Abstract
Background
Unaided attempts to quit smoking commonly fail during the first two weeks of the nicotine withdrawal syndrome. Alterations in dopamine (DA) signaling correlate with withdrawal from chronic nicotine exposure, but those changes have not been well characterized.
Methods
Mice were administered nicotine in their drinking water for 4 or 12 weeks. Then nicotine was withheld for 1 to 10 days while DA signaling was characterized using in vivo microdialysis or fast-scan cyclic voltammetry.
Results
Upon withdrawal of nicotine, the basal DA concentration in the nucleus accumbens decreased as measured by microdialysis. The length of time that the low basal DA state lasted depended on the length of the chronic nicotine treatment. Microdialysis indicated that acute re-exposure to nicotine during withdrawal temporarily reversed this hypodopaminergic state. Voltammetry measurements supported the microdialysis results by showing that nicotine withdrawal decreased tonic and phasic DA release. The basal DA concentration and tonic DA signals, however, were disproportionately lower than the phasic DA signals. Therefore, the phasic/tonic DA signaling ratio was increased during the withdrawal period.
Conclusions
The relative increase in the sensitivity of DA release to phasic stimulation suggests an increase in the signal-to-noise relationship of DA signaling during the withdrawal period. Therefore, the DA signal produced by acute nicotine re-exposure produces a DA response that may reinforce relapse to drug use (i.e., smoking). Because the basal DA concentration is low during withdrawal, therapies aimed at elevating the background DA signal represent a reasonable treatment strategy for nicotine dependent individuals attempting to quit.
Keywords: Chronic Nicotine, Tonic, Phasic, Dopamine, Withdrawal, Nucleus Accumbens
Introduction
Nicotine is the principle addictive component found in tobacco. The midbrain dopamine (DA) system mediates certain aspects of nicotine reinforcement and withdrawal (1, 2). Altered DA transmission following nicotine withdrawal may contribute to changes in motivation and in sensitivity to drug-associated stimuli, thus promoting relapse (3).
Acute nicotine exposure enhances DA release through a dynamic balance of activation and desensitization of nicotinic acetylcholine receptors (nAChRs) located mainly in the ventral tegmental area (VTA) and in the striatum (4–7). Through this action nicotine induces phasic burst firing from VTA DA neurons (8, 9). In addition, desensitization of striatal nAChRs strongly depresses tonic DA signals and favors DA release induced by phasic burst firing (10–12). This relative boost in phasic DA release in the target areas contributes to the nicotine-induced reinforcement of addictive behaviors (2, 13).
Early withdrawal (≈24 h) from common drugs of abuse leads to deficiencies in basal DA transmission that may initiate drug seeking and taking (14, 15). Withdrawal from chronic nicotine exposure also induces a hypofunctional DA state, which is reflected in decreased brain reward function (16). These studies support the hypothesis that a low DA state may induce drug seeking to reverse the nicotine-induced DA deficiencies. In agreement, the majority of people who attempt unaided to quit smoking relapse within the first 2 weeks (17, 18), suggesting that the early period following nicotine withdrawal is a critical time for relapse and, potentially, for intervention.
In this study, we examined changes in DA signaling 1–10 days after withdrawal from chronic nicotine exposure. Nicotine withdrawal significantly lowered the baseline DA concentration in vivo compared to controls, and acute re-exposure to nicotine temporarily increased the absolute DA to the control levels. The persistence of these alterations depended on the duration of the chronic nicotine treatment. Voltammetric recordings in the nucleus accumbens (NAc) showed that tonic DA signals were inhibited to a greater degree than phasic DA signals after nicotine withdrawal, increasing the relative phasic-to-tonic response during the withdrawal period.
Materials and Methods
Animals
Wild-type C57BL/6J mice aged 2–3 months (Jackson Laboratory, Bar Harbor, ME) had free access to food and water and were housed in accordance with the guidelines specified by the Institutional Animal Care and Use Committee at Baylor College of Medicine and the Public Health Service Policy on Humane Care and Use of Laboratory Animals.
Chronic Nicotine Administration and Withdrawal
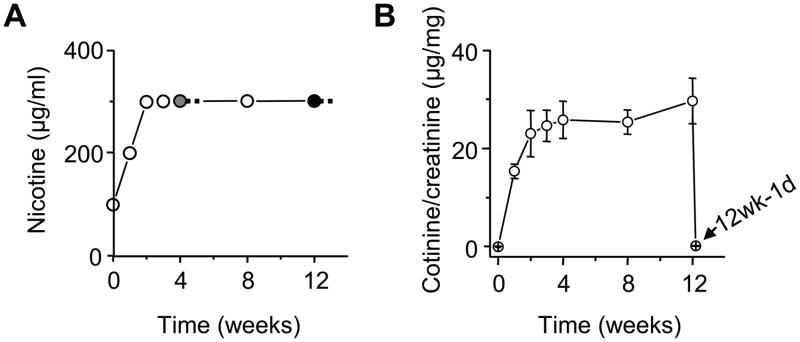
Nicotine hydrogen tartrate at a final concentration of 300 μg/ml was administered chronically to the mice in their home cage water supply with 2% saccharin using previously established methods (19, 20) (Figure 1A). The gray and black filled circles (Figure 1A) indicate the experiments occurred after 4 or 12 total weeks of nicotine treatment. Nicotine was withdrawn for 1, 5, and 10 days before the experiment.
Figure 1.
Nicotine self-administration via the drinking water. (A) The nicotine concentration in the home cage drinking water was increased over 2 wks to a final concentration of 300 μg/ml and maintained at that concentration for 4–12 wks depending on the experiment (gray filled circle = 4 wks of nicotine and withdrawal; black filled circle = 12 wks of nicotine and withdrawal). (B) The ratio of cotinine to creatinine in the urine is an indicator of the nicotine concentration experienced by the mice (n = 6–14) over the 12 wks and after 1 day of withdrawal (arrow, 12w-1d). After 1 day of withdrawal from 12 wks of nicotine treatment, the cotinine concentration rapidly fell back to the control level of 0 μg/mg.
Nicotine levels were estimated by cotinine (21), which has a longer half-life than nicotine (22). The concentrations of urinary cotinine were monitored by an enzyme-linked immunosorbent assay (ELISA, Bio-Quant, San Diego, CA 92121). The urinary cotinine concentration was adjusted for the urinary creatinine concentration to improve the accuracy (23). Thus, the urinary cotinine concentration was expressed as a ratio of the creatinine concentration.
