Abstract
Invasive aspergillosis usually affects immune-compromised patients and is common in diabetics. Proptosis, visual loss and ophthalmoplegia due to intra-orbital extension are common presentations. Three out of five patients in our series were immune-compromised. All the patients had visual loss and three patients presented with unilateral blindness. Three patients were treated by surgical debridement followed by Amphotericin B therapy. Two patients who had intra-cranial extension of the disease died during the treatment. Only one patient had improvement in vision following the treatment. High index of suspicion in immune-compromised patients, early diagnosis and prompt aggressive treatment is required to achieve clinical cure.
Keywords: Invasive aspergillosis, Sinonasal aspergillosis, Fungal sinusitis, Invasive fungal sinusitis
Introduction
Fungal diseases of sinuses are a diverse group of diseases with subtle presentation like allergic fungal sinusitis to invasive aspergillosis and fulminant form, rhinocerebral mucormycosis. Aspergillosis of the paranasal sinuses is a relatively infrequent but a distinct entity. Invasive aspergillosis with intra-orbital and intra-cranial spread usually is fatal and necessitates prompt diagnosis and treatment [1]. It usually involves the species Aspergillus fumigatus and Aspergillus flavus. The maxillary sinus is the most common sinus to be affected. Invasive cranio-orbital aspergillosis originating in the sphenoid sinus is rare and mostly occurs in immunocompromised patients with poor outcomes [2]. Invasive aspergillosis originating from the paranasal sinuses can cause an intra-cranial growth mainly along the skull base and larger vessels [3]. Orbital invasion readily occurs due to breach of thin bony partition of lamina papyracea leading to proptosis and gradual loss of vision. Invasion of surrounding tissues on radiology and histopathology is hallmark of the disease. The current study was undertaken to assess the clinico-radiological profile of the patients with invasive aspergillosis and the outcome of treatment. We report a case series of five patients with invasive rhino-orbital aspergillosis. All these patients had confirmed diagnosis of invasive aspergillosis on histopathology.
Materials and Methods
The study was conducted as a retrospective review of clinical, pathological and radiological case records of five patients who were admitted in our department between January 2006 and June 2008. Detailed history and clinical examination was performed in all the cases. History was sought for diabetes, immune-deficiency, chemotherapy and steroid use. Ophthalmologist was consulted for all the cases. Visual acuity and ocular movements were recorded. Neurosurgical opinion was sought in two patients who had intra-cranial extension. Contrast enhanced CT scan was done in all the cases to know the extent of disease. MR scans were done in two patients who had intra-cranial extension on CT scans. Routine haematological and biochemical tests were done for suspected immune-compromise. ELISA for HIV was done in all the cases. Endonasal biopy was taken in four patients, one patient underwent external biopsy as disease has invaded and fungated through the soft tissue of maxillary region. The patients following confirmation of diagnosis on histopathology were taken up for surgical debridement. Amphotericin B therapy was started in all the patients 7–10 days prior to surgery and continued after the surgery. Liposomal Amphotericin B was given in the dose of 1 mg/kg/day for a period of 4–6 weeks. Conventional Amphotericin B was given to a total dose of 3 g over 2–3 months (Table 1). Postoperative CT scan and nasal endoscopy was done every 3–4 months after the surgery to assess the recurrent disease. Oral itraconazole 400 mg per day for 8–12 months was given in patients having radiological evidence of residual/recurrent disease. End point of treatment was complete disappearance of lesions on contrast CT scan, with pre-operative CT scan taken as baseline. Patients were followed up for at least 1 year after completion of treatment. The data was analyzed in terms of clinical presentation, radiological features of disease, treatment received, visual and survival outcome.
Table 1.
