Synopsis
Esophageal high resolution manometry (HRM) improves the management of patients with non-obstructive dysphagia. It has increased the diagnostic yield for detecting achalasia and defined three clinically relevant achalasia subtypes. Esophagogastric junction (EGJ) outflow obstruction, defined as an impaired EGJ relaxation in association with some preserved peristalsis, might also represent an achalasia variant in some cases. Using the concept of distal latency, the criteria for defining distal esophageal spasm, have been revised as the occurrence of premature distal contractions. Finally, the combination of HRM and impedance monitoring allows for a functional definition of weak peristalsis associated with incomplete bolus transit.
Keywords: dysphagia, high resolution manometry, impedance
Introduction
Esophageal motility disorders may be an explanation of dysphagia in patients after exclusion of esophageal structural lesions by endoscopy and radiography and eosinophilic esophagitis by histology. The best defined motility disorder is achalasia; however other motility disorders such as diffuse esophageal spasm (DES), hypercontractile esophagus, and absent or weak peristalsis have also been reported with dysphagia 1.
Esophageal manometry characterizes the contractility of the esophagus to identify and classify motility disorders. High resolution manometry (HRM) with esophageal pressure topography (EPT) analysis is now the method of choice to assess esophageal contractile function 2. These techniques were initially described by Clouse in the 1990s 3. The concept of HRM is to overcome the limitations of conventional manometric systems by using advanced electronic technologies. The key to this development involved vastly increasing the number of pressure sensors on the manometric assembly. Pressure sensors are placed with sufficient proximity to each other so that, by interpolating between adjacent sensors, intraluminal pressure can be viewed as a continuum along the length of the entire esophagus and adjacent sphincters. When HRM is coupled with improved sensor design, such that each sensor is circumferentially sensitive and capable of high fidelity recordings, it also overcomes the fidelity and directionality limitations inherent in conventional water perfused systems. The final technological advance that facilitated the widespread application of HRM to clinical manometry was the development of sophisticated plotting algorithms to display the hugely expanded manometric dataset as colored EPT plots rather than as a multitude of overlapping line tracings 3–4. Together, these developments facilitate dynamic imaging of intra-esophageal pressure as a continuum along the length of the esophagus with pressure magnitude depicted by spectral color. Figure 1 depicts the typical pressure topography of both sphincters and the entire length of intervening esophagus during a swallow. The relative timing of sphincter relaxation, segmental esophageal contraction, as well as the position and length of pressure troughs between segments, are all readily demonstrated.
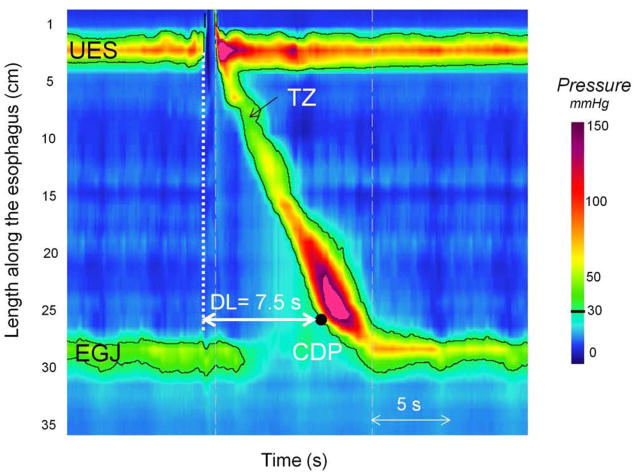
Figure 1.
Esophageal pressure topography (EPT) plot of normal swallow. The black line represents the 30-mmHg isobaric contour. Before swallowing, two high pressure zones are visualized: the upper esophageal sphincter (UES) and the esophago-gastric junction (EGJ). During swallowing, the pharyngeal contraction wave occurs and UES pressure decreases. In the esophageal body swallowing induces firstly a period of latency followed by peristaltic esophageal contraction. The proximal third of peristaltic esophageal contraction is separated from the 2 distal thirds by the transition zone (TZ). The contractile deceleration point (CDP, black dot) represents the inflexion point in the contractile front propagation. The EGJ relaxation starts just after swallowing. Distal latency time (DL) is measured from the onset of UES relaxation to the CDP.
The use of intraluminal impedance to monitor the bolus movement within the GI tract was first described by Silny in 1991 5. The technique is based on measurement of electrical impedance between closely placed electrodes mounted on an intraluminal probe. Impedance between each electrode pair depends on the nature of the luminal contents surrounding the electrodes. When the esophagus is empty, the impedance reflects the conductivity of the esophageal mucosa. Otherwise, it is indicative of surrounding intraluminal air (high impedance) or liquid (low impedance). With multiple pairs of impedance rings along the lumen of the esophagus, the spatial distribution and movement of air or liquid within the esophagus can be detected. Validation studies have verified that intraluminal impedance measurement has a high sensitivity and accuracy for tracking intra-esophageal bolus movement and monitoring reflux 6–7. However, it is important to note that the technique is not sensitive to the volume of the bolus or refluxate; 1.0 ml of residue potentially yields the same signal as 10 ml 8.
