Abstract
It is challenging to discuss the use of high risk organs with patients, in part due to lack of information on how patients view this topic. This study aimed to understand how patients think about organ quality, and to test formats for risk communication. Semi-structured interviews of 10 patients on the waiting list revealed limited understanding about the spectrum of organ quality, and reluctance to consider anything but the “best” organs. A computerized quantitative survey was then conducted using an interactive graph to elicit the risk of graft failure that patients would accept. Among 95 wait-listed patients completing the survey, 58% would only accept organs with ≤25% risk of graft failure at 3 years, while 18% would only accept the lowest risk possible (19% at 3 years). Risk tolerance was increased by presenting organ quality in reference to “average quality” rather than “best quality”, and by feedback about implications for organ supply. More than three-quarters of patients reported wanting an equal or dominant role in organ acceptance decisions. Men tended to prefer lower risk organs than women (mean acceptable risk 29% vs 35%, p=0.04), but risk tolerance was not associated with other demographic or clinical characteristics such as severity of liver disease. In summary, patients want to be involved in decisions about organ quality. Patients’ risk tolerance varies widely, and their acceptance of high risk organs can be facilitated by presenting risks of graft failure in reference to “average” organs and providing feedback about implications for organ supply.
Keywords: organ quality, liver transplantation, graft failure, risk tolerance, patient preferences
Introduction
Deceased donor liver allografts vary widely in quality. Donor characteristics such as age, cause of death, steatosis, and ischemia time can make the difference between a 20% rate of graft failure and a 40% rate of graft failure by 3 years after transplantation(1). Furthermore, organ quality appears likely to worsen in the future. The donor pool is aging, more donors have experienced stroke as the cause of brain death, and the federally funded Organ Donation Breakthrough Collaborative is promoting expansion of the donor pool with Extended Criteria Donor (ECD) organs which carry higher risks of graft failure(2). Thus, issues of organ quality are increasingly relevant for every liver transplant candidate.
Each time an organ offer is made, the patient and physician are faced with a difficult choice: to accept the offer, or wait in hopes that a better one will come along. For patients with end-stage liver disease, this decision could mean the difference between life and death. For physicians, this decision is fraught with uncertainty, requiring balancing of numerous patient and donor factors. Furthermore, communicating this complex risk/benefit tradeoff to the patient can be challenging. Ideally, discussions about organ quality should occur prospectively during the transplant evaluation(3). However, this is easier said than done in a busy transplant clinic. These discussions are made even more difficult by our limited understanding of how patients perceive organ quality. How much risk of graft failure are patients willing to accept, and what factors influence their decisions? What are the critical knowledge deficits and cognitive biases which must be addressed in physician-patient conversations?
In this study, we investigated how candidates for liver transplantation consider issues of organ quality. In addition to describing an overview of patients’ decision making process, we aimed to 1) test various presentation formats for communicating risks to patients, and 2) determine which patient factors might be associated with increased willingness to accept higher risk organs.
Methods
Given the limited prior knowledge about this subject, we employed a mixed methods approach consisting of qualitative and quantitative components(4). These methods are complementary: qualitative data is more rich and nuanced, and allows detection of findings that did not necessarily fit with researchers’ preconceived notions or hypotheses. Quantitative data, on the other hand, allows testing of specific inferential hypotheses.
Both portions of the study were performed among adult patients being seen in transplant clinic, who were already on the waiting list for liver transplantation. At our center, before placement on the waiting list patients all receive extensive education about the transplant system, including a half-day group education class. Additionally, each patient has at least one individual appointment with a transplant surgeon who routinely discusses the topic of organ quality. Specifically, in each one of these appointments the surgeon explains the concept that organ quality is on a spectrum, and describes organs at higher than average risk of graft failure such as those from donors after cardiac death (DCD).
