Abstract
Small heat shock proteins are ubiquitous in all three domains (Archaea, Bacteria and Eukarya) and possess molecular chaperone activity by binding to unfolded polypeptides and preventing aggregation of proteins in vitro. The functions of a small heat shock protein (S.so-HSP20) from the hyperthermophilic archaeon, Sulfolobus solfataricus P2 have not been described. In the present study, we used real-time polymerase chain reaction analysis to measure mRNA expression of S.so-HSP20 in S. solfataricus P2 and found that it was induced by temperatures that were substantially lower (60°C) or higher (80°C) than the optimal temperature for S. solfataricus P2 (75°C). The expression of S.so-HSP20 mRNA was also up-regulated by cold shock (4°C). Escherichia coli cells expressing S.so-HSP20 showed greater thermotolerance in response to temperature shock (50°C, 4°C). By assaying enzyme activities, S.so-HSP20 was found to promote the proper folding of thermo-denatured citrate synthase and insulin B chain. These results suggest that S.so-HSP20 promotes thermotolerance and engages in chaperone-like activity during the stress response.
Keywords: sHSP, S.so-HSP20, Sulfolobus solfataricus P2
Introduction
Archaea, one of the three major groups of life forms, are a highly diverse and abundant group that includes many “extremophiles” that thrive in harsh environments, such as hot springs, salt lakes, and submarine volcanic habitats (Madigan et al. 2000; Eckburg et al. 2003). Current knowledge on stress genes in archaea is considerably more limited than that for bacteria and eukaryotes. The stress response in archaea was first studied in 1980, but it was not until 1991 that an archaeal stress gene (hsp70) was first cloned and sequenced (Macario et al. 1991; Klumpp and Baumeister 1998). Since then, several studies have focused on stress genes and their expression in archaea (Macario et al. 1999), but many aspects of the archaeal stress response are yet to be studied. Sulfolobus solfataricus P2 is a very widely studied crenarchaeal organism that grows well in the low pH, high sulfur environment of volcanic hot springs. It is used as a model organism in archaeal research to study processes such as DNA replication, cell cycle, chromosomal integration, and RNA processing (She et al. 2001; Nicholas et al. 2004). The optimum temperature for S. solfataricus P2 is about 75°C, although it can live between 55°C and 90°C and in a pH range of 0.9–5.8, with its optimum pH being 2–3. The genome of S. solfataricus P2 was sequenced in 2001. It consists of a single circular chromosome of 2,992,245 base pairs, with 3,032 genes encoding 2,977 proteins (Ehrnsperger et al. 1997; Jakob et al. 1993; chang et al. 1996). Heat shock proteins (HSPs) are ubiquitously expressed cellular proteins that form a major, conserved protein family. Some HSPs are expressed constitutively under physiological conditions (Lindquist and Craig 1998; Haslbeck et al. 2005), while others are induced in response to chemical or physical stressors such as heat, heavy metals, oxidative stress, ethanol (Charles et al. 1996; Laszlo and Li 1993; Sun et al. 2007). Small heat shock proteins (sHSPs) are a family of stress-inducible molecular chaperones that range in size from 12 to 43 kDa (Arrigo 1998; Ehrnsperger et al. 1998; MacRae 2000; Van Montfort et al. 2001) and that form oligomers consisting 9 to 50 subunits (Boston et al. 1996; Waters et al. 1996; She et al. 2001). The ability of sHSPs to form oligomers contributes to their thermal stability and ability to avoid denaturation in response to high temperatures. sHSPs have chaperone-like activity in vitro and protect organisms from various stresses. At denaturing temperatures, sHSPs can prevent the aggregation of proteins by binding to, and forming a stable complex with, folding intermediates of their substrate proteins. In some cases, sHSPs can also promote renaturation of unfolded polypeptides (Brock et al. 1972; Esposito et al. 1998). In this study, we subcloned the coding sequence of the small heat shock protein, HSP20 (SSO2427) from the thermoacidophilic archaeon S. solfataricus P2 into a bacterial expression vector and produced recombinant protein for structural and functional analysis. To investigate the level of HSP20 mRNA in S. solfataricus P2, we measured its mRNA expression profile in response to variations in culture temperature, including cold shock. Escherichia coli transformed with HSP20 was protected from a thermal stress of 50°C and cold stress of 4°C. Purified S.so-HSP20 behaved as a molecular chaperone by inhibiting the thermal aggregation of citrate synthase and insulin B chain. These results indicate that HSP20 plays an important role in the response to thermal stress.
