Abstract
Strategies to decrease intracellular polyamine levels have been studied for their efficacy in reducing colorectal cancer (CRC) risk. A successful strategy combined agents that decreased polyamine synthesis by inhibiting ornithine decarboxylase with difluoromethylornithine (DFMO), and increased cellular export of polyamines by activating the spermidine/spermine acetyl transferase with non-steroidal anti-inflammatory drugs (NSAIDs). A Phase III trial treating resected adenoma patients with DFMO plus sulindac demonstrated marked reduction of metachronous adenomas, advanced adenomas and multiple adenomas compared to placebo. This combination regimen was well-tolerated, however there was a non-significant excess of cardiovascular events in the treatment arm compared to placebo as well as modest ototoxicity. Targeting this therapy to people elevated risk of CRC, and employing clinical and genetic predictors, should improve patient benefit and reduce risk of side effects to improve the acceptability of this strategy.
Keywords: DFMO, chemoprevention, colorectal cancer, polyamines
Introduction
Eflornithine (difluoromethylornithine or DFMO) was synthesized by scientists at the Merrell Dow Research Institute as an enzyme-activated irreversible inhibitor of ornithine decarboxylase (ODC), the first and rate-limiting enzyme in polyamine synthesis [1]. DFMO was ineffective as a single agent for cancer treatment [2], but was subsequently shown to be effective in other pathologies. Since then DFMO in combination with a non-steroidal anti-inflammatory drug (NSAID) has been shown to be safe and effective in chemoprevention of colorectal adenomas in people with prior colon polyps [3].
FDA-approved uses of DFMO
DFMO is a potent, enzyme-activated irreversible inhibitor of ODC [4], an essential enzyme in the polyamine synthesis pathway. DFMO is generally cytostatic in mammalian cells, causing a reduction in the rate of cell proliferation in the absence of cell death. However, in certain protozoans, DFMO is cytotoxic, possibly due to the combined inhibitory effect both on polyamine synthesis and on the production of an essential antioxidant, trypanothione.
DFMO (Ornidyl®) was developed as a treatment for forms of African sleeping sickness and an intravenously dosed form of this agent was approved by the US Food and Drug Administration under an orphan drug indication in 1990. The combination of DFMO (intravenous 400 mg/kg per day; every 12 hours for 7 days) and oral Nifurtimox was shown to be non-inferior to DFMO monotherapy (intravenous 400 mg/kg per day; every 6 hours for 14 days) and judged to be suitable for first-line treatment of human African trypanosomiasis [4].
Topical DFMO (Vaniqa®) was developed as a depilatory agent and received FDA approval for treatment of hirsutism in 2001 [5-7].
DFMO as a chemopreventive agent
DFMO inhibits the promotion and proliferation/progression stages of initiated cancer cells [8, 9] suggesting its use as a cancer chemotherapy. Treatment of carcinogen-exposed animals with DFMO has been reported to reduce tumour incidence in bladder, colon, esophagus, small and large intestine, liver, mammary gland, glandular stomach, skin and pancreas [10-15]. DFMO has also been shown to have an inhibitory effect on specific markers of cell proliferation and neoplasia in animal models of carcinogenesis and efficacy has been reported in a variety of target organs, including bladder, colon, small intestine, mammary gland, and skin [4, 12, 16-24]. Although DFMO inhibits the growth of tumour cells in vitro, in many animal models in vivo, prohibitively high doses of DFMO were required to inhibit malignant tumour growth in early chemotherapeutic trials [25-27].
When used as a single agent in patients with a variety of malignancies, DFMO did not significantly slow tumour growth or disease progression [28]. When DFMO was used to prevent recurrent gliomas, however, 45% of patients improved and suffered only modest toxicity [29]. It has been proposed that the lack of efficacy DFMO has demonstrated against established tumours is due to the availability of extracellular polyamines derived from the diet, the retroconversion pathway and gastrointestinal microbial flora [30]. A growing body of evidence suggests that proliferation only needs to return to normal, not be completely blocked, to inhibit the process of carcinogenesis. Very low, non-toxic doses of DFMO may slow growth to normal and/or inhibit stimulation of proliferation by various carcinogens [31] and has led to interest in DFMO as a cancer chemopreventive agent [2].
Human trials of DFMO alone have shown mixed results in cancer chemoprevention. Topical DFMO reduced by one-quarter the number of pre-malignant actinic keratoses as compared to placebo [32]. Treatment with oral DFMO (0.5 g/m2/day) for 4-5 years showed a trend toward protection against all non-melanoma skin cancers, and significantly decreased the number of basal cell carcinomas [33]. One year of 0.5 g/m2/day DFMO decreased prostate putrescine levels and the rate of prostate growth [34]. Oral DFMO at 0.125 and 0.5 g/m2 for 28 days did not cause regression of CIN 2-3 cervical lesions in affected women [35] nor did 1 g daily prevent recurrence of low-risk superficial bladder cancer [36]. Six months of 0.5 g/m2/day oral DFMO did not improve cytological measurements of hyperplasia or atypia found in random breast periareolar fine-needle aspirations compared to placebo [37]. A non-placebo-controlled trial showed that after 6 months of 0.5 g/m2/day of DFMO, expression of proliferation genes was decreased in samples from patients with Barrett's esophagus [38].
Therapeutic and prevention clinical trials of DFMO in colorectal cancer (CRC) have been conducted including pilot Phase IIa and IIb studies of DFMO in reducing polyamine content in colorectal tissue. These trials have been used to determine the dose of DFMO necessary to consistently lower polyamine content in colorectal tissue [25, 39-41]. This dose was validated in a subsequent prospective, randomized trial of three daily oral DFMO doses versus placebo in patients with prior colorectal polyps [40]. Efficacy of DFMO alone in polyp prevention has not been reported.
Rationale for combination of DFMO and NSAIDs in colon cancer chemoprevention
Polyamine concentrations have been found to increase during carcinogenesis [42] and an increase in ODC activity accompanies neoplastic transformation [43]. Thus, a variety of strategies to decrease intracellular polyamine levels have been studied for their efficacy in cancer prevention [44]. One successful strategy decreases polyamine synthesis by inhibiting ODC with DFMO, while increasing cellular export of polyamines by activating spermidine/spermine acetyl transferase (SAT1) with NSAIDs. This combination produces at least an additive reduction colon carcinogenesis in mouse models [10, 11]. An ODC allele associated with decreased promoter activity is associated with decreased risk of colorectal adenomas in patients taking aspirin [45], suggesting a beneficial effect of combinations of ODC inhibitors and NSAIDs for colorectal adenoma risk reduction. Several NSAIDs activate polyamine acetylation and export, but do so by unique transcriptional mechanisms. Sulindac activates SAT1 transcription via a peroxisomal proliferatoration activated receptor γ (PPARγ) element [46], while aspirin activates the SAT1 promoter via an NF-κB dependent mechanism [47]. Sulindac and celecoxib are both potent stimulators of SAT1 and inhibitors of intestinal carcinogenesis in the ApcMin/+ mouse model [48, 49].
