Abstract
To elucidate the early steps required during biosynthesis of a broad class of 7-deazapurine containing natural products, we have studied the reaction catalyzed by Escherichia coli QueD, a 6-pyruvoyl-5,6,7,8-tetrahydropterin synthase (PTPS) homolog possibly involved in queuosine biosynthesis. While mammalian PTPS homologs convert 7,8-dihydroneopterin triphosphate (H2NTP) to 6-pyruvoyltetrahydropterin (PPH4) in biopterin biosynthesis, E. coli QueD catalyzes the conversion of H2NTP to 6-carboxy-5,6,7,8-tetrahydropterin (CPH4). E. coli QueD can also convert PPH4 and sepiapterin to CPH4, allowing a mechanism to be proposed.
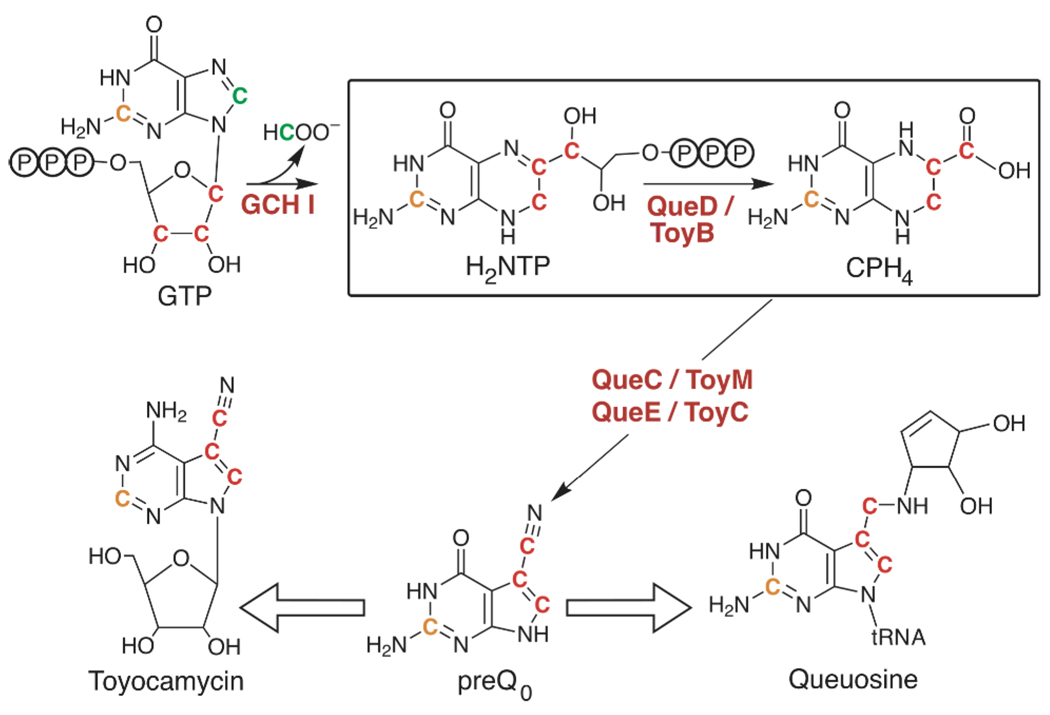
Pyrrolo[2,3-d]pyrimidines, collectively referred to as deazapurines, are found in a number of antibiotic secondary metabolites produced by species of Streptomyces (1), as well as the hypermodified tRNA base queuosine. Recent studies have led to identification of many of the genes that appear to be required for biosynthesis of deazapurine-containing secondary metabolites including queuosine and toyocamycin (3–7). These results indicate that the biosynthesis of deazapurine containing compounds as diverse as queuosine and toyocamycin begins with the conversion of GTP to 7,8-dihydroneopterin triphosphate (H2NTP) by GTP cyclohydrolase type I (GCH I), followed by the action of three additional enzymes: a 6-pyruvoyltetrahydropterin synthase (PTPS), a protein of the radical SAM superfamily (8), and an ExsB family homolog (see Fig. 1).
Figure 1.
Biosynthesis of the deazapurines toyocamycin and queuosine begins with the conversion of GTP to H2NTP by GCH I. The remaining steps leading to the central precursor, preQ0, are catalyzed by PTPS (QueD/ToyB), radical SAM superfamily (QueE/ToyC), and ExsB family (QueC/ToyM) homologs (protein designations Que and Toy names refer to those involved in queuosine and toyocamycin biosynthesis, respectively). In this study E. coli QueD (boxed reaction) was shown to convert H2NTP to 6-carboxy-5,6,7,8-tetrahydropterin (CPH4). The coloring scheme of the carbon atoms reflects the results of early radiotracer experiments on deazapurine biosynthesis (1, 2).