Materials and methods
Culturing S. solfataricus P2 at different temperature and cold-shock treatment
For the cultivation of S. solfataricus P2, a standard culture medium (yeast extract, 2.0 g; KH2PO4, 3.10 g; (NH4)2SO4, 2.50 g; MgSO4 × 7H2O, 0.20 g; CaCl2 × 2H2O, 0.25 g; Distilled water, 1,000.00 ml) was used. The pH of the medium was adjusted to 3.5 at room temperature with 10 N H2SO4 prior to autoclaving. S. solfataricus P2 was cultured at different temperatures (60°C, 75°C, 80°C). The cells were harvested during the log growth phase at an OD600 = 0.6. For cold-shock treatment, S. solfataricus P2 was exposed to 4°C for 2 h after culturing at 75°C.
RNA extraction
All samples were homogenized in Trizol Reagent (Invitrogen, CA, USA), and total RNA was prepared according to the manufacturer's instructions. Total RNA was quantified on a Genova UV/visible spectrophotometer at 260 nm.
Measurement of S.so-HSP20 expression by quantitative real-time PCR
Complementary DNA was synthesized from 2 μg of total RNA from each sample using random decamer primers and Murine Molony Leukemia Virus reverse transcriptase (Promega, Madison, WI, USA) in a 25-μl reaction. The expression pattern of S.so-HSP20 was analyzed in various samples using gene-specific primers HSP20RT-F, HSP20RT-R (Table 1) and the MiniOpticon TM System (Bio-Rad, USA). Each polymerase chain reaction (PCR) reaction mix contained 12.5 μl of Supermix, 10 μM primer HSP20RT-F, 10 M primer HSP20RT-R, and 1 μl RT-products. PCR products were detected using the iQ SYBR Green Supermix kit (Bio-Rad). A standard curve was constructed using tenfold serial dilutions (1:104, 1: 105, 1: 106, 1: 107, and 1: 108) of purified plasmids subcloned with S.so-HSP20 together with a non-template control. A standard curve for NusG (Samson et al. 2008, 2011) was constructed using a purified plasmid subcloned with the NusG gene fragment that had been amplified with primers NusGF and NusGR (Table 1). This plasmid was then diluted in a tenfold series (1:105, 1:106, 1:107, 1:108, and 1:109). All samples were analyzed in triplicate. Statistical significance was performed using the two-tailed paired Student's t test. S.so-HSP20 gene expression profiles obtained from real-time PCR were normalized with the NusG transcript to correct for differences in the starting amount of RNA and in the efficiency of the reverse transcription reactions. Melting curve analysis of the PCR reaction was performed to assess specificity.
Table 1.
Primers used in this work
| Primer | Sequence(5′-3′) |
|---|---|
| HSP20F | CGggatccATGCCCAAGAGGGAAGAAAAGGATA |
| HSP20R | CCGctcgagTTACTCAACTTTTATGTCAACACCA |
| NusGF | GTGGTTGGAGGGCAAGAAATAA |
| NusG | GGGACCAGAAATTACTTCTACTACATC |
| HSP20RT-F | CAGACGGTGTGCCGAAAATAG |
| HSP20RT-R | TAGCCTTAGCCGCCTTCTCG |
Lowercase letters indicate restriction endonuclease recognition sequences (BamHI, XhoI)
Genomic DNA extraction and plasmid purification
S. solfataricus P2 was cultured at 75°C for 2 days using a standard culture medium. Cells were collected from 30 ml of culture by centrifugation at 4,200×g for 10 min. S. solfataricus P2 genomic DNA and Plasmids (Table 2) were extracted and purified using EZ-10 Spin Column Genome DNA isolation Kit, EZ-10 Spin Column Plasmid Mini-Preps Kit and EZ-10 Spin Column Gel Extraction Kit (BIO BASIC INC). All samples were prepared according to the manufacturer's instructions and DNA concentration was measured using a Genova UV/visible spectrophotometer at 260 nm.
Table 2.