Qualitative methods
Semi-structured interviews of adult patients on the waiting list for liver transplantation at our center were conducted by the primary author (MLV). Patients were enrolled from the transplant clinic, with exclusion criterion being significant hepatic encephalopathy (West Haven grade 2 or higher) or inability to converse in English. This study was approved by our Institutional Review Board, and informed consent was obtained for the interview and audio recording. The questions began as open-ended, and grew progressively more focused on patients’ understanding of organ quality and preferences for accepting higher risk organs. Probes were used to elicit further detail. The interview also included questions about patients’ perceptions of their risk of dying on the waiting list, and their opinions about the organ allocation system. The interviews were recorded, transcribed, and imported into NVivo 7® (QSR International, Doncaster, Australia), a qualitative data analysis package which allows manipulation and organization of text from the transcripts. Analysis was performed using methods of qualitative description(5). First, codes representing analytically meaningful categories were assigned to segments of text. Second, these segments were organized together so that patterns and themes could emerge. Interviews were conducted until transcripts no longer revealed new, relevant information.
Quantitative methods
The findings from the qualitative portion of the study were then used to inform development of a quantitative web-based patient survey. Patients on the waiting list for liver transplantation were enrolled from the transplant clinic to complete the survey on a study laptop computer. Patients with limited computer literacy were assisted by a study coordinator trained in survey administration. Those with significant hepatic encephalopathy were excluded, as were subjects from the qualitative portion of the study. Among those approached, the participation rate was 82%. Informed consent was obtained from all subjects, and the entire protocol was approved by the Institutional Review Board.
The survey consisted of three sections: 1) education about the spectrum of organ quality and implications of graft failure, 2) elicitation of patient preferences about the level of graft failure risk they would accept, and 3) covariates hypothesized to influence patient decisions. These covariates included:
demographics and clinical characteristics including Model for End-stage Liver Disease (MELD) score
quality of life(6) (measured using a visual analog scale)
belief in control over one’s health, measured using a validated scale termed “locus of control” – which has been shown in other fields to affect risk-taking behavior(7)
a validated scale measuring trust in physicians(8)
knowledge of the MELD-based allocation system, measured by percentage of questions answered correctly
patients’ estimates of their risk of dying in the next 3 months while waiting for a transplant
their estimates of the probability of receiving a transplant in the next 3 months
expectations about survival and quality of life after transplantation
emotional distress of waiting for an organ, measured using a standardized scale termed the PANAS (positive affect/negative affect scale)(9), and
a validated scale of numeracy, which reflects patients’ comfort and ability to work with numbers (similar to the concept of literacy)(10)
Patients’ preferences were elicited by instructing them to imagine they are being offered a transplant right now and asking them to indicate what risk of graft failure at 3 years they would accept. Responses were recorded using an interactive graphical tool that visually represented how many organs would fail out of 100 in a pictograph format. An example of this preference elicitation tool is shown in Figure 1. The pictograph format was chosen because pictographs have been shown to improve understanding of risk information in other clinical settings(11, 12).
Figure 1.
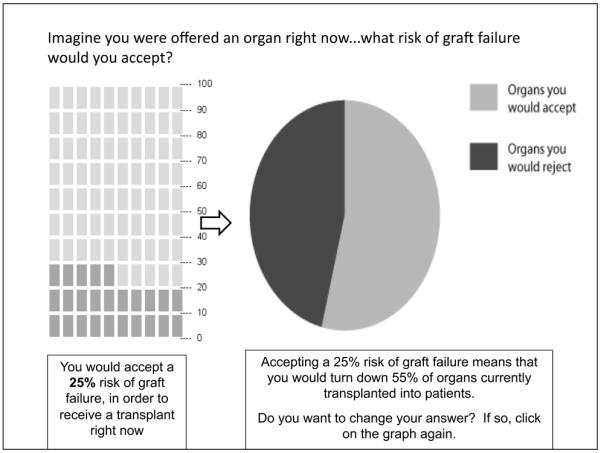
Tool for eliciting patient preferences about risk of graft failure
Using a computerized random number generator, patients were randomized to receive two different versions of this elicitation tool: one where the initial pictograph shows the risk of graft failure with “best quality” organs (19% at 3 years), versus a second version where the initial pictograph shows the risk of graft failure with “average quality” organs (25% at 3 years). This randomization was performed in order to determine whether patients’ responses are anchored to the initial graph they see. After the initial preference elicitation, patients were provided feedback regarding how their preferences would limit the number of organs available to them (see pie chart in Figure 1, which was not visible in the initial elicitation). They were then asked if they would like to revise their answer; data was collected pre-feedback and post-feedback to determine whether this feedback influenced preferences.