In conjunction with HRM, impedance monitoring allows tracking the swallowed bolus in relation to EPT. Although the impedance data are ideally also displayed in a topographic format, the validated criteria for bolus presence within a segment is of a 50% decrease in impedance while a 50% increase toward the baseline value correlates with bolus exit 9. Swallows can then be classified as having complete bolus transit if bolus entry is seen at the most proximal site and bolus exit is recorded in all distal impedance-measuring sites, or incomplete bolus transit, if bolus exit is not identified at one or more of the distal impedance-measuring sites 10.
Achalasia
Achalasia is both the best-defined esophageal motor disorder and the one with the most specific treatment making its accurate identification a key objective of clinical manometry. The manometric criteria for diagnosing achalasia are incomplete lower esophageal sphincter (LES) relaxation and absent peristalsis 11. One of the greatest gains realized with HRM over conventional manometry has been in refining the definition of both of these criteria with the net effect of greatly improved accuracy in the identification of the varied contractile patterns of achalasia.
It is a common misconception that the LES (EGJ) normally relaxes completely to intragastric pressure after swallowing. In fact, this is distinctly unusual and even abnormal. Rather, the EGJ relaxes to a value that is close to intragastric pressure for a certain amount of time during the post-deglutitive period. Considerable effort has been expended in using EPT to more precisely define these vague terms of ‘close to intragastric pressure’ and ‘certain amount of time’. Deglutitive EGJ relaxation occurs at a fixed time and place on EPT plots. Figure 2 illustrates the location and relaxation of the sphincter during bolus transit relative to the pharyngeal swallow. In most instances, EGJ relaxation is measured in the region spanning from 2 cm above the proximal aspect of the EGJ at rest to the proximal stomach for a 10 s period commencing with UES relaxation. In the setting of normal peristalsis, the window terminates with the arrival of the peristaltic contraction, but in the setting of failed peristalsis, an arbitrary 10-s cutoff is established, and in the setting of a premature distal esophageal contraction, a very brief window of opportunity exists. Note that if sphincter elevation exceeds 2 cm as evident by the position of the LES during the post-deglutitive contraction, the spatial limits for the measurement need to be adjusted accordingly. Once the spatial limits of the EGJ relaxation window are established, maximal EGJ pressure is then ascertained for each instant within the window; in essence, an e-sleeve measurement. The resultant data set then amounts to a history of EGJ residual pressure commencing at the instant of UES relaxation and ending either with the arrival of the esophageal contraction or 10 s later. However, it is overly simplistic to think of EGJ relaxation pressure as solely indicative of LES relaxation. Actually, at any one instant the e-sleeve pressure is the greatest of three possible contributions: LES pressure, crural diaphragm contraction, or intrabolus pressure as the swallowed water traverses the EGJ. Hence, the development of the EPT relaxation metric of the integrated relaxation pressure (IRP) 12. The IRP is measured within the deglutitive window capturing the axial movement of the LES and spanning from the time of initiation of the swallow until the arrival of the peristaltic contraction with the added stipulation that the relaxation pressure being reported represents the 4s period of lowest EGJ pressure after the swallow (Figure 2). Table 1 illustrates the added yield of the IRP compared to the nadir LES or EGJ pressure in the detection of impaired EGJ relaxation in a series of well-defined achalasia patients. This is of great significance because failing to detect impaired EGJ relaxation has the result of giving these patients an alternative diagnosis, most commonly misclassifying them as ineffective esophageal motility or DES 4, 13.
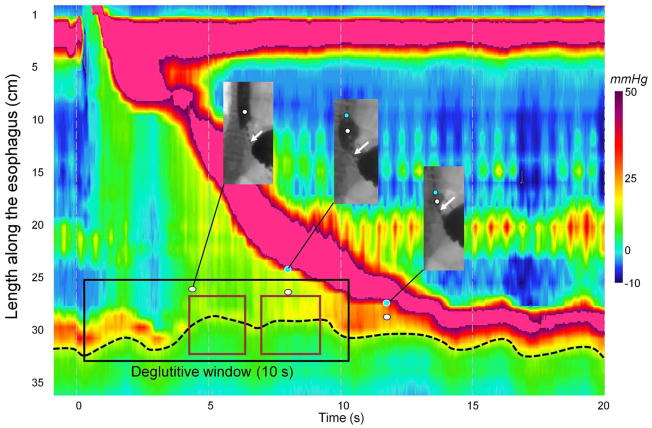
Figure 2.
Concomitant EPT and fluoroscopy during esophageal emptying. The fluoroscopic images in the windows are synchronized with the EPT plot. The white and blue dots indicate areas of intrabolus pressure and the onset of luminal closure respectively. The second image (at about time 8 s) is near the CDP, evident both by the transition of the fluoroscopic image to ampullary conformation and slowing of the luminal closure front. The maroon rectangles within the deglutitive relaxation window (black rectangle) indicate the time fragments used to compute the integrated relaxation pressure (IRP). The distal border of the esophagogastric junction is indicated by black dashed line on EPT and by white arrows on barium swallow.