PTPS catalyzes the conversion of H2NTP, produced by GCH I, to pyruvoyltetrahydropterin (PPH4) in the second step of tetrahydrobiopterin biosynthesis in mammals (see Scheme S1). PPH4 is subsequently converted to tetrahydrobiopterin (BH4) by sepiapterin reductase in an NADPH dependent reaction (9). In mammals, BH4 serves as a cofactor for such enzymes as phenylalanine hydroxylase and nitric oxide synthase. Curiously, PTPS homologs are widely present in prokaryotes, which are not known to produce biopterin. E. coli contains a single PTPS homolog (B2765 in E. coli W3110), which is variously annotated as ygcM, queD and sscR. Since queuosine is the only deazapurine produced by this organism, and studies on deazapurine biosynthetic pathways have implicated a PTPS homolog, it is possible that this protein is engaged in the biosynthesis of queuosine.
Studies of E. coli QueD by Park and coworkers (10) have established that the protein has an alternate activity, which they proposed to be the conversion of sepiapterin to 7,8-dihydropterin. However, these assays were carried out under aerobic conditions and products were detected by fluorescence after oxidative derivatization. We have reexamined the activity of QueD under strictly anaerobic conditions without derivatization and reveal that the product is 6-carboxy-5,6,7,8-tetrahydropterin (CPH4).
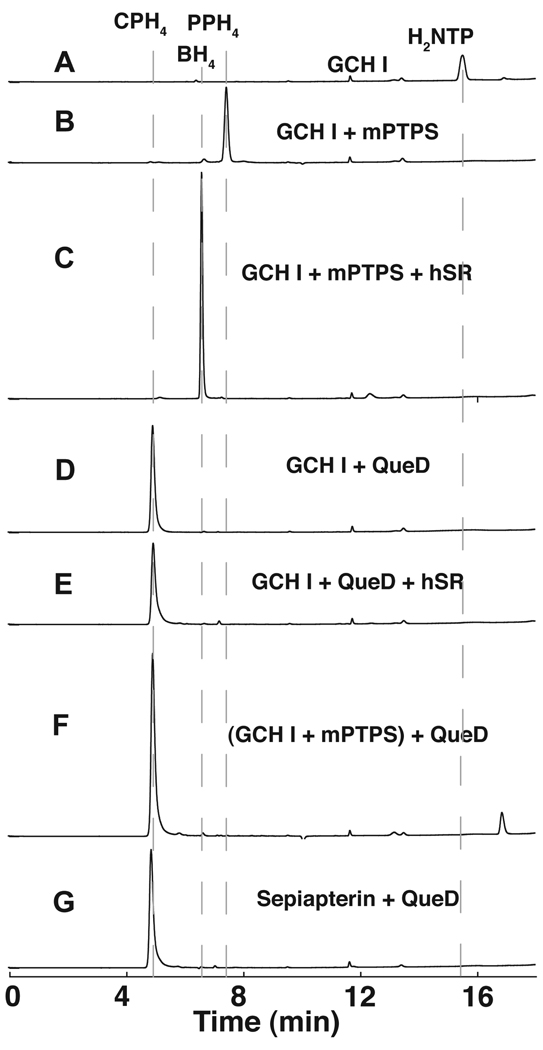
In all experiments described here, recombinant native QueD was assayed under anaerobic conditions (95% N2:5% H2) and the substrate (H2NTP) was generated using the E. coli GCH I homolog FolE, in situ. HPLC analysis indicated that under these conditions, H2NTP could be produced quantitatively from GTP (Fig. 2A). As a control, when the GCH I reaction is carried out in the presence of recombinant mouse PTPS (mPTPS), H2NTP was quantitatively converted to PPH4 (Fig. 2B) which elutes at 7.5 min and also has a UV-visible spectrum consistent with that expected for PPH4 (Fig. S1A). Furthermore, when recombinant human sepiapterin reductase (hSR) and NADPH are included, PPH4 is converted cleanly to BH4 (Fig. 2C), whose retention time (6.5 min) is identical to that of commercially obtained BH4 (data not shown). By contrast, when H2NTP is incubated with QueD, a peak at 4.9 min is observed whose retention time (Fig. 2D and 2E) and spectral properties (see Fig. S1C and D) do not change in the presence of hSR. Interestingly, when PPH4, generated enzymatically from the combined actions of GCH I and mPTPS, is combined with QueD, the peak for the product has identical retention time and spectral properties as that observed when H2NTP is mixed with QueD, suggesting that the identical product is formed (compare Fig. 2D and 2F). Moreover, the same product forms when sepiapterin is the substrate for QueD (see Fig. 2G and Fig S1F). These experiments clearly establish that the product of QueD differs from PPH4. Based on the UV-visible spectra of the compounds eluting from the HPLC column at 4.9 min the product of QueD is a tetrahydro-substituted pterin (Fig. S1C–F).
