Abstract
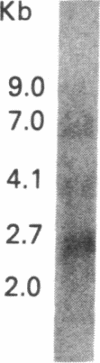
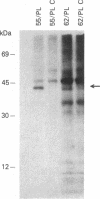
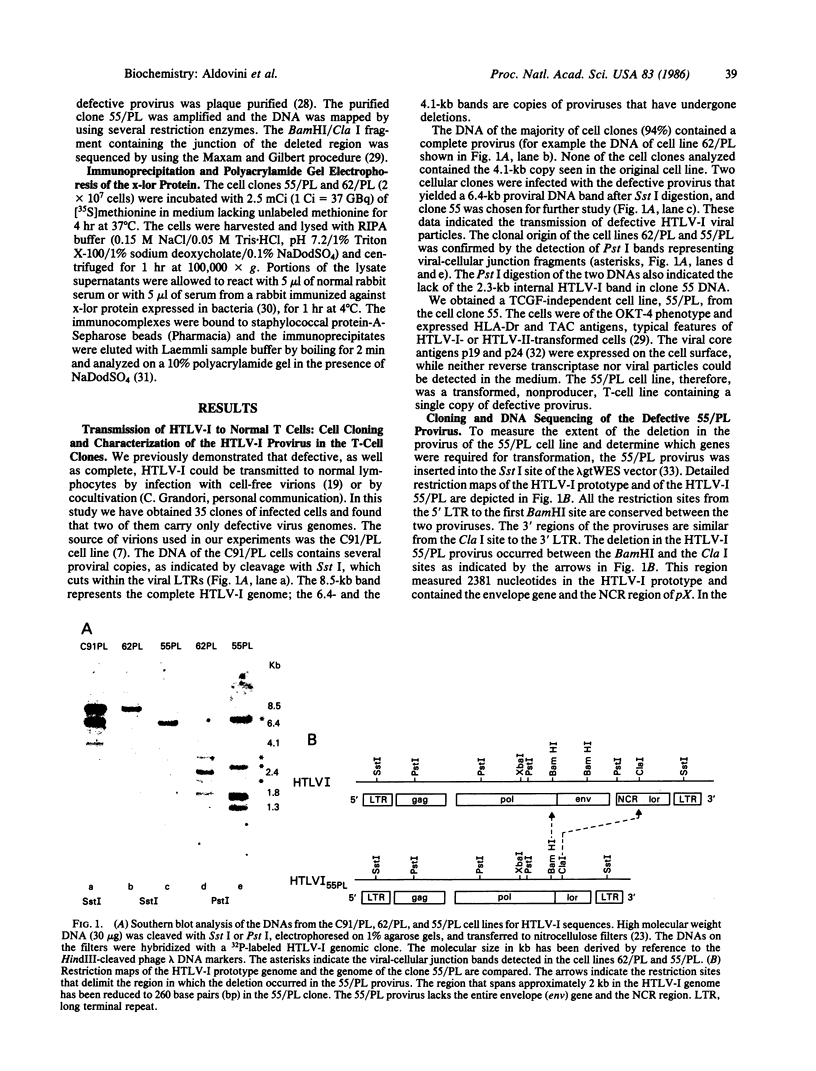
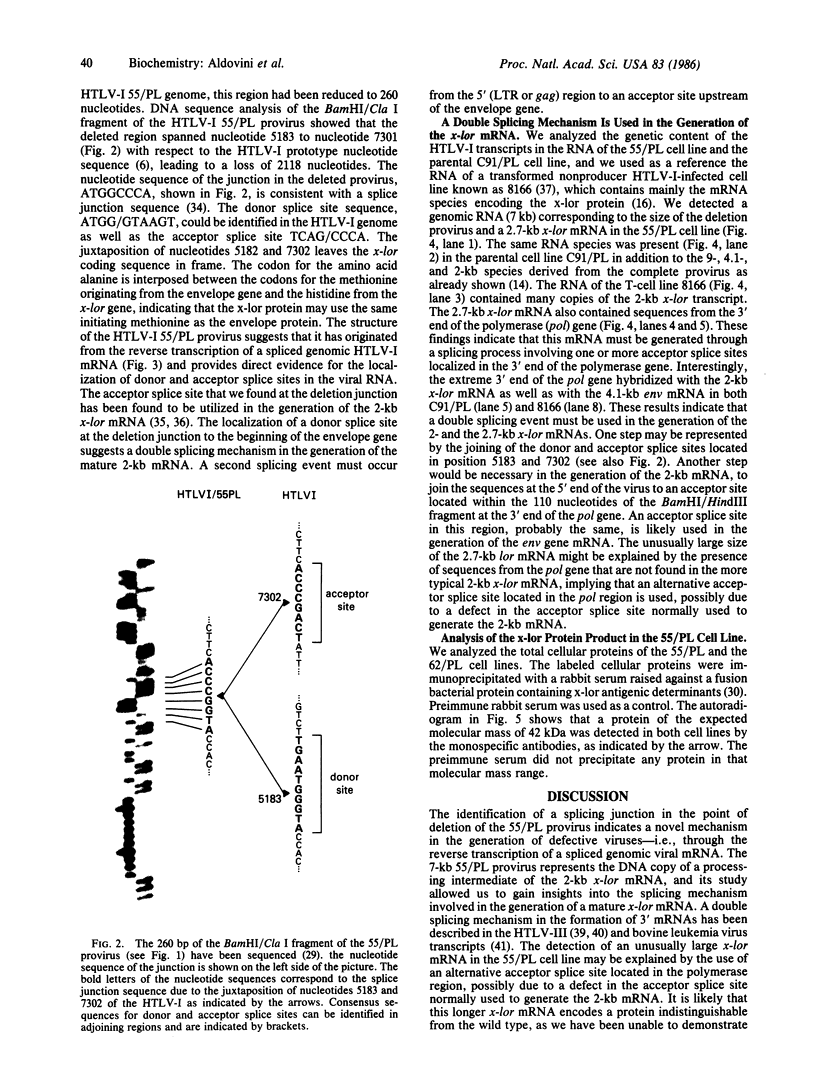
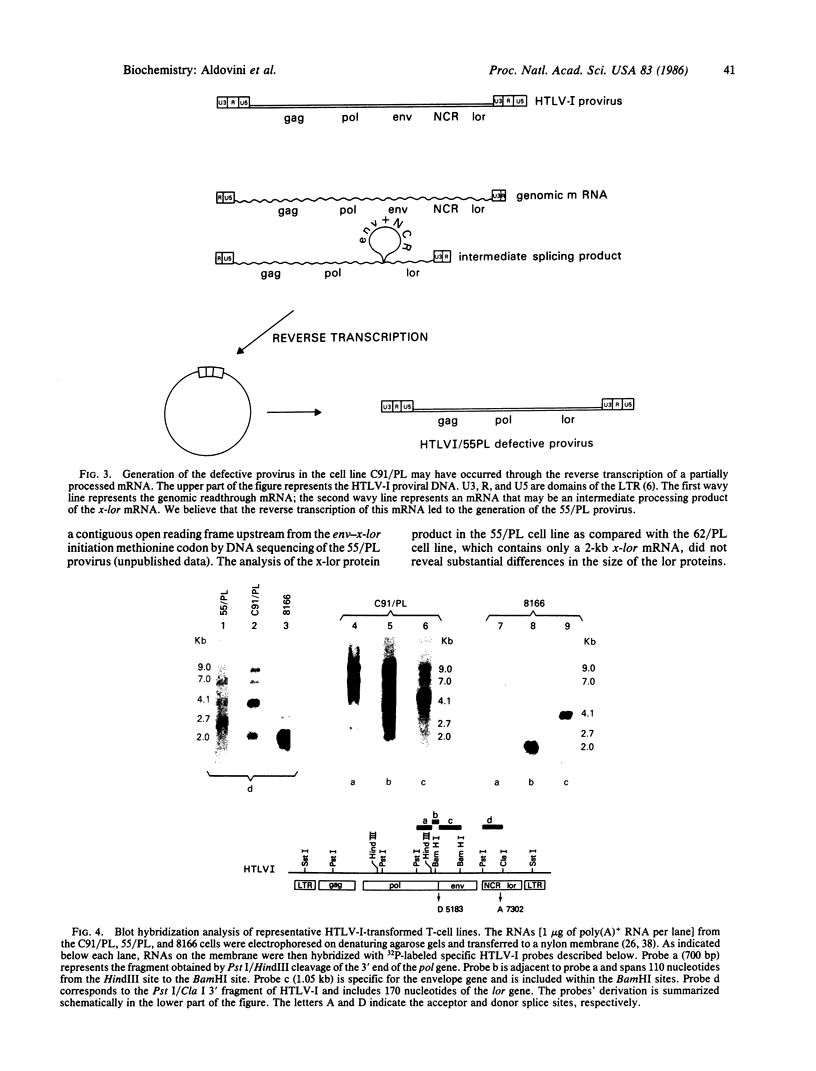
The genome of the human T-cell leukemia/lymphotropic virus type I (HTLV-I) contains a functional gene denominated x-lor that may be important in HTLV-I transformation of human T cells. To study the role of x-lor and other HTLV-I genes in cellular transformation, we obtained a transformed nonproducer human T-cell line containing a single defective HTLV-I provirus (HTLV-I 55/PL). This 7-kilobase provirus had undergone a deletion involving the entire envelope gene and the nonconserved region. The point of the deletion corresponded to the junction of a donor splice site, located between the polymerase gene and the envelope gene (nucleotide 5183), and the acceptor site for the mRNA of the x-lor gene (nucleotide 7302). The juxtaposition of nucleotides 5182 and 7302 brings the initiating methionine codon of the envelope gene immediately 5' to the x-lor region, leaving the DNA sequence in frame for expression of a protein product. This finding suggests that a double splicing mechanism is used to express the x-lor gene, and that the defective provirus 55/PL was generated through the reverse transcription of a partially spliced mRNA. Analysis of the x-lor mRNA of other HTLV-I-transformed cell lines revealed that a double splicing process is commonly used. Furthermore, since 55/PL can be faithfully transmitted and is able to immortalize recipient T cells, we can conclude that the envelope gene is not necessary for in vitro transformation by HTLV-I.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arya S. K., Guo C., Josephs S. F., Wong-Staal F. Trans-activator gene of human T-lymphotropic virus type III (HTLV-III). Science. 1985 Jul 5;229(4708):69–73. doi: 10.1126/science.2990040. [DOI] [PubMed] [Google Scholar]
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Blattner F. R., Williams B. G., Blechl A. E., Denniston-Thompson K., Faber H. E., Furlong L., Grunwald D. J., Kiefer D. O., Moore D. D., Schumm J. W. Charon phages: safer derivatives of bacteriophage lambda for DNA cloning. Science. 1977 Apr 8;196(4286):161–169. doi: 10.1126/science.847462. [DOI] [PubMed] [Google Scholar]
