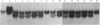Abstract
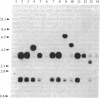
Species- and strain-specific spontaneously occurring tumors have been observed in rodents maintained under normal laboratory conditions. Elucidation of the molecular mechanisms associated with the development of these spontaneous tumors may provide a better understanding of tumor development associated with exposure to chemical carcinogens. In view of the high frequencies of oncogene activation shown in rodent tumors induced by known chemical carcinogens, we have investigated oncogene activation in spontaneous tumors of the B6C3F1 mouse and Fischer 344/N rat by DNA transfection techniques. A marked difference in the presence of activated oncogenes in spontaneous rat tumors versus spontaneous mouse liver tumors was observed in this study. All rat tumors tested failed to yield activated oncogenes (0/29), whereas 30% (3/10) of mouse hepatocellular adenomas and 77% (10/13) of hepatocellular carcinomas scored positive by DNA transfection. These transforming genes were identified as an activated Ha-ras gene in all the adenoma transfectants and in 8 of the 10 carcinoma transfectants. The two remaining hepatocellular carcinomas contained transforming genes that appear not to be members of the known ras gene family. The B6C3F1 mouse liver system might provide a very sensitive assay not only for assessing the potential of a chemical to activate a cellular proto-oncogene, but also for detecting various classes of proto-oncogenes that are susceptible to mutational activation.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen P. R., Tronick S. R., Aaronson S. A. Structural organization and biological activity of molecular clones of the integrated genome of a BALB/c mouse sarcoma virus. J Virol. 1981 Nov;40(2):431–439. doi: 10.1128/jvi.40.2.431-439.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balmain A., Pragnell I. B. Mouse skin carcinomas induced in vivo by chemical carcinogens have a transforming Harvey-ras oncogene. Nature. 1983 May 5;303(5912):72–74. doi: 10.1038/303072a0. [DOI] [PubMed] [Google Scholar]
- Balmain A., Ramsden M., Bowden G. T., Smith J. Activation of the mouse cellular Harvey-ras gene in chemically induced benign skin papillomas. Nature. 1984 Feb 16;307(5952):658–660. doi: 10.1038/307658a0. [DOI] [PubMed] [Google Scholar]
- Barbacid M., Lauver A. V., Devare S. G. Biochemical and immunological characterization of polyproteins coded for by the McDonough, Gardner-Arnstein, and Snyder-Theilen strains of feline sarcoma virus. J Virol. 1980 Jan;33(1):196–207. doi: 10.1128/jvi.33.1.196-207.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop J. M. Cellular oncogenes and retroviruses. Annu Rev Biochem. 1983;52:301–354. doi: 10.1146/annurev.bi.52.070183.001505. [DOI] [PubMed] [Google Scholar]
- Capon D. J., Chen E. Y., Levinson A. D., Seeburg P. H., Goeddel D. V. Complete nucleotide sequences of the T24 human bladder carcinoma oncogene and its normal homologue. Nature. 1983 Mar 3;302(5903):33–37. doi: 10.1038/302033a0. [DOI] [PubMed] [Google Scholar]
- Capon D. J., Seeburg P. H., McGrath J. P., Hayflick J. S., Edman U., Levinson A. D., Goeddel D. V. Activation of Ki-ras2 gene in human colon and lung carcinomas by two different point mutations. Nature. 1983 Aug 11;304(5926):507–513. doi: 10.1038/304507a0. [DOI] [PubMed] [Google Scholar]
- Eva A., Aaronson S. A. Frequent activation of c-kis as a transforming gene in fibrosarcomas induced by methylcholanthrene. Science. 1983 May 27;220(4600):955–956. doi: 10.1126/science.6302839. [DOI] [PubMed] [Google Scholar]
- Eva A., Tronick S. R., Gol R. A., Pierce J. H., Aaronson S. A. Transforming genes of human hematopoietic tumors: frequent detection of ras-related oncogenes whose activation appears to be independent of tumor phenotype. Proc Natl Acad Sci U S A. 1983 Aug;80(16):4926–4930. doi: 10.1073/pnas.80.16.4926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farber E. Cellular biochemistry of the stepwise development of cancer with chemicals: G. H. A. Clowes memorial lecture. Cancer Res. 1984 Dec;44(12 Pt 1):5463–5474. [PubMed] [Google Scholar]
- Fox T. R., Watanabe P. G. Detection of a cellular oncogene in spontaneous liver tumors of B6C3F1 mice. Science. 1985 May 3;228(4699):596–597. doi: 10.1126/science.3983645. [DOI] [PubMed] [Google Scholar]
- Furth M. E., Davis L. J., Fleurdelys B., Scolnick E. M. Monoclonal antibodies to the p21 products of the transforming gene of Harvey murine sarcoma virus and of the cellular ras gene family. J Virol. 1982 Jul;43(1):294–304. doi: 10.1128/jvi.43.1.294-304.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Guerrero I., Calzada P., Mayer A., Pellicer A. A molecular approach to leukemogenesis: mouse lymphomas contain an activated c-ras oncogene. Proc Natl Acad Sci U S A. 1984 Jan;81(1):202–205. doi: 10.1073/pnas.81.1.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrero I., Villasante A., Corces V., Pellicer A. Activation of a c-K-ras oncogene by somatic mutation in mouse lymphomas induced by gamma radiation. Science. 1984 Sep 14;225(4667):1159–1162. doi: 10.1126/science.6474169. [DOI] [PubMed] [Google Scholar]