Polyamines and colorectal cancer
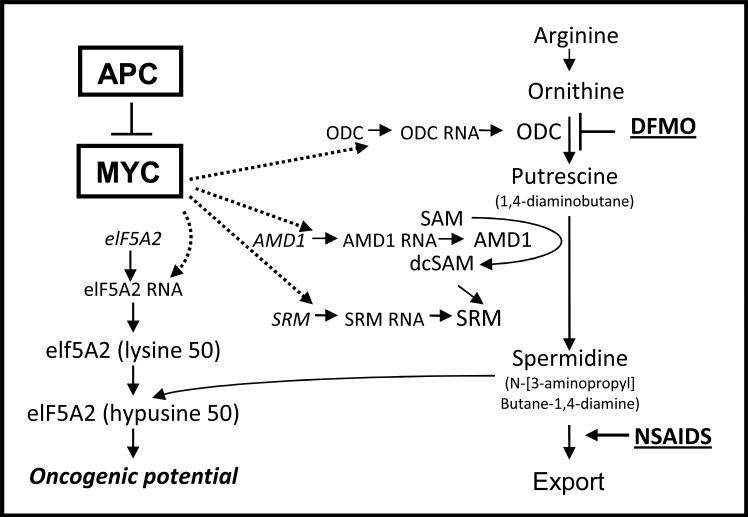
The polyamine synthesis pathway and its regulation (Figure 1) have been strongly linked to CRC [50-54]. The APC tumour suppressor gene, which is lost or mutated in the germline of patients with familial adenomatous polyposis (FAP) and in somatic epithelial cells in most neoplastic CRCs, regulates expression of a number of genes, including the c-MYC oncogene. MYC regulates a number of genes, including several in the polyamine pathway. DFMO and NSAIDs reduce polyamine synthesis and increase export, respectively. Polyamine levels are elevated in renal and colorectal carcinoma cells compared with their nonmalignant counterparts [55-57] and are essential for neoplastic transformation in vitro [55, 58, 59]. Levels of ODC, which catalyzes the conversion of ornithine to putrescine, are higher in neoplastic colorectal tissue compared to adjacent normal tissue [60, 61] and in biopsies of bladder transitional cell carcinoma and abnormal cervical cells [62]. High concentrations of polyamines are excreted in the urine of cancer patients compared with normal counterparts and these levels decrease upon successful therapy [63]. Inhibition of polyamine synthesis in mammalian cell lines by appropriate enzyme inhibitors or mutations leads to a virtual cessation of growth unless exogenous polyamines are provided [64]. There is increasing evidence that polyamines exert their effect on cellular transformation via stimulation of proto-oncogene expression [55, 59].
Figure 1.
APC regulates polyamines via the MYC oncogene. MYC transcriptionally activates a number of polyamine metabolic genes, including ornithine decarboxylase (ODC), which converts ornithine to putrescine, adenosylmethionine decarboxylase (AMD1), which provides the propylamine moiety for the longer chain amines, spermidine synthase (SRM), which adds the propylamine moiety to diaminobutane, and the eukaryotic translation initiation factor 5A2 (eIF5A2). A lysine in this protein is co-translationally modified with the butylamine moiety of spermidine to create the novel amino acid hypusine in eIF5A. The eIF5A2 protein has been shown to have oncogenic activity. DFMO decreases polyamine synthesis by inhibiting ODC activity. NSAIDs activate SAT1 which acetylates spermidine and spermine and targets them for export. Thus, DFMO and NSAIDs act in complement to reduce cellular and tissue polyamine contents. Modified from [3].
A specific association between the increase in polyamine synthesis and some primary and or secondary events involved in eukaryotic cellular growth and differentiation processes has been clearly identified. Polyamine biosynthesis has been associated with cell transformation, chemical-induced carcinogenesis, and experimental tumour cell proliferation. Experimental data indicate that inhibition of polyamine biosynthesis results in either a stimulatory or inhibitory effect on cellular differentiation depending on the model studied. Accordingly, DFMO treatment has resulted in opposite effects on cell differentiation in a variety of models and has been used to identify the various roles of polyamines in cellular growth, invasion, apoptosis and metastasis [17-21, 65].
Despite evidence that polyamines mediate normal and tumour cell growth, the precise role they play in regulation of cellular proliferation is unclear. These highly basic cations with a high affinity for nucleic acids may be involved in nucleic acid-mediated regulation of cellular proliferation and secretory activity [66, 67]. Polyamine depletion induced by DFMO inhibits DNA synthesis by slowing DNA elongation [68].
DFMO's inhibitory effect on cell proliferation and tumourigenesis involves a complex relationship between oncogenes, polyamine levels and ODC activity. The ODC gene is a transcriptional activation target of c-myc [69, 70] and DFMO decreases mRNA of the oncogenes N-myc in neuroblastoma cells and c-myc in human colon carcinoma cells [71]. Spermidine preferentially stimulates the transcription and expression of c-myc, while t c-fos was preferentially stimulated by putrescine [59]. These results suggest that polyamines play a feedback role in the regulation of expression of oncogenes at the level of transcription.
In vitro work suggested low oral bioavailability and short t1/2 of DFMO
DFMO inhibits ODC present in cultured cells, thus depleting intracellular polyamine levels. The addition of DFMO to rapidly proliferating rat hepatoma cells maximally blocked the increase in ODC activity and almost completely prevented the accumulation of putrescine and spermidine [22]. These effects were dose-dependent. Treating animals with DFMO inhibits ODC activity, especially in tissues and organs with rapidly dividing cells. Inhibition of ODC activity correlating to dose occurs in ventral prostate, thymus, and testis of male Sprague-Dawley rats given DFMO doses ranging from 12.5 to 400 mg/kg-bw intraperitonneally. Polyamine inhibition was greatest in the prostate, reaching 70% with 12.5 mg/kg-bw, 90% with 100 mg/kg-bw, and 96% with 200-400 mg/kg-bw.
In vivo efficacy seen at lower doses with less frequent dosing
Clinical translational studies have validated markers of polyamine metabolism in colorectal tissues [72]. Subsequent Phase II clinical studies investigated the dose-dependency of DFMO to suppress polyamine contents, especially putrescine levels and spermidine/spermine ratios, in colorectal mucosa [41, 73]. These studies evaluated DFMO doses adjusted for body size (dose in g DFMO per meter squared surface area = g/m2). The first study [41] used a dosede-escalation design determine the minimal DFMO dose to reduce tissue polyamine contents [41]. In this trial, oral liquid DFMO administered in doses in the range from 0.1-3.0 g/m2 per day for 28 days suppressed colorectal tissue polyamine contents in humans. In a second, randomized placebo-controlled Phase II trial oral liquid DFMO doses ranging from 0.075-0.4 g/m2 per day were evaluated and doses of 0.2 and 0.4 g/m2 per day for 6 or 12 months reduced putrescine, spermidine and spermidine/spermine ratios in rectal mucosal tissue in patients with prior colon polyps [73]. This reduction was statistically significant for putrescine and spermidine at 6 months and for all three parameters at 12 months. Especially the spermidine/spermine ratio has been validated as a biomarker of tissue polyamine contents [72]. Tissue spermine values were not expected to change, and did not; tissue polyamine values returned to placebo values after 3 months, but did not rebound.
