Abstract
Rapid transcription of the survival transcript, inducible heat shock protein 70 (hsp70), is critical for mounting cytoprotection against severe cellular stress, like elevated temperature. Previous investigations have demonstrated that exercise-induced expression of Hsp70 protein occurs in a fiber-specific pattern; however, the activation pattern of hsp70 mRNA expression remains unclear in skeletal muscle. Consequentially, the temporal localization of hsp70 mRNA was characterized via in situ hybridization (ISH) experiments examining fast-muscle, white vastus: 1, 3, 10, and 24 h after a single bout of intense treadmill running (1 h, 30 m/min, 6% grade) in rats. The role that the physiologic temperature stress associated with exercise (raising core body temperature to 40.0°C for 15 min (HS-40.0°C)) might play in inducing hsp70 mRNA expression was also explored. In skeletal muscle myofibers (SkM), hsp70 mRNA ISH signal was observed to be concentrated in a punctate manner that was associated with nuclei post-exercise. HS-40°C treatment produced minimal detectable hsp70 mRNA ISH signal in SkM. In large intermyofibrillar blood vessels (BV), peak hsp70 mRNA signal, distributed throughout the vessel wall, was observed 1 h post-exercise. In BV, no differences in hsp70 mRNA signal were observed between HS-40°C and EX-1 h. Results indicate that the majority of hsp70 mRNA is retained in a perinuclear localization in SkM post-exercise. They further suggest a muscle-type specific time course for peak hsp70 mRNA expression. This investigation suggests that the physiologic rise in core temperature associated with exercise per se is not the key stimulus responsible for inducing hsp70 mRNA transcription in SkM.
Keywords: Heat shock response, hsp70 mRNA, Regulation, Skeletal muscle, Blood vessel, Exercise
Introduction
Rapid transcription of the survival transcript, inducible heat shock protein 70 (also referred to as hsp70, hsp72, or HSPA1), is critical for mounting cytoprotection against severe cellular stress, like elevated temperature. In response to elevated temperature, the general splicing of precursor mRNA (Yost and Lindquist 1986) and global cap-dependent translation (Duncan et al. 1987) become repressed. However, enhanced hsp70 gene expression persists as part of an eukaryotically conserved phenomenon referred to as the heat shock response (see Wu 1995 for review). Synthesized Hsp70 protein assists in the refolding of denatured peptides, thereby minimizing proteolytic degradation so that the heat shock response may be attenuated. Enhanced Hsp70 protein expression is believed to play an important role in preserving skeletal (Lepore et al. 2000) and cardiac muscle (Karmazyn et al. 1990) function subsequent to injury (see Noble et al. 2010 for review).
The spatiotemporal localization of transcripts in the cytoplasm is important for coupling the synthesis of proteins to location of function (see Meignin and Davis 2010 for review). Skeletal myofibers are densely packed with myofibrils enveloped by sarcoplasmic reticulum, which is predominantly involved in calcium homeostasis. The sarcoplasmic reticulum contains microdomains of endoplasmic reticulum that are involved in the translation and processing of transcripts which encode proteins requiring compartmentalization to specific organelles within the myofiber (Volpe et al. 1992). In skeletal myofibers, ribosomes are localized to both the subsarcolemmal and intermyofibrillar cytoplasm (Horne and Hesketh 1990). Transcripts encoding proteins that are targeted to the endoplasmic reticulum, such as calsequestrin and dihydropyridine receptor mRNA, have been found to localize to the microfilament isotropic-band (I-band) in skeletal myofibers (Nissinen et al. 2005), which has been shown to correlate with microdomains of endoplasmic reticulum (Kaakinen et al. 2008). In contrast, myofiber housekeeping genes, such as various isoforms of myosin heavy chain mRNA, have been found to localize to the intermyofibrillar space (Dix and Eisenberg 1988). Subsarcolemmal localization was additionally common in both of the abovementioned cases. Hsp70 protein has been shown to be concentrated in a subsarcolemmal fashion (O’Neill et al. 2006), and localize to the nucleolus (Milarski and Morimoto 1989) and myofibrils (Paulsen et al. 2009) in response to stressful conditions. In a study by Neufer et al. (1996), in situ hybridization (ISH) of a radioisotope-labeled probe revealed enhanced hsp70 mRNA expression, which was concentrated in the periphery of skeletal myofibers in response to continuous low-frequency motor nerve stimulation. However, the precise cytoplasmic organization and trafficking of hsp70 mRNA in skeletal myofibers remains unclear, particularly in response to exercise.
Heat stress, commonly defined in animal models as whole-body heating at 42°C for 15 min (also known as heat shock) (Noble et al. 2010), represents a controlled stimulus for enhancing hsp70 mRNA expression. Given the extensive capillarization and proximity of neighboring myofibers in a skeletal muscle, the elevated body temperature associated with exercise is also thought to pose homogenous heat stress to all myofibers. Indeed, Watkins et al. (2007) observed minimal variability in intramuscular temperature as measured through probing the belly of vastus lateralis post-exercise (40 ± 0.2°C). However, various modes of exercise tend to induce Hsp70 protein expression in the phenotypically slower contracting and more oxidative myofibers of various skeletal muscles (O’Neill et al. 2006; Tarricone et al. 2008; Tupling et al. 2007), which creates a pattern of differential expression as compared to the neighboring faster and more glycolytic myofibers. In addition to elevated body temperature, the physiologic stress associated with exercise can pose selective homeostatic perturbations to recruited myofibers such as mechanical damage, hypoxia, lowered pH, impaired calcium homeostasis, reactive oxygen species generation, decreased ATP pools, glycogen depletion, and a rise in circulating catecholamines, which have all been implicated in contributing to the activation of the heat shock response (see Noble et al. 2008 for review). However, it is unknown whether slower and more oxidative myofibers experience greater Hsp70 protein expression in response to exercise as a result of preferential recruitment or a heightened sensitivity to the associated rise in body temperature. It is also unclear as to whether the fiber-specific induction of Hsp70 protein is a function of transcriptional control or a consequence of translational regulation.
During exercise, blood flow is increased to the working skeletal muscle and myocardium to deliver nutrients and remove waste products (Laughlin and Armstrong 1982). This increased blood flow is believed to present shear (Wang et al. 2007) and elevated temperature stress (Amrani et al. 1998) to the vasculature, which, in response, is believed to induce Hsp70 protein expression as a means of cytoprotection or, potentially, vascular regulation (see Noble et al. 2010 for review). Indeed, heat stress (Currie et al. 1988) and exercise (Paroo et al. 2002) have both been shown to enhance cardiac function post-injury through a mechanism that has been shown to be dependent on enhanced Hsp70 protein expression, which tends to be preferentially induced in the endothelial cells of the myocardium (Amrani et al. 1998; Leger et al. 2000). However, Hsp70 protein expression pattern in the vasculature is tissue-specific. For example, in the vasculature of skeletal muscle, enhanced Hsp70 protein expression has been observed to be predominantly localized throughout the smooth muscle portion post-exercise (Tarricone et al. 2008). While it is clear that the myocardium becomes protected in response to both exercise and heat stress per se, the role of elevated temperature in inducing Hsp70 protein expression in the vasculature of exercised skeletal muscle remains unknown.