Finally, a possible bias against “lower quality” organs was tested by presenting a discrete choice scenario with a logically “correct” answer: a) staying on the waiting list, with a 20% risk of dying, versus b) accepting a lower quality liver, with a 20% risk of dying but an improved quality of life.
Statistical analysis was performed using nonparametric methods, since preliminary analysis of the data revealed that patient preferences did not fit a normal distribution, and linear residuals demonstrated significant heteroskedasticity. Spearman correlation was used for interval independent variables, Mann-Whitney test for dichotomous independent variables, and Wilcoxon signed-rank test for paired samples. Given the exploratory nature of this analysis and the relatively few predictors of subject preferences on bivariable testing, multivariable regression was not done. Calculations were performed using Stata/SE 11.0 (Statacorp, College Station TX). The required sample for detecting medium-sized associations between patient factors and their preferences for organ quality was 84 subjects, assuming β=0.8 and two-tailed α=0.05(13).
Finally, we re-approached 20 patients who remained on the waiting list and had taken the initial survey at least 6 months prior, to determine whether their preferences remained stable over time. During this period we continued to recruit new participants; thus our final sample size was 95 subjects.
Results
Qualitative results
Several themes became apparent after interviewing ten wait-listed patients (4 men, 6 women, median MELD of 14). First, despite having received pre-listing education about the spectrum of organ quality, these waitlisted patients had very poor understanding of the subject. They would attempt to dichotomize organs into “good” versus “bad”, and assumed that any organ they would be offered would be “good.” Example quotes include “They’re not going to offer it if it’s not transplantable” and “Just knowing this hospital and its reputation, I would think they would be looking only at good quality organs.” This view was even held by one patient who had previously been called in for a transplant, which was canceled because of concern about the organ quality. Despite this experience, she indicated that when she is offered another one, “I’m sure it will be good – we should be checking them out first.” Most patients were initially resistant to receiving anything but the “best quality” organ. Example quotes include “Well, I’d like to get a liver from an 18 year-old football player”, “I would think that 5% risk (of graft failure) would be the norm, but I’m not sure I would want more than that”, and “If I’m going to be sliced and diced, I’d rather get something that will last me a long time.” These qualitative findings informed the design of the quantitative survey – specifically the emphasis on spectrum of organ quality and testing of methods for mitigating patients’ initial biases.
Quantitative results
Demographic characteristics are shown in Table 1 for the 95 waiting list patients who participated in this portion of the study. The median age was 55, 65% were male, and the median MELD score was 14. Participants had slightly lower mean lab MELD scores than our overall waitlisted population (13 vs 15, p<0.001) and were less likely to be Hispanic (1% vs 7%, p=0.02), but other demographic characteristics were representative.
Table 1.
Demographic characteristics of the 95 waiting list patients in the quantitative study
| Age – median (range) | 55 (19-70) |
|---|---|
| Gender - % male | 65% |
|
| |
| Race/ethnicity - % | |
| White | 87% |
| Black | 9% |
| Hispanic | 1% |
| Other | 3% |
|
| |
| Lab MELD score – median (range) | 13 (6-23) |
|
| |
| HCC exception - % | 8% |
|
| |
| Primary diagnosis - % | |
| Alcohol | 18% |
| Viral | 29% |
| Cryptogenic/fatty liver | 22% |
| Other | 31% |
Results of the various psychological metrics are shown in Table 2, which provide an overview of the group’s mental and emotional state at the time of the survey. Patients’ numeracy was similar to that in the general population, with a median score of 4.2 on a 1-6 scale(10). Their affect was on the positive end of the spectrum (median of 4 on a −27 to +27 scale). Their quality of life was moderately impaired, with a median score of 65 on a 0-100 analogue scale. Eighty percent of them expected that their quality of life would improve after transplant, by a median of 15 points. They perceived a relatively low chance of dying on the waiting list in the next 3 months (median 5% risk, range 0-80), and believed they had a median 10% chance (range 0-100) of receiving a transplant in the next 3 months. Their trust in physicians was high, with a median score of 5 on this 1-6 scale. In response to knowledge questions about factors that will influence their chance of receiving a transplant, 67% were answered correctly. In particular, 98% answered correctly that their MELD score would influence their chances of being transplanted, while only 67% responded that their chances would be influenced by the quality of organ they are willing to accept. Finally, this group had a relatively “external” locus of control, with a median score of 4 on a 1-6 scale – a higher number reflecting belief that their health was out of their control.