Carbone and colleagues compared the bioavailability of liquid and tablet forms of DFMO [74] and found no difference in mean area under the time-by-concentration curves, peak concentrations, time to peak concentration or in serum half-life. Oral DFMO doses from 0.2-0.4 g/m2 could be specified without adjustment for body size and effectively reduce tissue polyamine contents [73]. Since an average adult body surface area is ~2 m2, an oral DFMO dose of 0.25 g/m2 X 2 m2 = 0.5 g = 500 mg was chosen for further investigation.
Additional polyamine pathway targeting strategies
Induction of polyamine catabolism has also been a major drug target [75, 76]. The enzyme SAT1 can be induced by a compounds including NSAIDs and polyamine analogues and by elevated temperature, which has been tested in the treatment of melanoma [77]. Most NSAIDs (e.g. sulindac, ibuprofen, indomethacin) induce SAT1 by activating PPARγ, while aspirin (the classic NSAID) activates NKκB and its response elements in the promoter region of the SAT1 gene [47]. This may explain the non-COX-2 anti-tumor activity shown by sulindac sulfone, which lacks the COX inhibitory activity of sulindac sulfide. [46, 78, 79]. The anti-COX mediated effects of NSAIDs should not be forgotten, however. Indeed, selective COX-2 inhibitors (e.g. Celebrex®) reduce colorectal adenoma load in patients with FAP [80] and NSAIDs inhibit anti-apoptotic activity of bcl2 which is often over-expressed in colorectal adenomas, thus returning the possibility of appropriate programmed cell death to the cell [81].
Additional polyamine biosynthesis enzymes, AdoMetDC and spermidine/spermine synthase, have also been targeted for inhibition [44]. While the targeting agents have shown in vitro efficacy, in vivo effects have been less promising. Recognition that spermine must be converted to spermidine by polyamine oxidase (PaOx) in order to overcome cell growth inhibition by polyamine depletion has led to the development PaOx inhibitors, which are potent killers of cancer cells in vitro and show promise when used with DFMO in in vivo carcinogenesis models [82]. Other aspects of the polyamine metabolism pathway including polyamine uptake and efflux have also been targeted for cancer drug development. Uptake inhibitors have shown promise in vitro and have increased the efficacy of DFMO as an anti-cancer agent [83]. The activity of the translation initiation factor eIF5A is dependent on cellular spermidine levels, and inhibitors of the interaction allowing activation have been developed [84] and observed to induce apoptosis in colon cancer cells (April Childs and E.W. Gerner, unpublished).
Manipulating polyamine levels with artificial polyamine analogues (mimetics or antimetabolites) produces pleiotropic effects on cancer cells. Mimetics inhibit cell growth by acting like endogenous polyamines. Antimetabolites decrease intracellular polyamine levels by “superinducing” the polyamine catabolism enzyme SAT1 [76]. These can deplete cellular polyamines by upregulating catabolism, decreasing biosynthesis by negative feedback inhibition, or by competing with exogenous polyamines for uptake [76]. They can also bind to intracellular polyamine binding sites rendering them “non-functional”.
Clinical trials with DFMO
Three Phase II studies of DFMO have been completed in colon cancer chemoprevention. In the first trial, 45 subjects with a history of colorectal adenomatous polyps or other risk factors for CRC were administered 0.5 g DFMO/m2/day or placebo for one year. DFMO treatment was associated with a significant decrease putrescine and spermidine levels in rectosigmoid colonic mucosal biopsies, compared with samples from the placebo group at 3 and 12 months [85]. In another completed Phase II study, 111 patients who had undergone colonoscopy for removal of a colorectal adenoma were administered daily oral doses of DFMO ranging from 0.1 to 3.0 g/m2 for 4 weeks [39, 41, 86]. Subjects treated with doses as low as 0.25 g DFMO/m2/day for 4 weeks showed a statistically significant decrease in Spd:Spm ratios in rectal mucosal biopsy specimens. DFMO treatment was also associated with a decrease in rectal mucosal putrescine content for all dose groups down to 0.25 g/m2. In a third Phase II trial, 118 subjects with a prior history of resected colon polyps were administered DFMO (0.075, 0.20, or 0.40 g/m2/day) for one year with a 3-month follow-up [31]. After 6 months of treatment, doses of 0.2 and 0.4 g/m2/day reduced putrescine levels to ~34% and 10%, respectively, of those observed in the placebo group [40].
Combination trials with NSAIDs and DFMO
Adenomatous polyps are precursors to CRC and late surrogates in the carcinogenic pathway to the development of these malignancies. Etiologic and experimental investigations have established the critical nature of definable molecular changes of disease pathogenesis that lead to abnormal signaling pathways and defective growth control. Epidemiological studies have suggested that dietary and non-dietary factors affect the development of adenomatous polyps and colon cancer [87-89] and that regular NSAID use is associated with a decreased risk of CRC [90]. By combining DFMO with NSAIDs or other proven chemopreventive agents, it is hoped that prevention efforts may be more effective at lower doses, and thus with fewer side effects.
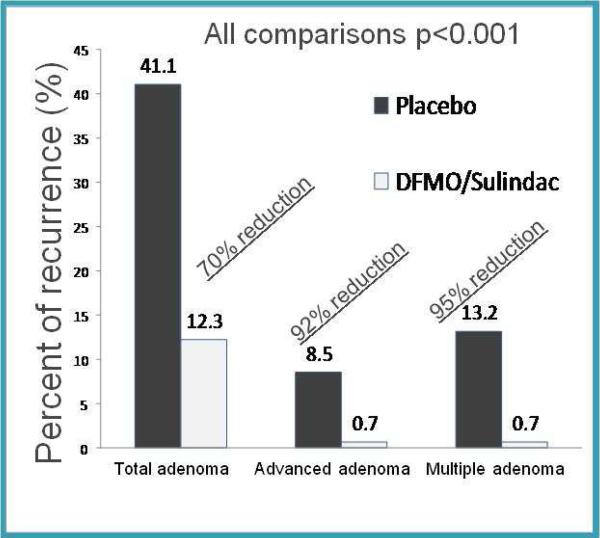
A phase III trial evaluated DFMO in combination with an NSAID to prevent metachronous adenomas in patients with prior colon polyps [3]. They found that the combination of oral DFMO (500 mg/day) and sulindac (150 mg/day) reduced total metachronous colorectal adenomas by 70% (p<0.001) and advanced and/or multiple adenomas by >9% (p<0.001) (Figure 2). The risk of malignancy correlates with the size, histologic type, and degree of dysplasia in the polyp [91]. Thus prevention of advanced adenomas (>1 cm, with villous or tubulovillous components, or with high-grade or severe dysplasia) translates into CRC prevention.
Figure 2.