- Haseman J. K., Huff J., Boorman G. A. Use of historical control data in carcinogenicity studies in rodents. Toxicol Pathol. 1984;12(2):126–135. doi: 10.1177/019262338401200203. [DOI] [PubMed] [Google Scholar]
- Kraus M. H., Yuasa Y., Aaronson S. A. A position 12-activated H-ras oncogene in all HS578T mammary carcinosarcoma cells but not normal mammary cells of the same patient. Proc Natl Acad Sci U S A. 1984 Sep;81(17):5384–5388. doi: 10.1073/pnas.81.17.5384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Land H., Parada L. F., Weinberg R. A. Cellular oncogenes and multistep carcinogenesis. Science. 1983 Nov 18;222(4625):771–778. doi: 10.1126/science.6356358. [DOI] [PubMed] [Google Scholar]
- Madaule P., Axel R. A novel ras-related gene family. Cell. 1985 May;41(1):31–40. doi: 10.1016/0092-8674(85)90058-3. [DOI] [PubMed] [Google Scholar]
- Nakano H., Yamamoto F., Neville C., Evans D., Mizuno T., Perucho M. Isolation of transforming sequences of two human lung carcinomas: structural and functional analysis of the activated c-K-ras oncogenes. Proc Natl Acad Sci U S A. 1984 Jan;81(1):71–75. doi: 10.1073/pnas.81.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padua R. A., Barrass N., Currie G. A. A novel transforming gene in a human malignant melanoma cell line. Nature. 1984 Oct 18;311(5987):671–673. doi: 10.1038/311671a0. [DOI] [PubMed] [Google Scholar]
- Parada L. F., Weinberg R. A. Presence of a Kirsten murine sarcoma virus ras oncogene in cells transformed by 3-methylcholanthrene. Mol Cell Biol. 1983 Dec;3(12):2298–2301. doi: 10.1128/mcb.3.12.2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulciani S., Santos E., Lauver A. V., Long L. K., Robbins K. C., Barbacid M. Oncogenes in human tumor cell lines: molecular cloning of a transforming gene from human bladder carcinoma cells. Proc Natl Acad Sci U S A. 1982 May;79(9):2845–2849. doi: 10.1073/pnas.79.9.2845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy E. P., Reynolds R. K., Santos E., Barbacid M. A point mutation is responsible for the acquisition of transforming properties by the T24 human bladder carcinoma oncogene. Nature. 1982 Nov 11;300(5888):149–152. doi: 10.1038/300149a0. [DOI] [PubMed] [Google Scholar]
- Santos E., Martin-Zanca D., Reddy E. P., Pierotti M. A., Della Porta G., Barbacid M. Malignant activation of a K-ras oncogene in lung carcinoma but not in normal tissue of the same patient. Science. 1984 Feb 17;223(4637):661–664. doi: 10.1126/science.6695174. [DOI] [PubMed] [Google Scholar]
- Schechter A. L., Stern D. F., Vaidyanathan L., Decker S. J., Drebin J. A., Greene M. I., Weinberg R. A. The neu oncogene: an erb-B-related gene encoding a 185,000-Mr tumour antigen. Nature. 1984 Dec 6;312(5994):513–516. doi: 10.1038/312513a0. [DOI] [PubMed] [Google Scholar]
- Shimizu K., Birnbaum D., Ruley M. A., Fasano O., Suard Y., Edlund L., Taparowsky E., Goldfarb M., Wigler M. Structure of the Ki-ras gene of the human lung carcinoma cell line Calu-1. Nature. 1983 Aug 11;304(5926):497–500. doi: 10.1038/304497a0. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Srivastava S. K., Yuasa Y., Reynolds S. H., Aaronson S. A. Effects of two major activating lesions on the structure and conformation of human ras oncogene products. Proc Natl Acad Sci U S A. 1985 Jan;82(1):38–42. doi: 10.1073/pnas.82.1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukumar S., Notario V., Martin-Zanca D., Barbacid M. Induction of mammary carcinomas in rats by nitroso-methylurea involves malignant activation of H-ras-1 locus by single point mutations. Nature. 1983 Dec 15;306(5944):658–661. doi: 10.1038/306658a0. [DOI] [PubMed] [Google Scholar]
- Tabin C. J., Bradley S. M., Bargmann C. I., Weinberg R. A., Papageorge A. G., Scolnick E. M., Dhar R., Lowy D. R., Chang E. H. Mechanism of activation of a human oncogene. Nature. 1982 Nov 11;300(5888):143–149. doi: 10.1038/300143a0. [DOI] [PubMed] [Google Scholar]
- Taparowsky E., Suard Y., Fasano O., Shimizu K., Goldfarb M., Wigler M. Activation of the T24 bladder carcinoma transforming gene is linked to a single amino acid change. Nature. 1982 Dec 23;300(5894):762–765. doi: 10.1038/300762a0. [DOI] [PubMed] [Google Scholar]
- Wigler M., Silverstein S., Lee L. S., Pellicer A., Cheng Y. c., Axel R. Transfer of purified herpes virus thymidine kinase gene to cultured mouse cells. Cell. 1977 May;11(1):223–232. doi: 10.1016/0092-8674(77)90333-6. [DOI] [PubMed] [Google Scholar]
- Yuasa Y., Gol R. A., Chang A., Chiu I. M., Reddy E. P., Tronick S. R., Aaronson S. A. Mechanism of activation of an N-ras oncogene of SW-1271 human lung carcinoma cells. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3670–3674. doi: 10.1073/pnas.81.12.3670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuasa Y., Srivastava S. K., Dunn C. Y., Rhim J. S., Reddy E. P., Aaronson S. A. Acquisition of transforming properties by alternative point mutations within c-bas/has human proto-oncogene. Nature. 1983 Jun 30;303(5920):775–779. doi: 10.1038/303775a0. [DOI] [PubMed] [Google Scholar]