney MT. IGF-dependent and IGF-independent actions of IGF-binding protein-1 and -2: implications for metabolic homeostasis. Trends Endocrinol.Metab. 2009;20:153–162. doi: 10.1016/j.tem.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 3.Maddux BA, Chan A, De Filippis EA, et al. IGF-binding protein-1 levels are related to insulin-mediated glucose disposal and are a potential serum marker of insulin resistance. Diabetes Care. 2006;29:1535–1537. doi: 10.2337/dc05-1367. [DOI] [PubMed] [Google Scholar]
- 4.Mogul HR, Marshall M, Frey M, et al. Insulin like growth factor-binding protein-1 as a marker for hyperinsulinemia in obese menopausal women. J.Clin.Endocrinol.Metab. 1996;81:4492–4495. doi: 10.1210/jcem.81.12.8954066. [DOI] [PubMed] [Google Scholar]
- 5.Morris DV, Falcone T. The relationship between insulin sensitivity and insulin-like growth factor-binding protein-1. Gynecol.Endocrinol. 1996;10:407–412. doi: 10.3109/09513599609023605. [DOI] [PubMed] [Google Scholar]
- 6.Heald AH, Cruickshank JK, Riste LK, et al. Close relation of fasting insulin-like growth factor binding protein-1 (IGFBP-1) with glucose tolerance and cardiovascular risk in two populations. Diabetologia. 2001;44:333–339. doi: 10.1007/s001250051623. [DOI] [PubMed] [Google Scholar]
- 7.Heald AH, Siddals KW, Fraser W, et al. Low circulating levels of insulin-like growth factor binding protein-1 (IGFBP-1) are closely associated with the presence of macrovascular disease and hypertension in type 2 diabetes. Diabetes. 2002;51:2629–2636. doi: 10.2337/diabetes.51.8.2629. [DOI] [PubMed] [Google Scholar]
- 8.Laughlin GA, Barrett-Connor E, Criqui MH, et al. The prospective association of serum insulin-like growth factor I (IGF-I) and IGF-binding protein-1 levels with all cause and cardiovascular disease mortality in older adults: the Rancho Bernardo Study. J.Clin.Endocrinol.Metab. 2004;89:114–120. doi: 10.1210/jc.2003-030967. [DOI] [PubMed] [Google Scholar]
- 9.Kalme T, Seppala M, Qiao Q, et al. Sex hormone-binding globulin and insulin-like growth factor-binding protein-1 as indicators of metabolic syndrome, cardiovascular risk, and mortality in elderly men. J.Clin.Endocrinol.Metab. 2005;90:1550–1556. doi: 10.1210/jc.2004-0762. [DOI] [PubMed] [Google Scholar]
- 10.Ruan W, Lai M. Insulin-like growth factor binding protein: a possible marker for the metabolic syndrome? Acta Diabetol. 2010;47:5–14. doi: 10.1007/s00592-009-0142-3. [DOI] [PubMed] [Google Scholar]
- 11.Pratley RE, Hagberg JM, Dengel DR, et al. Aerobic exercise training-induced reductions in abdominal fat and glucose-stimulated insulin responses in middle-aged and older men. J.Am.Geriatr.Soc. 2000;48:1055–1061. doi: 10.1111/j.1532-5415.2000.tb04780.x. [DOI] [PubMed] [Google Scholar]
- 12.Cononie CC, Goldberg AP, Rogus E, et al. Seven consecutive days of exercise lowers plasma insulin responses to an oral glucose challenge in sedentary elderly. J.Am.Geriatr.Soc. 1994;42:394–398. doi: 10.1111/j.1532-5415.1994.tb07487.x. [DOI] [PubMed] [Google Scholar]
- 13.Nindl BC, Alemany JA, Tuckow AP, et al. Effects of exercise mode and duration on 24-h IGF-I system recovery responses. Med.Sci.Sports Exerc. 2009;41:1261–1270. doi: 10.1249/MSS.0b013e318197125c. [DOI] [PubMed] [Google Scholar]
