Abstract
The decline in cognitive function that accompanies normal aging has a negative impact on the quality of life of the elderly and their families. Studies in humans and rodents show that spatial navigation and other hippocampus-dependent functions are particularly vulnerable to the deleterious effects of aging. However, reduced motor activity and alterations in the stress response that accompany normal aging can hinder the ability to study certain cognitive behaviors in aged animals. In an attempt to circumvent these potential confounds, we used a hippocampus-dependent object-place recognition task to show that long-term spatial memory is impaired in aged mice. Aged animals performed similarly to young adult mice on an object recognition task that does not rely on hippocampal function.
Keywords: Spatial memory, aging, mice, object recognition, hippocampus
1. Introduction
Over the past 30 years, considerable effort has been devoted to the identification and characterization of the neurological and molecular pathways that underlie age-related cognitive impairments. Studies in humans and rodents show that the hippocampus is particularly vulnerable to the effects of aging (Bach, et al., 1999, Davis, et al., 1993, Gallagher, et al., 2010), leading to impairments in episodic memory (Miller and O'Callaghan, 2005, Park and Reuter-Lorenz, 2009) and spatial navigation (Barnes, 1979, Gage, et al., 1984, Markowska, et al., 1989). However, confounding factors such as changes in motor activity and in the response to stress that accompany normal aging can complicate the interpretation of studies using the Morris water maze and other spatial navigation tasks (Sparkman and Johnson, 2008). The present studies focus on object memory tasks, which are ideal to study both short- and long-term memory formation because learning occurs within a single session (Baker and Kim, 2002, de Lima, et al., 2006, Oliveira, et al., 2010). Furthermore, object-based tasks take advantage of the innate tendency of mice to explore novel items in their environment, while inflicting little stress on the animals (Save, et al., 1992, Sharma, et al., 2010), and the anatomical substrates underlying object memory are well characterized (Ennaceur and Aggleton, 1997, Oliveira, et al., 2010, Roozendaal, et al., 2010).
2. Methods
2.1 Subjects
Young (2–4 months-old) and old (22–24 months-old) male C57BL/6NIA mice provided by the National Institute of Aging were used in all experiments. Food and water were provided ad libitum. Lights were maintained on a 12–12 light/dark cycle, and all experiments were performed in the light phase. Mice were singly housed a week before training. Animals were handled in the experimental room for 1 minute per day for 3 days prior to the first exposure to the training arena. All experiments were approved by the Institutional Animal Care and Use Committee of the University of Pennsylvania and conducted according to National Institutes of Health guidelines.
2.2 Object location memory task
Object location memory experiments were performed as described previously (Oliveira, et al., 2010) with minor modifications. On the day of training, mice were placed in the training arena for a total of four 10-minute sessions with an inter-session interval of 3 minutes during which mice were returned to their home cages. The first session consisted of a context habituation period without objects in the arena. During the next three sessions, mice were placed in the training arena with two distinct objects. The objects used were a glass bottle, and a metal tower (5”H × 2”W × 2”L). The objects and the arenas were wiped with 70% ethanol before each session. Either 3 minutes or 24 hours after training, mice were placed in the original training arena for 10 minutes with one object displaced to a new location (displaced object, DO), while the other object was not moved (non-displaced object, NDO). Exploration was recorded during training and testing on a digital camera for subsequent scoring of time spent exploring objects. Exploration of the objects was defined as the amount of time mice were oriented toward an object with its nose within 1 cm of it, and was scored by an experimenter blind to age.
2.3 Object recognition memory task
Object recognition memory experiments were performed in the same training arenas and using the same objects as previously described (Oliveira, et al., 2010). Mice were pre-exposed to the training context for 10 minutes on 3 consecutive days prior to object training. 24 hours after the last context exposure, mice were placed in the training arena with two identical objects and allowed to explore for 10 minutes. Twenty-four hours after training, mice were placed in the original training arena for 10 minutes with one familiar object and one novel object in the same locations used during training. Exploration was measured and recorded as described above.