The purpose of this investigation was to characterize the temporal localization of hsp70 mRNA post-exercise in skeletal myofibers and large intermyofibrillar blood vessels. The role that the physiologic temperature stress associated with exercise (40.0°C core body temperature) might play in inducing hsp70 mRNA expression was also explored. It was hypothesized that, in skeletal myofibers and large blood vessels, hsp70 mRNA would initially be localized in a perinuclear fashion before being distributed throughout the cytoplasm at later time points post-exercise. It was further hypothesized that both skeletal myofibers and large blood vessels would experience greater hsp70 mRNA expression in response to exercise than to physiologic heat stress per se.
Materials and methods
Animals and experimental procedures
Use and treatment of Sprague–Dawley rats was approved by the University of Western Ontario Council on Animal Care, which is in accordance with the guidelines of the Canadian Council on Animal Care. Adult male rats (8–11 weeks, ~386 g) were obtained from Charles River, Quebec. All animals were housed two per standard shoe box cage in a facility with constant temperature, humidity, and a 12:12-h light/dark cycle. All animals were fed (LabDiet 5P00) and watered ad libitum.
After being housed for 1 week, animals were randomly assigned to one of seven groups (n = 5 each): sedentary control group (CON); one of four acute exercise groups, which were each subjected to a single, 1 h bout of continuous intense treadmill running (30 m/min, 6% incline, in a 21°C environment), but sacrificed 1, 3, 10, or 24 h following completion of bout (EX-1 h, EX-3 h, EX-10 h, or EX-24 h, respectively); or one of two heat stress groups, which were subjected to full-body heating for 15 min at either 40.0°C (HS-40°C) or 42.0°C (HS-42°C) core body temperature and sacrificed 1 h following completion of treatment to capture hsp70 mRNA expression (Locke et al. 1995). All exercised rats were familiarized to treadmill running 2 and 4 days prior to the experimental bout as follows: 2 min at 15 m/min, 4 min at 24 m/min, 2 min at 30 m/min, and 2 min at 15 m/min (2% incline). Rats were encouraged to run with a gentle air blast from behind. Gentle tapping of the hindquarter was used as a secondary method of encouragement when necessary. The employed familiarization protocol and methods of encouragement do not induce a significant heat shock response (unpublished observations). Weight and rectal temperature were measured immediately prior to and following the exercise bout. Animals subjected to heat stress were anesthetized with isoflurane (20 ml/min) via a nosecone apparatus and placed on a heating pad set at ~55°C for an average of 29 ± 3 min or 37 ± 6 min (total heat load) for HS-40°C or HS-42°C, respectively. Upon reaching 40.0°C or 42.0°C, rectal temperature was maintained for 15 min (40.3 ± 0.2°C or 42.1 ± 0.1°C peak temperature) for HS-40°C or HS-42°C, respectively. Following removal of the nosecone, heat stressed animals were further revived with 10–15 ml of water administered orally. All animals completed their assigned experimental protocol.
Sample collection
All animals were anesthetized with sodium pentobarbital (6 mg per 100 g body weight) prior to sacrifice. Transcardial exsanguination and fixation-perfusion with 1× phosphate-buffered saline (PBS) (100 ml per 100 g body weight), followed by 4% paraformaldehyde (PFA) (200 ml per 100 g body weight), was performed to optimize tissue samples for ISH experiments (Wilkinson 1998). Fast-muscle, white portion of the vastus lateralis (WV), which contains predominantly type IIx and IIx/b myofibers (Armstrong and Phelps 1984) and expresses low resting levels of Hsp70 protein (Locke et al. 1991), and mixed-muscle plantaris (PLT), which contains all myofiber-types and high resting levels of hsp70 mRNA, were harvested and subsequently fixed in 4% PFA for a total of 3 h. Samples were cryoprotected in 15% sucrose/1× PBS until samples dropped, followed by 30% sucrose/1× PBS until samples dropped, and equilibrated in 1:1 15% sucrose/optimal cutting temperature (OCT) media for 1 h. All sample collection and processing was conducted at 4°C to minimize ribonuclease (RNase) activity. Samples were mounted in OCT by submersion into liquid nitrogen-cooled isopentane. Eight-micrometer cross sections were transferred to poly-l-lysine-coated, positively charged slides and left to air dry at room temperature for ~4 h. All sample collection, riboprobe manufacture, and ISH methods were performed in a RNase free environment.
Riboprobe manufacture
The hsp70 plasmid vector used in this investigation, UI-R-CM0-bji-h-10-0-UI.s1 (NCBI accession code BF395145.1), was used to create a novel riboprobe. In rodents, a single isoform of Hsp70 protein, is encoded by two hsp70 transcripts, hspa1a and hspa1b (also referred to as hsp70.1 and hsp70.2, respectively), which contain nearly identical open reading frames and 98.4% 5′ untranslated region sequence similarity. Sequencing results reveal that the plasmid insert used for this investigation aligns with both hsp70 transcript isoforms over a region spanning ~620 nucleotides within the open reading frame and 5′ untranslated region (99% identity, 100% coverage, e-value = 0, <1% gaps). hsp70 plasmid vector was used to transform XL1-Blue competent cells, which were subsequently plated and isolated from picked colonies. hsp70 plasmid was isolated using a QIAprep Spin Miniprep kit. T7/T3 promoting with NotI/EcoRI restriction digested template was used to transcribe sense and antisense riboprobe, respectively (Drysdale et al. 1997). For digestion reactions, 15 μl of plasmid eluate was incubated at 37°C for 2 h with: 5 μl of 10× REact three buffer (Invitrogen 16303-018), 2 μl of restriction enzyme, and 28 μl double deionized autoclaved water (addH2O). Templates were purified via aqueous extraction from a phenol preparation. For in vitro transcription reaction, 4 μl of sense or antisense template was incubated at 37°C for 90 min, with 1.5 μl addH2O, 4 μl nucleoside triphosphate substrate containing 13:7 uridine triphosphate (UTP)/digoxigenin-labeled UTP, 4 μl 10 mM DL-dithiothreitol, 0.5 μl RNaseOUT (Invitrogen 10777-019), 4 μl 5× transcription buffer, and 2 μl RNA polymerase T7 or T3 correspondingly. Riboprobe was purified from the previous reaction with deoxyribonuclease I (Invitrogen 18068015) at 37°C for 10 min, and pelleted out in 80 μl 1% sodium dodecyl sulfate in 1× Tris and ethylenediaminetetraacetic acid (EDTA) buffer (TE buffer), 10 μl 5 M ammonium acetate, and 500 μl −20°C 95% ethanol. Riboprobe was diluted to its working concentration of ~1:500–1:2,000 in hybridization (HYB) buffer (50% formamide, 5× saline sodium citrate (SSC), 1 mg/ml bulk RNA (Roche 10-109-223-001), 1× Denhardt’s solution, 5 mM EDTA, and 0.1% Tween-20). All intermediary DNA and RNA yield assessments were visualized via 1% agarose, ethidium bromide gel electrophoresis in 1× Tris base, acetic acid, and EDTA buffer (TAE buffer).