In Vivo Microdialysis and Dopamine Quantification
Microdialysis methodology were much like described previously (24). Guide cannulae were aimed at the medial nucleus accumbens (NAc) (Figure S1 in Supplement 1). The stereotaxic coordinates (relative to bregma) were 1.4 mm AP, 0.55 mm LAT, and 3.3 mm DV. After 3–5 days recovery from surgery, the microdialysis probe (CMA/7) (CMA/Microdialysis, Solna, Sweden) was perfused with artificial cerebral spinal fluid (149 mM NaCl, 2.8 mM KCl, 1.2 mM CaCl2, 1.2 mM MgCl, and 0.25 mM ascorbic acid, and 5.4 mM D-glucose) and implanted at least 14 hrs before the experiment. The perfusion flow rate was 0.5 μl/min overnight and, then, 2.0 μl/min beginning at least 1 hr prior to baseline sampling.
The DA content of the microdialysates was determined by HPLC: pump (Model 582; ESA, Inc., Chelmsford, MA, USA), autosampler (Model 542; ESA, Inc.), and a HR-80 × 3.2 mm column (3-μm particle size; ESA Inc.). A coulometric cell (5014B; ESA, Inc.) was connected to an ESA Coulochem II detector. Quantification of dialysate DA concentration was estimated relative to external standards (0–2 nM). The slope and the y-intercept were calculated for the standard curve, and then the microdialysate concentration was interpolated from the regression by using the slope-intercept equation.
Fast-scan Cyclic Voltammetry in Mouse Striatal Brain Slices
Horizontal slices (350 μm) containing the NAc shell were cut from nicotine-treated or control mice and studied at 34 ± 1°C in (mM) 125 NaCl, 2.5 KCl, 1.3 MgCl2, 2.5 CaCl2, 26 NaH2PO4, 1.25 NaHCO3, and 10.0 glucose saturated with 95% O2 and 5% CO2 (12).
Home-made carbon fiber (10 μm diameter, P55S, Amoco Polymers) microelectrodes were used for fast-scan cyclic voltammetry (12). Scans of the microelectrode potential (10 Hz) were from 0 mV to −400 mV to 1000 mV to −400 mV to 0 mV against a Ag/AgCl reference electrode at a rate of 300 mV/ms. An oxidation peak between 400 and 600 mV and a reduction peak between −200 and −250 mV was used to identify the DA signal. Intra-accumbens stimuli (1 ms duration, 0.6 mA constant current) were delivered using bipolar tungsten electrodes with 120–240 s between stimuli to ensure recovery of DA release unless trains of stimulation were used as indicated. The tip of the carbon-fiber recording electrode was centered about 200 μm away from the tips of the stimulating electrode. The voltammetric DA signal was obtained by digital subtraction and calibrated against standards of 0.5 to 5 μM DA. The DA signals were determined as amplitude (μM) or the area under the curve (μM × s).
Statistical Analysis
Dialysate DA concentrations (in nanomolar or percent of baseline values) were analyzed using analysis of variance (ANOVA) with repeated measures. DA concentrations were log transformed to maintain homogeneity of variance. The average of the three 20-min basal samples defined the baseline DA response, which we used to test between-subject and within-subject effects. DA concentrations measured by voltammetry were assessed by paired t test or one-way ANOVA. ANOVA was performed using the Manova in SPSS for Windows. Significance for all analyses was determined at p < 0.05.
Results
Alterations in Basal and Nicotine-induced DA Signals after Nicotine Withdrawal
Mice self-administered nicotine (plus saccharin) via their drinking water for 4 or 12 wks followed by withdrawal (Figure 1A), whereas the controls only drank saccharin water. The ratio of urinary cotinine concentration to creatinine concentration was used to estimate the nicotine exposure levels over the chronic treatment period (25). The cotinine/creatinine ratio reached peak values (≈24 μg/mg) after 3 wks of nicotine treatment and remained stable thereafter (Figure 1B). This estimated level of nicotine exposure is comparable to what a chronic smoker might experience (26).
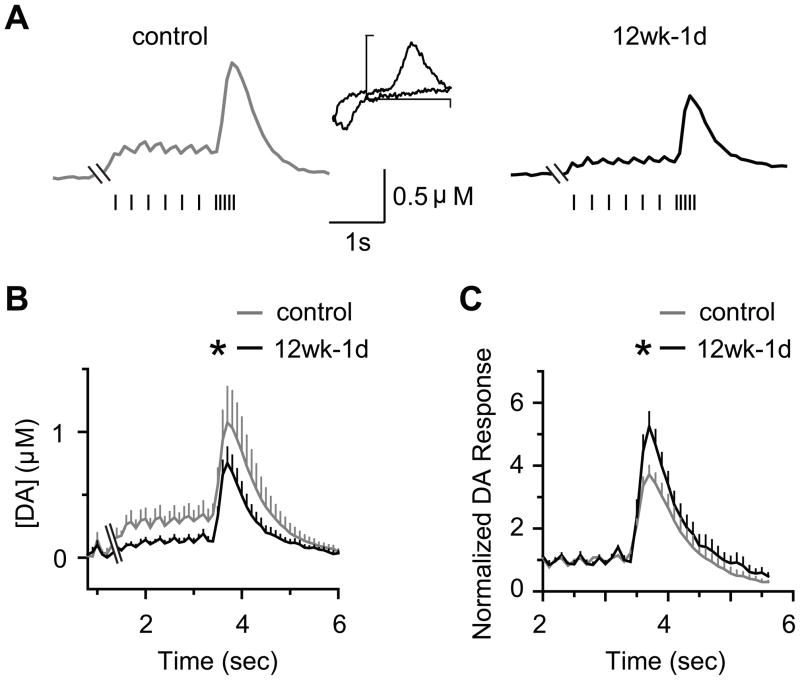
To examine the effect of the chronic nicotine treatment on basal and nicotine-induced DA signals during early withdrawal (1 day), we measured DA concentrations in the medial NAc (mainly the NAc shell) using in vivo microdialysis (Figure S1 in Supplement 1). The timeline for chronic nicotine treatment, nicotine withdrawal, and the microdialysis experiments are shown in Figure 2A. The mean basal DA concentration (Figure 2B,C) was significantly lower (p < 0.05) after 4 weeks (0.22 ± 0.03 nM, n = 7) and 12 weeks (0.24 ± 0.01 nM, n = 7) of nicotine treatment compared to the control (0.31 ± 0.02 nM, n = 9). There was no difference in basal DA levels between the 4-week and 12-week nicotine treatments after 1 day of withdrawal: F(1,12) = 0.73, p > 0.05.
Figure 2.