Strains and plasmids used in this work
| Strain or plasmid | Relevant genotype or features | Reference |
|---|---|---|
| Strains | ||
| Sulfolobus solfataricus p2 | Sulfolobus solfataricus DSMZ1617 | DSMZ |
| E.coli BL21(DE3) | E.coli B (DE3)[F−dcm ompT hsdSB (r−Bm−B)] | Studier et al. 1990 |
| E.coli P | E.coli BL21(DE3) with pET-28a | This work |
| E.coli H | E.coli BL21(DE3) with pET-28a-HSP20 | This work |
| E.coli HR | E.coli DH5α with pUCm-T-HSP-RT | This work |
| E.coli NusG | E.coli DH5α with pUCm-T-NusG-RT | This work |
| Plasmids | ||
| pET-28a-HSP20 | pET28a(+) with HSP20* | This work |
| pUCm-T-HSP-RT | pUCm-T with HSP-RT DNA | This work |
| pUCm-T-NusG | pUCm-T with NusG-RT DNA | This work |
*HSP20, NCBI reference sequence: NP_343781.1 (She et al. 2001)
Cloning, expression, and purification of recombinant S.so-HSP20
The open reading frame of S.so-HSP20 was amplified from the chromosomal DNA of S. solfataricus P2. The forward primer (HSP20F) contained a BamH I site (indicated lowercase, Table 1) and the reverse primer (HSP20R) contained a unique XhoI site (indicated lowercase, Table 1) with stop codon. The PCR product was digested with BamHI and XhoI, and ligated into the pET-28a vector (Novogen, USA) digested with BamHI and XhoI, generating pET-28a-S.so-HSP20, which encodes S.so-HSP20 with an N-terminal His6 extension. pET-28a-S.so-HSP20 was transformed into E. coli BL21 (DE3), and His6-S.so-HSP20 was induced by addition of isopropyl-β-d-Thiogalactoside (IPTG; 1-mM final concentration) to exponentially growing cultures of E. coli (pET -28a-S.so-HSP20) in Lauria-Bertani (LB) medium containing 50 μg/ml of kanamycin. His6-tagged protein was purified by Ni-NTA (Qiagen, USA) according to the manufacturer's instructions. S.so-HSP20 was purified to homogeneity, based on Coomassie blue staining after sodium dodecyl sulfate-polyacrylamide gel electrophoresis.
Thermotolerance experiments with transformed E. coli
For thermotolerance experiments, E. coli (pET-28a-S.so-HSP20) was cultured as described above. IPTG (1 mM) was added to mid-log phase cultures (OD600 = 0.8), and the cells were incubated at 30°C for 2 h. The cultures were incubated at 50°C for 0, 15, 30, 45, and 60 min and at 4°C for 1, 2, 3, and 4 d. The cultures were then diluted to 5.0 × 106 cells/ml and 50 μl samples were plated in triplicate on LB plates containing 50 μg/ml of kanamycin. The plates were incubated overnight at 37°C before scoring colony formation to determine the percentage of survivors. For each treatment, the proportion of surviving E. coli colonies was calculated by dividing the number of viable cells per plate in the treated samples versus untreated samples (no exposure to heat or cold shock). All samples were analyzed in triplicate. Statistical significance was performed using the two-tailed paired Student's t test.
Assay of molecular chaperone activity
Citrate synthase (Sigma, USA) was diluted with 40 mM HEPES (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid) KOH buffer at pH 7.5 to a final concentration of 150 nM in 1.0-ml cuvettes and heated at 43°C with 150 nM or 300 nM of purified S.so-HSP20 or at 45°C with 150 nM of purified S.so-HSP20. Aggregation of citrate synthase was monitored by measuring turbidity at 360 and 320 nm with a SPECTRA Max PLUS spectrophotometer every 5 and 10 min for 1 h. Bovine serum albumin (BSA) was used at 150 nM to evaluate non-specific protection of citrate synthase (Chen et al. 2007). Chaperone-like activity of S.so-HSP20 was determined by measuring its ability to prevent dithiothreitol (DTT)-induced aggregation of insulin B chain in 50 mM Tris–HCl (pH 7.4) buffer with 0.15 M NaCl for 25 min at 40°C. The DTT was used at a final concentration of 20 mM. The turbidity of insulin B chain was measured using a SPECTRA max PLUS spectrophotometer at 360 nm. The ratio of S.so-HSP20 to insulin concentration for the assay was 1:2.