2.3 Data analysis
Percent preference in the object location experiment was calculated as time spent exploring the DO relative to the total time spent exploring both objects (i.e., preference = DO/(NDO+DO)). For object recognition experiments, percent preference was calculated as time spent exploring the novel object relative to the total time exploring both objects (i.e. novel/(familiar + novel)). Repeated measures ANOVAs (RM-ANOVA) were used to compare age groups, during training and retention test sessions for both tasks, with session as a within subject factor and age as a between subject factor. Tukey post-hoc tests were used in the event of a significant session × age interaction. p<0.05 was considered significant.
3. Results
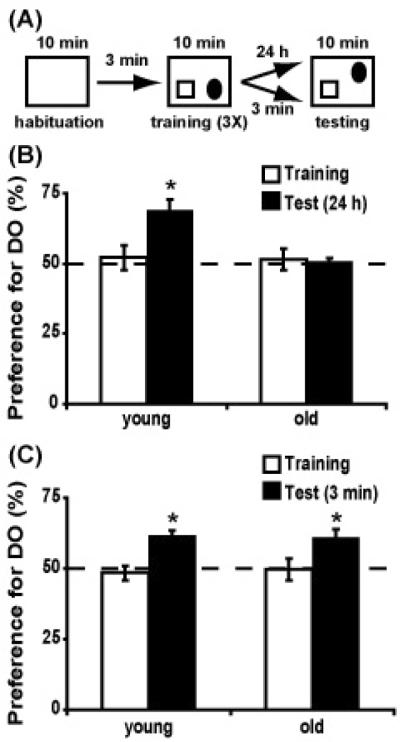
We assessed hippocampal function and spatial memory formation in aged mice by using a hippocampus-dependent object place recognition task (Mumby, et al., 2002, Oliveira, et al., 2010, Save, et al., 1992). Training consisted of three 10-minute exposures (3 minute inter-trial interval) to an arena that contained two distinct objects (Fig. 1A). Both groups of animals spent an equal amount of time exploring what would ultimately be the DO and NDO (RM-ANOVA, F(2,74)=0.18, p=0.83) where exploration of the objects gradually decreased over time (F(2,74)=33.98, p<0.001). Age had no effect on the time spent exploring the objects (F(1,18)=2.29, p=0.14) and total exploration times for sessions 1, 2 and 3 were 8.4 ± 1.0, 5.9 ± 1.3, 5.0 ± 0.6 seconds, for young mice, and 11.7 ± 0.8, 6.9 ± 0.6, 5.3 ± 0.7 seconds, for aged mice. Twenty-four hours after the last session, young and old mice were re-introduced to the training arena with one of the two objects displaced to a new location. The relative preference for the DO was calculated as the percentage of object exploration time dedicated to this object. We compared the preference for the DO for each age group during the last training session to the preference during the 24 hour test. The preference for the DO increased during the test for young mice, whereas old animals did not show a preference for either object during either sessions (session × age interaction: F(1,18)=5.15, p=0.036). Aged mice showed lower preference for the DO than young animals (post-hoc Tukey tests, p<0.05) only during the 24 hour retention test session, suggesting that aged mice have impaired spatial memory (Fig. 1 B). Next, we assessed short term memory, using the same training protocol in a different cohort of young and old mice. RM-ANOVA revealed that time spent exploring the objects decreased over the course of the three training sessions (F(2,78)=20.70, p<0.001) and the animals spent equal time exploring what would ultimately become the DO and NDO (F(2,78)=0.07, p=0.93), with no effect of age (F(2,38)=0.46, p=0.64). Three minutes after the last training session, the animals were returned to the training arena with one object displaced to a novel location. In this case, young and aged animals showed a preference for the DO (session: F(1,19)=20.49, p<0.001; session × age: F(1,19)=0.15, p=0.70), suggesting that acquisition of spatial memory was not affected by aging (Fig. 1C). Taken together, these results indicate that hippocampal function declines with age, causing impairments in the consolidation of long-term spatial memory.
Figure 1. Aged mice have impaired long-term memory for object location.
a) Diagram illustrating the experimental design for object place recognition. Animals were exposed to the context once for 10 minutes followed by three 10 minute training sessions with a 3 minute inter-trial interval. Testing occurred either 24 hours or 3 minutes after the last training session by moving one of the objects to a new location. b) Bar graphs showing the percent preference for the displaced object (DO) at 24 hours for young (n=10) and aged (n=10) animals. c) Graphs showing the percent preference for the DO at 3 minutes for young (n=10) and aged (n=11) mice. Dotted line denotes 50% (chance) preference. * p<0.05.