In situ hybridization
1× PBS-hydrated slides were prehybridized in 60°C HYB buffer and hybridized with either sense or antisense riboprobe at 60°C for ~22 h. Slides were stringently washed (50% formamide, 1× SSC, 0.1% Tween-20) at 60°C, 50°C, and room temperature for 15, 30, and 30 min, respectively, before equilibration in 1× maleic acid buffer–Tween-20 (MABT) twice for 30 min. Sections were blocked (1× MABT, 20% heat inactivated sheep serum, 2% blocking reagent (Roche 11-096-176-001)), and immuno-detected 1:1,500 with anti-digoxigenin antibody conjugated to alkaline phosphatase (AP) (Roche 11-093-274-910) in blocking solution at room temperature for ~18 h. Slides were equilibrated in stain buffer (0.1 M Tris base, 0.05 M MgCl2, 0.1 M NaCl, 0.1% Tween-20), and incubated with AP substrate BM Purple (Roche 11-442-074-001) at room temperature for ~20 h. Slides were cover-slipped with blue-fluorescent, nuclear counterstain, 4′,6-diamidino-2-phenylindole (DAPI), and 1:1 glycerol/1× PBS.
Imaging
All bright-field hsp70 mRNA signal and blue-fluorescent DAPI images were captured in a dark room using a Zeiss Axiovert S100 inverted microscope in combination with a Sony PowerHAD (DXC950-3CCD color) camera. The following objectives were used with the fixed brightness intensity and integration levels as follows: 16× (0,50 1 mm Plan-NEOFLUAR 44, 05, 31), bright-field light level 6 with no integration; 40× (1,30 Oil Plan-NEOFLUAR 44, 40, 50), bright-field light level 12 with no integration, blue fluorescence 100% intensity integrated 6×. All images were captured with fixed phase/contrast filter DIC.5-1.4. Bright-field images were not rendered. Blue-fluorescent images were rendered with ImageJ (property of NIH) to optimize brightness/contrast between the maximum and minimum emittance on the visual histogram and to eliminate red and green channels. A rendering tool was subsequently applied to increase maximum points of contrast (nuclei) by an additional 2%. Bright-field and blue-fluorescent images were stacked and Z-projected at maximum intensity to create a merged result.
Signal quantitation
The absolute number of myofibers (~102 per sample), punctate hsp70 mRNA ISH signal, and hsp70 mRNA ISH signal-free myofibers per fasciculus, were counted in WV cross sections (five randomly selected fasciculi per sample, two samples per animal) to produce single data-points. The criteria for counting punctate hsp70 mRNA signal were one point for each discretely detectable small dot within the boundary of a fasciculus, two points for each large dot, and three points for each distinct region of dark signal. Myofibers were considered to be hsp70 mRNA signal free under the condition that no signal was detectable within, or in direct contact with, the myofiber perimeter. The absolute number of large blood vessels and hsp70 mRNA ISH signal-free large blood vessels was counted (approximately three per sample, two samples per animal) in WV cross sections to produce single data-points.
Statistical analyses
All statistical analysis was performed using SigmaStat version 3.5. Body weight and temperature were analyzed using one-way variance analysis, in conjunction with Tukey’s post hoc test to determine differences between groups. Punctate hsp70 mRNA ISH signal in skeletal myofibers, signal-free skeletal myofibers, and signal-free large blood vessels were all analyzed using Kruskal–Wallis one-way variance analysis on ranks, in conjunction with Student Newman–Keuls post hoc test to determine differences between groups. Post hoc tests were only applied upon the finding of a significant F-ratio and H-test for one-way variance analysis and Kruskal–Wallis one-way variance analysis on ranks respectively (P < 0.001). All data are expressed as mean (n = 5) ± standard error (SE) and considered significantly different at P < 0.05.
Results
hsp70 mRNA temporal localization
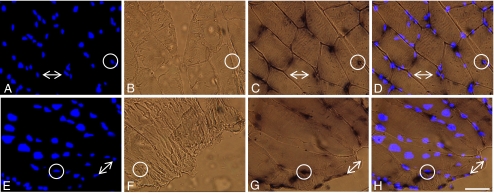
The temporal localization of hsp70 mRNA was investigated in the myofibers of the white portion of the vastus lateralis (WV) via in situ hybridization (ISH) experiments. Figure 1 demonstrates the response of the WV 1 h post-whole-body heating at 42°C (rectal temperature) for 15 min. HS-42°C peak temperature was significantly greater than all post-exercise peak temperatures (P < 0.05) (Table 1); 42°C heat stress is known to induce Hsp70 protein expression and therefore was used as a positive control treatment. Specificity of hsp70 mRNA signal was tested using a sense probe that had similar thermodynamic properties as the antisense probe (Wilkinson 1998). No samples examined in this investigation were observed to display a detectable sense signal (Fig. 1b, f).
Fig. 1.
Localization of hsp70 mRNA in cross (a, b, c, d) and longitudinal sections (e, f, g, h) of the fast, white portion of the vastus lateralis, 1 h post-exposure to the positive control stimulus, 42°C heat stress, (×40 magnification). a, e Blue fluorescence of nuclear counterstain, DAPI. b, f A negative control for the in situ hybridization, sense probe, did not produce any non-specific signal in all samples examined. c, g In situ hybridization of hsp70 mRNA with antisense probe produced a concentrated dark and punctate signal near the periphery of the myofiber, and a diffuse signal throughout the cytoplasm. d, h Merged images of a and c or e and g demonstrated that all punctate hsp70 mRNA signal were associated with the nuclear counterstain DAPI (circles). Differential punctate hsp70 mRNA signal was found among the nuclei of individual myofibers; also consistent for all treatment groups (arrows). Bar = 50 μm
Table 1.