- 14.Kanaley JA, Frystyk J, Moller N, et al. The effect of submaximal exercise on immuno- and bioassayable IGF-I activity in patients with GH-deficiency and healthy subjects. Growth Horm.IGF.Res. 2005;15:283–290. doi: 10.1016/j.ghir.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 15.Berg U, Enqvist JK, Mattsson CM, et al. Lack of sex differences in the IGF-IGFBP response to ultra endurance exercise. Scand.J.Med.Sci.Sports. 2008;18:706–714. doi: 10.1111/j.1600-0838.2007.00758.x. [DOI] [PubMed] [Google Scholar]
- 16.Koistinen H, Koistinen R, Selenius L, et al. Effect of marathon run on serum IGF-I and IGF-binding protein 1 and 3 levels. J.Appl.Physiol. 1996;80:760–764. doi: 10.1152/jappl.1996.80.3.760. [DOI] [PubMed] [Google Scholar]
- 17.Manetta J, Brun JF, Maimoun L, et al. The effects of intensive training on insulin-like growth factor I (IGF-I) and IGF binding proteins 1 and 3 in competitive cyclists: relationships with glucose disposal. J.Sports Sci. 2003;21:147–154. doi: 10.1080/0264041031000070895. [DOI] [PubMed] [Google Scholar]
- 18.Nindl BC, Alemany JA, Tuckow AP, et al. Circulating bioactive and immunoreactive IGF-I remain stable in women, despite physical fitness improvements after 8 weeks of resistance, aerobic, and combined exercise training. J.Appl.Physiol. 2010;109:112–120. doi: 10.1152/japplphysiol.00025.2010. [DOI] [PubMed] [Google Scholar]
- 19.Heald AH, Sharma R, Anderson SG, et al. Dietary intake and the insulin-like growth factor system: effects of migration in two related populations in India and Britain with markedly different dietary intake. Public Health Nutr. 2005;8:620–627. doi: 10.1079/phn2005729. [DOI] [PubMed] [Google Scholar]
- 20.Krauss RM, Eckel RH, Howard B, et al. AHA Dietary Guidelines: revision 2000: A statement for healthcare professionals from the Nutrition Committee of the American Heart Association. Circulation. 2000;102:2284–2299. doi: 10.1161/01.cir.102.18.2284. [DOI] [PubMed] [Google Scholar]
- 21.Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22:1462–1470. doi: 10.2337/diacare.22.9.1462. [DOI] [PubMed] [Google Scholar]
- 22.Matthews DR, Hosker JP, Rudenski AS, et al. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 23.Patsch JR, Karlin JB, Scott LW, et al. Inverse relationship between blood levels of high density lipoprotein subfraction 2 and magnitude of postprandial lipemia. Proc. Natl. Acad. Sci. U.S.A. 1983;80:1449–1453. doi: 10.1073/pnas.80.5.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weiss EP, Fields DA, Mittendorfer B, et al. Reproducibility of postprandial lipemia tests and validity of an abbreviated 4-hour test. Metabolism. 2008;57:1479–1485. doi: 10.1016/j.metabol.2008.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Manetta J, Brun JF, Maimoun L, et al. Effect of training on the GH/IGF-I axis during exercise in middle-aged men: relationship to glucose homeostasis. Am. J. Physiol Endocrinol. Metab. 2002;283:E929–E936. doi: 10.1152/ajpendo.00539.2001. [DOI] [PubMed] [Google Scholar]
- 26.Manetta J, Brun JF, Fedou C, et al. Serum levels of insulin-like growth factor-I (IGF-I), and IGF-binding proteins-1 and -3 in middle-aged and young athletes versus sedentary men: relationship with glucose disposal. Metabolism. 2003;52:821–826. doi: 10.1016/s0026-0495(03)00096-9. [DOI] [PubMed] [Google Scholar]