Table 2.
Results of psychological metrics. A higher score reflects better numeracy, more positive affect, better quality of life, more trust, more external locus of control, etc.
| Metric | Median score | Interpretation/comment |
|---|---|---|
| Numeracy | 4.2 | Scale goes from 1-6; scores are similar to other populations |
| PANAS (positive affect – negative affect scale) |
4 | Scale goes from −27 to +27; positive values reflect more positive than negative feelings |
| QOL visual analogue | 65 | Scale goes from 0-100; reflects moderate impairment in QOL |
| Perceived risk of dying on waitlist in next 3 months |
5% (range 0%-80%) | Patients perceived low chance of death on waitlist |
| Perceived chance of receiving transplant in next 3 months |
10% (range 0%-100%) | Patients perceived slightly higher chance of transplant |
| Trust in physicians | 5 | Scale goes from 1-6; reflects high trust in physicians |
| Knowledge | 67% items answered correctly | 97% understood their MELD score influence their chance of transplant; only 67% knew this about their willingness to accept high risk organs |
| Locus of control | 4 | Scale goes from 1-6; scores above 3 reflect perception that health is not in their control |
Risk preferences
After completing the preference elicitation tool, the mean risk of graft failure that patients would accept for a particular organ being offered was 32% at 3 years post-transplant. There was significant between-patient variability in risk preferences, as shown in Figure 2. The majority of patients preferred relatively low risk organs: despite feedback that stringent acceptance criteria could shrink the pool of organs available to them, 58% would only accept organs with 25% or less risk of graft failure at 3 years, while 18% would only accept the lowest risk possible (19% at 3 years). A bias against lower quality organs was demonstrated by their responses to the following scenario: subjects were asked to choose between a) continuing on the waiting list, with a 20% chance of dying, and b) accepting a lower quality liver, with 20% chance of dying but a quality of life improvement. In this discrete choice scenario with a logically “correct” answer (accepting the liver), 42% preferred to stay on the waiting list. Nonetheless, a sizable minority were willing to accept much higher risks, up to nearly 100% in some cases, in order to receive a transplant. Patients reported a strong desire to be involved in decisions about organ acceptance, with 83% wanting an equal or dominant role in the decision.
Figure 2.
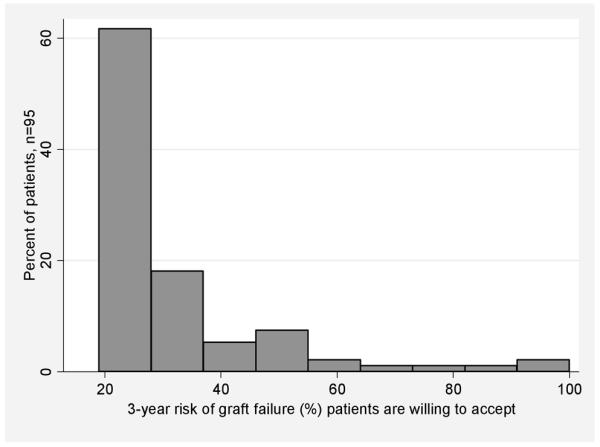
Histogram demonstrating variability between patients in terms of the 3-year risk of graft failure they are willing to accept. Preferences displayed are those given by patients after they had opportunity to reflect upon the implications for organ availability (pie chart in Figure 1).
Among the 20 patients re-approached after a mean of 16 months (range 6-30 months), group risk preferences were not significantly different (mean acceptable graft failure risk of 34% on initial survey, and 33% on re-approach, p=0.3). However, the individual preferences were not particularly stable, with only modest correlation between initial and re-approach values (spearman’s rho=0.24). Changes in preferences were not associated with either change in MELD score (which increased by mean of 1 point in this group) or time from initial survey to re-approach (p=0.1 and 0.9, respectively).