Animal age, body weight, and rectal temperature
| CON | EX-1 h | EX-3 h | EX-10 h | EX-24 h | HS-40°C | HS-42°C | |
|---|---|---|---|---|---|---|---|
| Age (weeks) | 8 | 8 | 11 | 11 | 11 | 11 | 8 |
| Body weight (g) | 340 ± 11a | 338 ± 4a | 417 ± 10 | 424 ± 8 | 420 ± 7 | 397 ± 5 | 336 ± 6a |
| Rectal temperature (°C) | |||||||
| Resting | 37.0 ± 0.1 | 37.4 ± 0.3 | 37.3 ± 0.2 | 37.8 ± 0.1 | 37.4 ± 0.2 | 36.9 ± 0.3 | 37.1 ± 0.2 |
| Post-exercise | 39.8 ± 0.3b | 39.0 ± 0.2b, c | 39.5 ± 0.1b | 40.0 ± 0.2b | |||
| Heat stress peak | 40.3 ± 0.2b | 42.1 ± 0.1b, d | |||||
| Heat stress load (min) | 29 ± 3 | 37 ± 6e | |||||
Values are mean (n = 5) ± SE and considered significant at P < 0.05
aP < 0.05, significantly lighter than EX-3 h, EX-10 h, EX-24 h, and HS-40°C; bP < 0.05, significantly elevated from all resting temperatures; cP < 0.05, significantly lower than EX-1 h and EX-24 h post-exercise temperatures and HS-40°C peak; dP < 0.05, significantly greater than post-exercise temperatures and HS-40 peak; eP < 0.05, significantly greater than HS-40°C heat stress load (total time on heating apparatus)
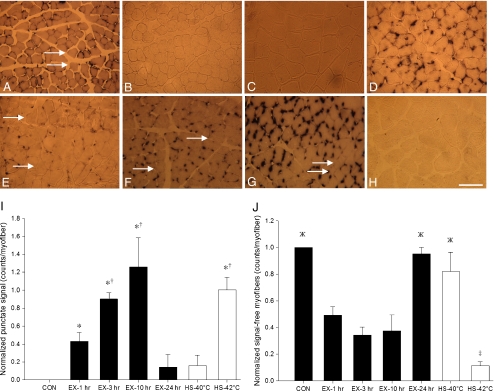
No detectable hsp70 mRNA ISH signal was observed in any skeletal myofiber from CON animals (Fig. 2b). In the skeletal myofibers of all HS-42°C samples, hsp70 mRNA signal was observed to be concentrated in a dark and punctate manner (Fig. 1c, g). In HS-42°C group, diffuse hsp70 mRNA ISH signal was also observed throughout the cytoplasm (Fig. 1g). Punctate hsp70 mRNA signal was primarily associated with the fluorescence of the nuclear counterstain, DAPI, near the periphery of sectioned myofibers (Fig. 1d, h). Mixed-muscle, PLT, harvested from CON group, was used to examine the constitutive localization of hsp70 mRNA signal, which was also observed to be punctate and associated with a perinuclear localization in myofibers (Fig. 2a). Punctate hsp70 mRNA signal derived from ISH experiments was quantitated at various time points post-exercise in myofibers from the WV. When expressed as counts per myofiber, hsp70 mRNA signal was significantly greater as early as 1 h post-exercise and continued to rise until 3 h post-exercise (P < 0.05) (Fig. 2i). However, the perinuclear localization of punctate hsp70 mRNA signal, although seemingly more intense, remained constant at these post-exercise time points (Fig. 2e–g). Nevertheless, diffuse, cytoplasmic hsp70 mRNA signal was also observed in PLT harvested from CON group and in the WV EX-1 h, EX-3 h, and EX-10 h groups. No significant differences in punctate hsp70 mRNA signal were found between EX-24 h and CON groups (Fig. 2i), as no detectable hsp70 mRNA signal was observed in four of five EX-24 h animals (P < 0.05) (Fig. 2h).
Fig. 2.
Temporal localization and temperature-related induction of hsp70 mRNA in situ hybridization signal in skeletal myofiber cross sections (×16 magnification). a Mixed-muscle, plantaris, taken from sedentary control animals, displayed constitutive hsp70 mRNA signal that was concentrated in a punctate fashion. b–h Fast-muscle, white vastus. b Sedentary control displayed no detectable hsp70 mRNA signal. e, f, g, and h follow the temporal localization of hsp70 mRNA signal at 1, 3, 10, and 24 h after a single bout of intense exercise, respectively. hsp70 mRNA signal was observed to be concentrated in a punctate fashion with a concomitant tend in rising counts of punctate signal per myofiber over time such that: b < e <f < g, before falling back to resting levels 24 h post-exercise treatment (h). Although not quantified, the intensity of punctate signal also appeared to rise up to 10 h post-exercise. c and d display hsp70 mRNA signal 1 h post-exposure to 40°C and 42°C heat stress, respectively; 40°C heat stress treatment (c) represents a temperature similar to that experienced during exercise, but it was observed to promote less punctate hsp70 mRNA signal than 1 h post-exercise (e) and 42°C heat stress (d). i The histogram illustrates the counts of punctate hsp70 mRNA signal per myofiber for the various treatment groups. *Significantly greater than CON, EX-24 h, and HS-40°C. †Significantly greater than EX-1 h. j The histograms illustrates the counts of hsp70 mRNA signal-free myofibers (arrows). ‡P < 0.05, significantly less than all other groups. ЖP < 0.05, significantly greater than EX-1 h, EX-3 h, and EX-10 h. All histogram values are normalized mean (n = 5) ± SE and considered significant at P < 0.05. In histograms, black represents control and exercise treatments, while white represents heat stress treatments. Bar = 125 μm
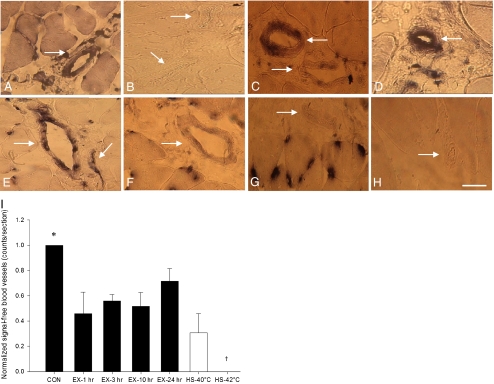
The localization of hsp70 mRNA was also investigated in the large blood vessels of WV via ISH experiments. No detectable hsp70 mRNA signal was observed in any large blood vessels of the CON group (Fig. 3b). In HS-42°C, all large blood vessels were observed to display hsp70 mRNA signal throughout the vessel wall (Fig. 3d). Large blood vessels from mixed-muscle, PLT, harvested from CON group were also observed to constitutively display hsp70 mRNA signal throughout the vessel wall (Fig. 3a). In contrast, and similar to what was observed in the myofibers, although hsp70 mRNA signal pattern was not evident in resting WV blood vessels (Fig. 3b), it was increased 1 h post-exercise (Fig. 3e). As determined by visual comparison to EX-1 h group, hsp70 mRNA ISH signal was observed to be reduced in large blood vessels at the later post-exercise time points (Fig. 3f–h). Although a proportion of large blood vessels did not display any detectable hsp70 mRNA signal, quantitation of signal-free vessels revealed no significant differences between any exercise treatment groups (Fig. 3i).
Fig. 3.