Risk of metachronous adenoma is reduced in people treated with DFMO and sulindac versus placebo. Percent recurrence in people treated with placebo are compared to those treated with both DFMO and sulindac. Data are summarized from [3]
Toxicity data for DFMO
Detailed safety data derive from 9 completed Phase I and Phase II chemoprevention clinical trials which were either open-label or subsequently unblinded. 359 subjects were involved in these studies, 56 in placebo or observation arms, and the rest receiving DFMO in doses ranging from 0.06 to 6.4 g/m2. The frequency and severity of adverse events did not have an obvious dose-response relationship when normalized to the number of subjects at each dose level. However, a comparison of the most frequent adverse events (AEs) reported in the placebo cohort versus DFMO-treated subjects reveals some differences in frequencies of specific types of AEs. For example, DFMO treatment was associated with a significantly higher-than-background occurrence of diarrhea (13.8% vs. 7.1%), hearing loss (12.9% vs. 5.3%), and stomatitis (4.8% vs. 0), and a slightly higher-than-background occurrence of nausea, headache, myalgia, emotional lability, and dizziness. Other AEs in the “most frequent” category for DFMO subjects occurred at the same or lower frequency than in placebo subjects (asthenia, skin rash, tinnitus, anemia, flatulence, vomiting, abdominal pain, and dyspepsia). For the placebo controls, no AEs with a severity grade higher than 2 were reported, whereas for DFMO-treated subjects, 10% of the AEs were grade 3 (and 2.7% were of unspecified grade). Results from early clinical trials suggested that ototoxicity might be a significant problem [92-96]. DFMO-associated ototoxicity was evidenced by tinnitus, hearing loss, and vertigo and these effects were reversed within three months of drug discontinuation [27, 96]. Ototoxicity was dose related and minimal toxicity was observed in patients who received a cumulative dose less than 150 g/m2 [93]. DFMO doses of ≤1 g/day for periods up to one year appear to be without significant ototoxicity in most patients [2, 95]. Most chemoprevention trials use doses in the range of 0.5-1.0 g/m2/day, equivalent to 12.5-25 mg/kg-bw/day for the average-size person.
Toxicity in combination trials
The overall, total GI and upper GI toxicity profile in patients in the Phase III trial taking low doses of DFMO and sulindac in combination was similar compared to the placebo group after three years treatment duration, although low power of the sample renders statistical tests inconclusive [3, 97, 98]. A non-significant but numerically greater number of cardiovascular events were observed in the treatment arm (16 events) compared to placebo (9 events) [3]. This numeric difference is concerning as the trial was not powered to accurately assess cardiovascular risk [98], but the increase in cardiovascular events was similar to an excess of patients with cardiovascular risk factors at baseline in the treatment compared to placebo arms [98-100]. While modest ototoxicity was attributed to treatment [3], further analysis found no significant difference in the proportion of patients in the treatment arm who experienced clinically significant hearing loss compared with the placebo group and a <2 dB difference in mean pure tone threshold for patients on treatment compared with placebo [97].
Conclusion & future directions
Tailoring treatment using pharmacogenetic information
Recent analyses of the Meyskens et al DFMO/Sulindac clinical trial indicate that ototoxicity affecting the DFMO treatment group may be associated with a specific germline single nucleotide polymorphism in the ODC1 promoter region that occurs in 5-6% of Caucasians [101]. Carriers of the ODC1A allele respond differently to DFMO and sulindac compared with GG genotype patients. ODC1A allele carriers experience less treatment-related benefit (i.e., less metachronous adenoma risk reduction) and a higher risk of ototoxicity, especially among the AA homozygotes (Table 1). Whether the A allele is a risk or protective allele may, therefore, depend on the tissue context or extent of polyamine inhibition. A major impediment to the translation of cancer chemoprevention research into clinical practice has been marginal agent efficacy and toxicities that exceed benefit [102, 103]. In this study, we identify genetic features that may be markers for both treatment benefit and toxicity. These results encourage evaluation in future polyamine inhibitory chemoprevention trials, as planned in the cooperative group setting.
Table 1.
Efficacy and toxicity of DFMO and sulindac vary based on individual ODC genotype.
| Placebo (n=111) | DFMO/sulindac (n=117) | ||||
|---|---|---|---|---|---|
| GG, No. (%) | GA or AA, No. (%) | GG, No. (%) | GA or AA, No. (%) | P | |
| Any adenoma recurrence | 22 (50) | 18 (34) | 7 (11) | 9 (21) | <0.001 |
| Any adverse event | |||||
| Cardiovascular eventsa | 8 (15) | 8 (14) | 13 (18) | 9 (20) | 0.30 |
| Gastrointestinal bleedingb | 4 (7) | 8 (14) | 9 (13) | 7 (15) | 0.54 |
| Hearing lossc | 10 (23) | 8 (17) | 14 (22) | 11 (27) | 0.25 |
Modified from Zell et al., (2010)
Coronary artery disease, myocardial infarction, cerebrovascular accident, congestive heart failure, chest pain
Rectal bleeding, upper gastrointestinal bleeding, hematochezia, or occult blood in the stool
≥15dB at ≥2 frequencies
S0820, “A Double Blind Placebo-Controlled Trial of Eflornithine and Sulindac to Prevent Recurrence of High Risk Adenomas and Second Primary Colorectal Cancers in Patients with Stage 0-III Colon Cancer, Phase III”
Even after surgical resection and chemotherapy, colon cancer patients remain at considerable risk for distant recurrence, second colonic tumour formation, and subsequent mortality. The Southwest Oncology Group (SWOG) will conduct a randomized Phase III trial (S0820, ClinicalTrials.gov Identifier NCT01349881; expected to open in the 4th quarter of 2011) that seeks to assess whether DFMO, sulindac or the combination reduce the occurrence of high-risk adenomas and new primary CRCs in patients with previously treated Stage 0-III colon cancer over three years. The recent Phase III trial involving adenoma patients indicates that the combination of DFMO plus sulindac is effective, but confers a modest risk of ototoxicity and the potential risk of cardiovascular toxicity [3, 97, 98]. Such considerations about safety are important to consider in the development of any chemoprevention trial, even when addressing a group at higher risk of CRC related events (i.e., colon cancer patients as opposed to colorectal adenoma patients). The proposed S0820 protocol includes a substantial translational component that should allow identification of key pharmacogenetic markers of drug toxicity that may be important in future prevention trials. The development of non-toxic drugs for tertiary prevention of recurrent malignant or pre-malignant lesions among optimally-treated colon cancer patients will be a major contribution to the field of cancer chemoprevention and public health. These conditions define the optimal window of time to begin a major Phase III combination trial of resected colon cancer patients.
Practice Points:
Dual targeting of the polyamine pathway with DFMO and NSAIDs significantly reduces risk of metachronous adenoma.
There is minimal toxicity from the low doses of DFMO and NSAIDs used in chemoprevention regimens.
Research Agenda:
Effective treatment targeting requires the identification of genetic features that are markers for both treatment benefit and toxicity.
Polyamine pathway targeting in prevention of CRC recurrence is promising, but needs to be tested.
Can a chemoprevention regimen postpone the need for colectomy in individuals with familial cancer syndromes (ie. HNPCC, FAP)?
Summary
Decreasing polyamine synthesis with DFMO while increasing polyamine export with NSAIDs significantly reduces intracellular polyamine levels. This combination markedly reduces occurrence of metachronous adenomas, advanced adenomas and multiple adenomas compared to placebo. This regimen is well-tolerated, however there was a non-significant excess of cardiovascular events in the treatment arm compared to placebo as well as modest ototoxicity. Understanding the complex relationship between oncogenes, polyamine levels and ODC activity may help to explain DFMO's inhibitory effect on cell proliferation and tumourigenesis and to allow efficient targeting of therapy. By understanding which people are most likely to benefit from treatment and/or most likely to be harmed, these people can targeted for cancer risk reduction therapy. Future studies will determine whether strategies targeting the polyamine pathway will contribute to prevention of colon cancer recurrence and/or delay of colectomy in people with genetic CRC syndromes.