Table 1.
Sensitivity of deglutitive esophago-gastric junction (EGJ) relaxation measures in detecting achalasia (from Ghosh et al, Am J Physiol Gastrointest Liver Physiol 2007 12).
| EGJ relaxation measure | Achalasia sensitivity (n=62) | False negative |
|---|---|---|
| Single sensor nadir (<7 mmHg) | 52% | 48% |
| High resolution nadir (<10 mmHg) | 69% | 31% |
| 4s integrated relaxation pressure (<15 mmHg) | 97% | 3% |
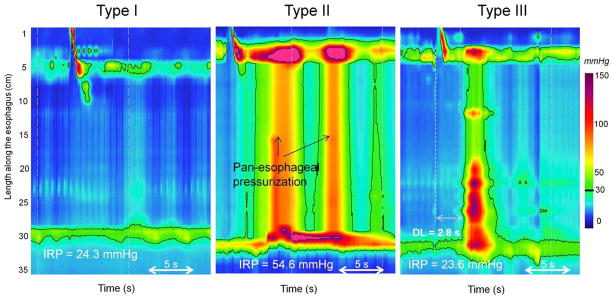
Apart from objectifying the definition of impaired deglutitive EGJ relaxation, EPT has also defined a clinically relevant sub-classification of achalasia based on the pattern of ‘absent peristalsis’ in the esophageal body 14. Absent peristalsis is not synonymous with an absence of pressurization or contractile activity. Rather, absent peristalsis accompanying impaired EGJ relaxation can occur in the setting of esophageal dilatation with negligible pressurization within the esophagus (Figure 3A), pan-esophageal pressurization (Figure 3B), or with some persistent contraction within the distal esophageal segment (Figure 3C). According to the Chicago Classification of EPT, the criteria for Type I (classic) achalasia are an IRP ≥15 mmHg and absent peristalsis; Type II (achalasia with esophageal pressurization) has an IRP ≥15 mmHg and at least 20% of swallows associated with panesophageal pressurization to >30 mmHg; and Type III achalasia has an IRP ≥15 mmHg and either a spastic contraction or a preserved peristaltic fragment with ≥20% of test swallows 14. Recent data suggest that classifying the etiology of the residual distal contraction in Type III achalasia is best accomplished by measuring its latency relative to UES relaxation 15. Premature contractions (latency <4.5 s) are indicative of spastic achalasia whereas normal latency contractions suggest a fragment of preserved peristalsis in the esophageal body. To add some perspective to the distribution of subtypes encountered, in a series of 99 consecutive patients with newly diagnosed achalasia, 21 had Type I, 49 had Type II, and 29 had Type III 14. Consequently, most of the patients in that series would not be diagnosed as achalasia with conventional manometric classification. The conventional diagnosis of ‘vigorous achalasia’ (although it never had a precise definition) would likely include some cases of both Type II and Type III achalasia, diagnoses with nearly opposite implications as detailed below.
Figure 3.
Achalasia subtypes on esophageal pressure topography (EPT). Type I is characterized by an elevated integrated relaxation pressure (mean IRP > 15 mmHg) associated with absent contractile activity and negligible esophageal pressurization. Type II is characterized by an elevated IRP, absent contractile activity and presence of pan-esophageal pressurization at 30-mm Hg isobaric contour. Type III is characterized by elevated IRP and at least 20% of persistent contractions that are either incomplete or premature. On this example the contraction is premature (distal latency (DL) < 4.5 s)).
The ultimate significance of identifying subtypes of achalasia is that it clarifies management and preliminary data suggest this to be the case. Logistic regression analysis of predictors of treatment benefit in a large consecutive series found pan-esophageal pressurization (Figure 3B) to be a predictor of good treatment response (dilation or myotomy) while spastic achalasia (Figure 3C) and pre-treatment esophageal dilatation were predictive of a relatively poor treatment response 14. Clearly, these nuances have not been utilized in prior reports of achalasia treatment outcomes. Given that the mix of achalasia subtypes within any reported case series likely impacts on the efficacy observed in that series, this calls into question the validity of the existing treatment data in the era of EPT. It is our suspicion that adopting these sub-classifications will likely strengthen the quality of future prospective studies of achalasia management, although this clearly requires further validation.
The impedance characteristics of achalasia are, as one would predict, incomplete bolus transit. Although that finding is supportive of the physiological defect associated with the disease, it has not as yet been shown to help in subtyping achalasia or in assessing the effectiveness of a rendered therapy.
EGJ Outflow Obstruction: Is it Achalasia?