Temporal localization and temperature-related induction of hsp70 mRNA in situ hybridization signal in large intermyofibrillar blood vessel (arrows) cross sections (×40 magnification). a Mixed-muscle, plantaris, taken from sedentary control animals, displayed constitutive hsp70 mRNA signal throughout the vessel wall. b–h Fast-muscle, white vastus. b Sedentary control displayed no detectable hsp70 mRNA signal in vessel wall. e, f, g, and h follow the temporal localization of hsp70 mRNA signal at 1, 3, 10, and 24 h after a single bout of intense exercise respectively. hsp70 mRNA signal was observed to be more intense in the vessel wall 1 h post-exercise treatment (e) as compared to b, f, g, and h. c and d display hsp70 mRNA signal 1 h post-exposure to 40°C and 42°C heat stress respectively. 40°C heat stress treatment (c), which represents a temperature similar to that experienced during exercise, was observed to promote similar hsp70 mRNA signal in vessel wall as compared to 1 h post-exercise (e), but less signal as compared to 42°C heat stress (d). i The histogram illustrates the counts of hsp70 mRNA signal-free large blood vessels for the various treatment groups. *P < 0.05, significantly greater than all treatments. †P < 0.05, significantly less than all treatments. Histogram values are normalized mean (n = 5) ± SE and considered significant at P < 0.05. In histograms, black represents control and exercise treatments, while white represents heat stress treatments. Bar = 50 μm
Temperature-related induction
ISH experiments revealed differential hsp70 mRNA signal between neighboring myofibers of the relatively homogenous WV (Fig. 2, arrows). Differential punctate hsp70 mRNA signal was further observed among the nuclei of individual myofibers for all treatment groups (Fig. 1, arrows). Differential signal between neighboring myofibers was quantitated by counting hsp70 mRNA signal-free myofibers. In WV, the population of hsp70 mRNA signal-free myofibers was not significantly different for EX-1 h, EX-3 h, and EX-10 h (Fig. 2j) despite the tendency for some fibers to be refractory to increases in hsp70 mRNA punctate signal over this time course (Fig. 2i). In contrast, HS-42°C treatment was observed to display significantly enhanced punctate hsp70 mRNA signal versus CON (Fig. 2i), with a concomitant significant loss of signal-free myofibers (P < 0.05) (Fig. 2j).
A physiologic heat stress group, HS-40°C, which was examined 1 h post-treatment, was utilized to investigate the role that elevated body temperature per se might play in the differential induction of hsp70 mRNA expression between neighboring skeletal myofibers post-exercise. The HS-40°C group experienced a peak rise in core body temperature that was not significantly different from post-exercise temperatures but significantly lower than the HS-42°C group (P < 0.05) (Table 1). In ISH experiments, three of five animals in the HS-40°C group produced no detectable hsp70 mRNA signal. Quantitation of hsp70 mRNA ISH signal-free myofibers from this physiologic heat stress group revealed the significant trend: HS-40°C > EX-1 h > HS-42°C (Fig. 2j), which correlated (R2 = 0.87) with the significant signal trend: HS-40°C < EX-1 h < HS-42°C (P < 0.05) (Fig. 2i).
The role that elevated body temperature might play in hsp70 mRNA expression in the large blood vessels of WV was also examined via ISH experiments. As determined by visual inspection, no differences in hsp70 mRNA ISH signal were observed between HS-40°C and EX-1 h groups (Fig. 3c, e). Quantitation of hsp70 mRNA ISH signal-free large blood vessels also revealed no significant differences between HS-40°C and EX-1 h groups (P < 0.05) (Fig. 3i). However, as determined by visual inspection, both HS-40°C and EX-1 h groups were observed to display less hsp70 mRNA signal as compared with HS-42°C group, which displayed no detectable hsp70 mRNA ISH signal-free large blood vessels (Fig. 3i).
Discussion
The main purpose of this study was to investigate the temporal localization of inducible hsp70 mRNA in skeletal myofibers and large intermyofibrillar blood vessels post-exercise. This was achieved by examining digoxigenin-labeled ISH of hsp70 mRNA in fast-muscle, WV, over the time course: 1, 3, 10, and 24 h post-exercise. WV, which contains 97% fast-glycolytic (FG) and 0% slow-oxidative (SO) myofibers (Armstrong and Phelps 1984), was selected for this investigation because it is not heavily recruited by rats under sedentary control conditions, and, as a result, has been shown to have low blood flow (Laughlin and Armstrong 1982) and Hsp70 protein content (Locke et al. 1991) at rest. hsp70 gene expression can be induced in the vasculature or skeletal myofibers by the shear stress associated with increased blood flow (Wang et al. 2007) or the signaling associated with myofiber recruitment, respectively (Noble et al. 2008). This low resting hsp70 mRNA content likely remains below the threshold for detection by the techniques used in this investigation, as no hsp70 mRNA ISH signal was observed in any skeletal myofibers (Fig. 2b) or large blood vessels (Fig. 3b) of WV under resting conditions. Therefore, detectable hsp70 mRNA signal, as observed in both skeletal myofibers and large blood vessels post-42°C heat stress, represents newly transcribed mRNA as compared to the resting condition. However, the skeletal myofibers and large blood vessels of muscles involved in rat low intensity ambulation such as walking, like mixed-PLT for example, which contains 9%, 50%, and 41% SO, fast-oxidative-glycolytic, and FG respectively (Armstrong and Phelps 1984), have been shown to experience greater blood flow (Laughlin and Armstrong 1982) and Hsp70 protein content (Locke et al. 1991; Tarricone et al. 2008) under resting conditions as compared to white muscle. Indeed, results demonstrated greater hsp70 mRNA ISH signal localized to the skeletal myofibers (Fig. 2a) and large blood vessels (Fig. 3a) of PLT under resting conditions as compared to WV. These differences between WV and PLT are consistent with the findings of Locke et al. (1991), which indicate that constitutive Hsp70 protein content of a skeletal muscle is positively related to SO myofiber abundance. For this reason, we chose to investigate the WV in the temporal localization experiments because of our ability to readily detect newly synthesized hsp70 mRNA.
It is well known that whole skeletal muscle hsp70 mRNA (Locke et al. 1995) and protein (Skidmore et al. 1995) expression increases early post-exercise as demonstrated through the use of whole sample homogenates. However, it remains unknown whether all myofibers of a skeletal muscle expresses hsp70 mRNA in response to exercise homogenously or in a more fiber-specific manner typical of protein distribution following exercise (O’Neill et al. 2006; Tarricone et al. 2008; Tupling et al. 2007). As a histological technique, ISH allows for the localization and semi-quantitative comparison of hsp70 transcript abundance between neighboring skeletal myofibers of a skeletal muscle. In this investigation, these data provide evidence for differential hsp70 mRNA expression between neighboring skeletal myofibers post-exercise versus more homogenous expression post-heat stress treatment. These findings support varying thresholds to the activation of the heat shock response, which may be a result of recruitment pattern or phenotype-specific sensitivity to the physiologic stress associated with exercise.