Acknowledgements
The authors would like to thank Corina Fuentes Mauss for assistance preparing the manuscript.
Role of the funding source
Work described in this review was supported by grants from the National Institutes of Health to EWG and colleagues, including CA047396, CA059024, CN075019, CA072008, CA088078, CA095960 and CA123065. CML was also supported by CA023074.
The study sponsors had no role in the collection, analysis and interpretation of data and in the writing of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
Dr. Laukaitis has no conflicts of interest to report. Dr. Gerner has an ownership interest in Cancer Prevention Pharmaceuticals, Inc., 1760 E. River Road, Suite 250, Tucson, AZ 85718.
Contributor Information
Christina M. Laukaitis, Department of Medicine, University of Arizona, Tucson, AZ 85724 USA claukaitis@azcc.arizona.edu Tel: (520)626-5845.
Eugene W. Gerner, Department of Cellular and Molecular Medicine, BIO5 Oro Valley, 1580 E. Hanley Blvd., University of Arizona, Tucson, AZ 85737 USA egerner@azcc.arizona.edu Tel: 520-626-2197.
Literature cited
- 1.Bey P, et al. Analogues of ornithine as inhibitors of ornithine decarboxylase. New deductions concerning the topography of the enzyme's active site. J Med Chem. 1978;21(1):50–5. doi: 10.1021/jm00199a009. [DOI] [PubMed] [Google Scholar]
- 2.Meyskens FL, Jr., Gerner EW. Development of difluoromethylornithine (DFMO) as a chemoprevention agent. Clin Cancer Res. 1999;5(5):945–51. [PubMed] [Google Scholar]
- 3.Meyskens FL, Jr., et al. Difluoromethylornithine plus sulindac for the prevention of sporadic colorectal adenomas: a randomized placebo-controlled, double-blind trial. Cancer Prev Res (Phila Pa) 2008;1(1):32–8. doi: 10.1158/1940-6207.CAPR-08-0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.White EL, et al. Screening of potential cancer-preventing chemicals for inhibition of induction of ornithine decarboxylase in epithelial cells from rat trachea. Oncol Rep. 1998;5(3):717–22. doi: 10.3892/or.5.3.717. [DOI] [PubMed] [Google Scholar]
- 5.Shapiro J, Lui H. Vaniqa--eflornithine 13.9% cream. Skin Therapy Lett. 2001;6(7):1–3, 5. [PubMed] [Google Scholar]
- 6.Hickman JG, Huber F, Palmisano M. Human dermal safety studies with eflornithine HCl 13.9% cream (Vaniqa), a novel treatment for excessive facial hair. Curr Med Res Opin. 2001;16(4):235–44. doi: 10.1185/030079901750176735. [DOI] [PubMed] [Google Scholar]
- 7.Wolf JE, Jr., et al. Randomized, double-blind clinical evaluation of the efficacy and safety of topical eflornithine HCl 13.9% cream in the treatment of women with facial hair. Int J Dermatol. 2007;46(1):94–8. doi: 10.1111/j.1365-4632.2006.03079.x. [DOI] [PubMed] [Google Scholar]
- 8.Hursting SD, et al. Mechanism-based cancer prevention approaches: targets, examples, and the use of transgenic mice. J Natl Cancer Inst. 1999;91(3):215–25. doi: 10.1093/jnci/91.3.215. [DOI] [PubMed] [Google Scholar]
- 9.Luk GD, Casero RA., Jr. Polyamines in normal and cancer cells. Adv Enzyme Regul. 1987;26:91–105. doi: 10.1016/0065-2571(87)90007-0. [DOI] [PubMed] [Google Scholar]
- 10.Jacoby RF, et al. Chemopreventive efficacy of combined piroxicam and difluoromethylornithine treatment of Apc mutant Min mouse adenomas, and selective toxicity against Apc mutant embryos. Cancer Res. 2000;60(7):1864–70. [PubMed] [Google Scholar]
- 11.Rao CV, et al. Chemoprevention of colon carcinogenesis by dietary administration of piroxicam, alpha-difluoromethylornithine, 16 alpha-fluoro-5-androsten-17-one, and ellagic acid individually and in combination. Cancer Res. 1991;51(17):4528–34. [PubMed] [Google Scholar]
- 12.Erdman SH, et al. APC-dependent changes in expression of genes influencing polyamine metabolism, and consequences for gastrointestinal carcinogenesis, in the Min mouse. Carcinogenesis. 1999;20(9):1709–13. doi: 10.1093/carcin/20.9.1709. [DOI] [PubMed] [Google Scholar]
- 13.Esmat AY, et al. Chemoprevention of prostate carcinogenesis by DFMO and/or finasteride treatment in male Wistar rats. Tumori. 2002;88(6):513–21. doi: 10.1177/030089160208800616. [DOI] [PubMed] [Google Scholar]
- 14.Gupta S, et al. Chemoprevention of prostate carcinogenesis by alpha- difluoromethylornithine in TRAMP mice. Cancer Res. 2000;60(18):5125–33. [PubMed] [Google Scholar]
- 15.Thompson HJ, et al. Effect of concentration of D,L-2-difluoromethylornithine on murine mammary carcinogenesis. Cancer Res. 1985;45(3):1170–3. [PubMed] [Google Scholar]
- 16.Ignatenko NA, et al. The chemopreventive agent alpha-difluoromethylornithine blocks Ki-ras-dependent tumor formation and specific gene expression in Caco-2 cells. Mol Carcinog. 2004;39(4):221–33. doi: 10.1002/mc.20008. [DOI] [PubMed] [Google Scholar]
- 17.Wallon UM, et al. Polyamine-dependent expression of the matrix metalloproteinase matrilysin in a human colon cancer-derived cell line. Mol Carcinog. 1994;11(3):138–44. doi: 10.1002/mc.2940110304. [DOI] [PubMed] [Google Scholar]
- 18.Alhonen-Hongisto L, Deen DF, Marton LJ. Time dependence of the potentiation of 1,3-bis(2-chloroethyl)-1-nitrosourea cytotoxicity caused by alpha-difluoromethylornithine-induced polyamine depletion in 9L rat brain tumor cells. Cancer Res. 1984;44(5):1819–22. [PubMed] [Google Scholar]
- 19.Choi W, et al. Combination of 5-fluorouracil and N1,N11-diethylnorspermine markedly activates spermidine/spermine N1-acetyltransferase expression, depletes polyamines, and synergistically induces apoptosis in colon carcinoma cells. J Biol Chem. 2005;280(5):3295–304. doi: 10.1074/jbc.M409930200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takahashi Y, Mai M, Nishioka K. alpha-difluoromethylornithine induces apoptosis as well as anti-angiogenesis in the inhibition of tumor growth and metastasis in a human gastric cancer model. Int J Cancer. 2000;85(2):243–7. [PubMed] [Google Scholar]
- 21.Takigawa M, et al. Tumor angiogenesis and polyamines: alpha-difluoromethylornithine, an irreversible inhibitor of ornithine decarboxylase, inhibits B16 melanoma-induced angiogenesis in ovo and the proliferation of vascular endothelial cells in vitro. Cancer Res. 1990;50(13):4131–8. [PubMed] [Google Scholar]