Effect of nicotine withdrawal on basal DA levels and nicotine-induced DA signals measured by microdialysis in the NAc. (A) The chronological order of nicotine treatment, nicotine withdrawal, and the experiments. (B) Dialysate DA concentrations at baseline and after an i.p. injection of saline and nicotine (1 mg/kg) following 1 day of withdrawal from 4 or 12 weeks (wk) of chronic nicotine treatment (n = 7–9). ANOVA revealed a group effect in both cases: control vs. 4-week, F(1, 14) = 6.35, p < 0.05; control vs. 12-week, F(1, 14) = 8.56, p < 0.05. A control injection of saline (i.p.) did not significantly alter baseline DA levels between the groups: F(2, 20) = 1.10, p > 0.05. (C) Nicotine withdrawal decreased basal DA levels compared to the control. * p < 0.05, one-way ANOVA. (D) The peak DA response to a nicotine challenge (1mg/kg, i.p.) was not different between the groups. (E) Normalization of the dialysate DA signals revealed that the response to nicotine was enhanced in both withdrawal groups compared to the control (n = 7–9), as assessed by a two-way ANOVA. 4-week vs. control, F(5, 65) = 3.39, p < 0.05*; 12-week vs. control, F(5, 70) = 3.21, p < 0.05*. (F) The peak nicotine-induced DA response (% of basal) was higher in both withdrawal groups than in the control. * p < 0.05 by t-test.
Acute administration of nicotine (1 mg/kg, i.p.) after 1-day of withdrawal increased the absolute DA concentrations to similar levels within 20 min of the injection across all treatments (Figure 2B,D). Because the nicotine-treated mice had lower baseline DA concentrations than the control mice, we normalized to compare the relative amplitudes of the nicotine-induced DA concentration changes (Figure 2E). Both the 4-week and 12-week withdrawal groups showed a significantly higher relative nicotine-induced DA response compared to the control mice (Figure 2E,F).
Modulation of Tonic and Phasic DA Signals after Nicotine Withdrawal
Fast-scan cyclic voltammetry measurements have shown that nicotine’s effect on DA release depends on neuronal firing patterns (12). To examine the influence of nicotine withdrawal, we used voltammetry to measure DA release evoked by stimulus trains applied to mouse brain slices containing the NAc shell. To mimic tonic firing (≈3 Hz) and phasic burst firing (≈20 Hz) of DA neurons in vivo, we applied a stimulus train using a combination of tonic (7 pulses delivered at 3 Hz) and phasic (5 pulses delivered at 20 Hz) stimulation. In control brain slices, tonic stimulation produced regular DA responses that elevated the baseline DA concentration, and phasic stimulation evoked a larger DA response that merged into a single transient (control, Figure 3A). In brain slices from mice chronically treated with nicotine (12 wks), there was a general reduction in DA release evoked by tonic and phasic stimulation after withdrawal (1 day) compared to the controls (Figure 3B). However, by normalizing the DA signals to the tonic plateau response, we found that the percent change in phasic DA signaling was enhanced after nicotine withdrawal compared to the controls (Figure 3C). This decrease in (tonic) DA release and the relative enhancement of phasic responses after withdrawal was consistent with the microdialysis data (Figure 2).
Figure 3.
Tonic (3 Hz) and phasic stimulation (5 pulses at 20 Hz) evoked DA release in slices from control and nicotine withdrawn mice measured by voltammetry. (A) Single DA traces evoked by tonic and then phasic stimulation in controls and after 1 day of withdrawal from 12 wks of nicotine treatment (12wk-1d). The first pulse of the tonic train is not shown because the first response from a train is initially large and not consistent with the train. Inset shows a representative voltammogram for DA (scale, 1.0 nA and 1.0 V). (B) Mean evoked DA release was significantly reduced after nicotine withdrawal (12wk-1d) compared to the control: group × time, F(48, 672) = 1.59, p < 0.05*, n = 7–9. (C) Normalization of the phasic DA signals to the tonic DA signals showed an enhanced response in the 12wk-1d group compared to the controls: group × time: F(21, 294) = 3.19; p < 0.05*, n = 7–9. Each point represents the mean ± SEM.
Ratio of Phasic-to-Tonic DA Signals Increases after Nicotine Withdrawal
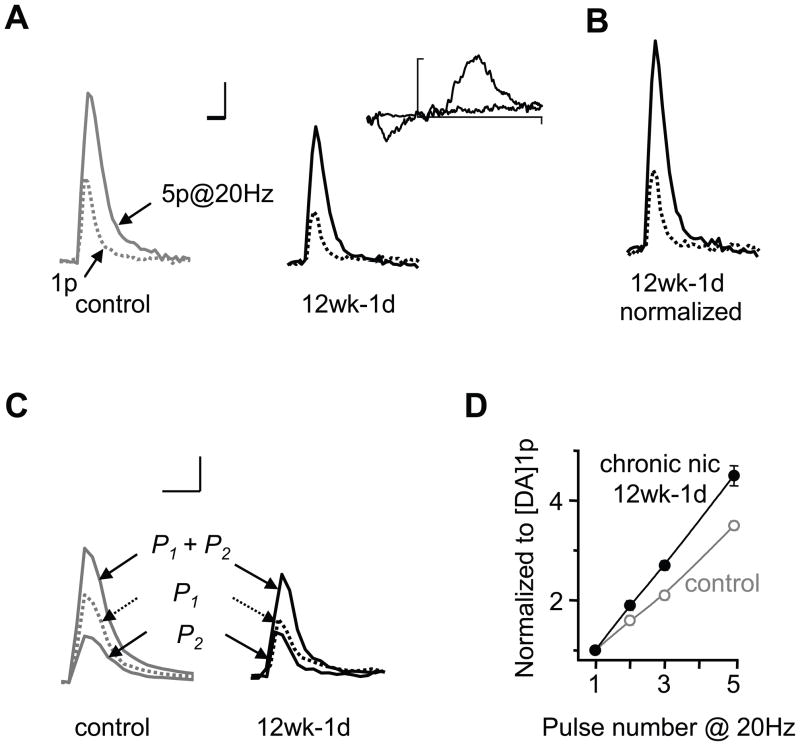
Previous studies demonstrated that acute nicotine regulates the phasic-to-tonic relationship of DA release (10, 11). To determine whether withdrawal from chronic nicotine exposure influences the DA signals evoked by tonic and phasic firing activity, we applied single stimulus pulses (1p) and phasic stimulation (5p at 20 Hz). In control NAc slices (Figure 4A), a single pulse (1p) stimulation evoked a smaller DA signal (0.21 ± 0.02 μM × s, n = 51) compared to phasic stimulation (0.70 ± 0.06 μM × s, n = 51). The ratio between these two measurements (i.e., the ratio of phasic-to-tonic DA release) was 3.4 ± 0.13 in control mice, indicating a potentiation of phasic evoked DA responses. After chronic nicotine treatment, the DA signals evoked by the single-pulse stimulus (0.13 ± 0.01 μM × s, n = 51) and the 5-pulse stimulus train (0.54 ± 0.05, n = 51) were both smaller than the control response (p < 0.01) (Figure 4A). However, normalization of the single-pulse evoked DA signal to the control 1p response revealed a robust frequency dependent facilitation (Figure 4B) as well as an increase in the ratio of phasic-to-tonic DA release (4.2 ± 0.15, p < 0.05). Thus, nicotine withdrawal revealed an overall decrease in DA release, but the relative response to phasic stimulation was greater when compared to the control.