In all cases where hsp70 mRNA ISH signal was detectable in skeletal myofibers, it was observed to be concentrated in a dark and punctate fashion. In a study by Neufer et al. (1996), ISH of a radioisotope-labeled hsp70 mRNA probe revealed enhanced transcript expression concentrated near the periphery of skeletal myofibers in response to continuous low-frequency motor nerve stimulation. The results of this investigation extend these observations, suggesting that nearly all concentrated, punctate hsp70 mRNA signals were associated with a perinuclear localization near the periphery of skeletal myofibers independent of expression level at different post-exercise time points (Fig. 1d, h). Therefore, punctate hsp70 mRNA signal likely represents nuclei that have transcribed this gene. However, of the nuclei population present in skeletal muscle, a proportion represents intermyofibrillar capillaries, stromal cells, monocytes, and satellite cells (Ten Broek et al. 2010). The methods used to fix tissue for ISH in the present investigation (Wilkinson 1998) hindered immunohistochemical analysis, thus limiting the ability to identify myonuclei and other proteins of interest, like myosin heavy chain isoforms or Hsp70 protein. Nevertheless, O’Neill et al. (2006) found that enhanced Hsp70 protein expression is concentrated in a subsarcolemmal fashion in skeletal myofibers post-hind limb overload stress. Since ribosomes are also localized in a subsarcolemmal fashion in skeletal myofibers (Horne and Hesketh 1990) and Hsp70 protein is thought to play a role in protecting the nucleolus during stress (Milarski and Morimoto 1989), it is possible that perinuclear concentrated hsp70 mRNA represents a potential site of translation in the skeletal myofiber. In addition to this perinuclear concentrated signal, diffuse cytoplasmic hsp70 mRNA signal was also observed independent of expression level at different post-exercise time points (Fig. 1g). This result indicates that a proportion of hsp70 mRNA is trafficked from a site of perinuclear concentration to a cytoplasmic distribution more rapidly than hypothesized after transcription. Since ribosomes are additionally localized to the intermyofibrillar cytoplasm (Horne and Hesketh 1990) and enhanced Hsp70 protein expression has been observed to localize to myofibrils post-exercise (Paulsen et al. 2009), it is possible that trafficking of hsp70 mRNA to the cytoplasm plays a role in regulating the site of translation. However, the precise mechanism involved in trafficking hsp70 mRNA remains unclear in skeletal myofibers.
hsp70 mRNA signal was not quantified via optical density in skeletal myofibers because it was not possible to determine the linearity of the signal. Instead, detectable punctate hsp70 mRNA signal was counted and normalized to the number of myofibers examined per sample. In WV, punctate hsp70 mRNA signal tended to progressively increase post-exercise such that resting condition<1 h<3 h<10 h, before returning to resting levels at the 24 h time point (Fig. 2i). Interestingly, the population of hsp70 mRNA ISH signal-free myofibers (which was quantitated by counting signal-free myofibers, normalized to the number of myofibers examined per sample) was unaltered over the 1, 3, and 10 h post-exercise time points (Fig. 2j). Given that differential punctate hsp70 mRNA signal was observed among the nuclei of individual skeletal myofibers at all these post-exercise time points (Fig. 1, arrows), results indicate that a static population of myofibers express hsp70 mRNA with a concomitant rise in transcriptionally active nuclei. Indeed, results support differential hsp70 mRNA ISH signal between neighboring skeletal myofibers of WV (Fig. 2, arrows). Watkins et al. 2007 provide evidence that neighboring myofibers in a skeletal muscle experience homogenous temperature stress in response to exercise. However, this observed pattern of differential hsp70 mRNA expression is more reflective of heterogeneous myofiber recruitment-based induction than homogenous heat stress per se. Various modes of exercise have been shown to induce Hsp70 protein expression in the phenotypically slower contracting and more oxidative myofibers of mixed-muscle, PLT (O’Neill et al. 2006; Tarricone et al. 2008), which produces a pattern of differential expression as compared to the neighboring faster and more glycolytic myofibers. Since WV is predominantly composed of type IIx and IIx/b skeletal myofibers, it may be the case that the more oxidative IIx myofibers preferentially induce hsp70 mRNA expression as a result of selective recruitment during exercise. Furthermore, 42°C heat stress was observed to significantly diminish the population of hsp70 mRNA signal-free skeletal myofibers as compared to exercise treatments (Fig. 2j), indicating that heat stress can indeed induce globalized hsp70 mRNA expression. However, 42°C heat stress represents a more severe stimulus than the physiologic rise in body temperature, 40°C per se, experienced during exercise. Ultimately, it remains a topic of debate whether some skeletal myofibers experience greater hsp70 mRNA expression in response to exercise as a result of preferential recruitment, or a heightened sensitivity to the associated temperature stress. Some fibers may be simply refractory to the exercise-induced rise in hsp70 mRNA expression.
Thus, the role that the physiologic temperature stress associated with exercise might play in inducing hsp70 mRNA expression was explored in skeletal myofibers by raising core body temperature of a group of animals to 40.0°C for 15 min and examining the ISH of hsp70 mRNA 1 h post-treatment (Table 1). The heat stress temperature of 40.0°C was similar to that experienced by all exercise groups at the end of a 1 h bout of treadmill running. However, results demonstrated that three of five animals subjected to HS-40°C treatment displayed no detectable hsp70 mRNA signal. One of the two animals which did respond to HS-40°C treatment displayed minimal hsp70 mRNA signal. Indeed, passive heating to 39.5 ± 0.2°C has been shown to have no effect on Hsp70 protein expression in human skeletal muscle (Morton et al. 2007). The results of this experiment support our hypothesis in that the physiologic rise in core body temperature associated with exercise did not induce hsp70 mRNA transcription in skeletal myofibers in the absence of exercise. However, the heat load (defined as the integral of time and temperature), experienced by both the 40.0°C heat stress and exercise groups, was not controlled for (Table 1). Nevertheless, it has been shown in skeletal muscle that in the absence of increased body temperature, exercise can induce Hsp70 protein expression (Skidmore et al. 1995). Further, it has been demonstrated that Hsp70 protein expression is more dependent on exercise intensity than the associated elevated body temperature per se (Milne and Noble 2002). The results of this investigation support that the physiologic temperature stress associated with exercise cannot alone account for the level of hsp70 mRNA expression seen in response to exercise. Ultimately, it may be a balance of myofiber recruitment and elevated temperature that fully activate hsp70 mRNA transcription in skeletal myofibers in response to exercise.
The temporal localization of hsp70 mRNA was also investigated in large intermyofibrillar blood vessels of WV via ISH experiments. For reasons identified above, the hsp70 mRNA signal in large blood vessels was not quantitated but instead compared via visual inspection by two independent observers. Results demonstrated elevated hsp70 mRNA signal localized throughout the wall of large blood vessels 1 h post-exercise (Fig. 3e) as compared to resting conditions (Fig. 3b) where all large blood vessels in WV were found to be signal free (Fig. 3i). Indeed, it has been shown that enhanced Hsp70 protein expression localizes to the smooth muscle portion of the vasculature in a variety of skeletal muscles post-exercise (Tarricone et al. 2008). Results also demonstrated a faster return to baseline of hsp70 mRNA signal in blood vessels compared to the myofibers, as the 3, 10, and 24 h post-exercise time points were reduced compared to 1 h post-exercise (Fig. 3f, g, h, e, respectively). In the myocardium, which predominantly expresses enhanced Hsp70 protein in the vasculature post-heat stress (Amrani et al. 1998; Leger et al. 2000), hsp70 mRNA expression has been shown to be maximally elevated up to 2 h post-42°C heat stress (Locke et al. 1995). Taken together, results from this experiment indicate that large blood vessels experience more rapid peak hsp70 mRNA expression as compared to the surrounding skeletal myofibers, which were found to display peak punctate hsp70 mRNA signal 10 h post-exercise (Fig. 2i). Further, a relatively static population of large blood vessels was observed to remain hsp70 mRNA signal free at all post-exercise time points examined (Fig. 3i). However, 42°C heat stress was observed to induce hsp70 mRNA signal throughout the wall of all large blood vessels examined (Fig. 3d). Together, these findings raise the question: what role might the physiologic temperature stress associated with exercise play in inducing hsp70 mRNA expression in the intermyofibrillar vasculature?