Although EPT goes a long way toward clarifying the diagnosis in many achalasia patients that would otherwise be classified as ‘nonspecific’ or misclassified to a non-achalasia diagnosis, there is still a group of patients with impaired EGJ relaxation failing to meet criteria for achalasia because they demonstrate some preserved peristalsis. Though not common, a series of 1000 consecutive patients studied with EPT included 16 such individuals with EGJ Outflow Obstruction exhibiting not only an IRP greater than 15 mmHg, but also preserved peristalsis and elevated intrabolus pressure above the EGJ during peristalsis 16. The finding of elevated intrabolus pressure is important because it validates the determination of impaired EGJ relaxation. From a physiological perspective, elevated intrabolus pressure is the consequence of that impaired relaxation. Nonetheless, EGJ Outflow Obstruction represents a heterogeneous group with some individuals having an incomplete expression of achalasia and others likely having an undetected mechanical cause of EGJ outflow obstruction such as hiatus hernia or esophageal stenosis. Consequently, it is a patient group that usually merits further intensive evaluation with imaging studies to exclude inflammatory or malignant etiologies, be that with computerized tomography or endoscopic ultrasound, before accepting it to be atypical achalasia.
Among the 16 patients with idiopathic EGJ Outflow Obstruction described above, 3 were noted to have hiatus hernias. In one of these instances it was the crural diaphragm rather than the LES that appeared to be the focus of resistance to bolus transit, suggesting this be the cause of dysphagia. A subsequent report specifically focused on the EGJ relaxation characteristics of patients with sliding hiatus hernia and dysphagia by selectively restricting the IRP measurement boundaries to the LES and crural diaphragm individually 17. A subset of 10 patients were found exhibiting a relative obstruction at the crural diaphragm with elevated intrabolus pressure extending through the LES, supporting the concept that sliding hiatus hernia could be responsible for dysphagia. Consequently, patients presenting with elevated EGJ relaxation pressure in the context of a small hiatus hernia require careful analysis of the discreet elements of the EGJ before making a diagnosis of achalasia.
Rethinking Spasm
Distal esophageal spasm (DES) is characterized by episodes of dysphagia and chest pain attributable to abnormal esophageal contractions in the setting of normal EGJ relaxation. Beyond that, there is little agreement. The pathophysiology and natural history of DES are ill-defined. In radiological publications, DES is commonly illustrated by tertiary contractions, a ‘corkscrew esophagus’, or a ‘rosary bead esophagus’, but in most instances these abnormalities are actually indicative of spastic achalasia. Manometrically, greatest consensus surrounds the concept of ‘simultaneous contractions’ either with a defining minimum of 30 mmHg or without defining amplitude 18–19. By ‘simultaneous contractions’ is meant that the upstroke of the pressure waves at adjacent recording sites (conventionally spaced 3–5 cm apart) occur at nearly the same instant. However, similar to the problems with the conventional manometric definition of achalasia, there is no distinction between pressure waves within the esophageal body attributable to intrabolus pressure or to contraction. Given these vagaries, it is likely that a heterogeneous group of patients have been diagnosed with DES and included in therapeutic trials of DES. Not surprisingly, none such studies have demonstrated efficacy.
Several nuances of defining DES emerge in EPT. First of all, there is a very important distinction to be made between a simultaneous contraction in the distal esophagus and simultaneous pressurization in the setting of EGJ Outflow Obstruction (Figure 4). The former fits with the concept of DES while the latter is simply a consequence of impaired EGJ relaxation, most commonly in the setting of achalasia. Consequently, much of what would be labeled DES on the basis of ‘simultaneous contractions’ in conventional manometry is actually achalasia 4, 14. Similarly, instances of ‘simultaneous contractions’ of low amplitude are almost invariably attributable to intrabolus pressure in the setting of failed peristaltic contractions of subtle obstructive phenomenon in the distal esophagus.
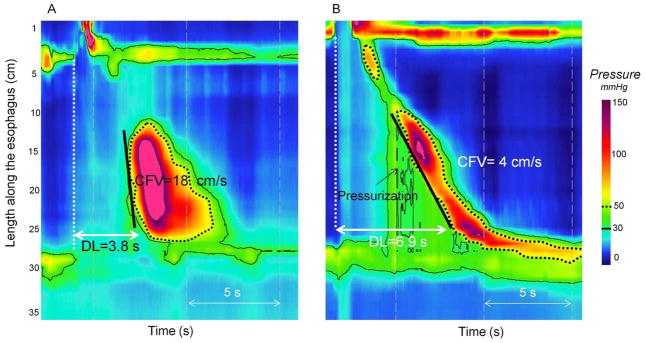
Figure 4.
Simultaneous contraction verses pressurization. The black line corresponds to 30-mmHg isobaric contour and the dashed one to 50-mmHg isobaric contour. Premature rapid contraction is represented on Panel A. Distal latency time (DL) and contractile front velocity (CFV) are measured at 30-mmHg isobaric contour. Note that 30- and 50-mmHg isobaric contours are parallel. The contraction on Panel B is characterized by distal compartmentalized pressurization. The 30- and 50-mmHg isobaric contours are not parallel. DL and CFV are measured at 50-mmHg to exclude the area of pressurization.