Figure 4.
Withdrawal from chronic nicotine treatment enhanced burst firing dependent facilitation. (A) Single DA traces show that both tonic (1p) and phasic stimuli (5p at 20 Hz) reduced DA release 1 day after withdrawal from 12 wks of nicotine treatment (12wk-1d) compared to control. Inset shows a representative voltammogram for DA (scale, 0.5 nA and 1.0 V). (B) Normalization of the 1p evoked signal (dashed trace) to the control showed that the DA response to phasic stimulation (solid trace) is greater after withdrawal. Scale bars represent 0.5 μM and 0.5 s. (C) Example DA traces show that paired pulses (P1+P2) given at 50 ms intervals (20 Hz) evoked smaller peaks after withdrawal compared to control. However, the peak evoked by the second pulse (P2) was similar between groups, resulting in a higher P2/P1 ratio after withdrawal. (D) Normalization of the DA signal (to the 1p evoked response) revealed an enhanced relationship between the pulse number (burst length) and the corresponding DA signal in the withdrawal group compared to the control (n = 45, 31). The relationship of burst length to DA release is y = 0.8 × + 0.05 (gray data) for control mice and is enhanced to y = 1.13 × −0.3 (black data) after withdrawal from nicotine.
The enhanced phasic-to-tonic DA responses (Figures 4A,B) after nicotine withdrawal indicate an alteration in the release properties. To examine the probability of evoked DA release before and after withdrawal, we applied paired-pulse stimulations (Figure 4C). Paired pulses evoked smaller DA signals (0.18 ± 0.03, n = 17) in the 12-week group after withdrawal (1 day) compared to the control group (0.32 ± 0.03, n = 45, p < 0.05). However, the paired pulse (P2/P1) ratio was higher after nicotine withdrawal (0.89 ± 0.12) compared to the control (0.59 ± 0.05; p < 0.001) (Figure 4C), suggesting lower initial probability of release and greater facilitation of release after withdrawal. In the NAc shell, the DA signal in the control was linearly dependent on the number of pulses given during a 20 Hz stimulus train (Figure 4D, gray data). Nicotine withdrawal increased the slope of this relationship (Figure 4D, black data), indicating an increased sensitivity to phasic burst length. That is, phasic bursts induced relatively more DA release during the withdrawal period, and the longer the burst the greater the enhancement of DA release.
Alterations in DA signals After Extended Periods of Nicotine Withdrawal
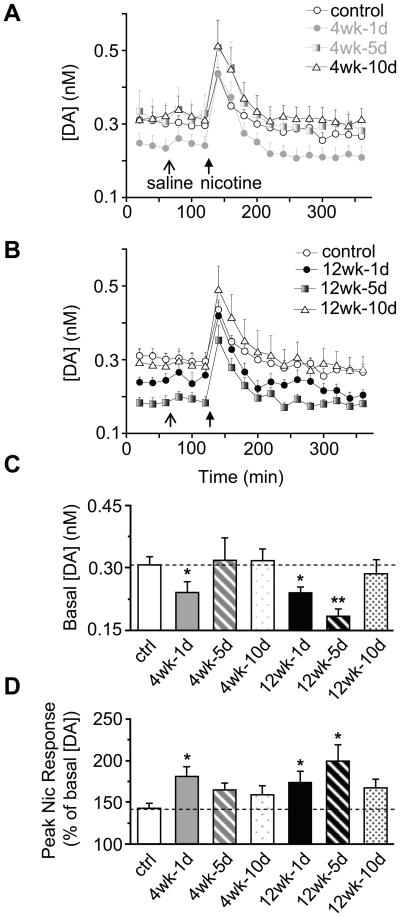
To determine the duration of the nicotine-induced withdrawal adaptations, we measured dialysate DA concentrations 1, 5, and 10 days after withdrawal from chronic nicotine treatment. After 4 wks of nicotine treatment, the baseline DA levels returned to control levels by day 5 of withdrawal (Figure 5A). In contrast, after 12 wks of nicotine treatment, the baseline DA levels did not return to normal until 10 days after withdrawal (Figure 5B). The baseline DA concentrations (Figure 5C) and the peak DA response to 1 mg/kg-nicotine (Figure 5D) are shown for each group at 1, 5, and 10 days after withdrawal. Interestingly, the baseline DA concentration (Figure 5C) was inversely related to the percent increase in DA concentration produced by the nicotine challenge (Figure 5D).
Figure 5.
Alterations in basal DA levels and nicotine-induced DA signals 1–10 days after nicotine withdrawal as measured by microdialysis. Data from Figure 2 are included for comparison. (A) Dialysate DA concentrations at baseline and after an i.p. injection of saline and nicotine (1 mg/kg) following 1–10 days of withdrawal from 4 wks of nicotine treatment. The baseline DA concentration returned to control levels by day 5: F(1, 14) = 0.04, p > 0.05. (B) DA concentrations after 12 wks of nicotine treatment. The baseline DA levels returned to control levels by day 10: F(1, 17) = 0.49, p > 0.05 . (C) Withdrawal from the 12 wks nicotine treatment produced a longer lasting reduction in basal DA concentration (at least 5 days) compared to the 4-wk treatment. DA levels recovered to control levels 10 days after withdrawal in all groups. * p < 0.05. (D) The increased sensitivity to nicotine challenge during withdrawal extended for at least 5 days after cessation of the 12-wk treatment, whereas this effect was only present after 1 day of withdrawal from the 4-wk treatment. * p < 0.05, by t-test. Dashed line indicates the control response. n = 7-9.
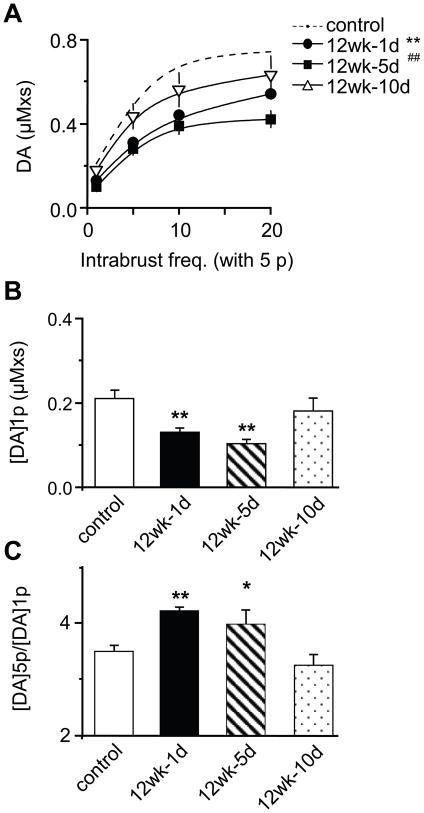
Voltammetric recordings further confirmed that 1 day and 5 days after withdrawal (from 12 wks of nicotine) DA signals were lower across all stimulation frequencies (1p, and 5p at 5–20 Hz) (Figure 6A). By day 10, the DA signals returned near to control levels (Figure 6B). During those periods when tonic (1p) DA signals were inhibited, there was a higher ratio of phasic-to-tonic DA release compared to the control (Figure 6B, C). After DA release to a single stimulus (1p) returned to control levels, then the phasic/tonic ratio also returned to normal (Figure 6B, C). This result suggests that the background DA signal indicates the sensitivity of DA release to phasic burst firing.