- Blin N., Stafford D. W. A general method for isolation of high molecular weight DNA from eukaryotes. Nucleic Acids Res. 1976 Sep;3(9):2303–2308. doi: 10.1093/nar/3.9.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen I. S., Quan S. G., Golde D. W. Human T-cell leukemia virus type II transforms normal human lymphocytes. Proc Natl Acad Sci U S A. 1983 Nov;80(22):7006–7009. doi: 10.1073/pnas.80.22.7006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Franchini G., Wong-Staal F., Gallo R. C. Human T-cell leukemia virus (HTLV-I) transcripts in fresh and cultured cells of patients with adult T-cell leukemia. Proc Natl Acad Sci U S A. 1984 Oct;81(19):6207–6211. doi: 10.1073/pnas.81.19.6207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelmann E. P., Franchini G., Manzari V., Wong-Staal F., Gallo R. C. Molecular cloning of a unique human T-cell leukemia virus (HTLV-IIMo). Proc Natl Acad Sci U S A. 1984 Feb;81(4):993–997. doi: 10.1073/pnas.81.4.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goh W. C., Sodroski J., Rosen C., Haseltine W. Expression of the x-lor gene of human T-cell leukemia virus I in Escherichia coli. J Virol. 1985 Aug;55(2):497–499. doi: 10.1128/jvi.55.2.497-499.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haseltine W. A., Sodroski J., Patarca R., Briggs D., Perkins D., Wong-Staal F. Structure of 3' terminal region of type II human T lymphotropic virus: evidence for new coding region. Science. 1984 Jul 27;225(4660):419–421. doi: 10.1126/science.6330894. [DOI] [PubMed] [Google Scholar]
- Kalyanaraman V. S., Sarngadharan M. G., Robert-Guroff M., Miyoshi I., Golde D., Gallo R. C. A new subtype of human T-cell leukemia virus (HTLV-II) associated with a T-cell variant of hairy cell leukemia. Science. 1982 Nov 5;218(4572):571–573. doi: 10.1126/science.6981847. [DOI] [PubMed] [Google Scholar]
- Kiyokawa T., Seiki M., Imagawa K., Shimizu F., Yoshida M. Identification of a protein (p40x) encoded by a unique sequence pX of human T-cell leukemia virus type I. Gan. 1984 Sep;75(9):747–751. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee T. H., Coligan J. E., Sodroski J. G., Haseltine W. A., Salahuddin S. Z., Wong-Staal F., Gallo R. C., Essex M. Antigens encoded by the 3'-terminal region of human T-cell leukemia virus: evidence for a functional gene. Science. 1984 Oct 5;226(4670):57–61. doi: 10.1126/science.6089350. [DOI] [PubMed] [Google Scholar]