Clinical features and treatment outcome of patients with invasive rhino-orbital aspergillosis
| Age/Sex | Immune-compromise | Proptosis | Visual loss | Ophthalm oplegia | IOE | ICE | Surgical treatment | Antifungal therapy | Survival outcome | Visual outcome | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Case 1 | 20/M | NO | + | PL(−ve) | + | + | + | Biopsy only | Lip Amph B | Died | − |
| Case 2 | 28/M | NO | + | PL(−ve) | + | + | − | Debridement + orbital exentration | Lip Amph B | Survived | − |
| Case 3 | 43/F | Yes, Ca breast, chemotherapy | − | PL(−ve) | + | + | − | Biopsy only | Amph B | Died | − |
| Case 4 | 48/M | Yes diabetes | + | Perception of hand movements | Restriction on medial gaze | + | + | Endonasal debridement | Amph B | Survived | Improved to finger counting at 3 m |
| Case 5 | 37/M | Yes diabetes | + | Perception of hand movements | + | + | − | Endonasal debridement | Lip Amph B | Survived | No improvement |
PL Perception of light, IOE Intra-orbital extension, ICE Intra-cranial extension, Lip. Amph B Liposomal Amphotericin B, Amph B Amphotericin B
Results
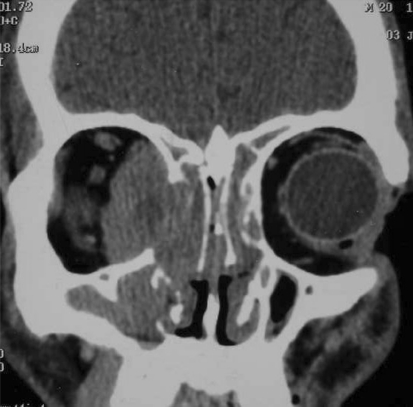
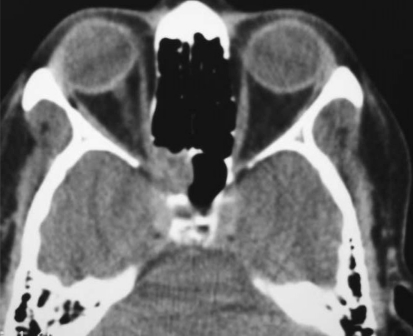
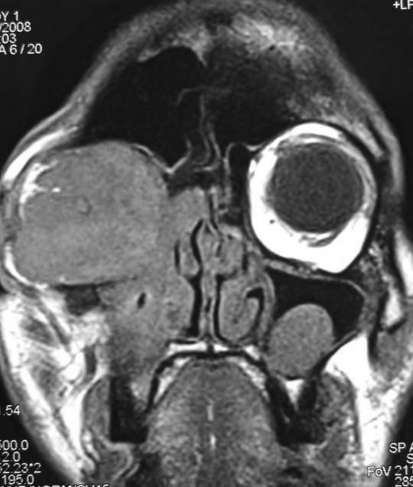
The age range of patients with invasive rhino-orbital aspergillosis was 20–48 years (mean 35.2 years). Two of the patients had uncontrolled diabetes, one patient a 43 years old lady was confirmed case of breast carcinoma and was receiving chemotherapy. This patient presented with loss of vision and facial pain of 2 weeks duration. Two patients did not have any immune-compromise. Proptosis was the presenting feature in all, except one patient undergoing chemotherapy. Three patients presented with unilateral blindness and two patients had visual loss to the extent of perception of hand movements only (Table 1). Four patients had ophthalmoplegia and one patient had restriction of ocular movements in convergence gaze only. On contrast CT scan Intra-orbital extension was present in all the patients (Fig. 1) and two patients had confirmed intra-cranial extensions on MRI Scan (Fig. 2). Four patients had involvement of maxillary, ethmoid and sphenoid sinuses. One patient on chemotherapy had isolated involvement of sphenoid and ethmoid sinuses (Fig. 3). Preoperative biopsy confirmed aspergillosis in all the cases. Endonasal surgical debridement was done in two patients. One patient who had gross proptosis and a massive intra-orbital extension with blind eye (Fig. 4), debridement was done by a lateral rhinotomy approach along with orbital exentration. Two patients underwent biopsy only. Fungal culture was done in all the cases and a positive fungal culture for aspergillus flavus was obtained in three patients. All patients received systemic antifungal therapy. Three patients received liposomal amphotericin B and two patients received conventional amphotericin B preparation. Liposomal amphotericin was given in the dose of 1 mg/kg/day. Conventional preparation was given up to a total dose of 3 g over 2–3 months. We found a significantly better tolerance and fewer side effects with liposomal preparation. Though the therapeutic dose can be achieved quickly, but in our experience it did not alter the survival outcome. Two patients died during the course of treatment, both these patients had intra-cranial extensions and died shortly after undergoing biopsy and started receiving antifungal therapy. One patient of breast carcinoma died due to systemic metastasis, other patient a 20 years old man, died due to embolic brain infarction and cavernous sinus thrombosis.