Figure 6.
Recovery of tonic and phasic DA release after nicotine withdrawal as measured by voltammetry. (A) The DA signal (area under the curve) evoked by a single pulse or by train stimulation (5p at 5–20 Hz) was significantly lower 1 day (n = 54) and 5 days (n = 17) after withdrawal compared to the control (n = 51). The DA signals recovered to the control levels by day 10 of withdrawal (n = 18). **, ## p < 0.01, one-way ANOVA test. (B) The tonic evoked DA concentration is inversely related to (C) the phasic-to-tonic DA ratio ([DA]5p/[DA]1p). The phasic-to-tonic ratio returned to normal after the single-pulse (1p) evoked DA signal recovered to control levels.* p < 0.05, ** p < 0.01.
High-affinity nAChRs Influence DA Release after Nicotine Withdrawal
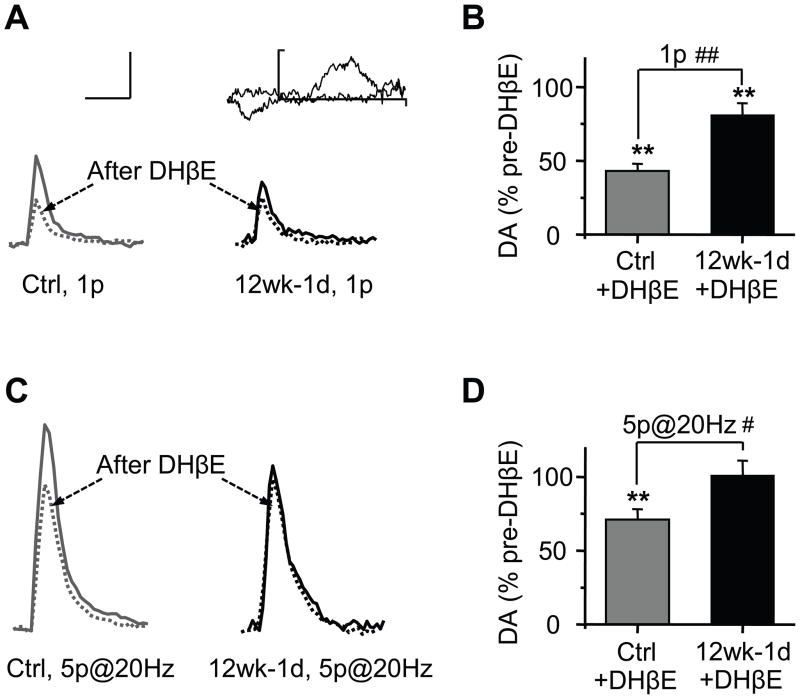
The high-affinity nAChRs (containing the β2 subunit) are the predominant nicotinic receptor subtype in both rodents and humans, and they influence DA neuron firing and DA release in the striatum (27–29). We examined the influence of the high-affinity nAChRs over tonic and phasic DA release during nicotine withdrawal. Inhibition of high-affinity nAChRs with DHβE (100 nM) in control slices decreased the single-pulse evoked DA signal by 57 ± 5 %, n = 16 (Figure 7A, B). By contrast, in slices from nicotine withdrawn mice, DHβE only reduced the single-pulse evoked signal by 19 ± 8 %, n = 17 (Figure 7A, B), which was significantly less than the control response (p < 0.01). Blockade of the high-affinity (mainly β2*) nAChRs only reduced the phasic evoked DA signal by 29 ± 7 % (Figure 7C, D) in the controls, but had almost no effect on phasic DA signals (1 ± 10 %) after nicotine withdrawal. These findings indicate that the influence of high-affinity nAChRs over NAc DA release decreased after chronic nicotine.
Figure 7.
Inhibition of tonic and phasic DA release by the high-affinity nAChRs after nicotine withdrawal. (A) Example DA traces evoked by a single pulse (1p) before (solid) and after (dashed) bath DHβE (0.1 μM) in the control (gray lines) and after (1 day) withdrawal from 12 wks nicotine treatment (12wk-1d, black lines). Inset shows a representative voltammogram for DA (scale, 0.25 nA and 1.0 V). (B) The inhibition of high-affinity nAChRs by DHβE significantly reduced DA release in both groups, however, the extent of inhibition was greater in the control group (n = 16–17). (C) Single DA traces evoked by phasic trains of 5 pulses (at 20 Hz) before and after DHβE (0.1 μM) in the control and after 12wk-1d. (D) The inhibition of β2* nAChRs by DHβE reduced train evoked DA release to 71%, but had no effect on the 12w-1d group (n = 16–17). Scale bars for panels A and C represent 0.2 μM and 1 s. ** p < 0.01; # p < 0.05; ## p < 0.01.
Discussion
In the present study, nicotine was self-administered via home cage drinking water. Based on the stable cotinine/creatinine measurements, nicotine was maintained at a consistent level that was comparable to that obtained by chronic daily smoking (60–300 nM) (26). Withdrawal from chronic nicotine treatment decreased the basal DA concentration in the NAc, and decreased tonic and phasic DA release in the NAc. The DA concentration decrease measured by microdialysis lasted longer after 12 weeks of nicotine treatment than after 4 weeks of treatment. Re-exposure to acute nicotine during the withdrawal period normalized the DA concentration to control levels for a short time. Voltammetric measurements in the NAc shell supported this finding by showing that tonic DA release was more strongly inhibited than phasic release during the withdrawal period.
After chronic nicotine, the stronger inhibition of tonic DA release enhanced the contrast between tonic and phasic DA signaling. It also switched the pattern of DA release so that it was more highly dependent on the number of spikes within a burst. That change increased the dependence of NAc DA release on bursts. Therefore, during the withdrawal period, phasic DA neuron activity of the kind induced by nicotine (9, 30) re-exposure induces DA signals that may make smokers in the withdrawal period more vulnerable to the reinforcing influence of nicotine (2).