- Lehrach H., Diamond D., Wozney J. M., Boedtker H. RNA molecular weight determinations by gel electrophoresis under denaturing conditions, a critical reexamination. Biochemistry. 1977 Oct 18;16(21):4743–4751. doi: 10.1021/bi00640a033. [DOI] [PubMed] [Google Scholar]
- Mamoun R. Z., Astier-Gin T., Kettmann R., Deschamps J., Rebeyrotte N., Guillemain B. J. The pX region of the bovine leukemia virus is transcribed as a 2.1-kilobase mRNA. J Virol. 1985 May;54(2):625–629. doi: 10.1128/jvi.54.2.625-629.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Miwa M., Shimotohno K., Hoshino H., Fujino M., Sugimura T. Detection of pX proteins in human T-cell leukemia virus (HTLV)-infected cells by using antibody against peptide deduced from sequences of X-IV DNA of HTLV-I and Xc DNA of HTLV-II proviruses. Gan. 1984 Sep;75(9):752–755. [PubMed] [Google Scholar]
- Morgan D. A., Ruscetti F. W., Gallo R. Selective in vitro growth of T lymphocytes from normal human bone marrows. Science. 1976 Sep 10;193(4257):1007–1008. doi: 10.1126/science.181845. [DOI] [PubMed] [Google Scholar]
- Mount S. M. A catalogue of splice junction sequences. Nucleic Acids Res. 1982 Jan 22;10(2):459–472. doi: 10.1093/nar/10.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muesing M. A., Smith D. H., Cabradilla C. D., Benton C. V., Lasky L. A., Capon D. J. Nucleic acid structure and expression of the human AIDS/lymphadenopathy retrovirus. Nature. 1985 Feb 7;313(6002):450–458. doi: 10.1038/313450a0. [DOI] [PubMed] [Google Scholar]
- Okamoto T., Josephs S. F., Kawanishi M., Wong-Staal F. Determination of a splice acceptor site of pX gene in HTLV-I infected cells. Virology. 1985 Jun;143(2):636–639. doi: 10.1016/0042-6822(85)90404-0. [DOI] [PubMed] [Google Scholar]
- Poiesz B. J., Ruscetti F. W., Gazdar A. F., Bunn P. A., Minna J. D., Gallo R. C. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7415–7419. doi: 10.1073/pnas.77.12.7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poiesz B. J., Ruscetti F. W., Reitz M. S., Kalyanaraman V. S., Gallo R. C. Isolation of a new type C retrovirus (HTLV) in primary uncultured cells of a patient with Sézary T-cell leukaemia. Nature. 1981 Nov 19;294(5838):268–271. doi: 10.1038/294268a0. [DOI] [PubMed] [Google Scholar]