- 22.Tome ME, Fiser SM, Gerner EW. Consequences of aberrant ornithine decarboxylase regulation in rat hepatoma cells. J Cell Physiol. 1994;158(2):237–44. doi: 10.1002/jcp.1041580205. [DOI] [PubMed] [Google Scholar]
- 23.Wan XS, et al. In vitro evaluation of chemopreventive agents using cultured human prostate epithelial cells. Oncol Rep. 2003;10(6):2009–14. [PubMed] [Google Scholar]
- 24.Zaletok S, et al. Role of polyamines in the function of nuclear transcription factor NF-kappaB in breast cancer cells. Exp Oncol. 2004;26(3):221–5. [PubMed] [Google Scholar]
- 25.Love RR, et al. Randomized phase I chemoprevention dose-seeking study of alpha-difluoromethylornithine. J Natl Cancer Inst. 1993;85(9):732–7. doi: 10.1093/jnci/85.9.732. [DOI] [PubMed] [Google Scholar]
- 26.Levin VA, et al. Phase III randomized study of postradiotherapy chemotherapy with alpha-difluoromethylornithine-procarbazine, N-(2-chloroethyl)-N'-cyclohexyl-N-nitrosurea, vincristine (DFMO-PCV) versus PCV for glioblastoma multiforme. Clin Cancer Res. 2000;6(10):3878–84. [PubMed] [Google Scholar]
- 27.Meyskens FL, et al. A phase II study of alpha-difluoromethylornithine (DFMO) for the treatment of metastatic melanoma. Invest New Drugs. 1986;4(3):257–62. doi: 10.1007/BF00179593. [DOI] [PubMed] [Google Scholar]
- 28.McCann PP, Pegg AE. Ornithine decarboxylase as an enzyme target for therapy. Pharmacol Ther. 1992;54(2):195–215. doi: 10.1016/0163-7258(92)90032-u. [DOI] [PubMed] [Google Scholar]
- 29.Levin VA, et al. Phase II study of 6-thioguanine, procarbazine, dibromodulcitol, lomustine, and vincristine chemotherapy with radiotherapy for treating malignant glioma in children. Neuro Oncol. 2000;2(1):22–8. doi: 10.1093/neuonc/2.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leveque J, et al. The gastrointestinal polyamine source depletion enhances DFMO induced polyamine depletion in MCF-7 human breast cancer cells in vivo. Anticancer Res. 1998;18(4A):2663–8. [PubMed] [Google Scholar]
- 31.Meyskens FL, Jr., Gerner EW. Development of difluoromethylornithine as a chemoprevention agent for the management of colon cancer. J Cell Biochem Suppl. 1995;22:126–31. doi: 10.1002/jcb.240590816. [DOI] [PubMed] [Google Scholar]
- 32.Alberts DS, et al. Chemoprevention of human actinic keratoses by topical 2-(difluoromethyl)-dl-ornithine. Cancer Epidemiol Biomarkers Prev. 2000;9(12):1281–6. [PubMed] [Google Scholar]
- 33.Bailey HH, et al. A randomized, double-blind, placebo-controlled phase 3 skin cancer prevention study of {alpha}-difluoromethylornithine in subjects with previous history of skin cancer. Cancer Prev Res (Phila) 2010;3(1):35–47. doi: 10.1158/1940-6207.CAPR-09-0096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Simoneau AR, et al. The effect of difluoromethylornithine on decreasing prostate size and polyamines in men: results of a year-long phase IIb randomized placebo-controlled chemoprevention trial. Cancer Epidemiol Biomarkers Prev. 2008;17(2):292–9. doi: 10.1158/1055-9965.EPI-07-0658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vlastos AT, et al. Results of a phase II double-blinded randomized clinical trial of difluoromethylornithine for cervical intraepithelial neoplasia grades 2 to 3. Clin Cancer Res. 2005;11(1):390–6. [PubMed] [Google Scholar]
- 36.Messing E, et al. Randomized prospective phase III trial of difluoromethylornithine vs placebo in preventing recurrence of completely resected low risk superficial bladder cancer. J Urol. 2006;176(2):500–4. doi: 10.1016/j.juro.2006.03.061. [DOI] [PubMed] [Google Scholar]
- 37.Fabian CJ, et al. A phase II breast cancer chemoprevention trial of oral alpha-difluoromethylornithine: breast tissue, imaging, and serum and urine biomarkers. Clin Cancer Res. 2002;8(10):3105–17. [PubMed] [Google Scholar]
- 38.Sinicrope FA, et al. Evaluation of difluoromethylornithine for the chemoprevention of Barrett's esophagus and mucosal dysplasia. Cancer Prev Res (Phila) 2011;4(6):829–39. doi: 10.1158/1940-6207.CAPR-10-0243. [DOI] [PubMed] [Google Scholar]
- 39.Boyle JO, et al. Polyamine contents in rectal and buccal mucosae in humans treated with oral difluoromethylornithine. Cancer Epidemiol Biomarkers Prev. 1992;1(2):131–5. [PubMed] [Google Scholar]
- 40.Meyskens FL, Jr., et al. Effect of alpha-difluoromethylornithine on rectal mucosal levels of polyamines in a randomized, double-blinded trial for colon cancer prevention. J Natl Cancer Inst. 1998;90(16):1212–8. doi: 10.1093/jnci/90.16.1212. [DOI] [PubMed] [Google Scholar]
- 41.Meyskens FL, Jr., et al. Dose de-escalation chemoprevention trial of alpha-difluoromethylornithine in patients with colon polyps. J Natl Cancer Inst. 1994;86(15):1122–30. doi: 10.1093/jnci/86.15.1122. [DOI] [PubMed] [Google Scholar]
- 42.Andersson G, Heby O. Polyamine and nucleic acid concentrations in Ehrlich ascites carcinoma cells and liver of tumor-bearing mice at various stages of tumor growth. J Natl Cancer Inst. 1972;48(1):165–72. [PubMed] [Google Scholar]
- 43.Russell DH, Levy CC. Polyamine accumulation and biosynthesis in a mouse L1210 leukemia. Cancer Res. 1971;31(3):248–51. [PubMed] [Google Scholar]
- 44.Basuroy UK, Gerner EW. Emerging concepts in targeting the polyamine metabolic pathway in epithelial cancer chemoprevention and chemotherapy. Journal of Biochemistry. 2006;139(1):27–33. doi: 10.1093/jb/mvj022. [DOI] [PubMed] [Google Scholar]
- 45.Martinez ME, et al. Pronounced reduction in adenoma recurrence associated with aspirin use and a polymorphism in the ornithine decarboxylase gene. Proc Natl Acad Sci U S A. 2003;100(13):7859–64. doi: 10.1073/pnas.1332465100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Babbar N, et al. Cyclooxygenase-independent induction of apoptosis by sulindac sulfone is mediated by polyamines in colon cancer. J Biol Chem. 2003;278(48):47762–75. doi: 10.1074/jbc.M307265200. [DOI] [PubMed] [Google Scholar]