The alterations in DA function after nicotine withdrawal was partly due to a decrease in the influence of the high-affinity (mainly β2*) nAChRs over DA release in the NAc. Normally, β2 in combination with α6 and α4 (30) on DA fibers and terminals differentially regulates tonic and phasic DA release in the striatum (10–12). After chronic nicotine treatment, nAChR regulation of DA release in the target area (i.e., NAc) decreased. Based on the literature, the following hypothesis may be reasonable. After chronic nicotine there is upregulation of mainly high affinity nAChRs of the α4β2* subtype (31, 32), which control burst firing in the VTA (30). In addition, release is decreased in the target area, in part, because other β2* nAChRs on DA terminals do not regulate release as usual (12). Based on the work of Exley and colleagues (28, 30) the β2* nAChR subtype in the NAc likely contains α6 possibly with or without α4 and other subtypes (33).
Decreased Influence of nAChRs After Nicotine Withdrawal
Withdrawal from chronic nicotine exposure (12 wks) decreased basal DA levels for at least 5 days as measured by in vivo microdialysis (34). A similar decrease in the basal firing rate of DA neurons after chronic nicotine exposure has been reported in brain slices and in vivo (32, 35). Consistent with these findings, Nashmi and colleagues showed that repeated nicotine exposure causes specific upregulation of high affinity nAChRs that are located on inhibitory GABA inputs at midbrain DA neurons, but not on DA neurons themselves (32). Increased GABA inhibition would result in an overall decrease in the basal spontaneous firing of DA neurons: i.e., tonic release would particularly decrease. Coupled with this hypothesized decrease in DA neuron firing, our voltammetric recordings showed a disproportionate inhibition of tonic release compared to release evoked by phasic bursts. The present study and others indicate that blockade of β2* nAChRs caused less inhibition of phasic DA signals than tonic signals (12). Together these findings suggest that β2* nAChRs have less influence over phasic DA release during the withdrawal period.
The α4 and/or α6 containing β2* nAChRs have emerged as critical subunits in the maintenance of nicotine self-administration and in the regulation of mesostriatal DA signaling (30, 33). Upregulation of nAChRs during chronic nicotine exposure can vary with the composition of these α subunits (36). Subtle differences in the affinity of DHβE for α containing nAChRs, suggests that DHβE may not block all high affinity nAChRs with equal efficacy (37). Previous work indicates that the concentration of DHβE used in the present study (100 nM) produces a near maximal inhibition of DA release as measured by voltammetry (29). Nevertheless, it remains possible that upregulation of less common β2* nAChRs or perhaps modifications in subunit composition, as a result of chronic nicotine exposure, could contribute to alterations in DA release during withdrawal.
Alternatively, the reduction in basal DA after chronic nicotine exposure could also arise from an increase in DA uptake from the extracellular space through changes in DA transporter (DAT) function (34, 38). Tonic background DA levels may be regulated more readily by DAT activity compared to phasic-evoked responses. However, we estimated reuptake changes by quantifying the rate of the falling phase for the evoked DA signals. We found that this measure (t90%) was not significantly different before (control, 0.63 ± 0.44 s) and after chronic nicotine treatment (0.71 ± 0.36 s), which is consistent with the work of others (33).
Alterations in Tonic and Phasic Dopamine Signals After Nicotine Withdrawal
In chronic nicotine treated mice, both tonic and phasic DA release were significantly lower than the control mice. This general decrease in DA function may contribute to motivational deficits such as anhedonia (39). However, an acute nicotine challenge during the withdrawal period produced a DA response that was relatively large, returning DA levels to near control values for a short time. Due to lower background DA levels during withdrawal, the DA target areas, such as the NAc shell, experience a greater relative change in DA concentration. Thus, the signal-to-noise relationship of the DA signal is higher after nicotine withdrawal than in the controls. Therefore, despite these deficits in basal DA function, the salience of the drug experience upon re-exposure may be greater.
Adaptations in DA signaling caused by chronic nicotine exposure alter the responses to nicotine (the drug) and possibly to nicotine-related stimuli. Previous studies demonstrate an inverse relationship between single action potential evoked release and the degree of frequency dependent facilitation (40). The desensitization of nAChRs reduces the probability of DA release to a single stimulation (29) and allows for the amplification of the DA signal across a wide range of burst frequencies (12, 40). In the present study, we found that long-term nicotine exposure especially decreased the tonic DA signals. However, there was a steeper increase in DA release associated with the burst length. That is, the positive correlation between the evoked DA concentration and the number of pulses within a burst was amplified during the withdrawal period.
Nicotine increases the average firing rate of DA neurons in vivo, but the major effect is an increase in burst firing (8, 12). Acute nicotine administration increases both the number and length of DA neuron phasic bursts. During the nicotine withdrawal period, the acute nicotine-induced bursts will produce a significant DA signal because the release process is more responsive to facilitation produced by burst firing. This result may explain why the low basal DA signal could rapidly increase in response to nicotine re-exposure. Thus, the reinforcing effects of nicotine during abstinence are particularly strong because nicotine burst firing causes an even stronger relative response than usual. This hypothesis is supported by recent studies showing that self-administration of nicotine, after chronic exposure, enhances burst firing activity in DA neurons in vivo (41).
These results contrast with a previous study showing that, after chronic treatment, an acute nicotine injection did not appear to increase DA activity (Rahman et al., 2004). This study was performed in Long-Evans rats, whereas our study employed C57 mice. Species related quantitative differences in DA function between rats and mice may account for the differences in the results (42, 43).
Influence of Other Neurotransmitter Systems
In addition to changes in DA function, nicotine exposure modulates a variety of other neurotransmitter and neuropeptide systems that influence DA transmission directly, but could also act in concert through independent mechanisms (44). For example, increased glutamate transmission onto VTA DA neurons is an important component of nicotine’s action that mediates enhanced firing activity and synaptic plasticity in DA neurons (4, 6, 7, 45, 46). Alterations in GABAergic and glutamatergic drive during nicotine withdrawal likely contribute to changes in tonic versus phasic DA neuron firing. In addition, nicotine’s widespread action within the central nervous system also recruits cholinergic, serotonin, and opioid systems (47–49). Thus, the behavioral reinforcement and withdrawal effects induced by nicotine arise from complex interactions between neurotransmitter systems, with DA having a key role.
These findings have implications for understanding how exposure to drug-associated cues or drug relapse may act upon and exploit existing pathological changes in DA function arising from long-term nicotine use. Future therapies aimed at restoring low tonic DA levels may represent an important therapeutic target for the treatment of nicotine addiction.
Supplementary Material
Acknowledgments
The authors are supported by grants from the National Institutes of Health, NIDA DA09411 and NINDS NS21229, and by the Cancer Prevention and Research Institute of Texas, and by the Diana Helis Henry Medical Research Foundation through its direct engagement in the continuous active conduct of medical research in conjunction with Baylor College of Medicine and the project, Genomic, Neural, Preclinical Analysis for Smoking Cessation, and the Cancer Program.