- 27.Heald AH, Cade JE, Cruickshank JK, et al. The influence of dietary intake on the insulin-like growth factor (IGF) system across three ethnic groups: a population-based study. Public Health Nutr. 2003;6:175–180. doi: 10.1079/PHN2002414. [DOI] [PubMed] [Google Scholar]
- 28.Ngo TH, Barnard RJ, Tymchuk CN, et al. Effect of diet and exercise on serum insulin, IGF-I, and IGFBP-1 levels and growth of LNCaP cells in vitro (United States) Cancer Causes Control. 2002;13:929–935. doi: 10.1023/a:1021911517010. [DOI] [PubMed] [Google Scholar]
- 29.Foster EB, Fisher G, Sartin JL, et al. Acute regulation of IGF-I by alterations in post-exercise macronutrients. Amino. Acids. 2011 doi: 10.1007/s00726-011-0837-y. [DOI] [PubMed] [Google Scholar]
- 30.Prior SJ, Joseph LJ, Brandauer J, et al. Reduction in midthigh low-density muscle with aerobic exercise training and weight loss impacts glucose tolerance in older men. J. Clin. Endocrinol. Metab. 2007;92:880–886. doi: 10.1210/jc.2006-2113. [DOI] [PubMed] [Google Scholar]
- 31.Dengel DR, Pratley RE, Hagberg JM, et al. Distinct effects of aerobic exercise training and weight loss on glucose homeostasis in obese sedentary men. J. Appl. Physiol. 1996;81:318–325. doi: 10.1152/jappl.1996.81.1.318. [DOI] [PubMed] [Google Scholar]
- 32.Powell DR, Allander SV, Scheimann AO, et al. Multiple proteins bind the insulin response element in the human IGFBP-1 promoter. Prog. Growth Factor Res. 1995;6:93–101. doi: 10.1016/0955-2235(95)00034-8. [DOI] [PubMed] [Google Scholar]
- 33.Lee PD, Conover CA, Powell DR. Regulation and function of insulin-like growth factor-binding protein-1. Proc. Soc. Exp. Biol. Med. 1993;204:4–29. doi: 10.3181/00379727-204-43630. [DOI] [PubMed] [Google Scholar]
- 34.Suikkari AM, Koivisto VA, Koistinen R, et al. Dose-response characteristics for suppression of low molecular weight plasma insulin-like growth factor-binding protein by insulin. J. Clin. Endocrinol. Metab. 1989;68:135–140. doi: 10.1210/jcem-68-1-135. [DOI] [PubMed] [Google Scholar]
- 35.Frost RA, Lang CH. Differential effects of insulin-like growth factor I (IGF-I) and IGF-binding protein-1 on protein metabolism in human skeletal muscle cells. Endocrinology. 1999;140:3962–3970. doi: 10.1210/endo.140.9.6998. [DOI] [PubMed] [Google Scholar]
- 36.Jones JI, Gockerman A, Busby WH, Jr., et al. Insulin-like growth factor binding protein 1 stimulates cell migration and binds to the alpha 5 beta 1 integrin by means of its Arg-Gly-Asp sequence. Proc. Natl. Acad. Sci. U.S.A. 1993;90:10553–10557. doi: 10.1073/pnas.90.22.10553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guilherme A, Torres K, Czech MP. Cross-talk between insulin receptor and integrin alpha5 beta1 signaling pathways. J. Biol. Chem. 1998;273:22899–22903. doi: 10.1074/jbc.273.36.22899. [DOI] [PubMed] [Google Scholar]
- 38.Lewitt MS, Hilding A, Ostenson CG, et al. Insulin-like growth factor-binding protein-1 in the prediction and development of type 2 diabetes in middle-aged Swedish men. Diabetologia. 2008;51:1135–1145. doi: 10.1007/s00125-008-1016-x. [DOI] [PubMed] [Google Scholar]