Abstract
The aim of this present work is to describe the nature and extent of fibrosis within muscle and to correlate this with the mouth opening (MO) in OSE patients and to prove our results in improvement of mouth opening in patients with OSMF with use of “muscle relaxants” along with other modalities of treatment . The study was conducted on 40 patients who visited our outpatient department with grade 3 (<19 mm) mouth opening. 20 of these patients underwent the routine treatment protocol of weekly injection of hyaluronidase with hydrocortisone and antioxidant capsules with added lycopene for 1 month. The remaining test subjects in addition to the routine injections and antioxidants were given skeletal muscle relaxants like thiocolchicoside or chlorzoxazone. The mouth opening (interincisal distance of maxillary and mandibular incisors at maximum possible mouth opening) was measured and graded as follows: grade 1 (>40 mm), grade 2 (20–39 mm) and grade 3 (<19 mm) with the help of vernier callipers after the study period of 1 month. 17 out of the 20 test patients who received muscle relaxants in addition to the routine protocol showed marked improvement with shift from grade 3 (<19 mm) to grade 1 (>40 mm) i.e. a greater than 20 mm improvement in mouth opening. Using muscle relaxants as a adjuvant therapy in the routine protocol of treatment of oral submucosal fibrosis will not only cater and halt the problem of fibrosis but also will take care of the muscle spasm and inflammation which also inadvertently contribute to the restricted mouth opening. We found excellent improvement on adding muscle relaxants to the routine protocol which was not just an objective but also a subjective success.
Keywords: Oral submucosal fibrosis (OSM), Mouth opening (MO)
Introduction
Oral submucous fibrosis (OSF) is a chronic progressive fibrosing disorder of the oral cavity. In OSF, the oral mucosa appears pale and blanched. Fibrous bands appear earlier in the course of disease, often symmetrically, in a vertical direction usually in the retromolar region and adjacent buccal mucosa. The clinical appearance varies from a localized pale white area to wide blanched region depending on the fibrosis. The site and extent of the fibrosis and its role in causation of trismus are determined by several factors including the anatomical and physiological integrity of the underlying musculature [1]. Hence the role of fibrosis in muscle warrants investigation. The muscle could be damaged primarily and repaired by fibrosis or progression of fibrosis inducing secondary muscle changes. The present work has been undertaken to describe the nature and extent of fibrosis within muscle and to correlate this with the mouth opening (MO) in OSE patients and to prove our results in improvement of mouth opening in patients with OSMF with use of “muscle relaxants” along with other modalities of treatment.
Materials and Methods
The study was conducted on 40 patients who visited our outpatient department with grade 3 (<19 mm) mouth opening. 20 of these patients underwent the routine treatment protocol of weekly injection of hyaluronidase with hydrocortisone and antioxidant capsules with added lycopene for 1 month.
The remaining test subjects in addition to the routine injections and antioxidants were given skeletal muscle relaxants like thiocolchicoside or chlorzoxazone.
The mouth opening (interincisal distance of maxillary and mandibular incisors at maximum possible mouth opening) was measured and graded as follows: grade 1 (>40 mm), grade 2 (20–39 mm) and grade 3 (<19 mm) with the help of vernier callipers after the study period of 1 month.
Results
16 out of the 20 patients who received routine treatment showed very mild improvement with a range of 5–7 mm mouth opening then previous status. Remaining four patients showed no significant improvement.
17 out of the 20 test patients who received muscle relaxants in addition to the routine protocol showed marked improvement with shift from grade 3 (<19 mm) to grade 1 (>40 mm) i.e. a greater than 20 mm improvement in mouth opening. The remaining three had mild improvement with a range of 5–7 mm.