Footnotes
Financial Disclosures
The authors reported no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Corrigall WA, Coen KM, Adamson KL. Self-administered nicotine activates the mesolimbic dopamine system through the ventral tegmental area. Brain Res. 1994;653:278–284. doi: 10.1016/0006-8993(94)90401-4. [DOI] [PubMed] [Google Scholar]
- 2.De Biasi M, Dani JA. Reward, addiction, withdrawal to nicotine. Annu Rev Neurosci. 2011;34:105–130. doi: 10.1146/annurev-neuro-061010-113734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mansvelder HD, McGehee DS. Long-term potentiation of excitatory inputs to brain reward areas by nicotine. Neuron. 2000;27:349–357. doi: 10.1016/s0896-6273(00)00042-8. [DOI] [PubMed] [Google Scholar]
- 5.Picciotto MR, Addy NA, Mineur YS, Brunzell DH. It is not "either/or": activation and desensitization of nicotinic acetylcholine receptors both contribute to behaviors related to nicotine addiction and mood. Prog Neurobiol. 2008;84:329–342. doi: 10.1016/j.pneurobio.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mansvelder HD, McGehee DS. Cellular and synaptic mechanisms of nicotine addiction. J Neurobiol. 2002;53:606–617. doi: 10.1002/neu.10148. [DOI] [PubMed] [Google Scholar]
- 7.Pidoplichko VI, Noguchi J, Areola OO, Liang Y, Peterson J, Zhang T, et al. Nicotinic cholinergic synaptic mechanisms in the ventral tegmental area contribute to nicotine addiction. Learn Mem. 2004;11:60–69. doi: 10.1101/lm.70004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mameli-Engvall M, Evrard A, Pons S, Maskos U, Svensson TH, Changeux JP, et al. Hierarchical control of dopamine neuron-firing patterns by nicotinic receptors. Neuron. 2006;50:911–921. doi: 10.1016/j.neuron.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 9.Zhang T, Zhang L, Liang Y, Siapas AG, Zhou FM, Dani JA. Dopamine signaling differences in the nucleus accumbens and dorsal striatum exploited by nicotine. J Neurosci. 2009;29:4035–4043. doi: 10.1523/JNEUROSCI.0261-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang H, Sulzer D. Frequency-dependent modulation of dopamine release by nicotine. Nat Neurosci. 2004;7:581–582. doi: 10.1038/nn1243. [DOI] [PubMed] [Google Scholar]
- 11.Rice ME, Cragg SJ. Nicotine amplifies reward-related dopamine signals in striatum. Nat Neurosci. 2004;7:583–584. doi: 10.1038/nn1244. [DOI] [PubMed] [Google Scholar]
- 12.Zhang L, Doyon WM, Clark JJ, Phillips PE, Dani JA. Controls of tonic and phasic dopamine transmission in the dorsal and ventral striatum. Mol Pharmacol. 2009;76:396–404. doi: 10.1124/mol.109.056317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sulzer D. How addictive drugs disrupt presynaptic dopamine neurotransmission. Neuron. 2011;69:628–649. doi: 10.1016/j.neuron.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weiss F, Parsons LH, Schulteis G, Hyytia P, Lorang MT, Bloom FE, et al. Ethanol self-administration restores withdrawal-associated deficiencies in accumbal dopamine and 5-hydroxytryptamine release in dependent rats. J Neurosci. 1996;16:3474–3485. doi: 10.1523/JNEUROSCI.16-10-03474.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shen RY. Ethanol withdrawal reduces the number of spontaneously active ventral tegmental area dopamine neurons in conscious animals. J Pharmacol Exp Ther. 2003;307:566–572. doi: 10.1124/jpet.103.053371. [DOI] [PubMed] [Google Scholar]
- 16.Epping-Jordan MP, Watkins SS, Koob GF, Markou A. Dramatic decreases in brain reward function during nicotine withdrawal. Nature. 1998;393:76–79. doi: 10.1038/30001. [DOI] [PubMed] [Google Scholar]
- 17.Ward KD, Klesges RC, Zbikowski SM, Bliss RE, Garvey AJ. Gender differences in the outcome of an unaided smoking cessation attempt. Addict Behav. 1997;22:521–533. doi: 10.1016/s0306-4603(96)00063-9. [DOI] [PubMed] [Google Scholar]
- 18.Shiffman S. Reflections on smoking relapse research. Drug Alcohol Rev. 2006;25:15–20. doi: 10.1080/09595230500459479. [DOI] [PubMed] [Google Scholar]
- 19.Brunzell DH, Russell DS, Picciotto MR. In vivo nicotine treatment regulates mesocorticolimbic CREB and ERK signaling in C57Bl/6J mice. J Neurochem. 2003;84:1431–1441. doi: 10.1046/j.1471-4159.2003.01640.x. [DOI] [PubMed] [Google Scholar]
- 20.Grabus SD, Martin BR, Batman AM, Tyndale RF, Sellers E, Damaj MI. Nicotine physical dependence and tolerance in the mouse following chronic oral administration. Psychopharmacology (Berl) 2005;178:183–192. doi: 10.1007/s00213-004-2007-3. [DOI] [PubMed] [Google Scholar]
- 21.King SL, Caldarone BJ, Picciotto MR. Beta2-subunit-containing nicotinic acetylcholine receptors are critical for dopamine-dependent locomotor activation following repeated nicotine administration. Neuropharmacology. 2004;47(Suppl 1):132–139. doi: 10.1016/j.neuropharm.2004.06.024. [DOI] [PubMed] [Google Scholar]
- 22.Dhar P. Measuring tobacco smoke exposure: quantifying nicotine/cotinine concentration in biological samples by colorimetry, chromatography and immunoassay methods. J Pharm Biomed Anal. 2004;35:155–168. doi: 10.1016/j.jpba.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 23.England LJ, Kendrick JS, Gargiullo PM, Zahniser SC, Hannon WH. Measures of maternal tobacco exposure and infant birth weight at term. Am J Epidemiol. 2001;153:954–960. doi: 10.1093/aje/153.10.954. [DOI] [PubMed] [Google Scholar]
- 24.Dong Y, Zhang T, Li W, Doyon WM, Dani JA. Route of nicotine administration influences in vivo dopamine neuron activity: habituation, needle injection, and cannula infusion. J Mol Neurosci. 2010;40:164–171. doi: 10.1007/s12031-009-9231-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thompson SG, Barlow RD, Wald NJ, Van Vunakis H. How should urinary cotinine concentrations be adjusted for urinary creatinine concentration? Clin Chim Acta. 1990;187:289–295. doi: 10.1016/0009-8981(90)90114-8. [DOI] [PubMed] [Google Scholar]
- 26.Pietila K, Ahtee L. Chronic nicotine administration in the drinking water affects the striatal dopamine in mice. Pharmacol Biochem Behav. 2000;66:95–103. doi: 10.1016/s0091-3057(00)00235-5. [DOI] [PubMed] [Google Scholar]