To address whether these deficits are specific to the hippocampus and spatial memory, we assessed object recognition in young and old mice. We and others have shown that object recognition becomes independent of the hippocampus if the task is conducted with limited spatial cues (Forwood, et al., 2005) or if the animals are familiarized to the context before training (Oliveira, et al., 2010). We removed spatial cues from the walls and the animals were familiarized to the training arena for three consecutive days prior to training. On the fourth day, animals were placed into the same training arena containing 2 identical objects and allowed to explore for ten minutes. Twenty-four hours later, mice were re-introduced to the context containing one familiar object and one novel object (Fig. 2A). The relative preference for the novel object was calculated as the percentage of object exploration time dedicated to this object. Both young and old mice showed a preference for the novel object 24 hours after training (session: F(1,29)=25.67, p<0.001; session × age: F(1,29)=0.21, p=0.65), suggesting that object recognition is unaffected by aging (Fig. 2B).
Figure 2. Aged mice show normal object recognition.
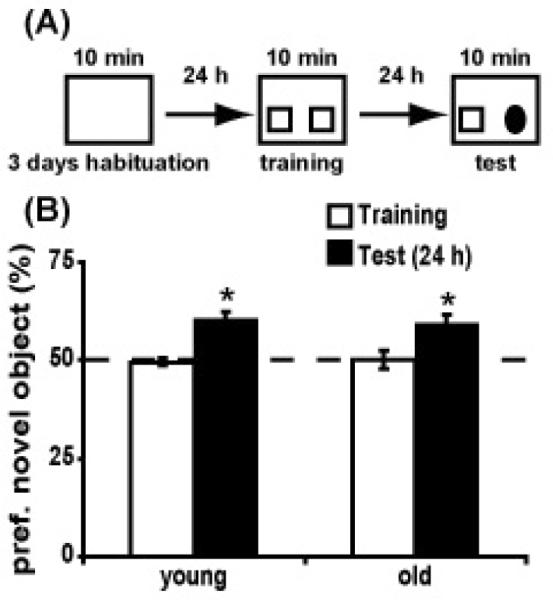
a) Diagram illustrating the experimental design for object recognition. Animals were familiarized to the training context 10 minutes per day for 3 days prior to training. On the 4th day, 2 identical objects were introduced for 10 minutes. 24 hours later the animals were tested for object recognition by replacing one of the familiar objects with a novel object. b) Bar graphs showing the percent preference for the novel object in young (n=16) and old (n=15) animals. Dotted line denotes 50% (chance) preference. * p<0.05.
4. Discussion
Using an object-place recognition task, we found that spatial memory consolidation is impaired in aged mice. Age-related cognitive decline can be difficult to characterize due to changes in motor activity (Gage, et al., 1984, Markowska, 1999) and stress-related physiology and behaviors (Sparkman and Johnson, 2008) that can alter performance in spatial tasks (e.g., Barnes and Morris water maze). Object-based tasks are advantageous because they are not physically demanding and they induce little stress. However, the requirement of the hippocampus for object-based memory (Forwood, et al., 2005, Oliveira, et al., 2010) and retention length (Roozendaal, et al., 2010) are known to vary with training protocol. For example, aging was found to impair both object-place and novel-object recognition tasks when using relatively short training sessions (Burke, et al., 2010, Murai, et al., 2007). Here we used two training protocols that produce robust long-term memory and allow for the dissociation of hippocampal involvement. Extended pre-exposure to the training context is known to reduce the role of the hippocampus in the novel object recognition task (Forwood, et al., 2005, Oliveira, et al., 2010). Thus, we provide a more direct examination of the effects of aging on spatial memory formation and consolidation.
Previous attempts to rescue age-induced memory deficits have involved systemic injections of memory-enhancing compounds, (Bach, et al., 1999, Davis, et al., 1993, de Lima, et al., 2008, de Lima, et al., 2005, Markowska, et al., 1994, Rech, et al.) often administered prior to training. A recent study found that injections of histone deacetylase (HDAC) inhibitors into the hippocampus prior to fear conditioning can rescue age-related contextual fear memory impairments (Peleg, et al., 2010). Our findings raise the interesting possibility of enhancing hippocampal function immediately after training in order to ameliorate age-induced spatial memory impairments.