An alternative metric for assessing propagation of peristalsis in EPT is the latency of the contraction in the distal esophagus. Behar and Biancani initially established the relationship between simultaneous contractions and reduced latency of contractions and proposed this to be indicative of impaired deglutitive inhibition as can be seen in DES 20. However, perhaps because it is cumbersome to measure, this concept never gained traction in conventional manometry. Two recently described tools in EPT analysis that improve the recognition of spasm are the Contractile Deceleration Point (CDP) and the Distal Contractile Latency (DL) (Figure 1). The CDP is the locus in the distal esophageal body characterized by a slowing of the deglutitive contraction as peristalsis terminates and ampullary emptying begins 21. Consequently, the identification of the Contractile Deceleration Point provides a reliable landmark (the endpoint) for measuring peristaltic velocity. The DL is a related measure in that times the occurrence of distal peristalsis relative to deglutitive upper sphincter relaxation. Together, these measures facilitate objective measurement of peristaltic velocity and provide a means for quantifying the latency of the distal contraction as a surrogate for inhibitory ganglionic integrity 20, 22–23 (Figure 4).
A recent study compared the performance of DL to propagation velocity in identifying DES in a series of 2000 patients studied with EPT. The major finding was that rapid contractile velocity was a very non-specific finding, rarely the defining feature of a clinically significant disorder unless accompanied by reduced DL 22. Tutuian et al similarly concluded that conventionally-defined DES identified a very heterogeneous population based on an assessment of bolus transit in 33 such patients with combined manometry and impedance 10. Reduced DL, however, was much better, both in terms of being a much less common and a much more homogeneous clinical entity. Affected patients almost uniformly had severe dysphagia. However, three quarters of these individuals were ultimately managed as an achalasia subtype (spastic achalasia) raising the question of whether or not spastic achalasia and ‘DES’ are not actually minor variations on the same theme of impaired inhibitory neuronal function in the distal esophagus.
A much more consistent pattern of abnormal contractility in EPT is of very vigorous contractions with normal deglutitive EGJ relaxation and propagation velocity. In such instances, the distal esophageal contraction can be characterized for the vigor of contraction using a newly developed measure, the Distal Contractile Integral (DCI). The DCI integrates the length, contractile amplitude, and duration of contraction of the distal esophageal segment contraction, expressed as mmHg-s-cm 24–25. Using data from control subjects, a mean DCI value greater than 5000 mmHg-s-cm exceeds the 95th percentile of normal. This threshold is used in EPT analysis as the equivalent of ‘Nutcracker Esophagus’ or ‘Hypertensive Peristalsis’. Even more extreme is a patient group with a single swallow with DCI >8,000 mmHg-s-cm, a magnitude never seen in normal subjects. These individuals are classified as having ‘Hypercontractile Esophagus’ in EPT and are characterized by normal propagation velocity, DCI > 8,000 mmHg-s-cm and no more than marginal abnormalities of the IRP. In many instances, the esophageal contraction is repetitive earning it the nickname ‘Jackhammer Esophagus’ in the latest iteration of the Chicago Classification of EPT 26. Although the full clinical spectrum of these patients is not yet understood, essentially all are symptomatic with dysphagia or chest pain. From a physiological perspective the abnormality is of hyperexcitability of the distal esophageal smooth muscle, establishing a clear distinction from the impaired inhibitory innervation characteristic of achalasia and ‘DES’. Consequently, given a plausible unifying pathophysiology, ‘Hypercontractile’ or ‘Jackhammer’ Esophagus’ is probably an appropriate target of future therapeutic trials.
Weak Peristalsis
One of the major clinical applications of manometry is to assess the integrity of peristalsis, either as part of an evaluation of dysphagia or in anticipation of antireflux surgery. Conventionally, this is done by measuring the distal peristaltic amplitude 18. The most commonly accepted metrics establishing normality are that peristaltic amplitude exceed 30 mmHg at recording sites 3 and 8 cm proximal to the lower esophageal sphincter (LES) 18 based on the observation that amplitudes less than 30 mmHg are frequently associated with bolus escape and incomplete bolus clearance on fluoroscopy 27. However, with the evolution of intraluminal impedance monitoring and EPT it has become apparent that these conventional metrics provide a very incomplete assessment of peristaltic integrity. Findings from multichannel intraluminal impedance recordings suggest that the 30 mmHg threshold value is too high in many instances 10 while EPT studies suggest that the arbitrary selection of two foci to measure pressure amplitude ignores much of the detail and variability inherent in the segmental architecture of the peristaltic contraction 4.
The most comprehensive assessment of peristaltic integrity is achieved by combining the technologies of HRM and high resolution intraluminal impedance monitoring. The combined study, called high resolution impedance manometry or HRIM depicts both EPT and bolus disposition on the same graphic (Figure 5). HRIM data show that failed peristalsis is uniformly associated with incomplete bolus transit. With respect to hypotensive peristalsis, the critical finding in EPT is of breaks in the 20 or 30 mmHg isobaric contour delineating the peristaltic contraction spanning from the UES to the EGJ 28–29. When 20 mmHg isobaric contour breaks exceed 5 cm in length, signifying that there is a 5 cm span of the esophagus with a peristaltic amplitude of less than 20 mmHg, they are uniformly associated with incomplete bolus transit at that site gauged by the high resolution impedance recording. When breaks are in the range of 2–5 cm they will variably be associated with IBT. Given these data, the frequency of occurrence of these three phenomena (failed peristalsis, large breaks in the 20 mmHg isobaric contour, and small breaks in the 20 mmHg isobaric contour) are indices of the adequacy of peristalsis for achieving esophageal bolus transit.