Impact of presentation format
The format of presenting information did have a significant impact on patient preferences. The absolute risk of graft failure patients were willing to accept was modified by the initial graphical presentation – those initially shown a graph with 19% risk of graft failure (“best” organ scenario) would accept up to 26% risk on average, while those initially shown a graph with 25% risk of graft failure (“average” organ scenario) would accept up to 29% risk on average (p=0.001). This experiment demonstrated the psychological bias of “anchoring” to the initial number seen. Patients were also influenced by feedback about organ availability. On the initial preference elicitation, the mean risk of graft failure at 3 years that patients would accept was 28%; this increased to 32% once they were given pie chart feedback (as shown in Figure 1) (p=0.003). Among the 67 patients who initially preferred organs with no more than 25% 3-year risk of graft failure, 13 (19%) indicated they would accept >25% risk after receiving this feedback. Conversely, among the 28 patients who initially would accept organs with more than 25% 3-year risk of graft failure, only 2 (7%) reduced their risk tolerance after receiving the feedback.
Correlates of risk preferences
Among patient demographic and clinical characteristics, only gender was associated with risk preferences, with men preferring lower risk organs than women (3-year graft failure risk tolerance after feedback 29% vs 35%, p=0.04). In particular, neither the patient’s lab MELD score nor MELD exception for hepatocellular carcinoma were significantly associated with their risk preferences (p=0.2 and 0.1, respectively), nor were their perceived chance of dying or getting a transplant. Among the psychological factors hypothesized to correlate with patient risk preferences, only belief in control over one’s health demonstrated a statistically significant association – patients with more “external” locus of control were more likely to accept higher risk organs (p=0.04). When asked directly what factors would influence their decisions about organ acceptance, the most common response was “quality of life” (85%) followed by “trust in transplant team” (79%).
Discussion
This study of patient decision making about organ quality in liver transplantation has three main findings. First, many patients entered discussions about organ quality with an inherent bias against acceptance of organs with higher risks of graft failure. This finding was demonstrated most clearly by their responses to the discrete choice scenario, where 42% would choose to stay on the waiting list rather than accepting a lower quality liver that would improve their quality of life and would not increase their chance of dying. This bias against “lower quality” organs could be partially mitigated by presenting organ quality in reference to “average quality” rather than “best quality”, and by feedback about implications for organ availability. Second, risk tolerance was highly variable between individuals and not particularly stable over time. Third, individual patient’s risk tolerance was associated with gender and beliefs about control over one’s health, but not with the severity of liver disease. While these findings need to be confirmed in future studies, they provide much-needed information about how to counsel patients on issues of organ quality.
How involved should patients be in decisions about organ acceptance? Some would argue that this decision is too complicated for patients to contemplate. On the contrary, we would contend that patient involvement is important for many reasons. First, from an ethical standpoint few would dispute that patients have the right to know the quality of organs they are receiving(3). Physicians routinely counsel patients about risks and benefits of medications and procedures, so organ quality should be no different. Second, from a legal standpoint there may be ramifications for transplant centers who do not adequately inform their patients(3). Third, from an outcomes standpoint, in the setting of renal transplantation patient involvement in decision-making is associated with improved survival(14) and improved medication compliance(15). Finally, the patients want to be involved. In our study, 83% of the patients reported that they would prefer an equal or even dominant role in deciding whether to accept a higher risk organ. Thus, it is incumbent upon the transplant community to respect patient autonomy by involving them in these discussions.
Despite these arguments, a number of logistical challenges remain. First, while general issues of organ quality can and should be discussed in advance(16), the real decision happens when an organ offer is made. This event often occurs in the middle of the night, under less than ideal circumstances for informed consent discussions. Second, while the primary decision should focus on risks and benefits for the individual patients, transplant physicians must also consider the impact on other patients on the waiting list, which can at times pose an ethical dilemma. Third, findings from our study suggest that patient preferences were significantly influenced by presentation format and were relatively unstable over time. Thus, we do not recommend that patients simply be asked to decide on their own (nor do patients want this). Instead, we suggest that patients be guided through the decision and provided with a clear recommendation which they can then accept or reject. Furthermore, patient preferences will need to be re-addressed frequently as their clinical condition changes. We are currently in the process of developing a patient education tool for this purpose, building upon our findings in this study as well as prior work which has demonstrated that complex risk communication is best done 1) using graphs, 2) using absolute rather than relative risks, and 3) providing contextual information to account for innumeracy(17-19). This education tool is intended as something patients can go through at home, so as to not interfere with flow in a busy transplant clinic, but rather to set the stage for when the actual organ offer occurs.