- 47.Babbar N, Gerner EW, Casero RA., Jr. Induction of spermidine/spermine N1-acetyltransferase (SSAT) by aspirin in Caco-2 colon cancer cells. Biochem J. 2006;394(Pt 1):317–24. doi: 10.1042/BJ20051298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ignatenko NA, et al. Dietary putrescine reduces the intestinal anticarcinogenic activity of sulindac in a murine model of familial adenomatous polyposis. Nutr Cancer. 2006;56(2):172–81. doi: 10.1207/s15327914nc5602_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ignatenko NA, et al. Combination chemoprevention of intestinal carcinogenesis in a murine model of familial adenomatous polyposis. Nutr Cancer. 2008;60(Suppl 1):30–5. doi: 10.1080/01635580802401317. [DOI] [PubMed] [Google Scholar]
- 50.Babbar N, Gerner EW. Polyamines as modifiers of genetic risk factors in human intestinal cancers. Biochem Soc Trans. 2003;31(2):388–92. doi: 10.1042/bst0310388. [DOI] [PubMed] [Google Scholar]
- 51.Gerner EW, Meyskens FL., Jr. Polyamines and cancer: old molecules, new understanding. Nat Rev Cancer. 2004;4(10):781–92. doi: 10.1038/nrc1454. [DOI] [PubMed] [Google Scholar]
- 52.Giardiello FM, et al. Ornithine decarboxylase and polyamines in familial adenomatous polyposis. Cancer Res. 1997;57(2):199–201. [PubMed] [Google Scholar]
- 53.Linsalata M, et al. Polyamine biosynthesis in relation to K-ras and p-53 mutations in colorectal carcinoma. Scand J Gastroenterol. 2004;39(5):470–7. doi: 10.1080/0036552031008755. [DOI] [PubMed] [Google Scholar]
- 54.Wallace HM, Caslake R. Polyamines and colon cancer. Eur J Gastroenterol Hepatol. 2001;13(9):1033–9. doi: 10.1097/00042737-200109000-00006. [DOI] [PubMed] [Google Scholar]
- 55.Seiler N, Atanassov CL, Raul F. Polyamine metabolism as target for cancer chemoprevention (review). Int J Oncol. 1998;13(5):993–1006. doi: 10.3892/ijo.13.5.993. [DOI] [PubMed] [Google Scholar]
- 56.Gerner EW, et al. Gastrointestinal tissue polyamine contents of patients with Barrett's esophagus treated with alpha-difluoromethylornithine. Cancer Epidemiol Biomarkers Prev. 1994;3(4):325–30. [PubMed] [Google Scholar]
- 57.Hixson LJ, et al. Sources of variability in estimating ornithine decarboxylase activity and polyamine contents in human colorectal mucosa. Cancer Epidemiol Biomarkers Prev. 1994;3(4):317–23. [PubMed] [Google Scholar]
- 58.Auvinen M, et al. Ornithine decarboxylase activity is critical for cell transformation. Nature. 1992;360(6402):355–8. doi: 10.1038/360355a0. [DOI] [PubMed] [Google Scholar]
- 59.Tabib A, Bachrach U. Role of polyamines in mediating malignant transformation and oncogene expression. Int J Biochem Cell Biol. 1999;31(11):1289–95. doi: 10.1016/s1357-2725(99)00098-9. [DOI] [PubMed] [Google Scholar]
- 60.Hixson LJ, et al. Ornithine decarboxylase and polyamines in colorectal neoplasia and mucosa. Cancer Epidemiol Biomarkers Prev. 1993;2(4):369–74. [PubMed] [Google Scholar]
- 61.Elitsur Y, et al. Polyamine levels, ornithine decarboxylase (ODC) activity, and ODC-mRNA expression in normal and cancerous human colonocytes. Life Sci. 1992;50(19):1417–24. doi: 10.1016/0024-3205(92)90260-v. [DOI] [PubMed] [Google Scholar]
- 62.Mitchell MF, et al. Polyamine measurements in the uterine cervix. J Cell Biochem Suppl. 1997;28-29:125–32. [PubMed] [Google Scholar]
- 63.Bachrach U. Polyamines as markers of malignancy. Prog Drug Res. 1992;39:9–33. doi: 10.1007/978-3-0348-7144-0_2. [DOI] [PubMed] [Google Scholar]
- 64.Pegg AE. Polyamine metabolism and its importance in neoplastic growth and a target for chemotherapy. Cancer Research. 1988;48(4):759–74. [PubMed] [Google Scholar]
- 65.Guo X, et al. Regulation of adherens junctions and epithelial paracellular permeability: a novel function for polyamines. Am J Physiol Cell Physiol. 2003;285(5):C1174–87. doi: 10.1152/ajpcell.00015.2003. [DOI] [PubMed] [Google Scholar]
- 66.Danzin C, et al. Effects of alpha-difluoromethylornithine, an enzyme-activated irreversible inhibitor or ornithine decarboxylase, on testosterone-induced regeneration of prostate and seminal vesicle in castrated rats. Biochem J. 1979;180(3):507–13. doi: 10.1042/bj1800507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Danzin C, et al. Effect of alpha-difluoromethylornithine, an enzyme-activated irreversible inhibitor of ornithine decarboxylase, on polyamine levels in rat tissues. Life Sci. 1979;24(6):519–24. doi: 10.1016/0024-3205(79)90173-5. [DOI] [PubMed] [Google Scholar]
- 68.Oredsson SM, Nicander B, Heby O. Implications for a reduced DNA-elongation rate in polyamine-depleted cells. Eur J Biochem. 1990;190(3):483–9. doi: 10.1111/j.1432-1033.1990.tb15599.x. [DOI] [PubMed] [Google Scholar]
- 69.Bello-Fernandez C, Packham G, Cleveland JL. The ornithine decarboxylase gene is a transcriptional target of c-Myc. Proc Natl Acad Sci U S A. 1993;90(16):7804–8. doi: 10.1073/pnas.90.16.7804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pena A, et al. Regulation of human ornithine decarboxylase expression by the c-Myc.Max protein complex. J Biol Chem. 1993;268(36):27277–85. [PubMed] [Google Scholar]
- 71.Celano P, et al. Effect of polyamine depletion on c-myc expression in human colon carcinoma cells. J Biol Chem. 1988;263(12):5491–4. [PubMed] [Google Scholar]
- 72.Hixson LJ, et al. Sources of variability in estimating ornithine decarboxylase activity and polyamine contents in human colorectal mucosa. Cancer Epidemiol Biomarkers Prev. 1994;3(4):317–23. [PubMed] [Google Scholar]
- 73.Meyskens FL, Jr., et al. Effect of alpha-difluoromethylornithine on rectal mucosal levels of polyamines in a randomized, double-blinded trial for colon cancer prevention. J Natl Cancer Inst. 1998;90(16):1212–8. doi: 10.1093/jnci/90.16.1212. [DOI] [PubMed] [Google Scholar]
- 74.Carbone PP, et al. Bioavailability study of oral liquid and tablet forms of alpha-difluoromethylornithine. Clinical Cancer Research. 2000;6(10):3850–4. [PubMed] [Google Scholar]
- 75.Wallace HM, Fraser AV. Inhibitors of polyamine metabolism: review article. Amino Acids. 2004;26(4):353–65. doi: 10.1007/s00726-004-0092-6. [DOI] [PubMed] [Google Scholar]
- 76.Wallace HM, Fraser AV, Hughes A. A perspective of polyamine metabolism. Biochem J. 2003;376(Pt 1):1–14. doi: 10.1042/BJ20031327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Harari PM, et al. Polyamine biosynthesis inhibitors combined with systemic hyperthermia in cancer therapy. Int J Radiat Oncol Biol Phys. 1990;19(1):89–96. doi: 10.1016/0360-3016(90)90139-b. [DOI] [PubMed] [Google Scholar]