Figure 2.
HPLC chromatograms of the QueD catalyzed reaction. The chromatograms correspond to: (A) H2NTP produced from GTP by E. coli GCH I (FolE); (B) PPH4 produced from GTP by FolE and mouse PTPS (mPTPS); (C) BH4 produced from GTP by FolE and mPTPS, and hSR with NADPH; (D) CPH4 produced from GTP with FolE and E. coli QueD; (E) was obtained under the same conditions as (D) but with hSR and NADPH; (F) was obtained by addition of QueD to a reaction mixture containing PPH4 prepared as in (B); (G) trace obtained when sepiapterin is a substrate for QueD. Refer to Supplementary Materials for the Experimental Procedures.
To identify the molecular formula of the unknown QueD product we repeated the QueD reaction as in Fig. 2D with unlabeled GTP, [U-13C10,15N5]-GTP and analyzed the reactions by fourier transform ion cyclotron resonance mass spectrometry (FT-ICR MS) for both positively and negatively charged ions (See Supplementary Figure S2 and S3). [U-13C10,15N5]-GTP is 15 amu heavier than unlabeled GTP. Since GCH I removes C-8 of GTP as formate (11), a genuine QueD product would be expected to show a mass difference of ≤14 amu with uniformly labeled GTP relative to the unlabeled control. Indeed, we observe a [M+H]+ ion with m/z 212.0778 and an [M−H]− ion with m/z 210.0642, in positive and negative ion modes, respectively. These peaks are absent from the corresponding spectra with labeled GTP. Instead, [M+H]+ and [M−H]− ions with a m/z of 224.0868 and 222.0732 are observed in positive and negative ion modes, respectively. Moreover, the MS of a solution containing samples of both the unlabeled and labeled GTP reaction mixtures shows both ions; a control reaction lacking GTP does not show any of the peaks. The high resolution mass measurements are consistent with a molecular formula of (C7H9N5O3), which when taken in the context of the UV-visible spectral data, are consistent with identification of 6-carboxy-5,6,7,8-tetrahydropterin (CPH4) as the product of the QueD reaction.
The identity of CPH4 was confirmed by tandem MS/MS experiments where the positively and negatively charged molecular ions for the product of QueD, observed with unlabeled and labeled GTP, were isolated in the quadrupole and underwent collision-induced fragmentation (Fig. S4). The ions exhibit loss of HCOOH or CO2, under positive and negative detection, respectively, further confirming the presence of a carboxy moiety.
While CPH4 is not commercially available, pterins can be reduced to 5,6,7,8-tetrahydropterin with reducing agents such as NaBH4 (12). Commercially available 6-carboxypterin was reduced with NaBH4 and the reaction mixture was subjected to HPLC analysis as described in Supplementary Procedures. The product of the reduction has a retention time and UV-visible spectrum identical to the QueD product; moreover, the two co-elute from the HPLC column in a co-injection experiment (Fig. S5), confirming formation of CPH4 by E. coli QueD.
Alignment of E. coli QueD with eukaryotic PTPS proteins, which catalyze the conversion of H2NTP to PPH4, reveals the conservation of amino acid residues that have been shown to be involved in substrate binding and catalysis in the well characterized rat PTPS homolog (Fig. S6) (13, 14). These include three His residues that coordinate an essential zinc metal ion. Inductively coupled plasma optical emission spectroscopy (ICP-OES) analysis of purified, recombinant QueD used in the experiments described in this work revealed the presence of 1.1 equivalents of Zn per monomer. Based on these observations, together with the fact that QueD is capable of utilizing H2NTP, PPH4 and sepiapterin as substrate, a plausible scheme for formation of CPH4 is proposed (Figure 3). In this scheme it is assumed that the initial rearrangements involved in the conversion of H2NTP to PPH4 by QueD are similar to those proposed for the mammalian PTPS (13, 14) and have not been depicted in the Figure. PPH4 can tautomerize to sepiapterin, the hydrate of which undergoes carbon-carbon bond cleavage by an aldolase-like mechanism, followed by a tautomerization, to yield CPH4. The proposed scheme predicts the formation of acetaldehyde as a leaving group. Indeed, when the QueD reaction mixture is quenched with acidic 2,4-dinitrophenylhydrazine and analyzed by HPLC, a new peak is observed that has the same retention time as the hydrazone adduct of acetaldehyde (see Fig. S7).
Figure 3.
Proposed scheme for conversion of H2NTP to 6-carboxy-5,6,7,8-tetrahydropterin and acetaldehyde. Compounds in red have been demonstrated to be turned over by QueD to 6-carboxytetrahydropterin.