This study was limited by its single-center nature, and the inclusion of relatively few patients with very high MELD scores. The unstable nature of high-MELD patients provided logistical challenges in capturing them for enrollment, as they often missed clinic appointments due to being admitted to the hospital, and either died or received a transplant quickly. Therefore, the findings in this study cannot necessarily be generalized to patients with different socio-demographic characteristics and more severe liver disease. Another limitation was the use of a preference elicitation tool that has not been previously validated. At the time this study began in 2008, no literature existed on this topic in liver transplantation. Recently, another group has conducted a study on patient willingness to accept ECD organs, which is currently in press(20). Although that study dichotomized organs into “standard” versus “ECD”, they found similar results in that a large proportion of patients expressed reluctance to consider ECD organs. Strengths of our study included the mixed methods approach, and the testing of various presentation formats. These design features allowed us to develop an understanding of how best to communicate with patients, rather than simply describing their preferences. Finally, it is important to note that both of these studies focused on organ-specific risk of graft failure, not disease transmission. The literature in kidney transplantation(21, 22) would suggest that patients hold similar biases about disease transmission risk.
In summary, patients express strong desire to be involved in decisions about organ quality, and demonstrate widely varying levels of risk tolerance. We found that risk tolerance was not associated with disease severity, but was significantly influenced by presentation format. These findings suggest that when discussing organ quality with patients, the risks of graft failure should be presented in reference to “average” quality organs, and feedback should be provided about the relationship between organ quality and supply. Future work to develop validated patient education tools will aid transplant physicians in these discussions.
Acknowledgments
This work was supported by the Robert Wood Johnson Foundation, the American Gastroenterological Association and by NIH K23DK085204 (MLV). Dr. Zikmund-Fisher is supported by a career development award from the American Cancer Society (MRSG-06-130-01-CPPB).
Abbreviations
- (ECD)
Extended Criteria Donor
- (MELD)
Model for End-stage Liver Disease
References
- 1.Feng S, Goodrich NP, Bragg-Gresham JL, Dykstra DM, Punch JD, DebRoy MA, et al. Characteristics associated with liver graft failure: the concept of a donor risk index. Am J Transplant. 2006;6(4):783–90. doi: 10.1111/j.1600-6143.2006.01242.x. [DOI] [PubMed] [Google Scholar]
- 2.Klein AS, Messersmith EE, Ratner LE, Kochik R, Baliga PK, Ojo AO. Organ donation and utilization in the United States, 1999-2008. Am J Transplant. 10(4 Pt 2):973–86. doi: 10.1111/j.1600-6143.2009.03008.x. [DOI] [PubMed] [Google Scholar]
- 3.Halpern SD, Shaked A, Hasz RD, Caplan AL. Informing candidates for solid-organ transplantation about donor risk factors. N Engl J Med. 2008;358(26):2832–7. doi: 10.1056/NEJMsb0800674. [DOI] [PubMed] [Google Scholar]
- 4.Mixed methods. SAGE Publications; Thousand Oaks, CA: 2006. [Google Scholar]
- 5.Patton MQ. Qualitative Research and Evaluation Methods. 3 ed. Sage Publications; Thousand Oaks, California: 2002. [Google Scholar]
- 6.Bryce CL, Angus DC, Switala J, Roberts MS, Tsevat J. Health status versus utilities of patients with end-stage liver disease. Qual Life Res. 2004;13(4):773–82. doi: 10.1023/B:QURE.0000021685.83961.88. [DOI] [PubMed] [Google Scholar]