Fig. 1.
Invasive aspergillosis with intra-orbital extension
Fig. 2.
Axial MRI scan with intra-orbital and intra-cranial extension
Fig. 3.
Sphenoid sinus involvement in patient on chemotherapy
Fig. 4.
MRI scan with massive intra-orbital extension of aspergillosis
Post-operative follow-up was done in all the three survivors. Post-operative CT scans were done 3–4 months after the debridement, with pre-operative CT scans taken as baseline; study was repeated every 3–4 months thereafter. The goal of CT scan was to identify and treat the recurrent disease while it was localized. Nasal endoscopy was done in the same sitting to look for recurrent disease. One patient (case 2) had complete clearance of the disease and was under regular follow-up and did not have any recurrence. Two patients (case 4, 5) had recurrent disease on CT scans in the post-operative period after Amphotericin B therapy. These patients received oral itraconazole 400 mg a day and CT scan was repeated every 3–4 months. The treatment was discontinued after 8–12 months when there was no radiological evidence of disease.
Discussion
De shazo et al. attributes the first description of fungal sinusitis to Plaignaud in 1791 and initial reports of fungal sinusitis to Mackenzie in 1893 [4]. In 1965, Hora et al. described clinical presentation of invasive and non invasive form of aspergillosis: the non invasive form has thick dark greenish yellow allergic mucin in the sinuses and has good prognosis after removal; the rarer invasive form was associated with pain and caused tissue invasion, which can be mistaken as malignancy [5]. Aspergillosis originating from the nose and paranasal sinuses can cause an intra-orbital and intra-cranial growth mainly along the skull base and larger vessels. The patients present with symptoms like proptosis, facial swelling, ophthalmoplegia, loss of vision, and hypoaesthesia of the ophthalmic and maxillary nerve. Computed tomography and MRI usually show extensive sino-orbital and skull base lesions [3]. Only small series of patients with this infection have been described; the radiographic diagnosis of cerebral and craniofacial aspergillosis has varied and has been relatively nonspecific. CT scan is able to demonstrate the extent of disease in paranasal sinuses, orbit and intra-cranial extension accurately. Patients with cerebral aspergillosis have multiple lesions, an irregular ring of contrast enhancement, and hypointensity of the ring on T2-weighted MR images. Patients with cortical and subcortical hypodensities on CT scanning or hyperintensities on MR imaging are consistent with cerebral cortical and subcortical infarction. Abnormal enhancement of the optic nerve and sheath with infiltrating enhancing soft tissue within the intra-orbital fat is seen in intraorbital lesions [6, 7]. The differential diagnosis of invasive aspergillosis include benign and malignant neoplasms, syphilis, tuberculosis, sarcoidosis, Wegner’s granulomatosis, lymphoma, mucopyocele, allergic fungal sinusitis and rhinoscleroma [8].
The possibility of opportunistic infections by saprophytic fungi must be considered in all immune-compromised patients, as it may endanger both vision and survival. Immediate diagnosis and therapy are essential. Diagnosis is reached by histopathology, fungal mount and culture. Histopathology show acute angled branching septate fungal hyphae with tissue invasion. A. fumigatus is the most common organism in immunocompetent patients [7] though A. flavus was the most common organism isolated in our series.
The standard treatment of rhino-orbito-cerebral aspergillosis is wide and aggressive surgical debridement followed by systemic antifungal therapy. Local irrigation with antifungal agents has been advocated by some authors. Hyperbaric oxygen has been tried in some studies without any distinct and proven advantage in terms of disease control and survival. Washburn has noted that invasive fungal sinusitis frequently recurs despite surgical debridement and recommended a prolonged course of amphotericin B exceeding 2 g for adults after surgery [9]. If persistent or recurrent disease develops, itraconazole 200–400 mg per day may be added [10]. Fungal cultures are essential because not all fungi are sensitive to amphotericin B or itraconazole. Regular post-operative follow-up is recommended in all the cases. Contrast CT scan and nasal endoscopy is recommended to look for recurrent disease every 3–4 months. Early diagnosis of recurrent disease requires prolonged systemic antifungal chemotherapy [9].