- 27.Grady SR, Salminen O, Laverty DC, Whiteaker P, McIntosh JM, Collins AC, et al. The subtypes of nicotinic acetylcholine receptors on dopaminergic terminals of mouse striatum. Biochem Pharmacol. 2007;74:1235–1246. doi: 10.1016/j.bcp.2007.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Exley R, Clements MA, Hartung H, McIntosh JM, Cragg SJ. Alpha6-containing nicotinic acetylcholine receptors dominate the nicotine control of dopamine neurotransmission in nucleus accumbens. Neuropsychopharmacology. 2008;33:2158–2166. doi: 10.1038/sj.npp.1301617. [DOI] [PubMed] [Google Scholar]
- 29.Zhou FM, Liang Y, Dani JA. Endogenous nicotinic cholinergic activity regulates dopamine release in the striatum. Nat Neurosci. 2001;4:1224–1229. doi: 10.1038/nn769. [DOI] [PubMed] [Google Scholar]
- 30.Exley R, Maubourguet N, David V, Eddine R, Evrard A, Pons S, et al. Distinct contributions of nicotinic acetylcholine receptor subunit {alpha}4 and subunit {alpha}6 to the reinforcing effects of nicotine. Proc Natl Acad Sci U S A. 2011;108:7577–7582. doi: 10.1073/pnas.1103000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vallejo YF, Buisson B, Bertrand D, Green WN. Chronic nicotine exposure upregulates nicotinic receptors by a novel mechanism. J Neurosci. 2005;25:5563–5572. doi: 10.1523/JNEUROSCI.5240-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nashmi R, Xiao C, Deshpande P, McKinney S, Grady SR, Whiteaker P, et al. Chronic nicotine cell specifically upregulates functional alpha 4* nicotinic receptors: basis for both tolerance in midbrain and enhanced long-term potentiation in perforant path. J Neurosci. 2007;27:8202–8218. doi: 10.1523/JNEUROSCI.2199-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Perez XA, O'Leary KT, Parameswaran N, McIntosh JM, Quik M. Prominent role of alpha3/alpha6beta2* nAChRs in regulating evoked dopamine release in primate putamen: effect of long-term nicotine treatment. Mol Pharmacol. 2009;75:938–946. doi: 10.1124/mol.108.053801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rahman S, Zhang J, Engleman EA, Corrigall WA. Neuroadaptive changes in the mesoaccumbens dopamine system after chronic nicotine self-administration: a microdialysis study. Neuroscience. 2004;129:415–424. doi: 10.1016/j.neuroscience.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 35.Tan H, Bishop SF, Lauzon NM, Sun N, Laviolette SR. Chronic nicotine exposure switches the functional role of mesolimbic dopamine transmission in the processing of nicotine's rewarding and aversive effects. Neuropharmacology. 2009;56:741–751. doi: 10.1016/j.neuropharm.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 36.Walsh H, Govind AP, Mastro R, Hoda JC, Bertrand D, Vallejo Y, et al. Up-regulation of nicotinic receptors by nicotine varies with receptor subtype. J Biol Chem. 2008;283:6022–6032. doi: 10.1074/jbc.M703432200. [DOI] [PubMed] [Google Scholar]
- 37.Khiroug SS, Khiroug L, Yakel JL. Rat nicotinic acetylcholine receptor alpha2beta2 channels: comparison of functional properties with alpha4beta2 channels in Xenopus oocytes. Neuroscience. 2004;124:817–822. doi: 10.1016/j.neuroscience.2004.01.017. [DOI] [PubMed] [Google Scholar]
- 38.Middleton LS, Cass WA, Dwoskin LP. Nicotinic receptor modulation of dopamine transporter function in rat striatum and medial prefrontal cortex. J Pharmacol Exp Ther. 2004;308:367–377. doi: 10.1124/jpet.103.055335. [DOI] [PubMed] [Google Scholar]
- 39.Volkow ND, Fowler JS, Wang GJ, Goldstein RZ. Role of dopamine, the frontal cortex and memory circuits in drug addiction: insight from imaging studies. Neurobiol Learn Mem. 2002;78:610–624. doi: 10.1006/nlme.2002.4099. [DOI] [PubMed] [Google Scholar]
- 40.Exley R, Cragg SJ. Presynaptic nicotinic receptors: a dynamic and diverse cholinergic filter of striatal dopamine neurotransmission. Br J Pharmacol. 2008;153(Suppl 1):S283–297. doi: 10.1038/sj.bjp.0707510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Caille S, Guillem K, Cador M, Manzoni O, Georges F. Voluntary nicotine consumption triggers in vivo potentiation of cortical excitatory drives to midbrain dopaminergic neurons. J Neurosci. 2009;29:10410–10415. doi: 10.1523/JNEUROSCI.2950-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ford CP, Mark GP, Williams JT. Properties and opioid inhibition of mesolimbic dopamine neurons vary according to target location. J Neurosci. 2006;26:2788–2797. doi: 10.1523/JNEUROSCI.4331-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lammel S, Hetzel A, Hackel O, Jones I, Liss B, Roeper J. Unique properties of mesoprefrontal neurons within a dual mesocorticolimbic dopamine system. Neuron. 2008;57:760–773. doi: 10.1016/j.neuron.2008.01.022. [DOI] [PubMed] [Google Scholar]
- 44.Ikemoto S, Qin M, Liu ZH. Primary reinforcing effects of nicotine are triggered from multiple regions both inside and outside the ventral tegmental area. J Neurosci. 2006;26:723–730. doi: 10.1523/JNEUROSCI.4542-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jin Y, Yang K, Wang H, Wu J. Exposure of nicotine to ventral tegmental area slices induces glutamatergic synaptic plasticity on dopamine neurons. Synapse. 2011;65:332–338. doi: 10.1002/syn.20850. [DOI] [PubMed] [Google Scholar]
- 46.Jones IW, Wonnacott S. Precise localization of alpha7 nicotinic acetylcholine receptors on glutamatergic axon terminals in the rat ventral tegmental area. J Neurosci. 2004;24:11244–11252. doi: 10.1523/JNEUROSCI.3009-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Drews E, Zimmer A. Modulation of alcohol and nicotine responses through the endogenous opioid system. Prog Neurobiol. 2010;90:1–15. doi: 10.1016/j.pneurobio.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 48.Maskos U. The cholinergic mesopontine tegmentum is a relatively neglected nicotinic master modulator of the dopaminergic system: relevance to drugs of abuse and pathology. Br J Pharmacol. 2008;153(Suppl 1):S438–445. doi: 10.1038/bjp.2008.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Seth P, Cheeta S, Tucci S, File SE. Nicotinic--serotonergic interactions in brain and behaviour. Pharmacol Biochem Behav. 2002;71:795–805. doi: 10.1016/s0091-3057(01)00715-8. [DOI] [PubMed] [Google Scholar]
- 50.Paxinos G, Franklin KBJ. The Mouse Brain in Stereotaxic Coordinates. San Diego, CA: Academic Press; 2001. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.