- 7.Wallston KA, Wallston BS, DeVellis R. Development of the Multidimensional Health Locus of Control (MHLC) Scales. Health education monographs. 1978;6(2):160–70. doi: 10.1177/109019817800600107. [DOI] [PubMed] [Google Scholar]
- 8.Hall MA. Researching medical trust in the United States. Journal of health organization and management. 2006;20(5):456–67. doi: 10.1108/14777260610701812. [DOI] [PubMed] [Google Scholar]
- 9.Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol. 1988;54(6):1063–70. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- 10.Zikmund-Fisher BJ, Smith DM, Ubel PA, Fagerlin A. Validation of the Subjective Numeracy Scale: effects of low numeracy on comprehension of risk communications and utility elicitations. Med Decis Making. 2007;27(5):663–71. doi: 10.1177/0272989X07303824. [DOI] [PubMed] [Google Scholar]
- 11.Hawley ST, Zikmund-Fisher B, Ubel P, Jancovic A, Lucas T, Fagerlin A. The impact of the format of graphical presentation on health-related knowledge and treatment choices. Patient Educ Couns. 2008;73(3):448–55. doi: 10.1016/j.pec.2008.07.023. [DOI] [PubMed] [Google Scholar]
- 12.Zikmund-Fisher BJ, Ubel PA, Smith DM, Derry HA, McClure JB, Stark A, et al. Communicating side effect risks in a tamoxifen prophylaxis decision aid: the debiasing influence of pictographs. Patient Educ Couns. 2008;73(2):209–14. doi: 10.1016/j.pec.2008.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. L. Erlbaum Associates; Hillsdale, N.J.: 1988. [Google Scholar]
- 14.Stack AG, Martin DR. Association of patient autonomy with increased transplantation and survival among new dialysis patients in the United States. Am J Kidney Dis. 2005;45(4):730–42. doi: 10.1053/j.ajkd.2004.12.016. [DOI] [PubMed] [Google Scholar]
- 15.Baines LS, Joseph JT, Jindal RM. Compliance and late acute rejection after kidney transplantation: a psycho-medical perspective. Clin Transplant. 2002;16(1):69–73. doi: 10.1034/j.1399-0012.2002.00111.x. [DOI] [PubMed] [Google Scholar]
- 16.Ross LF, Zenios S, Thistlethwaite JR., Jr. Shared decision making in deceased-donor transplantation. Lancet. 2006;368(9532):333–7. doi: 10.1016/S0140-6736(06)69078-8. [DOI] [PubMed] [Google Scholar]
- 17.Fagerlin A, Ubel PA, Smith DM, Zikmund-Fisher BJ. Making numbers matter: present and future research in risk communication. Am J Health Behav. 2007;31(Suppl 1):S47–56. doi: 10.5555/ajhb.2007.31.supp.S47. [DOI] [PubMed] [Google Scholar]
- 18.Zikmund-Fisher BJ, Fagerlin A, Roberts TR, Derry HA, Ubel PA. Alternate methods of framing information about medication side effects: incremental risk versus total risk of occurrence. J Health Commun. 2008;13(2):107–24. doi: 10.1080/10810730701854011. [DOI] [PubMed] [Google Scholar]
- 19.Fagerlin A, Zikmund-Fisher BJ, Ubel PA. “If I’m better than average, then I’m ok?”: Comparative information influences beliefs about risk and benefits. Patient Educ Couns. 2007;69(1-3):140–4. doi: 10.1016/j.pec.2007.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rodrigue JR, Hanto DW, Curry MP. Patients’ Willingness to Accept Expanded Criteria Donor Liver Transplantation. Am J Transplant. 2011 doi: 10.1111/j.1600-6143.2011.03592.x. [DOI] [PubMed] [Google Scholar]
- 21.Duan KI, Englesbe MJ, Volk ML. Centers for Disease Control ‘high-risk’ donors and kidney utilization. Am J Transplant. 2010;10(2):416–20. doi: 10.1111/j.1600-6143.2009.02931.x. [DOI] [PubMed] [Google Scholar]
- 22.Ros RL, Kucirka LM, Govindan P, Sarathy H, Montgomery RA, Segev DL. Patient attitudes toward CDC high infectious risk donor kidney transplantation: inferences from focus groups. Clin Transplant. 2011 doi: 10.1111/j.1399-0012.2011.01469.x. [DOI] [PubMed] [Google Scholar]