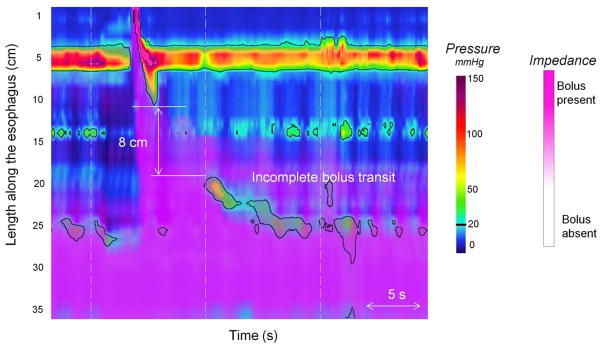
Figure 5.
High resolution manometry combined with impedance. The black line represents the 20-mmHg isobaric contour (IBC). Impedance data are displayed by overlaid pink colorization with the pink shading indicative of areas on the topography plots with retained bolus. The swallow is associated with a large proximal break (>5 cm) at 20-mmHg IBC. The break is responsible of a bolus escape as attested by the persistence of the pink shadow.
A recent study examined the relationship between these putative measures of weak peristalsis (failed peristalsis, larger breaks and small breaks in the 20 mmHg isobaric contour) and non-obstructive dysphagia29. The major aims were to establish normal limits of peristaltic integrity in EPT terms based on a systematic analysis of a large series of control subjects, and to develop a classification scheme for weak peristalsis based on a comparison between control subjects and a cohort of patients with unexplained non-obstructive dysphagia intended for use in clinical EPT studies. The major findings were that the segmental architecture of peristalsis was highly stereotyped among subjects as were defects in that architecture associated with incomplete bolus transit for individual subjects: large (>5 cm) and small (2–5 cm) breaks in the 20 mmHg isobaric contour of the peristaltic contraction. Although encountered in only about a third of the 113 patients studied, frequent large and small breaks in the 20 mmHg isobaric contour were significantly more common in the dysphagia patients than in control subjects. Failed peristalsis, the other mechanism of IBT observed in the HRIM studies occurred no more frequently in the dysphagia population than in the control subjects. Based on these observations, an EPT classification of weak peristalsis has been proposed based on the occurrence of breaks in the 20 mmHg isobaric contour wherein weak peristalsis with large breaks is defined by these occurring with >20% of swallows and weak peristalsis with small breaks defined by these occurring with >30% of swallows (Table 2).
Table 2.
Proposed classification of peristaltic integrity in EPT. Isobaric contour pressure is referenced to atmospheric. Note that an individual may have more than one diagnosis.
| Diagnosis | Diagnostic Criteria (all with normal EGJ relaxation) |
|---|---|
| Absent peristalsis | 100% of swallows with failed peristalsis |
| Frequent failed peristalsis† | >30%, but <100% of swallows with failed peristalsis |
| Weak peristalsis with large peristaltic defects | >20% of swallows with >5 cm breaks in the 20 mmHg isobaric contour |
| Weak peristalsis with small peristaltic defects | >30% of swallows with 2–5 cm breaks in the 20 mmHg isobaric contour |
Although statistically exceeding the 95th percentile of normal, this finding has not been shown to correlate with non-obstructive dysphagia
Summary: the Evolving Chicago Classification of EPT
The preceding description of distal esophageal motility disorders in terms of EPT is a concise summation of an evolving process that has unfolded during the past six or seven years as part of the International High Resolution Manometry Working Group. The evolving classification is referred to as the Chicago Classification and is being specifically developed to facilitate the interpretation of clinical EPT studies in clinical practice. The Chicago Classification has been, and will continue to be, an evolutionary process, molded first by published evidence and secondly by group experience when suitable evidence is lacking. The most recent iteration of his classification emerged from a meeting of the International High Resolution Manometry Working Group that occurred in Ascona Switzerland in April, 2011 and is currently in the process of being published. The essential details of this are outlined in Table 3. Moving forward, we anticipate continuing this process with increased emphasis placed on natural history studies and outcome data based on the developing classification.
Table 3.