- Popovic M., Sarin P. S., Robert-Gurroff M., Kalyanaraman V. S., Mann D., Minowada J., Gallo R. C. Isolation and transmission of human retrovirus (human t-cell leukemia virus). Science. 1983 Feb 18;219(4586):856–859. doi: 10.1126/science.6600519. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Robert-Guroff M., Nakao Y., Notake K., Ito Y., Sliski A., Gallo R. C. Natural antibodies to human retrovirus HTLV in a cluster of Japanese patients with adult T cell leukemia. Science. 1982 Feb 19;215(4535):975–978. doi: 10.1126/science.6760397. [DOI] [PubMed] [Google Scholar]
- Robert-Guroff M., Ruscetti F. W., Posner L. E., Poiesz B. J., Gallo R. C. Detection of the human T cell lymphoma virus p19 in cells of some patients with cutaneous T cell lymphoma and leukemia using a monoclonal antibody. J Exp Med. 1981 Dec 1;154(6):1957–1964. doi: 10.1084/jem.154.6.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salahuddin S. Z., Markham P. D., Wong-Staal F., Franchini G., Kalyanaraman V. S., Gallo R. C. Restricted expression of human T-cell leukemia--lymphoma virus (HTLV) in transformed human umbilical cord blood lymphocytes. Virology. 1983 Aug;129(1):51–64. doi: 10.1016/0042-6822(83)90395-1. [DOI] [PubMed] [Google Scholar]
- Seiki M., Hattori S., Yoshida M. Human adult T-cell leukemia virus: molecular cloning of the provirus DNA and the unique terminal structure. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6899–6902. doi: 10.1073/pnas.79.22.6899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiki M., Hikikoshi A., Taniguchi T., Yoshida M. Expression of the pX gene of HTLV-I: general splicing mechanism in the HTLV family. Science. 1985 Jun 28;228(4707):1532–1534. doi: 10.1126/science.2990031. [DOI] [PubMed] [Google Scholar]
- Shaw G. M., Gonda M. A., Flickinger G. H., Hahn B. H., Gallo R. C., Wong-Staal F. Genomes of evolutionarily divergent members of the human T-cell leukemia virus family (HTLV-I and HTLV-II) are highly conserved, especially in pX. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4544–4548. doi: 10.1073/pnas.81.14.4544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimotohno K., Miwa M., Slamon D. J., Chen I. S., Hoshino H., Takano M., Fujino M., Sugimura T. Identification of new gene products coded from X regions of human T-cell leukemia viruses. Proc Natl Acad Sci U S A. 1985 Jan;82(2):302–306. doi: 10.1073/pnas.82.2.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slamon D. J., Shimotohno K., Cline M. J., Golde D. W., Chen I. S. Identification of the putative transforming protein of the human T-cell leukemia viruses HTLV-I and HTLV-II. Science. 1984 Oct 5;226(4670):61–65. doi: 10.1126/science.6089351. [DOI] [PubMed] [Google Scholar]