hsp70 mRNA expression was explored in the large blood vessels of WV post-40.0°C heat stress via ISH experiments. In contrast to skeletal myofibers, no difference in hsp70 mRNA signal or signal-free large blood vessels was detected 1 h post-40.0°C heat stress or exercise (Fig. 3c, e, respectively). This result indicates that the physiologic temperature stress associated with exercise can account for a large degree of hsp70 mRNA signal produced in the large blood vessels of WV in response to exercise. It is possible that during exercise and heat stress treatments, both shear and temperature stress increase to collectively promote the induction of hsp70 mRNA transcription in the vasculature. Indeed, increase in blood flow has been measured to the WV during intense exercise (Laughlin and Armstrong 1982) and cardiac output is elevated in response to heat stress independent of exercise (Horowitz 2002). Lastly, the vasculature is well supplied with receptors that would be activated by the release of adrenergic hormones which occurs both with exercise and heat shock (Horowitz 2002).
In conclusion, the spatial organization of transcripts in the cell can be tightly coupled with the localization of translation and protein function (Meignin and Davis 2010). The results of this investigation indicate that in the skeletal myofibers of fast-muscle, WV, hsp70 mRNA was concentrated in a perinuclear fashion independent of the rise and attenuation of the heat shock response (Fig. 2d, h). This perinuclear localization of hsp70 mRNA may play a role in rapid translation of Hsp70 protein and thus nucleolar protection post-exercise. In comparison to skeletal myofibers, the large intermyofibrillar blood vessels of WV were observed to reach peak hsp70 mRNA expression in a shorter timeframe. In the literature, it remains unclear whether skeletal myofibers experience greater Hsp70 protein expression in response to exercise as a result of preferential recruitment, or phenotype-specific sensitivity to the associated rise in body temperature. The results of this investigation indicate that the physiologic temperature stress associated with exercise, 40°C heat stress per se, is sufficient to induce hsp70 mRNA expression in the large intermyofibrillar blood vessels of WV (Fig. 3d), but not the surrounding skeletal myofibers (Fig. 2d). Ultimately, this investigation supports that the physiologic rise in core temperature associated with exercise per se is not a sufficient stimulus to induce hsp70 mRNA transcription in skeletal myofibers in the absence of recruitment. Further, factors associated with the increase in hsp70 mRNA transcription may differ between the vasculature and the myofibers.
Acknowledgments
The authors thank Dr. Steven J. Deimling and Dr. Thomas A. Drysdale for their outstanding help with techniques in molecular cloning and in situ hybridization as well as Bonaldo et al. 1996 for generously donating the hsp70 construct used in this investigation. This study was supported by grants from the National Science and Engineering Research Council of Canada (8170-05) and the Canadian Institutes of Health Research (CCT-83029) to EG Noble.
References
- Amrani M, Latif N, Morrison K, Gray CC, Jayakumar J, Corbett J, et al. Relative induction of heat shock protein in coronary endothelial cells and cardiomyocytes: implications for myocardial protection. J Thorac Cardiovasc Surg. 1998;115:200–209. doi: 10.1016/S0022-5223(98)70458-1. [DOI] [PubMed] [Google Scholar]
- Armstrong RB, Phelps RO. Muscle fiber type composition of the rat hindlimb. Am J Anat. 1984;171:259–272. doi: 10.1002/aja.1001710303. [DOI] [PubMed] [Google Scholar]
- Currie RW, Karmazyn M, Kloc M, Mailer K. Heat-shock response is associated with enhanced postischemic ventricular recovery. Circ Res. 1988;63:543–549. doi: 10.1161/01.res.63.3.543. [DOI] [PubMed] [Google Scholar]
- Dix DJ, Eisenberg BR. In situ hybridization and immunocytochemistry in serial sections of rabbit skeletal muscle to detect myosin expression. J Histochem Cytochem. 1988;36:1519–1526. doi: 10.1177/36.12.3057072. [DOI] [PubMed] [Google Scholar]
- Drysdale TA, Patterson KD, Saha M, Krieg PA. Retinoic acid can block differentiation of the myocardium after heart specification. Dev Biol. 1997;188:205–215. doi: 10.1006/dbio.1997.8623. [DOI] [PubMed] [Google Scholar]
- Duncan R, Milburn SC, Hershey JW. Regulated phosphorylation and low abundance of HeLa cell initiation factor eIF-4F suggest a role in translational control. Heat shock effects on eIF-4F. J Biol Chem. 1987;262:380–388. [PubMed] [Google Scholar]
- Horne Z, Hesketh J. Immunological localization of ribosomes in striated rat muscle. Evidence for myofibrillar association and ontological changes in the subsarcolemmal myofibrillar distribution. Biochem J. 1990;268:231–236. doi: 10.1042/bj2680231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz M. From molecular and cellular to integrative heat defense during exposure to chronic heat. Comp Biochem Physiol. 2002;131:475–483. doi: 10.1016/s1095-6433(01)00500-1. [DOI] [PubMed] [Google Scholar]
- Kaakinen M, Papponen H, Metsikko K. Microdomains of endoplasmic reticulum within the sarcoplasmic reticulum of skeletal myofibers. Exp Cell Res. 2008;314:237–245. doi: 10.1016/j.yexcr.2007.10.009. [DOI] [PubMed] [Google Scholar]
- Karmazyn M, Mailer K, Currie RW. Acquisition and decay of heat-shock-enhanced postischemic ventricular recovery. Am J Physiol. 1990;259:H424–H431. doi: 10.1152/ajpheart.1990.259.2.H424. [DOI] [PubMed] [Google Scholar]
- Laughlin MH, Armstrong RB. Muscular blood flow distribution patterns as a function of running speed in rats. Am J Physiol. 1982;243:H296–H306. doi: 10.1152/ajpheart.1982.243.2.H296. [DOI] [PubMed] [Google Scholar]
- Leger JP, Smith FM, Currie RW. Confocal microscopic localization of constitutive and heat shock-induced proteins HSP70 and HSP27 in the rat heart. Circulation. 2000;102:1703–1709. doi: 10.1161/01.cir.102.14.1703. [DOI] [PubMed] [Google Scholar]