The Chicago Classification of esophageal motility
| DIAGNOSIS | DIAGNOSTIC CRITERIA |
|---|---|
| Achalasia | |
| Type I achalasia | Classic achalasia: mean IRP > upper limit of normal, 100% failed peristalsis |
| Type II achalasia | Achalasia with esophageal compression: mean IRP > upper limit of normal, no normal peristalsis, panesophageal pressurization with ≥20% of swallows |
| Type III achalasia | Mean IRP > upper limit of normal, no normal peristalsis, preserved fragments of distal peristalsis or premature (spastic) contractions with ≥20% of swallows |
| EGJ outflow obstruction | Mean IRP > upper limit of normal, some instances of intact peristalsis or weak peristalsis with small breaks such that the criteria for achalasia are not met† |
| Motility Disorders | (patterns not observed in normal individuals) |
| Distal esophageal spasm | Normal mean IRP, ≥20% premature contractions |
| Hypercontractile (Jackhammer) esophagus | At least one swallow DCI > 8,000 mmHg-s-cm with single peaked or multipeaked contraction†† |
| Absent peristalsis | Normal mean IRP, 100% of swallows with failed peristalsis |
| Peristaltic abnormalities | (defined by exceeding statistical limits of normal) |
| Weak peristalsis with large peristaltic defects | Mean IRP <15 mmHg and >20% swallows with large breaks in the 20 mmHg isobaric contour (>5 cm in length) |
| Weak peristalsis with small peristaltic defects | Mean IRP <15 mmHg and >30% swallows with small breaks in the 20 mmHg isobaric contour (2–5 cm in length) |
| Frequent failed peristalsis | >30%, but <100% of swallows with failed peristalsis |
| Rapid contractions with normal latency | Rapid contraction with ≥20% of swallows, DL >4.5 s |
| Hypertensive peristalsis (nutcracker esophagus) | Mean DCI > 5,000 mmHg-s-cm, but not meeting criteria for hypercontractile esophagus |
| Normal | Not achieving any of the above diagnostic criteria |
May be a variant form of achalasia, indicative of wall stiffness consequent from an infiltrative disease, or manifestation of hiatal hernia in which case it can be subtyped to CD or LES
The locus of the multipeaked contraction can be in either of the distal two contractile segments or very rarely in the LES, but is usually this is in the third contractile segment. May coexist with EGJ outflow obstruction.
Acknowledgments
This work was supported by Grant No R01DK56033 from the National Institute of Health
Footnotes
Conflict of interest: SR has served as consultant for Given Imaging
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pandolfino JE, Kahrilas PJ. AGA technical review on the clinical use of esophageal manometry. Gastroenterology. 2005;128(1):209–224. doi: 10.1053/j.gastro.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 2.Fox MR, Bredenoord AJ. Oesophageal high-resolution manometry: moving from research into clinical practice. Gut. 2008;57(3):405–423. doi: 10.1136/gut.2007.127993. [DOI] [PubMed] [Google Scholar]
- 3.Clouse RE, Staiano A, Alrakawi A. Development of a topographic analysis system for manometric studies in the gastrointestinal tract. Gastrointest Endosc. 1998;48(4):395–401. doi: 10.1016/s0016-5107(98)70010-0. [DOI] [PubMed] [Google Scholar]
- 4.Clouse RE, Staiano A, Alrakawi A, et al. Application of topographical methods to clinical esophageal manometry. Am J Gastroenterol. 2000;95(10):2720–2730. doi: 10.1111/j.1572-0241.2000.03178.x. [DOI] [PubMed] [Google Scholar]
- 5.Silny J. Intraluminal multiple electrical impedance procedure for measurement of gastrointestinal motility. J Gastroenterol Motil. 1991;3:151–162. [Google Scholar]
- 6.Sifrim D, Silny J, Holloway RH, et al. Patterns of gas and liquid reflux during transient lower oesophageal sphincter relaxation: a study using intraluminal electrical impedance. Gut. 1999;44(1):47–54. doi: 10.1136/gut.44.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Silny J, Knigge KP, Fass J, et al. Verification of the intraluminal multiple electrical impedance measurement for the recording of gastrointestinal motility. J Gastrointest Motil. 1993;5:107–122. [Google Scholar]
- 8.Kahrilas PJ. Will impedence testing rewrite the book on GERD? Gastroenterology. 2001;120(7):1862–1864. doi: 10.1053/gast.2001.25290. [DOI] [PubMed] [Google Scholar]
- 9.Tutuian R, Vela MF, Balaji NS, et al. Esophageal function testing with combined multichannel intraluminal impedance and manometry: multicenter study in healthy volunteers. Clin Gastroenterol Hepatol. 2003;1(3):174–182. doi: 10.1053/cgh.2003.50026. [DOI] [PubMed] [Google Scholar]
- 10.Tutuian R, Castell DO. Combined multichannel intraluminal impedance and manometry clarifies esophageal function abnormalities: study in 350 patients. Am J Gastroenterol. 2004;99(6):1011–1019. doi: 10.1111/j.1572-0241.2004.30035.x. [DOI] [PubMed] [Google Scholar]