Clinical success can be achieved by aggressive debridement and intravenous antifungal agents. Radical procedures like orbital exenteration must be considered in all cases [11]. Two out of five patients in our series died after diagnosis of the disease, during the antifungal treatment, before any surgical debridement could be undertaken in these patients. Advances in antimicrobial therapy, hyperbaric oxygen therapy and treatment of the underlying disease has not significantly changed the outcome of the disease which is fatal most of the times.
Conclusions
Invasive rhino-orbital aspergillosis is a distinct fungal infection of the paranasal sinuses with potential of intra-orbital and intra-cranial extension. Early diagnosis by histopathology and contrast CT scan is required for appropriate surgical debridement, followed by antifungal treatment by Amphotericin B. Patients should be followed up regularly by imaging study and nasal endoscopy for detection of residual or recurrent disease, which requires prolonged systemic antifungal therapy. Despite adequate treatment the disease has high mortality.
References
- 1.Yoon JS, Park HK, Cho NH, Lee SY. Outcomes of three patients with intra-cranially invasive sino-orbital aspergillosis. Ophthal Plast Reconstr Surg. 2007;23(5):400–406. doi: 10.1097/IOP.0b013e31814db448. [DOI] [PubMed] [Google Scholar]
- 2.Akhaddar A, Gazzaz M, Albouzidi A, Lmimouni B, Elmostarchid B, Boucetta M. Invasive Aspergillus terreus sinusitis with orbitocranial extension: case report. Surg Neurol. 2008;69(5):490–495. doi: 10.1016/j.surneu.2007.02.059. [DOI] [PubMed] [Google Scholar]
- 3.Knipping S, Holzhausen HJ, Koesling S, Bloching M. Invasive aspergillosis of the paranasal sinuses and the skull base. Pneumonol Alergol Pol. 2008;76(5):400–406. [PubMed] [Google Scholar]
- 4.DeShazo RD, O’Brian M, Chapin K, et al. A new classification and diagnostic criteria for invasive fungal sinusitis. Arch Otolaryngol Head Neck Surg. 1997;123:1181–1188. doi: 10.1001/archotol.1997.01900110031005. [DOI] [PubMed] [Google Scholar]
- 5.Hora JF. Primary aspergillosis of paranasal sinuses and associated area. Laryngoscope. 1965;75:768–773. doi: 10.1288/00005537-196505000-00004. [DOI] [PubMed] [Google Scholar]
- 6.Ashdown BC, Tien RD, Felsberg GJ. Aspergillosis of the brain and paranasal sinuses in immunocompromised patients: CT and MR imaging findings. AJR Am J Roentgenol. 1994;162(1):155–159. doi: 10.2214/ajr.162.1.8273655. [DOI] [PubMed] [Google Scholar]
- 7.Kameswaran M, al-Wadei A, Khurana P, Okafor BC. Rhinocerebral aspergillosis. J Laryngol Otol. 1992;106(11):981–985. doi: 10.1017/S0022215100121528. [DOI] [PubMed] [Google Scholar]
- 8.Sarti EJ, Blaugrund SM, Lin PT, et al. Paranasal sinus diseases with intra-cranial extension: aspergillosis versus malignancy. Laryngoscope. 1988;98:632–635. doi: 10.1288/00005537-198806000-00011. [DOI] [PubMed] [Google Scholar]
- 9.Washburn RG, Kennedy DW, Begley MG, et al. Chronic fungal sinusitis in apparently normal hosts. Medicine. 1998;67:231–247. doi: 10.1097/00005792-198807000-00004. [DOI] [PubMed] [Google Scholar]
- 10.Zieske LA, Kopke RD, Hsmill R. Dematiaceous fungal sinusitis. Otolaryngol Head Neck Surg. 1991;105:567–577. doi: 10.1177/019459989110500408. [DOI] [PubMed] [Google Scholar]
- 11.Arndt S, Aschendorff A, Echternach M, Daemmrich TD, Maier W. Rhino-orbital-cerebral mucormycosis and aspergillosis: differential diagnosis and treatment. Eur Arch Otorhinolaryngol. 2009;266(1):71–76. doi: 10.1007/s00405-008-0692-y. [DOI] [PubMed] [Google Scholar]