Control subjects
| Patient serial no. (control) | Pretreatment MO (mm) | Posttreatment MO (mm) |
|---|---|---|
| 1 | 18 | 24 |
| 2 | 15 | 21 |
| 3 | 14 | 13 |
| 4 | 16 | 23 |
| 5 | 18 | 24 |
| 6 | 17 | 24 |
| 7 | 16 | 21 |
| 8 | 14 | 20 |
| 9 | 15 | 20 |
| 10 | 18 | 17 |
| 11 | 17 | 23 |
| 12 | 18 | 24 |
| 13 | 16 | 22 |
| 14 | 15 | 15 |
| 15 | 14 | 21 |
| 16 | 16 | 23 |
| 17 | 16 | 16 |
| 18 | 18 | 23 |
| 19 | 14 | 21 |
| 20 | 17 | 23 |
Test subjects
| Patient serial no. (control) | Pretreatment MO (mm) | Posttreatment MO (mm) |
|---|---|---|
| 1 | 17 | 37 |
| 2 | 15 | 36 |
| 3 | 16 | 38 |
| 4 | 14 | 35 |
| 5 | 15 | 20 |
| 6 | 16 | 40 |
| 7 | 17 | 37 |
| 8 | 18 | 25 |
| 9 | 15 | 34 |
| 10 | 14 | 36 |
| 11 | 16 | 42 |
| 12 | 15 | 32 |
| 13 | 17 | 23 |
| 14 | 18 | 36 |
| 15 | 15 | 34 |
| 16 | 16 | 34 |
| 17 | 15 | 35 |
| 18 | 14 | 37 |
| 19 | 16 | 36 |
| 20 | 18 | 38 |
Discussion
OSE has been graded histologically into four stages, depending on fibroblastic response, hyalinization and inflammation [2]. Binnie and Cawson [3] have reported a homogenous collagenous subepithelial zone along with degeneration of muscle fibers. Oliver and Radden [4] reported the presence of dense collagen bundles that were randomly oriented and extended into the underlying striated muscles. The electron microscopic features of muscle fibers of OSF patients revealed partial to complete loss of plasma membrane, filled with large pools of homogenous material and often surrounded with edematous fluid [5].
A common finding in tissue fibrosis is that stromal fibroblasts become ‘activated’ myofibroblasts and express α-smooth muscle actin (SMA). The cytokine TGF-β1 is considered to have a central role in inducing this myofibroblastic phenotype, and its expression is increased in numerous fibrotic conditions.
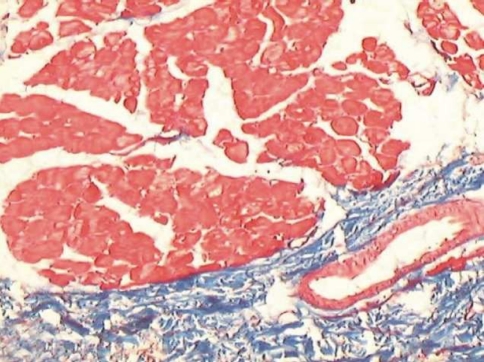
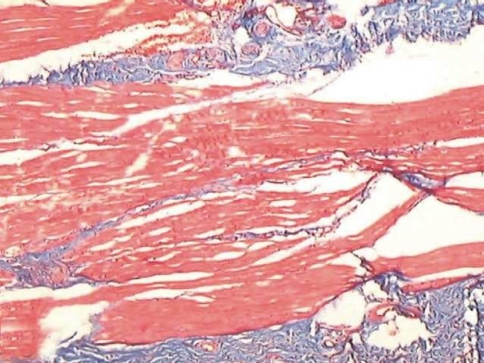
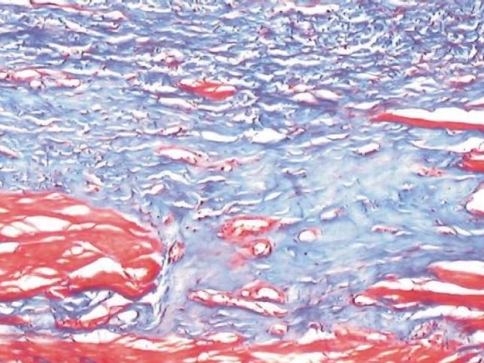
Muscle involvement in OSF has been studied previously using special stains. Gupta and Hamner and Mehta have used Van Gieson, a special stain for collagen [6, 7]. Though there are more specific and sensitive special stains like Phosphotungstic Acid Hematoxylin (PTAH) to observe changes in muscle fibers, Masson’s trichrome stain offered a simultaneous contrast color to the collagen fibers along with ME and MB. The collagen was stained blue while the muscle took a brilliant red color [8]. This colour contrast facilitated a better visual discrimination between muscle and collagen. The technique is also simple and easily reproducible.
Normal striated muscles are aligned as fascicles, each of which is invested by a thin fibrous connective sheath known as perimysium. Within this sheath nerves, arteries, arterioles and vein travel. Each muscle fiber normally appears to be invested by endomysium, a mesenchymal matrix made of collagen, elastic and reticulin fibers to provide support to the vasculature of the fascicles.