- Sodroski J. G., Goh W. C., Rosen C. A., Salahuddin S. Z., Aldovini A., Franchini G., Wong-Staal F., Gallo R. C., Sugamura K., Hinuma Y. trans-Activation of the human T-cell leukemia virus long terminal repeat correlates with expression of the x-lor protein. J Virol. 1985 Sep;55(3):831–835. doi: 10.1128/jvi.55.3.831-835.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sodroski J. G., Rosen C. A., Haseltine W. A. Trans-acting transcriptional activation of the long terminal repeat of human T lymphotropic viruses in infected cells. Science. 1984 Jul 27;225(4660):381–385. doi: 10.1126/science.6330891. [DOI] [PubMed] [Google Scholar]
- Sodroski J., Rosen C., Goh W. C., Haseltine W. A transcriptional activator protein encoded by the x-lor region of the human T-cell leukemia virus. Science. 1985 Jun 21;228(4706):1430–1434. doi: 10.1126/science.2990028. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Staal S. P., Hartley J. W., Rowe W. P. Isolation of transforming murine leukemia viruses from mice with a high incidence of spontaneous lymphoma. Proc Natl Acad Sci U S A. 1977 Jul;74(7):3065–3067. doi: 10.1073/pnas.74.7.3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wachsman W., Golde D. W., Temple P. A., Orr E. C., Clark S. C., Chen I. S. HTLV x-gene product: requirement for the env methionine initiation codon. Science. 1985 Jun 28;228(4707):1534–1537. doi: 10.1126/science.2990032. [DOI] [PubMed] [Google Scholar]
- Wachsman W., Shimotohno K., Clark S. C., Golde D. W., Chen I. S. Expression of the 3' terminal region of human T-cell leukemia viruses. Science. 1984 Oct 12;226(4671):177–179. doi: 10.1126/science.6091270. [DOI] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Rossi A., Aldovini A., Franchini G., Mann D., Gallo R. C., Wong-Staal F. Clonal selection of T lymphocytes infected by cell-free human T-cell leukemia/lymphoma virus type I: parameters of virus integration and expression. Virology. 1985 Jun;143(2):640–645. doi: 10.1016/0042-6822(85)90405-2. [DOI] [PubMed] [Google Scholar]