Acknowledgments
This work was supported by the grants from NIA (5P01AG017628-09 to T.A., Principal Investigator Allan Pack) and NHLBI (2T32HL007953-11A1 to M.W., Principal Investigator Allan Pack) and NSF (to P.H.). We also thank Drs. Robbert Havekes and Joshua Hawk for their input on the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Statement: Mathieu E Wimmer, Pepe J Hernandez, Jennifer M Blackwell and Ted Abel do not have any conflicts of interest to disclose.
References
- Bach ME, Barad M, Son H, Zhuo M, Lu YF, Shih R, Mansuy I, Hawkins RD, Kandel ER. Age-related defects in spatial memory are correlated with defects in the late phase of hippocampal long-term potentiation in vitro and are attenuated by drugs that enhance the cAMP signaling pathway. Proc Natl Acad Sci U S A. 1999;96(9):5280–5. doi: 10.1073/pnas.96.9.5280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker KB, Kim JJ. Effects of stress and hippocampal NMDA receptor antagonism on recognition memory in rats. Learn Mem. 2002;9(2):58–65. doi: 10.1101/lm.46102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes CA. Memory deficits associated with senescence: a neurophysiological and behavioral study in the rat. J Comp Physiol Psychol. 1979;93(1):74–104. doi: 10.1037/h0077579. [DOI] [PubMed] [Google Scholar]
- Burke SN, Wallace JL, Nematollahi S, Uprety AR, Barnes CA. Pattern separation deficits may contribute to age-associated recognition impairments. Behav Neurosci. 2010;124(5):559–73. doi: 10.1037/a0020893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis S, Markowska AL, Wenk GL, Barnes CA. Acetyl-L-carnitine: behavioral, electrophysiological, and neurochemical effects. Neurobiol Aging. 1993;14(1):107–15. doi: 10.1016/0197-4580(93)90030-f. [DOI] [PubMed] [Google Scholar]
- de Lima MN, Dias CP, Torres JP, Dornelles A, Garcia VA, Scalco FS, Guimaraes MR, Petry RC, Bromberg E, Constantino L, Budni P, Dal-Pizzol F, Schroder N. Reversion of age-related recognition memory impairment by iron chelation in rats. Neurobiol Aging. 2008;29(7):1052–9. doi: 10.1016/j.neurobiolaging.2007.02.006. [DOI] [PubMed] [Google Scholar]
- de Lima MN, Laranja DC, Caldana F, Bromberg E, Roesler R, Schroder N. Reversal of age-related deficits in object recognition memory in rats with l-deprenyl. Exp Gerontol. 2005;40(6):506–11. doi: 10.1016/j.exger.2005.03.004. [DOI] [PubMed] [Google Scholar]
- de Lima MN, Luft T, Roesler R, Schroder N. Temporary inactivation reveals an essential role of the dorsal hippocampus in consolidation of object recognition memory. Neurosci Lett. 2006;405(1–2):142–6. doi: 10.1016/j.neulet.2006.06.044. [DOI] [PubMed] [Google Scholar]
- Ennaceur A, Aggleton JP. The effects of neurotoxic lesions of the perirhinal cortex combined to fornix transection on object recognition memory in the rat. Behav Brain Res. 1997;88(2):181–93. doi: 10.1016/s0166-4328(97)02297-3. [DOI] [PubMed] [Google Scholar]
- Forwood SE, Winters BD, Bussey TJ. Hippocampal lesions that abolish spatial maze performance spare object recognition memory at delays of up to 48 hours. Hippocampus. 2005;15(3):347–55. doi: 10.1002/hipo.20059. [DOI] [PubMed] [Google Scholar]
- Gage FH, Dunnett SB, Bjorklund A. Spatial learning and motor deficits in aged rats. Neurobiol Aging. 1984;5(1):43–8. doi: 10.1016/0197-4580(84)90084-8. [DOI] [PubMed] [Google Scholar]