Results
S.so-HSP20 mRNA expression in S. solfataricus P2 at different growth temperatures and under cold-shock conditions
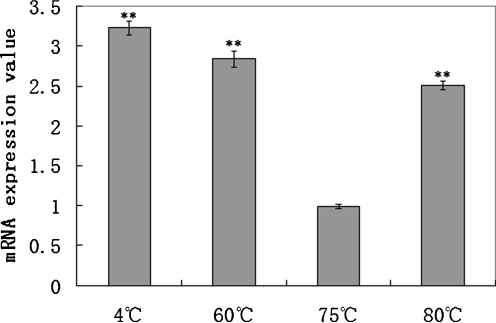
The expression of S.so-HSP20 mRNA in S. solfataricus P2 was measured at different culture temperatures (Fig. 1). S.so-HSP20 mRNA expression was strongly increased at culture temperatures far from the optimal growth temperature of 75°C. The increases in S.so-HSP20 mRNA expression at temperatures that were lower (60°C) or higher (80°C) than the optimum culture temperature (75°C) were 2.83- and 2.5-fold, respectively. S.so-HSP20 mRNA expression also increased 3.23-fold in response to cold-shock treatment (4°C).
Fig. 1.
S.so-HSP20 mRNA expression in S. solfataricus P2 analyzed by real-time PCR. The level of S.so-HSP20 mRNA was measured in cells cultured at the indicated growth temperature or cold shock at 4°C for 2 h, and normalized against NusG (Internal control) levels. The mRNA expression value at 75°C was set to 1 and the other values were normalized against this. Data are representative of three trials and were expressed as the mean±SEM. Statistical significance was performed using the two-tailed paired Student's t test. (**p < 0.01)
Survival after thermal stress in E. coli expressing S.so-HSP20
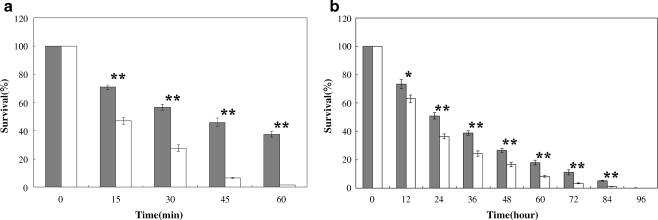
To determine whether S.so-HSP20 could enhance the thermotolerance of E. coli, the effect of heat stress on the growth of E. coli cells transformed with S.so-HSP20 was examined. E. coli expressing S.so-HSP20 was more resistant to heat stress than cells containing only the pET-28a vector. As shown in Fig. 2, after exposure to 50°C, the survival of cells transformed with pET-28a only dropped to about 1.5% at 1 h, whereas that of the cells with pET-28a-S.so-HSP20 was 37.9%. Transformation with S.so-HSP20 also protected E. coli from exposure to cold (4°C), with survival recorded as 18.8% after 3-days cold exposure, compared to just 1.2% in E. coli transformed with pET-28a only. These results show that expression of S.so-HSP20 in E. coli cells increases cellular thermotolerance.
Fig. 2.
Thermotolerance assays were performed at 50°C (a) and 4°C (b). E. coli P (with pET-28a) and E. coli H (with pET-28a-S.so-HSP20) were cultured as described and subjected to 50°C treatment for up to 60 min and 4°C treatment for up to 4 days. After heat or cold treatment, samples were taken at the time indicated, diluted, and immediately plated on LB plates with kanamycin. Survival of E. coli H cells (black boxes) and E. coli P cells (open boxes) are shown, and are expressed as a percentage of the survival obtained in LB plates. Data are representative of three trials and were expressed as the mean±SEM. Statistical significance was performed using the two-tailed paired Student's t test. (*p < 0.05; **p < 0.01)
S.so-HSP20 exhibits chaperone activity in vitro
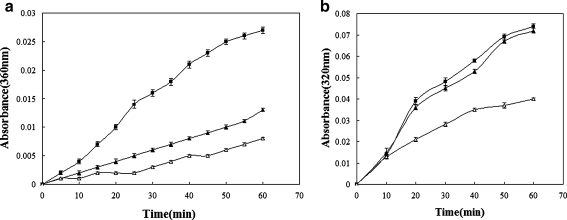
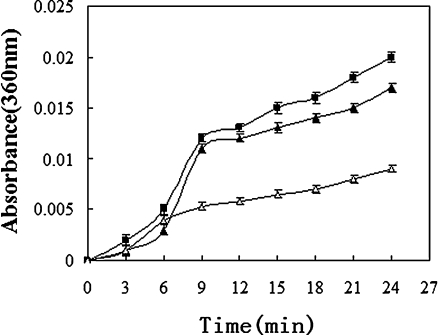
A key characteristic of molecular chaperones is their ability to suppress the aggregation of non-native proteins. To test whether S.so-HSP20 exhibits chaperone activity, we performed thermal unfolding assays using citrate synthase and insulin B chain as model substrates (Figs. 3 and 4). Purified S.so-HSP20 effectively protected citrate synthase against heat-induced denaturation. At concentrations of 150 or 300 nM, S.so-HSP20 reduced heat-induced turbidity resulting from incubation at 43°C for 60 min by 48.1% and 70.4%, respectively (Fig. 3a). Similarly, heat-induced turbidity resulting from a 60-min incubation at 45°C was reduced by S.so-HSP20 by 38.3% (Fig. 3b). Heat-induced turbidity of the insulin B chain was reduced by S.so-HSP20 by 47% after 24 min at 40°C (Fig. 4). These results demonstrate that S.so-HSP20 exhibits chaperone activity in vitro.