Under normal situations, the injured muscle initiates the repair of myofibres by the proliferation of satellite cells present in endo and perimysium. These are reserve pool of stem cells that can generate differentiated myocyte after injury. Inflammatory cells appear soon, becoming a source for pro-inflammatory cytokines. The satellite cells are activated by growth factors. These cells proliferate into myocytes and fuse to form multinucleated myofibres [9]. The same inflammatory mediators also stimulate the fibroblasts to proliferate and help in the healing process. Successful functional repair of injured muscle is characterized by ME regeneration while impairment denotes that it is replaced by fibrosis.
Rajendran et al. studied the histopathology of OST from buccal mucosa using light and electron microscope. They described fibroblasts, bindles of collagen, mast cells, macrophages and subepithelial fibrosis in the lamina propria. They also observed that the fibrosis in some cases was extending deep into MB under light microscope. They observed focal myofibrillar lysis, hypercontraction of myofibres and extensive fatty infiltration between muscle bindles under electron microscope [10]. Gupta et al. studied histopathologically the palatal muscle of OSF patients and reported degenerative changes in ME. 20.8% of their cases exhibited degenerative changes either as loss of cross striations or edema.
Muscle changes were investigated ultrastructurally in two groups of patients having oral submucous fibrosis. One group was from patients with no evidence of restricted mouth opening and the other was from patients with restricted mouth opening. Electronmicroscopically, the majority of muscle fibres taken from the first group appeared normal with only occasional muscle fibres showing accumulation of homogeneous material and compression of the sarcomeres closest to this material. In contrast, the tissues from patients with restricted mouth opening showed severe changes and necrosis in a high proportion of muscle fibres. The necrotic muscle fibres exhibited complete loss of their plasma membrane, but in which the outline was maintained by an intact basal lamina. It is suggested from this study that restricted mouth opening in submucous fibrosis might depend not only on the sub-epithelial fibrosis but also on the extent of muscle degeneration.
The light microscopic study of OSF revealed varying degrees of alterations involving the muscle fibers as the disease progresses. We consider that the damage to the ME appear more as consequences of fibrosis rather than by a direct injury to the muscle by the areca nut products. The factors other than fibrosis that restrict MO in OSF cases need to be explored.
Incisional biopsy from right buccal mucosa opposite to premolar-molar junction was taken and tissues were processed for routine haemotoxylin and eosin staining and Masson’s Trichrome staining for histopathological study (Figs. 1, 2, 3).
Fig. 1.
Muscle bundles seen separate from the ongoing submucosal fibrosis
Fig. 2.
Muscle fibres getting distorted with fibrosis involving the muscle zone
Fig. 3.
Fibrosis invading the muscle zone with atrophy of muscle bundles
The fibrosis was graded as
Stage 1: Fibrosis limiting to laminapropriaalone
Stage 2: Fibrosis involving superficial region of muscle bundle
Stage 3: Fibrosis involving deeper regions of MB
Stage 4: MB replaced by fibrosis.
This instilled in us an idea of using muscle relaxants as a adjuvant therapy in the routine protocol of treatment of oral submucosal fibrosis which will not only cater and halt the problem of fibrosis but also will take care of the muscle spasm and inflammation which also inadvertently contribute to the restricted mouth opening. We found excellent improvement on adding muscle relaxants to the routine protocol which was not just an objective but also a subjective success.