- 78.Piazza GA, et al. Sulindac sulfone inhibits azoxymethane-induced colon carcinogenesis in rats without reducing prostaglandin levels. Cancer Res. 1997;57(14):2909–15. [PubMed] [Google Scholar]
- 79.Piazza GA, et al. Apoptosis primarily accounts for the growth-inhibitory properties of sulindac metabolites and involves a mechanism that is independent of cyclooxygenase inhibition, cell cycle arrest, and p53 induction. Cancer Res. 1997;57(12):2452–9. [PubMed] [Google Scholar]
- 80.Steinbach G, et al. The effect of celecoxib, a cyclooxygenase-2 inhibitor, in familial adenomatous polyposis. N Engl J Med. 2000;342(26):1946–52. doi: 10.1056/NEJM200006293422603. [DOI] [PubMed] [Google Scholar]
- 81.Levy GN. Prostaglandin H synthases, nonsteroidal anti-inflammatory drugs, and colon cancer. Faseb J. 1997;11(4):234–47. [PubMed] [Google Scholar]
- 82.Niiranen K, et al. Targeted disruption of spermidine/spermine N1-acetyltransferase gene in mouse embryonic stem cells. Effects on polyamine homeostasis and sensitivity to polyamine analogues. J Biol Chem. 2002;277(28):25323–8. doi: 10.1074/jbc.M203599200. [DOI] [PubMed] [Google Scholar]
- 83.Belting M, et al. Tumor attenuation by combined heparan sulfate and polyamine depletion. Proc Natl Acad Sci U S A. 2002;99(1):371–6. doi: 10.1073/pnas.012346499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cracchiolo BM, et al. Eukaryotic initiation factor 5A-1 (eIF5A-1) as a diagnostic marker for aberrant proliferation in intraepithelial neoplasia of the vulva. Gynecol Oncol. 2004;94(1):217–22. doi: 10.1016/j.ygyno.2004.03.018. [DOI] [PubMed] [Google Scholar]
- 85.Love RR, et al. A randomized, placebo-controlled trial of low-dose alpha-difluoromethylornithine in individuals at risk for colorectal cancer. Cancer Epidemiol Biomarkers Prev. 1998;7(11):989–92. [PubMed] [Google Scholar]
- 86.Meyskens FL, Jr., et al. Enhancement of regression of cervical intraepithelial neoplasia II (moderate dysplasia) with topically applied all-trans-retinoic acid: a randomized trial. J Natl Cancer Inst. 1994;86(7):539–43. doi: 10.1093/jnci/86.7.539. [DOI] [PubMed] [Google Scholar]
- 87.Giovannucci E. Modifiable risk factors for colon cancer. Gastroenterol Clin North Am. 2002;31(4):925–43. doi: 10.1016/s0889-8553(02)00057-2. [DOI] [PubMed] [Google Scholar]
- 88.Giovannucci E. Obesity, gender, and colon cancer. Gut. 2002;51(2):147. doi: 10.1136/gut.51.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jacobs ET, Thompson PA, Martinez ME. Diet, gender, and colorectal neoplasia. J Clin Gastroenterol. 2007;41(8):731–46. doi: 10.1097/MCG.0b013e3180338e56. [DOI] [PubMed] [Google Scholar]
- 90.Flossmann E, Rothwell PM. Effect of aspirin on long-term risk of colorectal cancer: consistent evidence from randomised and observational studies. Lancet. 2007;369(9573):1603–13. doi: 10.1016/S0140-6736(07)60747-8. [DOI] [PubMed] [Google Scholar]
- 91.Muto T, Bussey HJ, Morson BC. The evolution of cancer of the colon and rectum. Cancer. 1975;36(6):2251–70. doi: 10.1002/cncr.2820360944. [DOI] [PubMed] [Google Scholar]
- 92.Creaven PJ, Pendyala L, Petrelli NJ. Evaluation of alpha-difluoromethylornithine as a potential chemopreventive agent: tolerance to daily oral administration in humans. Cancer Epidemiol Biomarkers Prev. 1993;2(3):243–7. [PubMed] [Google Scholar]
- 93.Croghan MK, Aickin MG, Meyskens FL. Dose-related alpha-difluoromethylornithine ototoxicity. Am J Clin Oncol. 1991;14(4):331–5. doi: 10.1097/00000421-199108000-00012. [DOI] [PubMed] [Google Scholar]
- 94.Jansen C, et al. An animal model of hearing loss from alpha-difluoromethylornithine. Arch Otolaryngol Head Neck Surg. 1989;115(10):1234–7. doi: 10.1001/archotol.1989.01860340088024. [DOI] [PubMed] [Google Scholar]
- 95.Loprinzi CL, et al. Toxicity evaluation of difluoromethylornithine: doses for chemoprevention trials. Cancer Epidemiol Biomarkers Prev. 1996;5(5):371–4. [PubMed] [Google Scholar]
- 96.Pasic TR, Heisey D, Love RR. alpha-difluoromethylornithine ototoxicity. Chemoprevention clinical trial results. Arch Otolaryngol Head Neck Surg. 1997;123(12):1281–6. doi: 10.1001/archotol.1997.01900120031004. [DOI] [PubMed] [Google Scholar]
- 97.McLaren CE, et al. Longitudinal assessment of air conduction audiograms in a phase III clinical trial of difluoromethylornithine and sulindac for prevention of sporadic colorectal adenomas. Cancer Prev Res (Phila) 2008;1(7):514–21. doi: 10.1158/1940-6207.CAPR-08-0074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zell JA, et al. Associations of a polymorphism in the ornithine decarboxylase gene with colorectal cancer survival. Clin Cancer Res. 2009;15(19):6208–16. doi: 10.1158/1078-0432.CCR-09-0592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zell JA, et al. Risk of cardiovascular events in a randomized placebo-controlled, double-blind trial of difluoromethylornithine plus sulindac for the prevention of sporadic colorectal adenomas. Cancer Prev Res (Phila Pa) 2009;2(3):209–12. doi: 10.1158/1940-6207.CAPR-08-0203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Solomon SD, et al. Cardiovascular risk of celecoxib in 6 randomized placebo-controlled trials: the cross trial safety analysis. Circulation. 2008;117(16):2104–13. doi: 10.1161/CIRCULATIONAHA.108.764530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zell JA, et al. Ornithine Decarboxylase-1 Polymorphism, Chemoprevention With Eflornithine and Sulindac, and Outcomes Among Colorectal Adenoma Patients. J Natl Cancer Inst. 2010 doi: 10.1093/jnci/djq325. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lippman SM. The dilemma and promise of cancer chemoprevention. Nat Clin Pract Oncol. 2006;3(10):523. doi: 10.1038/ncponc0609. [DOI] [PubMed] [Google Scholar]
- 103.Psaty BM, Potter JD. Risks and benefits of celecoxib to prevent recurrent adenomas. N Engl J Med. 2006;355(9):950–2. doi: 10.1056/NEJMe068158. [DOI] [PubMed] [Google Scholar]