- Lepore DA, Hurley JV, Stewart AG, Morrison WA, Anderson RL. Prior heat stress improves survival of ischemic-reperfused skeletal muscle in vivo. Muscle Nerve. 2000;23:1847–1855. doi: 10.1002/1097-4598(200012)23:12<1847::AID-MUS8>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Locke M, Noble EG, Atkinson BG. Inducible isoform of HSP70 is constitutively expressed in a muscle fiber type specific pattern. Am J Physiol. 1991;261:C774–C779. doi: 10.1152/ajpcell.1991.261.5.C774. [DOI] [PubMed] [Google Scholar]
- Locke M, Noble EG, Tanguay RM, Feild MR, Ianuzzo SE, Ianuzzo CD. Activation of heat-shock transcription factor in rat heart after heat shock and exercise. Am J Physiol. 1995;268:C1387–C1394. doi: 10.1152/ajpcell.1995.268.6.C1387. [DOI] [PubMed] [Google Scholar]
- Meignin C, Davis I. Transmitting the message: intracellular mRNA localization. Curr Opin Cell Biol. 2010;22:112–119. doi: 10.1016/j.ceb.2009.11.011. [DOI] [PubMed] [Google Scholar]
- Milarski KL, Morimoto RI. Mutational analysis of the human HSP70 protein: distinct domains for nucleolar localization and adenosine triphosphate binding. J Cell Biol. 1989;109:1947–1962. doi: 10.1083/jcb.109.5.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milne KJ, Noble EG. Exercise-induced elevation of HSP70 is intensity dependent. J Appl Physiol. 2002;93:561–568. doi: 10.1152/japplphysiol.00528.2001. [DOI] [PubMed] [Google Scholar]
- Morton JP, Maclaren DP, Cable NT, Campbell IT, Evans L, Bongers T, et al. Elevated core and muscle temperature to levels comparable to exercise do not increase heat shock protein content of skeletal muscle of physically active men. Acta Physiol (Oxf) 2007;190:319–327. doi: 10.1111/j.1748-1716.2007.01711.x. [DOI] [PubMed] [Google Scholar]
- Neufer PD, Ordway GA, Hand GA, Shelton JM, Richardson JA, Benjamin IJ, et al. Continuous contractile activity induces fiber type specific expression of HSP70 in skeletal muscle. Am J Physiol. 1996;271:C1828–C1837. doi: 10.1152/ajpcell.1996.271.6.C1828. [DOI] [PubMed] [Google Scholar]
- Nissinen M, Kaisto T, Salmela P, Peltonen J, Metsikko K. Restricted distribution of mRNAs encoding a sarcoplasmic reticulum or transverse tubule protein in skeletal myofibers. J Histochem Cytochem. 2005;53:217–227. doi: 10.1369/jhc.4A6431.2005. [DOI] [PubMed] [Google Scholar]
- Noble EG, Milne KJ, Melling CW. Heat shock proteins and exercise: a primer. Appl Physiol Nutr Metab. 2008;33:1050–1065. doi: 10.1139/H08-069. [DOI] [PubMed] [Google Scholar]
- Noble EG, Melling CWJ, Milne KJ. HSP, exercise and skeletal muscle. In: Asea AA, Calderwood SK, editors. Heat shock proteins and whole body physiology. New York: Springer; 2010. pp. 285–316. [Google Scholar]
- O’Neill DET, Aubrey FK, Zeldin DA, Michel RN, Noble EG. Slower skeletal muscle phenotypes are critical for constitutive expression of Hsp70 in overloaded rat plantaris muscle. J Appl Physiol. 2006;100:981–987. doi: 10.1152/japplphysiol.00831.2005. [DOI] [PubMed] [Google Scholar]
- Paroo Z, Haist JV, Karmazyn M, Noble EG. Exercise improves postischemic cardiac function in males but not females: consequences of a novel sex-specific heat shock protein 70 response. Circ Res. 2002;90:911–917. doi: 10.1161/01.RES.0000016963.43856.B1. [DOI] [PubMed] [Google Scholar]
- Paulsen G, Lauritzen F, Bayer ML, Kalhovde JM, Ugelstad I, Owe SG, et al. Subcellular movement and expression of HSP27, alphaB-crystallin, and HSP70 after two bouts of eccentric exercise in humans. J Appl Physiol. 2009;107:570–582. doi: 10.1152/japplphysiol.00209.2009. [DOI] [PubMed] [Google Scholar]
- Skidmore R, Gutierrez JA, Guerriero V, Jr, Kregel KC. HSP70 induction during exercise and heat stress in rats: role of internal temperature. Am J Physiol. 1995;268:R92–R97. doi: 10.1152/ajpregu.1995.268.1.R92. [DOI] [PubMed] [Google Scholar]
- Tarricone E, Scapin C, Vitadello M, Esposito F, Margonato V, Milano G, et al. Cellular distribution of Hsp70 expression in rat skeletal muscles. Effects of moderate exercise training and chronic hypoxia. Cell Stress Chaperon. 2008;13:483–495. doi: 10.1007/s12192-008-0048-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broek RW, Grefte S, den Hoff JW. Regulatory factors and cell populations involved in skeletal muscle regeneration. J Cell Physiol. 2010;224:7–16. doi: 10.1002/jcp.22127. [DOI] [PubMed] [Google Scholar]
- Tupling AR, Bombardier E, Stewart RD, Vigna C, Aqui AE. Muscle fiber type-specific response of Hsp70 expression in human quadriceps following acute isometric exercise. J Appl Physiol. 2007;103:2105–2111. doi: 10.1152/japplphysiol.00771.2007. [DOI] [PubMed] [Google Scholar]
- Volpe P, Villa A, Podini P, Martini A, Noiw A, Panzeri MC, et al. The endoplasmic reticulum- sarcoplasmic reticulum connection: distribution of endoplasmic reticulum markers in the sarcoplasmic reticulum of skeletal muscle fibers. Proc Natl Acad Sci USA. 1992;89:6142–6146. doi: 10.1073/pnas.89.13.6142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XL, Fu A, Raghavakaimal S, Lee HC. Proteomic analysis of vascular endothelial cells in response to laminar shear stress. Proteomics. 2007;7:588–596. doi: 10.1002/pmic.200600568. [DOI] [PubMed] [Google Scholar]
- Watkins AM, Cheek DJ, Harvey AE, Goodwin JD, Blair KE, Mitchell JB. Heat shock protein (HSP-72) levels in skeletal muscle following work in heat. Aviat Space Environ Med. 2007;78:901–905. [PubMed] [Google Scholar]
- Wilkinson DG. In situ hybridization: a practical approach. Oxford: Oxford University Press; 1998. [Google Scholar]
- Wu C. Heat shock transcription factors: structure and regulation. Annu Rev Cell Dev Biol. 1995;11:441–469. doi: 10.1146/annurev.cb.11.110195.002301. [DOI] [PubMed] [Google Scholar]
- Yost HJ, Lindquist S. RNA splicing is interrupted by heat shock and is rescued by heat shock protein synthesis. Cell. 1986;45:185–193. doi: 10.1016/0092-8674(86)90382-X. [DOI] [PubMed] [Google Scholar]