References
- 1.Rajendran R. Oral submucous fibrosis, etiology, pathogenesis and future research. Bull World Health Organ. 1994;72(6):985–996. [PMC free article] [PubMed] [Google Scholar]
- 2.Pindborg JJ, Sirsat SM. Oral submucous fibrosis oral. Surg Oral Med Oral Path. 1966;22:764–779. doi: 10.1016/0030-4220(66)90367-7. [DOI] [PubMed] [Google Scholar]
- 3.Binnie WA, Cawson RA. Anew ultra structural finding in oral submucous fibrosis. Br J Dermatol. 1972;86:286. doi: 10.1111/j.1365-2133.1972.tb02230.x. [DOI] [PubMed] [Google Scholar]
- 4.Oliver AJ, Radden BG. Oral submucous fibrosis. Case report and review of literature. Aust Dent J. 1992;37(1):31–34. doi: 10.1111/j.1834-7819.1992.tb00830.x. [DOI] [PubMed] [Google Scholar]
- 5.El-Labban NG, Canniff JP. Ultrastructural findings of muscle degeneration in oral submucous fibrosis. J Oral Pathol. 1985;14:709–717. doi: 10.1111/j.1600-0714.1985.tb00550.x. [DOI] [PubMed] [Google Scholar]
- 6.Gupta SC, Khanna S, Singh M, Singh PA. Histological changes to palatal and paratubal muscle in oral submucous fibrosis. J Laryngol Otol. 2000;114:947–950. doi: 10.1258/0022215001904428. [DOI] [PubMed] [Google Scholar]
- 7.Hamner JC, III, Mehta FS, Pindborg ff, Daftary DK. Altered staining reaction of connective tissue in 53 submucous fibrosis patients. J Dent Res. 1971;50(2):388–392. doi: 10.1177/00220345710500024601. [DOI] [PubMed] [Google Scholar]
- 8.Bancroft JD, Stevens A. Theory and practice of histological techniques. 4. Hong Kong: Churchill Livingston; 1996. p. 129. [Google Scholar]
- 9.Kumar V, Abbas AK, Fausto N, editors. Robbins and cotran—pathologic basis of disease. 7. Pennsylvania: Saunders, Elseiver Inc; 2004. p. 94. [Google Scholar]
- 10.Rajendran R, Radhakrishnan NS, Kartha CC. Light and Electron microscopic studies on oral submucous fibrosis. J Indian Dent Assoc. 1993;64(5):157–162. [Google Scholar]
- 11.Pindorg JJ. Oral submucous fibrosis: a review. Ann Acad Med Singapore. 1989;18:603–607. [PubMed] [Google Scholar]
- 12.Rajendran R. Oral submucous fibrosis. J Oral Maxillofac Pathol. 2003;7(1):1–4. doi: 10.4103/0973-029X.86678. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 13.Ranganathan K, Devi MU, Joshua E, Kiran Kumar K, Saraswathi TR. Oral submucous fibrosis: a case-control study in Chennai, South India. J Oral Pathol Med. 2004;33:274–277. doi: 10.1111/j.0904-2512.2004.00116.x. [DOI] [PubMed] [Google Scholar]
- 14.Chang YC, Tai KW, Chang MII, Chou LS, Chou MY. Cytotoxic and non-genotoxic effects of arecoline on human buccal fibroblasts in vitro. J Oral Pathol Med. 1998;27(2):68–71. doi: 10.1111/j.1600-0714.1998.tb02096.x. [DOI] [PubMed] [Google Scholar]
- 15.Boucher BJ, Mannan N. Metabolic effects of the consumption of areca catechu. Addict Biol. 2002;7:103–110. doi: 10.1080/13556210120091464. [DOI] [PubMed] [Google Scholar]
- 16.Kumar V, Abbas AK, Fausto N, editors. Robbins and Cotran—pathologic basis of disease. 7. Pennsylvania: Saunders, Elseiver Inc; 2004. pp. 96–97. [Google Scholar]
- 17.Hsu HJ, Chang KL, Yang YR, Stitch TY. The effects of arecoline on the release of cytokines using cultured peripheral blood mononuclear cells from patients with oral mucous diseases. Kaohsiung J Med Set. 2001;17(4):175–182. [PubMed] [Google Scholar]
- 18.Haque ME, Harris M, Meghji S, Barrett AW. Immunolocalization of cytokines and growth factors in oral submucous fibrosis. Cytokine. 1998;10(9):713–719. doi: 10.1006/cyto.1997.0342. [DOI] [PubMed] [Google Scholar]
- 19.Haque MF, Meghji S, Khitab U, Harris M. Oral submucous fibrosis patients have altered levels of cytokine production. J Oral Pathol Med. 2000;29(3):123–128. doi: 10.1034/j.1600-0714.2000.290304.x. [DOI] [PubMed] [Google Scholar]
- 20.Rooban T, Saraswathi TR, Al Zainab FH, Devi U, Eligabeth J, Ranganathan K (2005) A light microscopic study of fibrosis involving muscle in oral submucous fibrosis. Indian J Dent Res 16:131 flammatory cells or as atrophy [DOI] [PubMed]