The side-chain cleavage activity of QueD, which results in the formation of CPH4, fulfills the requirement for loss of carbons C4′ and C5′ (GTP numbering) necessary for conversion of GTP to preQ0, a known intermediate in queuosine biosynthesis. The details surrounding two additional transformations required for deazapurine biosynthesis, which are catalyzed by a radical SAM superfamily and an ExsB family homolog (see Figure 1), remain to be elucidated. However, we hypothesize that CPH4 serves as substrate in one of them. Another interesting question posed by the results presented here is how prokaryotic PTPS homologs that are involved in deazapurine biosynthesis can catalyze the production of a product which is distinct from that produced by mammalian homologs, even though sequence alignments reveal very few differences between them. Additional studies aimed at addressing the molecular basis for the observed activities, as well as mechanistic studies of this fascinating protein are underway and will be reported in due course.
Supplementary Material
ACKNOWLEDGMENT
The authors acknowledge Alberto Rascón for the E. coli FolE (GCH I) protein.
Footnotes
RM wishes to acknowledge Science Foundation Arizona for a SfAZ Graduate Fellowship. Support from the National Institutes of Health (NIH) to VB (R01 GM 72623) and NCRR 1S10RR 23029 to Vicki H. Wysocki for acquisition of the FT ICR-MS are gratefully acknowledged. In addition, the research of V.B. is supported (in part) by a Career Award in Biomedical Sciences from the Burroughs Wellcome Fund.
SUPPORTING INFORMATION PARAGRAPH
Materials and Detailed experimental procedures, UV-visible spectra of compounds shown in the HPLC traces in Fig. 2, FT-ICR MS data, comparison of QueD reaction product with synthetic CPH4, multiple sequence alignment of PTPS homologs, and acetaldehyde assay are available free of charge on the internet at: http://pubs.acs.org.
REFERENCES
- 1.Suhadolnik RJ. Nucleoside antibiotics. New York: Wiley-Interscinece; 1970. Pyrrolopyrimidine nucleosides; pp. 298–353. [Google Scholar]
- 2.Kuchino Y, Kasai H, Nihei K, Nishimura S. Nuc. Acids. Res. 1976;3:393–398. doi: 10.1093/nar/3.2.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McCarty RM, Bandarian V. Chem Biol. 2008;15:790–798. doi: 10.1016/j.chembiol.2008.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Phillips G, El Yacoubi B, Lyons B, Alvarez S, Iwata-Reuyl D, de Crecy-Lagard V. J Bacteriol. 2008 doi: 10.1128/JB.00874-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reader JS, Metzgar D, Schimmel P, de Crecy-Lagard V. J. Biol. Chem. 2004;279:6280–6285. doi: 10.1074/jbc.M310858200. [DOI] [PubMed] [Google Scholar]
- 6.Van Lanen SG, Reader JS, Swairjo MA, de Crecy-Lagard V, Lee B, Iwata-Reuyl D. Proc Natl Acad Sci U S A. 2005;102:4264–4269. doi: 10.1073/pnas.0408056102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gaur R, Varshney U. J. Bacteriol. 2005;187:6893–6901. doi: 10.1128/JB.187.20.6893-6901.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sofia HJ, Chen G, Hetzler BG, Reyes-Spindola JF, Miller NE. Nucleic Acids Res. 2001;29:1097–1106. doi: 10.1093/nar/29.5.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Auerbach G, Nar H. Biol Chem. 1997;378:185–192. [PubMed] [Google Scholar]
- 10.Woo HJ, Hwang YK, Kim YJ, Kang JY, Choi YK, Kim CG, Park YS. FEBS Lett. 2002;523:234–238. doi: 10.1016/s0014-5793(02)02997-6. [DOI] [PubMed] [Google Scholar]
- 11.Burg AW, Brown GM. J. Biol. Chem. 1968;243:2349–2358. [PubMed] [Google Scholar]
- 12.Pfleiderer W. Chemistry of naturally occurring pterins. In: Blakley RL, Benkovic SJ, editors. Folates and pterins. New York: John Wiley & Sons; 1985. pp. 44–114. [Google Scholar]
- 13.Burgisser DM, Thony B, Redweik U, Hess D, Heizmann CW, Huber R, Nar H. J Mol Biol. 1995;253:358–369. doi: 10.1006/jmbi.1995.0558. [DOI] [PubMed] [Google Scholar]
- 14.Ploom T, Thony B, Yim J, Lee S, Nar H, Leimbacher W, Richardson J, Huber R, Auerbach G. J. Mol. Biol. 1999;286:851–860. doi: 10.1006/jmbi.1998.2511. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.