Fig. 3.
S.so-HSP20 prevents heat-induced denaturation of citrate synthase. Purified S.so-HSP20 was incubated at 43°C (a) with 150 nM citrate synthase, and turbidity was measured at 360 nm. S.so-HSP20 concentrations were 0.0 nM (black square), 150 nM (black triangle), 300 nM (open triangle). S.so-HSP20 was also incubated at 45°C (b) with 150 nM citrate synthase, and turbidity was measured at 320 nm. The S.so-HSP20 concentration was 0.0 nM (black triangle) or 150 nM (open triangle). BSA at 150 nM (black square) was used as a control. Data are representative of three trials and were expressed as the mean±SEM
Fig. 4.
S.so-HSP20 has chaperone-like activity. Chaperone-like activity of S.so-HSP20 was determined by measuring turbidity during DTT-induced aggregation of the insulin B chain (0.2 mg/ml) for 24 min at 40°C; insulin B chain alone (black triangle), insulin B chain in the presence of 0.2 mg/ml BSA (black square), or the insulin B chain in the presence of 0.1 mg/ml S.so-HSP20 (open triangle). Data are representative of three trials and were expressed as the mean±SEM
Discussion
In the present study, we examined the thermotolerance and molecular chaperone properties of S. solfataricus P2 sHSP, HSP20. The coding region of the HSP20 gene of the thermoacidophilic archaeon, S. solfataricus P2, was cloned into a plasmid vector and overexpressed in bacteria. The recombinant protein was used for structural and functional analysis. We analyzed mRNA expression of HSP20 in response to different culture temperatures. Many sHSPs are not constitutively expressed, and their expression is induced specifically in response to stress conditions, such as elevated temperatures. We found that the expression of S.so-HSP20 mRNA is markedly increased following culture of S. solfataricus P2 at temperatures far from the optimal growth temperature. This result suggests that S.so-HSP20 mRNA expression is stress-induced, and may protect S. solfataricus P2 from stress. Expression of S.so-HSP20 in E. coli also protected these cells from temperature stress including cold shock. E.coli HSPs have been suggested to play a major role in protecting cells from thermal killing. The function of S.so-HSP20 in E. coli may be to promote thermotolerance by enhancing protein synthesis in E. coli (including HSPs) or by protecting E. coli proteins from heat denaturation through its chaperone activity. We demonstrated chaperone activity for S.so-HSP20 of S. solfataricus P2 at physiological and heat shock temperatures. The ability of S.so-HSP20 to protect against heat-induced aggregation of citrate synthase (Keisuke et al. 2001; Ding et al. 2008; Martin et al. 2008; Angelo et al. 2004) and insulin B chain (Azzoni et al. 2004; Satoru 2006) indicates that S.so-HSP20 possesses chaperone activity and the ability to recognize and bind unfolded proteins to prevent their aggregation in vitro. The relationship between cellular thermotolerance and S.so-HSP20 expression in an E. coli expression system has not previously been reported. The results of this study provide evidence that S.so-HSP20 can protect E. coli from thermostress and behave as a chaperone in vitro. Future studies will develop an S.so-HSP20 expression system in Sacharomyses cerevisiae, and will assess the ability of S.so-HSP20 to regulate apoptosis.