- Gallagher M, Bakker A, Yassa MA, Stark CE. Bridging neurocognitive aging and disease modification: targeting functional mechanisms of memory impairment. Curr Alzheimer Res. 2010;7(3):197–9. doi: 10.2174/156720510791050867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markowska AL. Sex dimorphisms in the rate of age-related decline in spatial memory: relevance to alterations in the estrous cycle. J Neurosci. 1999;19(18):8122–33. doi: 10.1523/JNEUROSCI.19-18-08122.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markowska AL, Koliatsos VE, Breckler SJ, Price DL, Olton DS. Human nerve growth factor improves spatial memory in aged but not in young rats. J Neurosci. 1994;14(8):4815–24. doi: 10.1523/JNEUROSCI.14-08-04815.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markowska AL, Stone WS, Ingram DK, Reynolds J, Gold PE, Conti LH, Pontecorvo MJ, Wenk GL, Olton DS. Individual differences in aging: behavioral and neurobiological correlates. Neurobiol Aging. 1989;10(1):31–43. doi: 10.1016/s0197-4580(89)80008-9. [DOI] [PubMed] [Google Scholar]
- Miller DB, O'Callaghan JP. Aging, stress and the hippocampus. Ageing Res Rev. 2005;4(2):123–40. doi: 10.1016/j.arr.2005.03.002. [DOI] [PubMed] [Google Scholar]
- Mumby DG, Gaskin S, Glenn MJ, Schramek TE, Lehmann H. Hippocampal damage and exploratory preferences in rats: memory for objects, places, and contexts. Learn Mem. 2002;9(2):49–57. doi: 10.1101/lm.41302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murai T, Okuda S, Tanaka T, Ohta H. Characteristics of object location memory in mice: Behavioral and pharmacological studies. Physiol Behav. 2007;90(1):116–24. doi: 10.1016/j.physbeh.2006.09.013. [DOI] [PubMed] [Google Scholar]
- Oliveira AM, Hawk JD, Abel T, Havekes R. Post-training reversible inactivation of the hippocampus enhances novel object recognition memory. Learn Mem. 2010;17(3):155–60. doi: 10.1101/lm.1625310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park DC, Reuter-Lorenz P. The adaptive brain: aging and neurocognitive scaffolding. Annu Rev Psychol. 2009;60:173–96. doi: 10.1146/annurev.psych.59.103006.093656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peleg S, Sananbenesi F, Zovoilis A, Burkhardt S, Bahari-Javan S, Agis-Balboa RC, Cota P, Wittnam JL, Gogol-Doering A, Opitz L, Salinas-Riester G, Dettenhofer M, Kang H, Farinelli L, Chen W, Fischer A. Altered histone acetylation is associated with age-dependent memory impairment in mice. Science. 2010;328(5979):753–6. doi: 10.1126/science.1186088. [DOI] [PubMed] [Google Scholar]
- Rech RL, de Lima MN, Dornelles A, Garcia VA, Alcalde LA, Vedana G, Schroder N. Reversal of age-associated memory impairment by rosuvastatin in rats. Exp Gerontol. 2010;45(5):351–6. doi: 10.1016/j.exger.2010.02.001. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, Hernandez A, Cabrera SM, Hagewoud R, Malvaez M, Stefanko DP, Haettig J, Wood MA. Membrane-associated glucocorticoid activity is necessary for modulation of long-term memory via chromatin modification. J Neurosci. 2010;30(14):5037–46. doi: 10.1523/JNEUROSCI.5717-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Save E, Poucet B, Foreman N, Buhot MC. Object exploration and reactions to spatial and nonspatial changes in hooded rats following damage to parietal cortex or hippocampal formation. Behav Neurosci. 1992;106(3):447–56. [PubMed] [Google Scholar]
- Sharma S, Rakoczy S, Brown-Borg H. Assessment of spatial memory in mice. Life Sci. 2010;87(17–18):521–36. doi: 10.1016/j.lfs.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparkman NL, Johnson RW. Neuroinflammation associated with aging sensitizes the brain to the effects of infection or stress. Neuroimmunomodulation. 2008;15(4–6):323–30. doi: 10.1159/000156474. [DOI] [PMC free article] [PubMed] [Google Scholar]