- 11.Pandolfino JE, Kahrilas PJ American Gastroenterological A. American Gastroenterological Association medical position statement: Clinical use of esophageal manometry. Gastroenterology. 2005;128(1):207–208. doi: 10.1053/j.gastro.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 12.Ghosh SK, Pandolfino JE, Rice J, et al. Impaired deglutitive EGJ relaxation in clinical esophageal manometry: a quantitative analysis of 400 patients and 75 controls. Am J Physiol Gastrointest Liver Physiol. 2007;293(4):G878–885. doi: 10.1152/ajpgi.00252.2007. [DOI] [PubMed] [Google Scholar]
- 13.Fox M, Hebbard G, Janiak P, et al. High-resolution manometry predicts the success of oesophageal bolus transport and identifies clinically important abnormalities not detected by conventional manometry. Neurogastroenterol Motil. 2004;16(5):533–542. doi: 10.1111/j.1365-2982.2004.00539.x. [DOI] [PubMed] [Google Scholar]
- 14.Pandolfino JE, Kwiatek MA, Nealis T, et al. Achalasia: A New Clinically Relevant Classification by High-Resolution Manometry. Gastroenterology. 2008;135(5):1526–1533. doi: 10.1053/j.gastro.2008.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McCarthy ST, Cluley JD, Roman S, et al. Spastic achalasia phenotypes in esophageal pressure topography (EPT): not all spasm is the same. Gastroenterology. 2011;140(5 Suppl1):S-77. [Google Scholar]
- 16.Scherer JR, Kwiatek MA, Soper NJ, et al. Functional esophagogastric junction obstruction with intact peristalsis: a heterogeneous syndrome sometimes akin to achalasia. J Gastrointest Surg. 2009;13(12):2219–2225. doi: 10.1007/s11605-009-0975-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pandolfino JE, Kwiatek MA, Ho K, et al. Unique features of esophagogastric junction pressure topography in hiatus hernia patients with dysphagia. Surgery. 2010;147(1):57–64. doi: 10.1016/j.surg.2009.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spechler SJ, Castell DO. Classification of oesophageal motility abnormalities. Gut. 2001;49(1):145–151. doi: 10.1136/gut.49.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dalton CB, Castell DO, Hewson EG, et al. Diffuse esophageal spasm. A rare motility disorder not characterized by high-amplitude contractions. Dig Dis Sci. 1991;36(8):1025–1028. doi: 10.1007/BF01297441. [DOI] [PubMed] [Google Scholar]
- 20.Behar J, Biancani P. Pathogenesis of simultaneous esophageal contractions in patients with motility disorders. Gastroenterology. 1993;105(1):111–118. doi: 10.1016/0016-5085(93)90016-6. [DOI] [PubMed] [Google Scholar]
- 21.Pandolfino JE, Leslie E, Luger D, et al. The contractile deceleration point: an important physiologic landmark on oesophageal pressure topography. Neurogastroenterol Motil. 2010;22(4):395–400. doi: 10.1111/j.1365-2982.2009.01443.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pandolfino JE, Roman S, Carlson D, et al. Distal esophageal spasm in high resolution esophageal pressure topography: defining clinical phenotypes. Gastroenterology. 2011 doi: 10.1053/j.gastro.2011.04.058. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roman S, Lin Z, Pandolfino JE, et al. Distal Contraction Latency: A Measure of Propagation Velocity Optimized for Esophageal Pressure Topography Studies. Am J Gastroenterol. 2011;106(3):443–451. doi: 10.1038/ajg.2010.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ghosh SK, Pandolfino JE, Zhang Q, et al. Quantifying esophageal peristalsis with high-resolution manometry: a study of 75 asymptomatic volunteers. Am J Physiol Gastrointest Liver Physiol. 2006;290(5):G988–997. doi: 10.1152/ajpgi.00510.2005. [DOI] [PubMed] [Google Scholar]
- 25.Pandolfino JE, Ghosh SK, Rice J, et al. Classifying esophageal motility by pressure topography characteristics: a study of 400 patients and 75 controls. Am J Gastroenterol. 2008;103(1):27–37. doi: 10.1111/j.1572-0241.2007.01532.x. [DOI] [PubMed] [Google Scholar]
- 26.Roman S, Lin Z, Kwiatek MA, et al. Jackhammer esophagus: a symptomatic phenotype of hypertensive contraction in high resolution esophageal pressure topography (EPT) Gastroenterology. 2011;140(5 Supplement 1):S-231. [Google Scholar]
- 27.Kahrilas PJ, Dodds WJ, Hogan WJ. Effect of peristaltic dysfunction on esophageal volume clearance. Gastroenterology. 1988;94(1):73–80. doi: 10.1016/0016-5085(88)90612-9. [DOI] [PubMed] [Google Scholar]
- 28.Bulsiewicz WJ, Kahrilas PJ, Kwiatek MA, et al. Esophageal pressure topography criteria indicative of incomplete bolus clearance: a study using high-resolution impedance manometry. Am J Gastroenterol. 2009;104(11):2721–2728. doi: 10.1038/ajg.2009.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roman S, Lin Z, Kwiatek MA, et al. Weak peristalsis in esophageal pressure topography: classification and association with dysphagia. Am J Gastroenterol. 2011;106(2):349–356. doi: 10.1038/ajg.2010.384. [DOI] [PMC free article] [PubMed] [Google Scholar]