Acknowledgments
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- Angelo K, Burr GA, John JH. Molecular chaperone function of the Rana catesbeiana small heat shock protein, hsp30. Compar Biochem Phys Part A. 2004;139:175–182. doi: 10.1016/j.cbpb.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Arrigo AP. Small stress proteins: chaperones that act as regulators of intracellular redox state and programmed cell death. Biol Chem. 1998;379:19–26. [PubMed] [Google Scholar]
- Azzoni AR, Tada SF, Rosselli LK, et al. Expression and purification of a small heat shock protein from the plant pathogen Xylella fastidiosa. Protein Expr Purif. 2004;33(2):297–303. doi: 10.1016/j.pep.2003.10.007. [DOI] [PubMed] [Google Scholar]
- Boston RS, Viitanen PV, Vierling E. Molecular chaperones and protein folding in plants. Plant Mol Biol. 1996;32:191–222. doi: 10.1007/BF00039383. [DOI] [PubMed] [Google Scholar]
- Brock T, Brock K, Belly R, et al. Sulfolobus: A new genus of sulfur-oxidizing bacteria living at low pH and high temperature. Arch Microbiol. 1972;84:54–68. doi: 10.1007/BF00408082. [DOI] [PubMed] [Google Scholar]
- Chang Z, Primm TP, Jakana J, et al. Mycobacterium tuberculosis 16-kDa antigen (Hsp 16.3) functions as an oligomeric structure in vitro to suppress thermal aggregation. J Biol Chem. 1996;271:7218–7223. doi: 10.1074/jbc.271.12.7218. [DOI] [PubMed] [Google Scholar]
- Charles JC, Stefan WR, Angela F, et al. Photodynamic therapy-mediated oxidative stress can induce expression of heat shock proteins. Cancer Res. 1996;56:2355–2360. [PubMed] [Google Scholar]
- Chen T, Villeneuve TS, Garant KA, et al. Functional characterization of artemin, a ferritin homolog synthesized in artemia embryos during encystment and diapause. FEBS J. 2007;274:1093–1101. doi: 10.1111/j.1742-4658.2007.05659.x. [DOI] [PubMed] [Google Scholar]
- Ding X, Lv ZM, Zhao Y, et al. MTH1745, a protein disulfide isomerase-like protein from thermophilic archaea, Methanothermobacter thermoautotrophicum involving in stress response. Cell Stress and Chaperones. 2008;13:239–246. doi: 10.1007/s12192-008-0026-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckburg PB, Lepp PW, Relman DA. Archaea and their potential role in human disease. Infect Immun. 2003;71(2):591–596. doi: 10.1128/IAI.71.2.591-596.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrnsperger M, Graber S, Gaestel M, et al. Binding of non-nativeprotein to Hsp25 during heat shock creates a reservoir of folding intermediates for reactivation. EMBO J. 1997;16:221–229. doi: 10.1093/emboj/16.2.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrnsperger M, Hergersberg C, Wienjues U, et al. Stabilization of proteins and peptides in diagnostic immuno-logical assays by the molecular chaperone hsp25. Anal Biochem. 1998;259:218–225. doi: 10.1006/abio.1998.2630. [DOI] [PubMed] [Google Scholar]
- Esposito L, Sica F, Sorrentino G, et al. Protein crystal growth in the advanced protein crystallization facility on the LMS mission: a comparison of Sulfolobus solfataricus alcohol dehydrogenase crystals grown on the ground and in microgravity. Biol Crystallogr. 1998;54:386–390. doi: 10.1107/S0907444997011992. [DOI] [PubMed] [Google Scholar]
- Haslbeck M, Franzmann T, Weinfurtner D, et al. Some like it hot: the structure and unction of small heat-shock proteins. Nat Struct Mol Biol. 2005;12:842–846. doi: 10.1038/nsmb993. [DOI] [PubMed] [Google Scholar]
- Jakob U, Gaestel M, Engel K, et al. Small heat shock proteins are molecular chaperones. J Biol Chem. 1993;268:1517–1520. [PubMed] [Google Scholar]
- Keisuke U, Takao Y, Tadashi M, et al. Small heat shock protein of a hyperthermophilic archaeum, Thermococcus sp. strain KS- 1, exists as a spherical 24 mer and its expression is highly induced under heat-stress conditions. J Biosci Bioeng. 2001;92(2):161–166. doi: 10.1263/jbb.92.161. [DOI] [PubMed] [Google Scholar]
- Klumpp M, Baumeister W. The thermosome: archetype of group II chaperonins. FEBS J. 1998;430:73–77. doi: 10.1016/S0014-5793(98)00541-9. [DOI] [PubMed] [Google Scholar]
- Laszlo A, Li GC. Effect of amino acid analogs on the development of thermotolerance and on thermotolerant cells. J Cell Physiol. 1993;154:419–432. doi: 10.1002/jcp.1041540226. [DOI] [PubMed] [Google Scholar]
- Lindquist S, Craig EA. The heat-shock proteins. Annu Rev Genet. 1998;22:631–677. doi: 10.1146/annurev.ge.22.120188.003215. [DOI] [PubMed] [Google Scholar]
- Macario AJ, Dugan CB, Conway ME. A dnaK homolog in the archaebacterium Methanosarcina mazei S6. Gene. 1991;108(1):133–137. doi: 10.1016/0378-1119(91)90498-Z. [DOI] [PubMed] [Google Scholar]
- Macario AJL, Lange M, Ahring BK, et al. Stress genes and proteins in the Archaea. Microbiol Mol Biol Rev. 1999;63:923–967. doi: 10.1128/mmbr.63.4.923-967.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacRae TH. Structure and function of small heat shock/a-crystallin proteins: established concepts and emerging ideas. Cell Mol Life Sci. 2000;57:899–913. doi: 10.1007/PL00000733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madigan MT, Martinko JM, Parker J (2000) Prokaryotic diversity: the Archaea. In: Brock biology of microorganisms. Prentice-Hall, Inc., Upper Saddle River, pp 546–572
- Martin H, Andreas K, Johannes B, et al. Structural dynamics of archaeal small heat shock proteins. J Mol Biol. 2008;378:362–374. doi: 10.1016/j.jmb.2008.01.095. [DOI] [PubMed] [Google Scholar]
- Nicholas PR, Isabelle D, Magnus L, et al. Identification of two origins of replicationin the single chromosome of the archaeon Sulfolobus solfataricus. Cell. 2004;116:25–38. doi: 10.1016/S0092-8674(03)01034-1. [DOI] [PubMed] [Google Scholar]
- Samson RY, Obita T, Freund SM, et al. A role for the ESCRT system in cell division in Archaea. Science. 2008;322:1710–1713. doi: 10.1126/science.1165322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samson RY, Obita T, Hodgson B, et al. Molecular and structural basis of ESCRT-III recruitment to membranes during Archaeal cell division. Mol Cell. 2011;41:186–196. doi: 10.1016/j.molcel.2010.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoru T. Analytical assays of human HSP27 and thermal-stress survival of Escherichia coli cells that overexpress it. Biochem Biophys Res Commun. 2006;341:1252–1256. doi: 10.1016/j.bbrc.2006.01.090. [DOI] [PubMed] [Google Scholar]
- She Q, Singh RK, Confalonieri F, et al. The complete genome of the crenarchaeon Sulfolobus solfataricus P2. Proc Natl Acad Sci USA. 2001;98:7835–7840. doi: 10.1073/pnas.141222098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studier FW, Rosenberg AH, Dunn JJ, et al. Use of T7 RNA polymerase to direct expression of cloned genes. Meth Enzymol. 1990;185:60–89. doi: 10.1016/0076-6879(90)85008-C. [DOI] [PubMed] [Google Scholar]
- Sun XK, Fontaine J-M, Bartl I, et al. Induction of Hsp22 (HspB8) by estrogen and the metalloestrogen cadmium in estrogen receptor–positive breast cancer cells. Cell Stress and Chaperones. 2007;12(4):307–319. doi: 10.1379/CSC-276.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Montfort R, Slingsby C, Vierling E. Structure and function of the small heat shock protein/a-crystallin family of molecular chaperones. Adv Protein Chem. 2001;59:105–156. doi: 10.1016/S0065-3233(01)59004-X. [DOI] [PubMed] [Google Scholar]
- Waters ER, Lee GJ, Vierling E. Evolution, structure and function of the small heat shock proteins in plants. J Exp Bot. 1996;47:325–338. doi: 10.1093/jxb/47.3.325. [DOI] [Google Scholar]